Abstract
In preparation for a larger trial, the Water, Sanitation, and Hygiene (WASH) Benefits pilot study enrolled 72 villages and 499 subjects in two closely related randomized trials of WASH interventions in rural western Kenya. Intervention households received hardware and promotion for one of the following: water treatment, sanitation and latrine improvements, handwashing with soap, or the combination of all three. Interventions were clustered by village. A follow-up survey was conducted 4 months after intervention delivery to assess uptake. Intervention households were significantly more likely than controls to have chlorinated stored water (36–60 percentage point increases), covers over latrine drop holes (55–75 percentage point increases), less stool visible on latrine floors (16–47 percentage point reductions), and a place for handwashing (71–85 percentage point increases) with soap available (49–66 percentage point increases). The high uptake in all arms shows that combined interventions can achieve high short-term adoption rates if well-designed.
Introduction
Illness early in life can have long-term effects on child growth and development.1 Water, sanitation, and hygiene (WASH) interventions have been found to reduce diarrheal and respiratory diseases among children, but conclusive evidence on the relative health benefits of these interventions is lacking.2 Furthermore, few studies have evaluated these interventions in combination to assess how benefits might aggregate,3 and few studies have been able to measure objective health outcomes instead of caregiver-reported outcomes.4
The main WASH Benefits study (http://www.washbenefits.net), a multiarm, cluster, randomized, controlled trial presently being conducted in both Bangladesh and Kenya, is designed to address many of the shortcomings just described. The study's scientific objectives are to (1) determine if WASH interventions aid in early child development, (2) determine if the combination of WASH interventions is more beneficial than a single intervention alone, and (3) determine if the combination of WASH interventions plus nutrient supplements is more beneficial than any of the interventions or supplements alone. The complete main trial protocol along with its objectives and rationale have been published separately5; the main study was registered at www.clinicaltrials.gov (NCT01590095 [Bangladesh] and NCT01704105 [Kenya]), and the pilot study was not separately registered. In preparation for the much larger randomized trial, the WASH Benefits project enrolled subjects in two separate but closely aligned pilot cluster, randomized trials of WASH interventions in rural western Kenya.
The scientific objective of the pilot study in Kenya was to determine if we could achieve high uptake of complementary hardware and behavior change interventions designed to be locally appropriate for our study area. An additional objective was to pilot implementation and logistics for our larger study, which is currently underway. To meet these objectives, we spent time developing innovative hardware and focusing on behavior changes that would facilitate habitual behavior by respondents in the larger WASH Benefits trial and provide helpful empiric uptake data to others designing similar studies.
Typically, WASH studies have focused on one type of intervention in each study. Efforts to improve water quality in rural areas have often consisted of point-of-use household water treatment (HWT), because source water quality improvement is often inadequate due to recontamination.6 A common form of HWT is providing household chlorine, which generally has low uptake7 and has been criticized for being unsustainable or ineffective.8–10 The chlorine dispenser, described below, makes chlorine available at the source for point-of-collection treatment, with the goal of making habit formation easier relative to point-of-use chlorination by more cost-effectively providing access to chlorine to the entire community.
Evaluations of sanitation interventions are rare and often have methodological shortcomings, with the exception of one of the first randomized, controlled trials to evaluate sanitation campaigns, which is currently underway.11 Improper disposal of child feces is a potentially important vector for disease because of increased pathogen transmission from contact with sibling's feces, but it has received relatively little attention in the literature.12 We developed a feces scooping tool intended to be easier to use than the common local practice of using a heavy garden hoe. A similar piloting process in Bangladesh found comparable preferences for a similar scooping tool.13 We also developed a plastic slab flooring with tightly fitting lid for the latrine, which helped to improve child safety inside the latrine itself (Van Schoyck G, unpublished data).
It has been shown that handwashing with soap can improve caregiver-reported child health.14,15 However, the best way to achieve consistent handwashing may be different depending on the setting. Tippy-tap handwashing stations have often been used to make access to soap and water convenient when running water is not available, but local preferences and conditions likely matter when considering design.16 With this in mind, we developed a dual tippy-tap,17 with independent levers for soapy and plain water, with the expectation that this could reduce the concern of theft of bar soap, which was reported anecdotally in early piloting as a key barrier to regular handwashing.
It is common practice to have complimentary hardware and behavior change components based on theory and multiple constructs for successful implementation of water, sanitation, hygiene, or combined WASH interventions.18 In addition to the hardware described above, a comprehensive behavior change package using techniques recently found to be effective in India19 was deployed by trained health promoters called intervention assistants (IAs) with the aim of promoting and sustaining behavior change among intervention households.
Materials and Methods
In November of 2011, the WASH Benefits Kenya study enrolled 72 villages and 499 subjects in two separate but closely aligned pilot randomized trials in rural western Kenya for a pre-determined length of 6 months. The pilot was clustered at the village level, because (1) interventions may spill over from neighbors to recipients in terms of both behavior and disease vectors and (2) the behavior change component of the interventions is only feasible at the village level (therefore, it facilitates implementation logistics). However, outcomes and objectives pertain to the individual respondents. The pilot study was both implemented and evaluated by Innovations for Poverty Action (IPA) in Kenya. Approval for the study was obtained from institutional review boards (IRBs) at both the University of California, Berkeley and the Kenyan Medical Research Institute (KEMRI) as well as from all levels of Kenyan government: local, provincial, and national. Informed consent was obtained from all individual participants as well as representatives of clusters (village elders) before randomization.
Study sites and enrollment.
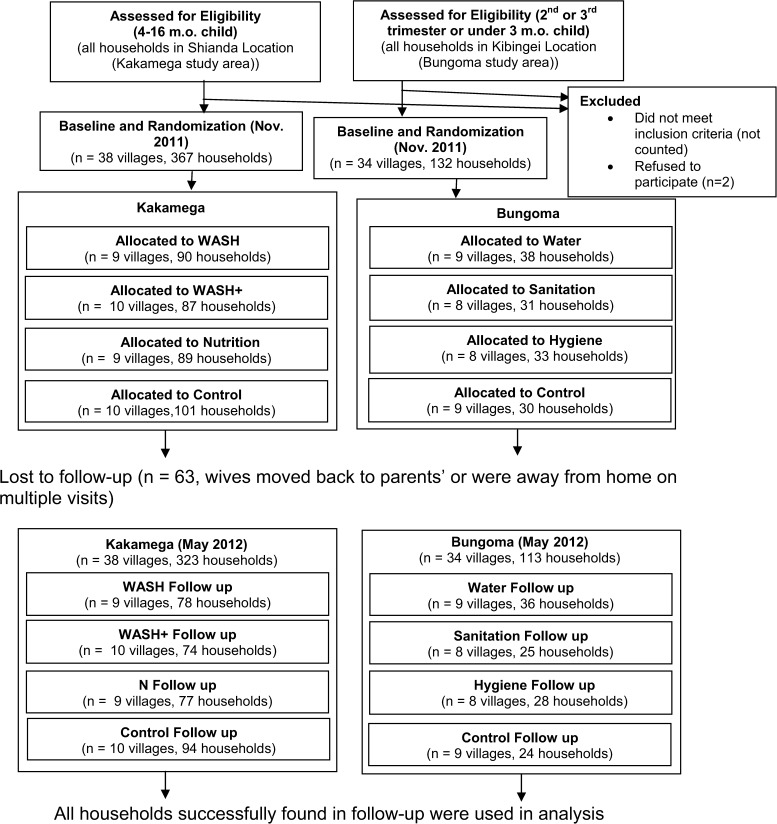
The study simultaneously enrolled respondents into two separate trials: caregivers of 4- to 16-month-old children in the first study area (all 38 villages in Shianda Location near the town of Kakamega) and pregnant women in their second or third trimester and caregivers of children under 3 months of age in the second study area (all 34 villages in Kibingei Location near the town of Bungoma). Age of children and pregnancy status were the only eligibility requirements. We attempted to enroll as respondents all eligible caregivers in the 72 study villages; no changes to eligibility criteria were made after trial commencement. The sample size of two locations (a Kenyan administrative unit) was chosen to allow training and piloting in both of our project offices (in the towns of Bungoma and Kakamega) as opposed to a more deliberate design based on power calculation and predicted effect sizes. The region chosen is agriculturally similar to the rest of western Kenya, with mostly smallholder maize and sugar cane farmers. Several families, often blood relatives, typically live together in a compound with separate housing and cooking structures for each family unit. Because of subtle differences in region as well as age of child eligibility, outcomes compared across study areas should not be interpreted as causal effects of the intervention. Within study area, because interventions were randomized by village, causal interpretation is appropriate.
Each of the study areas had a separate trial, with four possible intervention assignments as described in Figure 1. The Kakamega villages were assigned to (1) combined WASH interventions (WASH), (2) combined WASH and nutrition interventions (WASH+), (3) nutrition intervention (N), or (4) control. Bungoma villages were assigned to (1) water intervention (W), (2) sanitation intervention (S), (3) hygiene intervention (H), or (4) control. Villages were randomized into one of four interventions for their respective region after baseline data collection and enrollment. Separately for each of the two regions, each village was assigned a randomly generated number using Stata, version 12 (Stata Corporation, College Station, TX), and intervention assignments were made to villages in ascending numerical order. Randomization and assignment of clusters to interventions were conducted by G.C., who had no personal ties to any of the villages and had not seen any baseline data at the time of randomization. Assignment of individuals to clusters was done before randomization by having village elders define the boundaries of their village and specify in which village all potentially eligible respondents lived. No stratification or matching was used for randomization. Respondents and survey enumerators could not know intervention assignment at baseline, because the intervention assignment randomization did not take place until after the survey was complete. The nature of the interventions made blinding impossible at follow-up.
Figure 1.
Participant recruitment and follow-up during the WASH Benefits Kenya Pilot, 2011–2012.
Starting in late January of 2012, 2 months after enrollment, WASH interventions were delivered to respondents according to the treatment status of their village. However, delivery of the nutrition interventions (both alone and in combination) was delayed until April of 2012. The nutrition intervention consisted of lipid-based nutrient supplements (LNSs) obtained from Nutriset in France20 and was originally intended to be delivered at the same time as the WASH interventions, but despite approval for all parts of the study from the appropriate IRBs, obtaining Kenyan government approval delayed LNS implementation. Because of this significant delay, most analyses of the Kakamega trial treat the N arm as the same as the control arm and the WASH+ arm as the same as the WASH arm. However, Supplemental Table 1 presents results for each of the Kakamega intervention arms separately.
Interventions.
All interventions were delivered at the village level. All villages, including controls, received at least monthly visits from health promoters trained to promote behavior change specific to their treatment arm, which was facilitated by the interventions that they received. That is, respondents received messaging relating only to the intervention arm to which they were assigned. In addition, promoters weighed mother and child and measured child middle-upper arm circumference (MUAC). Weight and MUAC measurements were collected not as an outcome measure but to give the health promoters in control villages a concrete task that justified repeated household visits.
Health promoters, working to increase behavior change and demand for interventions, were selected from among each village's population and chosen by eligible respondents (who were themselves excluded from selection as health promoters) with some limited input by village elders. They were given a small monthly monetary appreciation of 1,500 Kenyan Shillings for their assistance, which is three-quarters of the salary of official Kenyan community health workers. Health promoters also liaised with IPA to assist in replacing broken or stolen hardware and keep chlorine dispensers full. Control respondent visits contained no messaging concerning WASH and pertained only to monitoring child growth. Thus, comparisons of interventions and controls estimate a treatment effect that does not include the effect of visits by health promoters per se and instead, can be attributed to intervention-related behavior change. Respondents were surveyed again in May of 2012 (4 months after intervention delivery).
Dilute chlorine was selected for the water intervention on the basis of its low cost, local availability, and the research group's prior experience achieving high and sustained take-up in a nearby area during another research project.21 Dilute chlorine, branded as WaterGuard, was widely promoted and distributed by the manufacturer in Kenya (Population Services International, Washington, DC), and therefore, it was familiar to the study population and could be considered an intervention that had already been scaled up. Turbidity is not a major concern in this part of Kenya, where most people rely on groundwater or rainwater for drinking; thus, filters were not seen to have a significant advantage over chlorine. Dilute chlorine is highly effective against bacterial and viral pathogens that cause diarrheal illness (e.g., rotavirus, norovirus, pathogenic Escherichia coli, and Vibrio cholerae); however, it does not inactivate Cryptosporidium parvum, which has been shown to be a cause of moderate to severe diarrhea in a nearby part of Kenya (this knowledge was generated after the interventions were selected for these pilot studies and the main trial).22 In water villages, the intervention consisted of installing chlorine dispensers at respondents' reported water sources within the village (usually a protected spring, well, or other source of groundwater), with an average rate of 3.3 dispensers per water-treated village. One turn of the knob on the chlorine dispenser releases the appropriate amount (3 mL) of 1.25% sodium hypochlorite solution to treat 20 L water. The chlorine dispensers used in the study cost 5,000 Kenyan shillings each (approximately $62.50).
For those households in water-treated villages who were unlikely to benefit from chlorine dispensers in their village (namely those who had piped water or reported that their primary and secondary water sources were located in a different, non-water treatment village), promoters regularly provided 150-mL bottles of WaterGuard-brand chlorine for point-of-use water treatment (each bottle cost 20 Kenyan shillings or approximately $0.25). These conditions applied to 9% of households in the water-treated arms. Interventions in all other arms were delivered only to study households or compounds.
Sanitation compounds received a feces disposal sani-scooper tool akin to a dustpan with a metal paddle (one for each household in the compound, which cost 180 Kenyan shillings each or approximately $2.25), a plastic child potty (one for each household in the compound with a child under 3 years old, which cost 85 Kenyan shillings each or approximately $1.07), and improvements to their existing latrine (consisting of a plastic latrine slab with a built-in drop-hole cover if the latrine floor was not concrete and simple mud walls, roof, and door if not present) or construction of a new latrine if they had none (which cost 1,750 Kenyan shillings or approximately $21.88 for the slab and up to 19,000 Kenyan shillings or approximately $237.50 for a new latrine). Of the recipients in the sanitation villages who had latrines, 27% were scheduled to receive improvements to the latrine door, 25% were scheduled to receive improvements to the latrine floor (this is separate from the plastic slabs and more structural in nature), 9% were scheduled to receive improvements to the latrine roof, and 32% were scheduled to receive improvements to the latrine walls. New latrines constructed by the project had unlined pits. As with all of the interventions, encouraging the use of the latrines and cleaning tools by the health promoter was a major component of the intervention package. It should be noted that latrine construction led to harm befalling a resident of a respondent's compound. A recently constructed pit latrine became unstable during heavy rains and collapsed under the weight of an adult, who sustained a mild leg injury in the fall. The incident was reported to the IRBs.
Hygiene households received two locally manufactured dual tippy-tap handwashing stations: one for near their latrine and one for their cooking area. Each cost 650 Kenyan shillings or approximately $8.13. (Dual indicates two separate pedal-controlled jugs: one with soapy water and one with plain water.) A limited quantity of two small sachets of powdered detergent was provided for the initial soapy water (valued at 5 Kenyan shillings or approximately $0.06). Respondents in nutrition arms were provided with two 10-g sachets of LNS per day for each of their children 6 to 24 months of age, although delivery had only begun a month before the follow-up survey (the LNS produced specifically for this study cost 3 Euro per kilogram or approximately $4.05, and it was not sold commercially). Figure 2 includes pictures of the intervention hardware.
Figure 2.
Intervention hardware.
The engagement strategy for the behavior change component of the interventions targeted first the respondents/primary caregivers of study children with monthly visits and second, their siblings and fathers. Each monthly visit took approximately 40–60 minutes. The behavior change material was comprised of visit scripts that included activities, such as songs, interactive games, and visual aids (calendars, cue cards, and picture sheet). The behavior change messages delivered through these engagements and materials balanced the need to promote the targeted behavior, regardless of the hardware provided and the hardware as a facilitator for the desired behavior. The behavior change component was developed based on theory (health belief model, theory of planned behavior,23 and social cognitive theory24) and formative research by local staff familiar with the study area.25 We identified the need to pilot context-specific constructs in the visit scripts and visual aid materials across intervention arms to provide the basis for targeted behavior change messaging. The constructs piloted in this study included convenience, self-efficacy, perceived benefits, perceived susceptibility, social norms, aspiration, disgust, nurture, and addressing barriers and facilitators.
The water intervention's primary behavior change messages focused on facilitating treatment of drinking water with chlorine at all times and storage in a covered container. Handling was also mentioned to a lesser extent. Because the chlorine dispenser was a novel piece of hardware, there was additional emphasis on convenient use at the point of collection and the prevention of recontamination by chlorination. The sanitation intervention's primary behavior change messages emphasized preventing fecal contamination of the environment and safe removal of feces (human and animal) from the environment facilitated by the potty, sani-scooper, and latrine. The sanitation behavior change messages also focused on contamination pathways, behaviors that could lead to exposure, and motivators and barriers of the targeted behaviors. The hygiene intervention's primary messages emphasized handwashing with soap at critical times defined as after fecal contact (e.g., after defecation and after cleaning a child who has defecated) and before handling food (e.g., before preparing food, eating, or feeding a child). The nutrition intervention's behavior change messages focused on supplementary and complimentary feeding practices, including use of LNS. For all of the intervention arms, the behavior change messages were simple but addressed the complex nature of the exposure pathways and prevention/mitigation of fecal exposure. The combined WASH arm used the same strategies, constructs, and messages as the single arms but had an added emphasis on integration and the synergistic nature of the three interventions. The behavior change messaging will be described in more detail in a forthcoming paper.
Data collection and definition of outcomes.
Primary data were generated from household surveys conducted by trained IPA enumerators in November of 2011 (baseline) and May and June of 2012 (follow-up). Effort was made to keep the survey visits unannounced, although enforcement was almost certainly imperfect. Our primary outcomes were adoption indicators of improved WASH behaviors and all measured at the household level, but they were not pre-specified; a small number (approximately 12) of additional but similar adoption measures that were not reported have been tested and does not change the conclusions of the paper. We measured this in ways that both do and do not specifically require our hardware. The pilot study also collected information about child illness and growth from all study households, but because the pilot study was not powered to compare different arms on these outcomes, the information (aggregated over study arms) was used only to check sample size assumptions for the main randomized trial and is not presented in this article.
For the water intervention, enumerators collected samples of household stored water and tested it for the presence of free and total chlorine using Hach color wheels (Loveland, CO) immediately after leaving the household. We report the detection of at least 0.2 mg/L chlorine. They also collected self-reported information on how, if at all, the water currently stored in the house had been treated. Households in villages with chlorine dispensers were also asked questions to ascertain correct use of the dispenser: self-report of always using the dispenser, the correct number of turns of the chlorine release knob, and whether they reported using the chlorine for other purposes (e.g., for laundry or cleaning). Name and identification of reported primary water sources were matched to our list of sources where dispensers were installed to learn the percentage of households accessing sources with dispensers.
For the sanitation intervention, enumerators asked if respondents believed that contact with feces posed a threat to their health and what the respondent had done, if anything, to dispose of the most recent child defecation. Enumerators observed latrines to record if the drop hole was covered or if stool was visible on the floor. Enumerators also discretely observed the compound to check for human and animal feces. To evaluate use of the sanitation intervention hardware, respondents were asked about use of the sani-scooper (to see if they used it for anything other than feces disposal), and they were also asked to produce the parts (so that the enumerator could observe whether they had been stored together).
For the hygiene intervention, enumerators asked respondents to name the times that they wash their hands; of the critical times (after defecation, before eating, before preparing food, before feeding a child, and after cleaning a child's anus), we counted the number of these responses volunteered by the respondent without prompting. Enumerators asked respondents to show their method of handwashing, and enumerators observed whether the respondent had a dedicated location for handwashing and whether soap was available. Before the handwashing demonstration, the mother and her youngest child's palms and fingerpads were observed for visible dirt. For handwashing hardware adoption, enumerators checked for the presence of water and soapy water at both of the study-installed tippy-taps.
Analysis.
We compared intervention uptake at follow-up in treated households with control households using ordinary least squares regression, with SEs clustered at the village level. All analyses were conducted using the originally assigned intervention status (intention to treat). No subgroup analyses have been undertaken. Both unadjusted and adjusted analyses are presented. Adjusted analyses control for a set of pre-specified baseline characteristics that were possible confounders or could reduce the variability in the outcomes: tin roof ownership, respondent age by quartile, Kiswahili and English literacy, number of households in the compound, and number of children under 3 years old in the compound. For questions that were directly related to our hardware (as opposed to the more general behavior questions described above), we calculated the percentage of treated households reporting a given response. We also investigated the question of loss to follow-up with ordinary least squares analysis by testing for differential attrition by regressing loss to follow-up on indicator variables of treatment assignment.
Results
Baseline.
As shown in Table 1, at baseline, in Kakamega, respondents reported that 20% of children 0–36 months old suffered from diarrhea the week before the survey. Of children under 3 years old, 55% were reportedly being breastfed, and therefore, this may be an overstatement of the population prevalence of diarrhea if healthy breastfed children meet the standard definition of diarrhea. Of children over 18 months (only 4% of whom were being breastfed), 7% were reported to have had diarrhea in the previous week. Only 3% of households reported chlorinating the water currently stored in their home, whereas 12% reported ever treating their water with chlorine. No adults reported open defecation, whereas 55% reported appropriate disposal of child feces. The vast majority of households used a multipurposed basin for handwashing (94%), but only 12% of respondents had soap and water accessible for handwashing.
Table 1.
Characteristics of respondents and households at baseline (mean and SD)
| Variable | WASH | WASH+ | N | Kakamega control | P value (F test) | W | S | H | Bungoma control | P value (F test) |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample size (N) | 90 | 87 | 87 | 101 | 38 | 31 | 32 | 30 | ||
| Respondent age (years) | 26.10 (6.15) | 27.14 (8.07) | 26.19 (7.68) | 25.99 (5.74) | 0.67 | 26.11 (5.49) | 24.26 (5.54) | 25.55 (6.42) | 27.21 (8.81) | 0.37 |
| Kiswahili literacy | 0.80 (0.40) | 0.76 (0.43) | 0.89 (0.32) | 0.83 (0.38) | 0.15 | 0.89 (0.31) | 0.87 (0.34) | 0.76 (0.44) | 0.70 (0.47) | 0.14 |
| English literacy | 0.66 (0.48) | 0.68 (0.47) | 0.82 (0.39) | 0.71 (0.45) | 0.07* | 0.71 (0.46) | 0.65 (0.49) | 0.67 (0.48) | 0.63 (0.49) | 0.91 |
| Any secondary schooling | 0.23 (0.43) | 0.25 (0.44) | 0.27 (0.45) | 0.19 (0.39) | 0.56 | 0.43 (0.50) | 0.32 (0.48) | 0.27 (0.45) | 0.13 (0.35) | 0.06* |
| Own home | 0.99 (0.11) | 0.92 (0.27) | 1.00 (0.00) | 1.00 (0.00) | 0.00† | 0.92 (0.27) | 0.97 (0.18) | 0.94 (0.24) | 0.93 (0.25) | 0.88 |
| Tin roof | 0.44 (0.50) | 0.60 (0.49) | 0.48 (0.50) | 0.47 (0.50) | 0.17 | 0.84 (0.37) | 0.90 (0.30) | 0.88 (0.34) | 0.87 (0.35) | 0.91 |
| Number of households in compound | 1.91 (1.15) | 2.08 (1.26) | 2.24 (1.70) | 2.34 (1.62) | 0.21 | 1.92 (1.71) | 1.77 (1.06) | 1.61 (1.17) | 1.66 (1.23) | 0.77 |
| Latrine in compound | 0.86 (0.35) | 0.83 (0.38) | 0.84 (0.37) | 0.76 (0.43) | 0.32 | 0.70 (0.46) | 0.70 (0.47) | 0.66 (0.48) | 0.67 (0.48) | 0.97 |
| Know/has used ORS | 0.43 (0.50) | 0.36 (0.48) | 0.48 (0.50) | 0.41 (0.49) | 0.39 | 0.47 (0.51) | 0.58 (0.50) | 0.48 (0.51) | 0.40 (0.50) | 0.58 |
| Proper disposal of child feces | 0.52 (0.50) | 0.63 (0.49) | 0.58 (0.50) | 0.48 (0.50) | 0.19 | 0.68 (0.48) | 0.33 (0.48) | 0.23 (0.43) | 0.11 (0.32) | 0.00† |
| Latrine drop hole covered | 0.00 (0.00) | 0.03 (0.16) | 0.03 (0.16) | 0.00 (0.00) | 0.22 | 0.03 (0.18) | 0.04 (0.19) | 0.08 (0.28) | 0.04 (0.20) | 0.85 |
| Chlorinated stored water (SR) | 0.04 (0.21) | 0.01 (0.11) | 0.04 (0.21) | 0.01 (0.10) | 0.26 | 0.05 (0.23) | 0.06 (0.25) | 0.03 (0.17) | 0.03 (0.18) | 0.90 |
| Children sleep under mosquito nets (SR) | 0.99 (0.11) | 0.98 (0.16) | 1.00 (0.00) | 1.00 (0.00) | 0.25 | 0.84 (0.37) | 0.84 (0.37) | 0.78 (0.42) | 0.90 (0.31) | 0.69 |
| Respondent hands: no visible dirt | 0.70 (0.46) | 0.74 (0.44) | 0.69 (0.47) | 0.63 (0.49) | 0.47 | 0.76 (0.43) | 0.65 (0.49) | 0.73 (0.45) | 0.70 (0.47) | 0.75 |
| Youngest child hands: no visible dirt | 0.55 (0.50) | 0.73 (0.45) | 0.63 (0.49) | 0.71 (0.46) | 0.12 | 0.69 (0.48) | 0.67 (0.52) | 0.56 (0.53) | 0.60 (0.51) | 0.92 |
| Total number of children < 3 years old in compound | 1.74 (0.97) | 1.95 (1.40) | 1.88 (1.36) | 2.52 (2.01) | 0.00† | 1.46 (1.30) | 1.39 (1.09) | 1.30 (1.05) | 1.43 (1.45) | 0.96 |
| Age (months) of respondent's youngest child < 3 years old | 9.29 (3.89) | 9.38 (3.67) | 9.65 (4.29) | 9.77 (4.07) | 0.83 | 8.71 (11.79) | 10.37 (11.06) | 11.08 (11.07) | 8.59 (10.65) | 0.86 |
| All of respondent's children < 3 years old (N) | 96 | 102 | 110 | 124 | 30 | 25 | 23 | 24 | ||
| Age (months) | 10.49 (6.01) | 11.84 (7.61) | 12.78 (8.32) | 12.76 (8.42) | 0.11 | 10.64 (11.74) | 13.77 (12.46) | 12.64 (11.97) | 11.32 (11.93) | 0.78 |
| Gender (male) | 0.61 (0.49) | 0.54 (0.50) | 0.50 (0.50) | 0.48 (0.50) | 0.26 | 0.43 (0.50) | 0.64 (0.49) | 0.64 (0.49) | 0.43 (0.51) | 0.25 |
| Fever last week | 0.40 (0.49) | 0.24 (0.43) | 0.26 (0.44) | 0.31 (0.47) | 0.06* | 0.03 (0.16) | 0.14 (0.36) | 0.08 (0.28) | 0.15 (0.36) | 0.26 |
| Diarrhea last week | 0.29 (0.45) | 0.27 (0.45) | 0.19 (0.39) | 0.23 (0.42) | 0.33 | 0.08 (0.28) | 0.06 (0.24) | 0.03 (0.16) | 0.15 (0.36) | 0.26 |
| Diarrhea case definition last week | 0.10 (0.30) | 0.14 (0.35) | 0.08 (0.27) | 0.19 (0.39) | 0.09* | 0.08 (0.28) | 0.03 (0.17) | 0.05 (0.23) | 0.06 (0.24) | 0.82 |
| Blood in stool last week | 0.00 (0.00) | 0.03 (0.17) | 0.02 (0.13) | 0.01 (0.09) | 0.30 | 0.00 (0.00) | 0.03 (0.17) | 0.03 (0.16) | 0.00 (0.00) | 0.58 |
| Cough last week | 0.41 (0.49) | 0.38 (0.49) | 0.25 (0.44) | 0.31 (0.46) | 0.06* | 0.16 (0.37) | 0.23 (0.43) | 0.21 (0.41) | 0.15 (0.36) | 0.80 |
| Congestion last week | 0.55 (0.50) | 0.59 (0.49) | 0.55 (0.50) | 0.52 (0.50) | 0.76 | 0.13 (0.34) | 0.17 (0.38) | 0.30 (0.46) | 0.18 (0.39) | 0.31 |
| Wheezing last week | 0.22 (0.42) | 0.21 (0.41) | 0.15 (0.36) | 0.19 (0.39) | 0.59 | 0.03 (0.16) | 0.00 (0.00) | 0.11 (0.31) | 0.03 (0.17) | 0.12 |
| Rash last week | 0.17 (0.38) | 0.21 (0.41) | 0.14 (0.34) | 0.17 (0.38) | 0.60 | 0.05 (0.23) | 0.03 (0.17) | 0.08 (0.28) | 0.00 (0.00) | 0.38 |
| Bruise last week | 0.03 (0.17) | 0.08 (0.27) | 0.07 (0.26) | 0.03 (0.18) | 0.25 | 0.00 (0.00) | 0.03 (0.17) | 0.00 (0.00) | 0.00 (0.00) | 0.39 |
Cells contain the mean value and SD of the observed variable in a given treatment arm (W, S, H, etc.) or the P value for an F test for joint significance on a set of dummy variables for treatment arms. Values are not adjusted for clustering. All observations are at the household level, except for child age, gender, and 1-week disease recall questions, which are asked about for up to two children under 36 months cared for by the respondent. SR = self-reported data; ORS = oral rehydration salts.
P < 0.1.
P < 0.01.
In Bungoma, only 8% of respondents' children under 3 years old were reported as having diarrhea in the last week. Only 5% of households reported chlorinating the water currently stored, whereas 14% reported ever chlorinating water. Only 34% of respondents with children under 3 years old reported appropriate disposal of child feces, and 29% of respondents had soap and water accessible for handwashing.
Uptake.
Because our primary outcomes in these pilot trials are uptakes of interventions, we first report measures of uptake in several ways that could be reasonably accomplished without the physical hardware provided by IPA and compare these measures between intervention respondents and control respondents at follow-up. Table 2 shows a summary of uptakes of the interventions, which are similar in both unadjusted and adjusted analyses. In the combined treatment arms in Kakamega, intervention households saw an increase in chlorination of household stored water by 36 percentage points (95% confidence interval [95% CI] = 26–47 percentage points), an increase in latrine drop holes covered by 55 percentage points (95% CI = 47–63 percentage points), a decrease in stool visible on the floor of latrines by 16 percentage points (95% CI = −30 percentage points to −2 percentage points), and an increase in soap available for handwashing by 49 percentage points (95% CI = 38–61 percentage points).
Table 2.
Analysis of uptake of WASH interventions and promoter visits—proportional differences from control
| Uptake variable | Kakamega (combined vs. control) | Bungoma (treatment vs. control only) | ||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Water intervention uptake and behavior change | ||||
| Free chlorine detected (95% CI) | 0.364 (0.258, 0.470)* | 0.373 (0.271, 0.474)* | 0.600 (0.336, 0.864)* | 0.554 (0.250, 0.859)* |
| Control mean (N) | 0.012 (284) | 0.012 (284) | 0.143 (56) | 0.143 (53) |
| Total chlorine detected (95% CI) | 0.410 (0.307, 0.513)* | 0.415 (0.315, 0.515)* | 0.667 (0.327, 1.007)* | 0.592 (0.289, 0.894)* |
| Control mean (N) | 0.012 (284) | 0.012 (284) | 0.190 (56) | 0.190 (53) |
| Filter use (95% CI) | −0.066 (−0.137, 0.005) | −0.067 (−0.140, 0.006) | −0.091 (−0.228, 0.046) | −0.046 (−0.129, 0.038) |
| Control mean (N) | 0.122 (304) | 0.122 (303) | 0.091 (57) | 0.091 (54) |
| Sanitation intervention uptake and behavior change | ||||
| Child feces risk belief (95% CI) | 0.054 (−0.042, 0.150) | 0.059 (−0.045, 0.163) | 0.005 (−0.166, 0.176) | −0.003 (−0.262, 0.257) |
| Control mean (N) | 0.785 (321) | 0.785 (321) | 0.875 (49) | 0.875 (47) |
| Child feces disposed (95% CI) | 0.471 (0.372, 0.571)* | 0.478 (0.372, 0.584)* | 0.213 (−0.008, 0.435) | 0.104 (−0.213, 0.420) |
| Control mean (N) | 0.181 (323) | 0.181 (323) | 0.667 (49) | 0.667 (47) |
| Drop hole covered (95% CI) | 0.553 (0.472, 0.634)* | 0.548 (0.470, 0.625)* | 0.750 (0.604, 0.896)* | 0.742 (0.475, 1.010)* |
| Control mean (N) | 0.023 (304) | 0.023 (304) | 0.000 (44) | 0.000 (42) |
| Stool visible in latrine (95% CI) | −0.161 (−0.297, −0.024) | −0.169 (−0.304, −0.034) | −0.400 (−0.632, −0.168) | −0.469 (−0.674, −0.264) |
| Control mean (N) | 0.420 (304) | 0.420 (304) | 0.400 (45) | 0.400 (43) |
| Human feces in compound (95% CI) | −0.074 (−0.132, −0.016) | −0.079 (−0.135, −0.024) | −0.043 (−0.192, 0.106) | −0.043 (−0.317, 0.230) |
| Control mean (N) | 0.106 (317) | 0.106 (316) | 0.083 (49) | 0.083 (47) |
| Any feces in compound (95% CI) | −0.053 (−0.147, 0.040) | −0.085 (−0.186, 0.016) | 0.007 (−0.229, 0.242) | 0.078 (−0.188, 0.345) |
| Control mean (N) | 0.862 (323) | 0.862 (322) | 0.833 (49) | 0.833 (47) |
| Hygiene intervention uptake and behavior change | ||||
| HW critical times (of five; 95% CI) | 0.547 (0.251, 0.843)* | 0.565 (0.301, 0.829)* | 0.494 (−0.348, 1.336) | 0.935 (−0.437, 2.306) |
| Control mean (N) | 2.160 (323) | 2.160 (323) | 2.292 (52) | 2.292 (41) |
| Have place for HW (95% CI) | 0.855 (0.804, 0.905)* | 0.838 (0.777, 0.899)* | 0.708 (0.430, 0.987)* | 0.676 (0.281, 1.072)* |
| Control mean (N) | 0.043 (323) | 0.043 (323) | 0.042 (52) | 0.042 (41) |
| Have soap for HW (95% CI) | 0.493 (0.375, 0.610)* | 0.493 (0.371, 0.615)* | 0.661 (0.444, 0.877)* | 0.614 (0.288, 0.940)* |
| Control mean (N) | 0.191 (323) | 0.191 (323) | 0.125 (52) | 0.125 (41) |
| Mother's hands have no visible dirt (95% CI) | 0.132 (0.035, 0.228)* | 0.128 (0.023, 0.233)* | 0.077 (−0.214, 0.369) | 0.236 (−0.054, 0.526) |
| Control mean (N) | 0.787 (323) | 0.787 (323) | 0.708 (52) | 0.708 (41) |
| Child's hands have no visible dirt (95% CI) | 0.057 (−0.050, 0.164) | 0.072 (−0.037, 0.182) | −0.205 (−0.475, 0.066) | −0.114 (−0.642, 0.414) |
| Control mean (N) | 0.570 (322) | 0.570 (322) | 0.955 (42) | 0.955 (32) |
| Health promoter uptake and behavior change (any treatment vs. control) | ||||
| Respondent knows promoter's name (95% CI) | 0.044 (−0.007, 0.094) | 0.055 (0.003, 0.108)* | 0.087 (−0.074, 0.248) | 0.045 (−0.043, 0.134) |
| Control mean (N) | 0.923 (319) | 0.923 (319) | 0.913 (110) | 0.913 (96) |
| Promoter booklet available (95% CI) | −0.107 (−0.287, 0.074) | −0.106 (−0.293, 0.081) | −0.044 (−0.214, 0.126) | −0.061 (−0.222, 0.100) |
| Control mean (N) | 0.914 (307) | 0.914 (307) | 0.913 (107) | 0.913 (94) |
| Trusts promoter information highly (95% CI) | −0.017 (−0.063, 0.030) | −0.008 (−0.052, 0.035) | −0.027 (−0.120, 0.065) | 0.015 (−0.088, 0.119) |
| Control mean (N) | 0.964 (319) | 0.964 (319) | 0.958 (111) | 0.958 (98) |
| Ranks promoter as highly committed (95% CI) | 0.032 (−0.042, 0.106) | 0.036 (−0.039, 0.112) | 0.089 (−0.121, 0.299) | 0.139 (−0.065, 0.343) |
| Control mean (N) | 0.867 (307) | 0.867 (307) | 0.750 (107) | 0.750 (93) |
| Considers promoter visits worth time (95% CI) | −0.001 (−0.019, 0.017) | 0.002 (−0.014, 0.018) | −0.023 (−0.067, 0.022) | −0.020 (−0.073, 0.033) |
| Control mean (N) | 0.994 (319) | 0.994 (319) | 1.000 (111) | 1.000 (97) |
| Enumerator fixed effects | NO | YES | NO | YES |
| Controls | NO | YES | NO | YES |
Estimated ordinary least squares (OLS) coefficients on intervention status with 95% CIs computed with SEs adjusted for clustering at the village level are shown. Interventions in the Kakamega columns were a combination of WASH hardware. Interventions in the Bungoma columns are the relevant treatments (W, S, or H separately) compared with the control group. HW = handwashing.
Estimates significantly different from zero.
In the single treatment arms in Bungoma, detection of chlorination of household stored water increased by 60 percentage points (95% CI = 34–86 percentage points) in the water arm. Latrine drop holes were covered 75 percentage points more (95% CI = 60 −90 percentage points) and stool was visible on the latrine floor 47 percentage points less (95% CI = −63 percentage points to −17 percentage points) in the sanitation arm. In the hygiene arm, 66 percentage points more of the households had soap for handwashing (95% CI = 44–88 percentage points). Supplemental Table 2 shows uptakes of the water interventions without the households who were given WaterGuard in the home. Point estimates of chlorination rates are slightly higher, but there are no significant differences. Supplemental Table 3 additionally controls for baseline appropriate disposal of child feces, because significant differences existed across intervention arm for this variable, which is seen in Table 1. None of the results change significantly.
Table 2 also shows an analysis of respondents' attitudes regarding visits by the promoter. There are few significant differences between treatment and control arms. In control arms, where promoters had no hardware to promote and no health messages other than monitoring of child growth, all but one caregiver reported that visits were worth their time, and 92% knew the name of their promoter.
In addition to measures of uptake that do not directly depend on IPA's hardware, we also measured use of the hardware itself in relevant intervention arms. Table 3 shows a summary of these results. We see that self-reported use of all of the hardware is high (95–100% for sani-scoopers and up to 65% for using chlorine dispensers every time that water is drawn) and that enumerator-observed indicators of use, although lower, are still high (72–85% for having both soap and water present at either tippy-tap).
Table 3.
Uptake of provided hardware and behavior change
| Variable | Kakamega combined arms (%) | N | Bungoma single arms (%) | N |
|---|---|---|---|---|
| Chlorine dispenser (W) | ||||
| Self-reported always use | 55.6 | 162 | 65.7 | 35 |
| Know correct operation | 91.9 | 149 | 97.1 | 34 |
| Admit to inappropriate use | 7.5 | 120 | 32.1 | 28 |
| Primary source has dispenser | 82.2 | 152 | 77.8 | 36 |
| Sani-scooper (S) | ||||
| Self-reported use | 94.7 | 152 | 100.0 | 25 |
| Parts stored together | 73.4 | 128 | 66.7 | 18 |
| No agricultural use for tool | 87.2 | 164 | 92.0 | 25 |
| Tippy-tap (H) | ||||
| Soap and water at both | 57.4 | 148 | 55.6 | 27 |
| Water at both | 83.8 | 148 | 74.1 | 27 |
| Soap and water at either | 71.6 | 148 | 85.2 | 27 |
Percentage of respondents in the relevant arm reporting behavior change or use of hardware provided by IPA as part of the intervention is shown. The relevant single arms are shown for the Bungoma trial, whereas the combined arms, which received all hardware, are shown for the Kakamega trial.
Loss to follow-up.
Of 499 respondents at baseline, we were able to survey 436 (87%) in repeated attempts at follow-up 6 months later. Attrition in most cases was caused by respondents moving to another village, and we did not track respondents outside the village. Although this level of attrition is cause for concern, we tested for differential attrition across treatment arms and found only limited evidence, which is documented in Table 4. When viewed collectively, subjects in treated arms were no more likely to be lost than those in the control arms. When analyzed separately by region, subjects from the arms promised LNS (WASH+ and N) were 7–8% points more likely to be lost to follow-up.
Table 4.
Loss to follow-up by treatment arm
| Treatment arm | All arms | Kakamega | Bungoma |
|---|---|---|---|
| WASH | 0.034 (0.047) | 0.064 (0.045) | |
| WASH+ | 0.05 (0.038) | 0.080* (0.035) | |
| Nutrition | 0.036 (0.036) | 0.066† (0.034) | |
| Hygiene | 0.052 (0.046) | −0.048 (0.082) | |
| Water | −0.047 (0.036) | −0.147† (0.077) | |
| Sanitation | 0.094 (0.100) | −0.006 (0.122) | |
| Constant | 0.099‡ (0.024) | 0.069‡ (0.020) | 0.200‡ (0.072) |
| F statistic | 2.18 | 2.38 | 2.56 |
| P value | 0.046 | 0.089 | 0.072 |
| Observations | 499 | 367 | 132 |
| R2 | 0.009 | 0.01 | 0.03 |
Indicator variables of attrition (loss to follow-up at main end-line study) regressed on treatment arm with robust SEs clustered by village in parentheses are shown. The excluded groups in the first regression are both control groups, and the appropriate single control group is excluded in the second and third specifications. F statistic indicates the test of all indicators equaling zero.
P < 0.05.
P < 0.1.
P < 0.01.
Discussion
These pilot trials for the WASH Benefits study in western Kenya showed that reasonably high uptake of interventions is attainable when combined with intensive encouragement, even for packages of combined WASH interventions; few randomized studies providing empiric data evaluating such a combined approach in conjunction with single interventions exist.3 A 36 percentage point increase in detection of free chlorine in combined WASH arms compared with the control group and a 60 percentage point increase in the water-only treatment arms are both significant improvements over the status quo. The 17 percentage point reduction in visible stool in the latrine coupled with the 55 percentage point increase in drop holes being covered show that the sanitation interventions were also successfully adopted in the combined WASH arms.
The uptake of our tippy-taps in the hygiene arms is consistent with an explanation that extreme ease of use and habit formation are the keys to adoption. We saw an 86 percentage point increase in having a dedicated location for handwashing in the combined treatment arms, but only a 49 percentage point increase in the presence of soap. Our tippy-tap was designed to become the designated place for handwashing, but we provided only a few small starter packets of detergent that were not expected to last for the duration of the pilot study. Based on lower prevalence of soap in respondents' tippy-taps relative to prevalence of water supply, it seems that restocking the tippy-tap with soap is a barrier to sustained use, and therefore, we decided to regularly provide respondents with the necessary soap for the duration of the main WASH Benefits study. Respondents in the combined arms were observed to have cleaner hands and could report to enumerators more of the critical times for handwashing with soap.
Although we did not randomize different types of hardware within an intervention type (for example, by comparing source chlorine dispensers with filters or one type of tippy-tap with another and analyzing uptake levels across different interventions), we are confident that, by conveniently locating interventions near where they should ideally be used (the chlorine dispenser immediately next to the water source, the tippy-taps next to the latrine and the cooking place, the drop hole cover in the latrine, and the potty someplace easily accessible) along with using locally developed behavior change communication material, we were able to overcome existing psychological barriers and achieve desirable WASH-related habit formation.
One important issue is the practicality of the interventions themselves. A full cost–benefit analysis will be conducted in the main WASH Benefits trial, but it became clear in the pilot that implementation of latrine improvements is a challenging and time-consuming task. Enumerators had to be trained to uniformly evaluate the conditions of latrines while collecting baseline data; then, different staff had to return with the appropriate improvement supplies, and conditions of the latrines had often changed in the intervening 2 months (e.g., the latrine had become full or a door had been removed) or the implementing staff viewed the needs of the latrine differently than the initial survey staff. This was far less of a problem with interventions such as the child potty, which was the same for every eligible respondent. Contracting with local laborers to construct new latrines also required significant time and expenditure. Because of these difficulties, we decided not to improve existing latrine structures in the main WASH Benefits study beyond the installation of the plastic slab flooring. We will dig new latrines for those who do not have a latrine at baseline or whose latrine was likely to fill up before the conclusion of the study. Similarly, determining whether each respondent's water source would be treated with a dispenser and providing them with WaterGuard only when dispenser installation was infeasible were undesirable complications of the water intervention. In the main trial, all water intervention households will receive bottled WaterGuard, and water sources in the village will receive chlorine dispensers.
Limitations.
Because of staffing, time, and cost limitations, we were unable to randomize all intervention arms across both study areas (Kakamega and Bungoma). Training of promoters required the trainees from several villages (all of the villages assigned to a given intervention arm) to travel to a central location, such as a church or small hotel that IPA rented for the day, and we had to minimize the travel distance to maximize attendance and instruction time while keeping costs down. Intervention delivery staff had to be trained to deliver each type of intervention, and we felt that training for all six of the intervention arms in each of our study areas was not attainable. Also, several of our interventions are not intended for infants (e.g., newborns do not defecate into potties and should not be fed LNS). Although this will not be a hindrance in the main WASH Benefits study that will last 2 years, because of the short duration of our pilot study, we chose an eligibility criteria of 4–16 months of age in the Kakamega study area to better assess use of interventions clearly aimed at toddlers, whereas in Bungoma, we used eligibility criteria closer to those that will be used in the main WASH Benefits study (pregnant women in first or second trimester and additionally, for the pilot study, caregivers of children under 3 months of age) to get practice with enrollment protocols. Unfortunately, this means that we cannot compare results in the combined arms (Kakamega) with the results of the single arms (Bungoma) using a straightforward randomized design. The combined arms are all in one study area, whereas the single arms are all in another study area, and one can see from Table 1 that important characteristics are not the same (e.g., tin roof ownership is higher and diarrhea rates are lower in Bungoma). Several measures of uptake (e.g., detection of free chlorine in stored household water increased 60 percentage points compared with 36 percentage points) seem to be higher in the single-treated arm than the combined arms. However, this pattern is not consistent across all interventions (e.g., cleanliness of mother's hands or knowledge of critical times for handwashing), and one should take caution in comparing across the two regions, because the cross-geographical analysis is confounded.
We remain concerned that the combined interventions might reduce intervention uptake, because differential uptake would complicate interpretation of the health effects of single versus combined interventions. Based on this, in the main study, we decided to implement cutoffs for respondents per promoter, above which we would train a second promoter. The cutoffs are lower in combined villages in an attempt to make the amount of work required by a promoter more equal.
Our inability to blind respondents to their treatment status is certainly of interest, but the issue is present in much of the literature, and we are unaware of a practical solution. There is evidence that the manner or frequency of follow-up surveys can affect outomes,26 but any response to the relatively intensive contact with the promoters should be controlled for by the promoter visits in the control arms. Although self-reported outcomes are subject to a number of biases, direct observation can also influence the outcomes of interest. Given the high expense of enumerator travel to field sites, we opted for self-reported outcomes of behaviors coupled with field officer reports of observable conditions (e.g., presence of chlorine in the water, presence of stool in the latrine, and presence of water and soap at the handwashing station) during surveys.
Another concern was our loss to follow-up, which was fairly high for a 6-month study (13%), despite up to three attempts to find each respondent at home. One possible explanation for slightly higher attrition rates in villages assigned to nutrition interventions could be because of disappointment caused by delays in delivery, although in the field, we worked to not overpromise interventions before they were actually available. For the forthcoming larger main study, we are taking the problem of attrition seriously and have changed the exclusion criteria to eliminate those likely to move, such as renters, who, although fairly uncommon in the pilot (3% of the sample), were lost to follow-up at a rate of 45% compared with 14% for respondents who own their home. We are also more strongly engaging our promoters to help track respondents on an ongoing basis rather than waiting to identify them at infrequent survey rounds.
Generalizability.
The interventions and messaging provided in our intervention are relatively intensive and likely beyond the budgets of local governments, which might consider these interventions at scale. However, the materials are comparable with those provided by well-resourced non-governmental organizations (NGOs) in the region. Also, the WASH Benefits trial is an efficacy study, which is intended to provide the first evidence on whether WASH interventions can affect objective outcomes, such as linear growth. As such, the research team deemed it appropriate to use relatively intensive interventions and encouragement, which are not necessarily scalable, to improve the chances that we will have take-up high enough to lead to changes in child growth. The area of Kenya chosen for the study is populated by smallholder sugarcane and maize farmers, and there is plentiful rainfall during most of the year. It is similar to much of western Kenya and may be externally valid for other tropical developing countries with similar characteristics. However, caution should be taken, because differences across our study areas may exist, although they are only 60 km apart. It should also be noted that self-reported open defecation among adults is generally low and that latrine ownership is high (70–80%) in our study areas. Reaction to latrine improvement and child sanitation hardware and behavior change messaging may be significantly different in areas with different levels of access to sanitation facilities and/or adult sanitation behaviors. Also, water sources in our study areas are not very turbid, and reaction to (and appropriateness of) chlorine dispensers may be different in areas where turbidity could interfere with the efficacy of the chlorine. Water availability is also clearly an important determinant of the appropriateness of our handwashing station design.
Despite these limitations, these pilot studies provide evidence that the WASH interventions being used in the main WASH Benefits study are likely to achieve high uptake in the study population. The main study will provide evidence about whether these specific interventions improve child health.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Miles Kirby, George Odhiambo, Christine Stewart, and the Innovations for Poverty Action (IPA) field team for their excellent assistance.
Footnotes
Financial support: This work was funded by a grant to the University of California Berkeley from the Bill and Melinda Gates Foundation.
Authors' addresses: Garret Christensen, CEGA, UC Berkeley, Berkeley, CA, E-mail: garret@berkeley.edu. Holly N. Dentz Department of Nutrition, University of California, Davis, CA, E-mail: hdentz@ucdavis.edu. Clair Null, Mathematica Policy Research, Washington, DC, E-mail: cnull@mathematica-mpr.com. Amy J. Pickering, Department of Civil and Environmental Engineering, Stanford University, Stanford, CA, E-mail: amyjanel@stanford.edu. Tomoé Bourdier, Agricultural and Resource Economics, University of California, Davis, CA, E-mail: bourdier@primal.ucdavis.edu. Benjamin F. Arnold and John M. Colford Jr, Division of Epidemiology, University of California, Berkeley, CA, E-mails: benarnold@berkeley.edu and jcolford@ucberkeley.edu.
References
- 1.Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. Proc Natl Acad Sci USA. 2006;103:498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM., Jr Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 3.Waddington H, Snilstveit B, White H, Fewtrell L. Water, Sanitation and Hygiene Interventions to Combat Childhood Diarrhoea in Developing Countries. International Initiative for Impact Evaluation; 2009. 2009. http://www.3ieimpact.org/media/filer_public/2012/05/07/17.pdf Available at. Accessed August 8, 2014. [Google Scholar]
- 4.Dangour AD, Watson L, Cumming O, Boisson S, Che Y, Velleman Y, Cavill S, Allen E, Uauy R. Interventions to improve water quality and supply, sanitation and hygiene practices, and their effects on the nutritional status of children. Cochrane Database Syst Rev. 2013;8:CD009382. doi: 10.1002/14651858.CD009382.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold BF, Null C, Luby SP, Unicomb L, Stewart CP, Dewey KG, Ahmed T, Ashraf S, Christensen G, Clasen T, Dentz HN, Fernald LCH, Haque R, Hubbard AE, Kariger P, Leontsini E, Lin A, Njenga SM, Pickering AJ, Ram PK, Tofail F, Winch P, Colford JM., Jr Cluster-randomized controlled trials of individual and combined water, sanitation, hygiene, and nutritional interventions in rural Bangladesh and Kenya: the WASH Benefits Study design and rationale. BMJ Open. 2013;3:e003476. doi: 10.1136/bmjopen-2013-003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kremer M, Leino J, Miguel E, Zwane AP. spring cleaning: rural water impacts, valuation, and property rights institutions. Q J Econ. 2011;126:145–205. doi: 10.1093/qje/qjq010. [DOI] [PubMed] [Google Scholar]
- 7.Rosa G, Clasen T. Estimating the scope of household water treatment in low- and medium-income countries. Am J Trop Med Hyg. 2010;82:289–300. doi: 10.4269/ajtmh.2010.09-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter PR. Household water treatment in developing countries: comparing different intervention types using meta-regression. Environ Sci Technol. 2009;43:8991–8997. doi: 10.1021/es9028217. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt W-P, Cairncross S. Household water treatment in poor populations: is there enough evidence for scaling up now? Environ Sci Technol. 2009;43:986–992. doi: 10.1021/es802232w. [DOI] [PubMed] [Google Scholar]
- 10.Makutsa P, Nzaku K, Ogutu P, Barasa P, Ombeki S, Mwaki A, Quick RE. Challenges in implementing a point-of-use water quality intervention in rural Kenya. Am J Public Health. 2001;91:1571–1573. doi: 10.2105/ajph.91.10.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clasen T, Boisson S, Routray P, Cumming O, Jenkins M, Ensink JHJ, Bell M, Freeman MC, Peppin S, Schmidt W-P. The effect of improved rural sanitation on diarrhoea and helminth infection: design of a cluster- randomized trial in Orissa, India. Emerg Themes Epidemiol. 2012;9:7. doi: 10.1186/1742-7622-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gil A, Lanata C, Kleinau E, Penny M. Children's feces disposal practices in developing countries and interventions to prevent diarrheal diseases: a literature review. Environ Health Proj USAID. 2004 http://www.ehproject.org/PDF/Strategic_papers/SR11-Child%20Excreta%20Format.pdf Available at. Accessed February 8, 2014. [Google Scholar]
- 13.Sultana R, Mondal UK, Rimi NA, Unicomb L, Winch PJ, Nahar N, Luby SP. An improved tool for household faeces management in rural Bangladeshi communities. Trop Med Int Health. 2013;18:854–860. doi: 10.1111/tmi.12103. [DOI] [PubMed] [Google Scholar]
- 14.Luby SP, Agboatwalla M, Feikin DR, Painter J, Billhimer W, Altaf A, Hoekstra RM. Effect of handwashing on child health: a randomised controlled trial. Lancet. 2005;366:225–233. doi: 10.1016/S0140-6736(05)66912-7. [DOI] [PubMed] [Google Scholar]
- 15.Greene LE, Freeman MC, Akoko D, Saboori S, Moe C, Rheingans R. Impact of a school-based hygiene promotion and sanitation intervention on pupil hand contamination in western Kenya: a cluster randomized trial. Am J Trop Med Hyg. 2012;87:385–393. doi: 10.4269/ajtmh.2012.11-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devine J. Beyond tippy-taps: the role of enabling products in scaling up and sustaining handwashing. Waterlines. 2010;29:304–314. [Google Scholar]
- 17.Biran A. Enabling Technologies for Handwashing with Soap: A Case Study on the Tippy-Tap in Uganda. World Bank Water and Sanitation Program. 2011. https://wsp.org/sites/wsp.org/files/publications/uganda-tippy-tap-hwws.pdf Available at. Accessed March 6, 2014. [Google Scholar]
- 18.Briscoe C, Aboud F. Behaviour change communication targeting four health behaviours in developing countries: a review of change techniques. Soc Sci Med. 2012;75:612–621. doi: 10.1016/j.socscimed.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Biran A, Schmidt W-P, Varadharajan KS, Rajaraman D, Kumar R, Greenland K, Gopalan B, Aunger R, Curtis V. Effect of a behaviour-change intervention on handwashing with soap in India (SuperAmma): a cluster-randomised trial. Lancet Glob Health. 2014;2:e145–e154. doi: 10.1016/S2214-109X(13)70160-8. [DOI] [PubMed] [Google Scholar]
- 20.Arimond M, Zeilani M, Jungjohann S, Brown KH, Ashorn P, Allen LH, Dewey KG. Considerations in developing lipid-based nutrient supplements for prevention of undernutrition: experience from the International Lipid-Based Nutrient Supplements (iLiNS) Project. Matern Child Nutr. 2013 doi: 10.1111/mcn.12049. doi:10.1111/mcn.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahuja A, Kremer M, Zwane AP. Providing safe water: evidence from randomized evaluations. Annu Rev Resour Econ. 2010;2:237–256. [Google Scholar]
- 22.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 23.Fishbein M, Yzer MC. Using theory to design effective health behavior interventions. Commun Theory. 2003;13:164–183. [Google Scholar]
- 24.Bandura A. Social Foundations of Thought and Action. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 25.US Department of Health and Human Services . Theory at a Glance: A Guide for Health Promotion Practice. Ed National Institutes of Health NIH Publication 05-3896. Washington, DC: National Institutes of Health (NIH); 2005. [Google Scholar]
- 26.Zwane AP, Zinman J, Dusen EV, Pariente W, Null C, Miguel E, Kremer M, Karlan D, Hornbeck R, Gine X, Duflo E, Devoto F, Crepon B, Banerjee A. Being surveyed can change later behavior and related parameter estimates. Proc Natl Acad Sci USA. 2011;108:1821–1826. doi: 10.1073/pnas.1000776108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.