Abstract
We describe the laboratory-confirmed etiologies of illness among participants in a hospital-based febrile illness cohort study in northern Tanzania who retrospectively met Integrated Management of Adolescent and Adult Illness District Clinician Manual (IMAI) criteria for septic shock, severe respiratory distress without shock, and severe pneumonia, and compare these etiologies against commonly used antimicrobials, including IMAI recommendations for emergency antibacterials (ceftriaxone or ampicillin plus gentamicin) and IMAI first-line recommendations for severe pneumonia (ceftriaxone and a macrolide). Among 423 participants hospitalized with febrile illness, there were 25 septic shock, 37 severe respiratory distress without shock, and 109 severe pneumonia cases. Ceftriaxone had the highest potential utility of all antimicrobials assessed, with responsive etiologies in 12 (48%) septic shock, 5 (14%) severe respiratory distress without shock, and 19 (17%) severe pneumonia illnesses. For each syndrome 17–27% of participants had etiologic diagnoses that would be non-responsive to ceftriaxone, but responsive to other available antimicrobial regimens including amphotericin for cryptococcosis and histoplasmosis; anti-tuberculosis therapy for bacteremic disseminated tuberculosis; or tetracycline therapy for rickettsioses and Q fever. We conclude that although empiric ceftriaxone is appropriate in our setting, etiologies not explicitly addressed in IMAI guidance for these syndromes, such as cryptococcosis, histoplasmosis, and tetracycline-responsive bacterial infections, were common.
Introduction
In resource-limited settings that often lack laboratory capacity for the diagnostic evaluation of illnesses, algorithmic patient management by syndrome classification is an important tool for healthcare workers. Such syndromic management approaches were first widely promulgated for children in the Integrated Management of Childhood Illness (IMCI).1–3 In 2011, the World Health Organization (WHO) issued Integrated Management of Adolescent and Adult Illness District Clinician Manual (IMAI).4 Although multiple studies have assessed the performance and impact of IMCI at the district and referral hospital level,5–8 studies are needed to assess the performance and impact of IMAI recommendations.
The IMAI recommended antibacterial therapy for emergency situations is ceftriaxone, or ampicillin plus gentamicin if ceftriaxone is not available. For septic shock and severe respiratory distress (SRD) without shock, IMAI recommends empiric antibacterials. Although substantial guidance is given on the broad differential diagnosis of septic shock, one can assume most providers at district hospitals in resource-limited settings would generally have recourse to the endorsed emergency antibacterial agents, ceftriaxone or ampicillin plus gentamicin. In malaria-endemic areas or in patients who have traveled to malaria-endemic areas, empiric antimalarials are also advised while awaiting the results of a malaria diagnostic test. For severe pneumonia, IMAI recommends empiric ceftriaxone plus one of three recommended macrolides (i.e., azithromycin, clarithromycin, or erythromycin), with ampicillin plus gentamicin offered as an alternative to ceftriaxone and doxycycline or a respiratory fluoroquinolone offered as alternatives to a macrolide. For those suspected or confirmed to have human immunodeficiency virus (HIV) infection who present with severe pneumonia, IMAI also advises empiric treatment of Pneumocystis pneumonia and consideration of pulmonary tuberculosis.
Using patient-level data from an etiology of febrile illness cohort study undertaken among hospitalized adolescents and adults in the Kilimanjaro Region of northern Tanzania,9–11 we sought to describe the laboratory-confirmed infectious etiologies among participants who met IMAI criteria for septic shock, SRD without shock, and severe pneumonia. We then assessed the potential utility of selected antimicrobial agents, including antimicrobials that feature prominently in IMAI guidance, for treatment of these syndromes. In addition, for those enrolled who died in-hospital, we similarly described the laboratory-confirmed etiologies and assessed the potential use of selected antimicrobial agents.
Methods and Materials
Setting.
Moshi (population 184,000) is the administrative center of the Kilimanjaro Region (population 1.6 million) in northern Tanzania.12 It lies 890 m above mean sea level. The annual weather pattern is characterized by a long rainy period March–May and a short rainy period November–December.13 Malaria transmission intensity is low.14 In 2007–2008, the estimated prevalence of HIV infection in the Northern Zone of Tanzania among men and women 15–45 years of age were 1.8% and 3.2%, respectively.15 Kilimanjaro Christian Medical Centre (KCMC) is a consultant referral hospital with 458 inpatient beds serving the Northern Zone of Tanzania. Mawenzi Regional Hospital (MRH) is Kilimanjaro's regional hospital, and has 300 inpatient beds. In 2008, KCMC admitted 22,099 patients and MRH admitted 21,763 patients. Both hospitals are located in Moshi.
Participants and procedures.
Participants were febrile adolescent and adult inpatients at KCMC and MRH who were prospectively enrolled into the fever cohort study from 17 September 2007 through 31 August 2008. The IMAI syndrome definitions were retrospectively applied to identify participants within this cohort who met criteria for septic shock, SRD without shock, and severe pneumonia (see below in “Syndrome criteria”). Adolescent and adult were defined according to IMAI guidance as persons >10 years of age. All patients with oral temperatures at the time of screening ≥ 38.0°C were eligible to participate in the study. Details of study procedures have been previously described.9 In brief, after obtaining informed consent, the following information and procedures were recorded on standardized study case report forms by trained study team clinical officers: the study clinical officers' history and physical examination, including vital signs at time of enrollment; sterile venipuncture; chest radiograph; urine collection; recording of provisional and discharge diagnoses given by hospital clinical team, as classified by the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). The venous blood was apportioned to aerobic blood culture (10 mL for participants ≥ 13 years; 4 mL for participants < 13 years), mycobacterial blood culture (5 mL), complete blood count, examination for blood parasites, HIV antibody testing, and archived aliquots of plasma and serum for further investigations at reference laboratories. Adequate blood volume for blood cultures was defined as the recommended goal volume ± 20%. For those found to be HIV seropositive, CD4-positive T-lymphocyte count and serum cryptococcal antigen titer were measured.
Laboratory methods and case definitions for etiologic diagnoses.
Laboratory methods and the case definitions used to define etiologic diagnoses have been previously described.9–11,16–20 In brief, a confirmed etiologic diagnosis required detection of Plasmodium spp. on peripheral blood smear (≥ 500 trophozoites/μL),10,11 nucleic acid detection in serum (dengue, chikungunya, or flaviviruses),19 serum antigen detection (Cryptococcus neoformans> 1:8),21 urine antigen detection (Histoplasma capsulatum,20 Streptococcus pneumoniae, Legionella pneumophila serogroup 1),10,11 ≥ 4-fold rise between acute and convalescent serology (microscopic agglutination tests [MAT] for Brucella spp.,18 and Leptospira spp.,17 and immunofluorescent antibody [IFA] for Coxiella burnetii, Rickettsia conorii, and Rickettsia typhi),16 or isolation of clinically relevant bacteria, mycobacteria, or fungi from blood culture.10,11 Illness was attributed to tuberculosis based solely on isolation of Mycobacterium tuberculosis complex from blood culture (i.e., bacteremic disseminated tuberculosis),22 and not based on a clinical diagnosis of tuberculosis or positive acid-fast bacilli smear or culture of respiratory specimens, which were not collected as part of the febrile illness cohort study.
For those who died in-hospital without one of the previous laboratory-confirmed diagnoses, U.S. Centers for Disease Control and Prevention (CDC) definitions for probable cases of the following bacterial infections were used, as these participants had typically died before the convalescent serum collection required to meet the confirmed CDC case definition: brucellosis, acute reciprocal antibody titer to Brucella spp. ≥ 160; Q fever, acute reciprocal antibody titer ≥ 128 to C. burnetii phase II antigen; spotted fever group rickettsioses, acute reciprocal antibody titer to R. conorii ≥ 64.23 Probable leptospirosis was defined as an acute MAT reciprocal antibody titer to Leptospira spp. ≥ 800.24,25
Syndrome criteria.
We used IMAI criteria for the three clinical syndromes4 and retrospectively applied them to participants from the febrile illness cohort study using information that was prospectively collected on the standardized study case report forms at the time of enrollment.
Septic shock.
The IMAI defines septic shock as a patient in whom infection is suspected who has systolic blood pressure < 90 mmHg, and one or more of the following: pulse > 100/minute, respiratory rate > 24/minute, temperature < 36.0°C or > 38.0°C.
Severe respiratory distress without shock.
The IMAI defines SRD without shock as a patient with a respiratory rate > 30/minute or oxygen saturation < 90%, systolic blood pressure ≥ 90 mmHg, and suspected pneumonia or acute lung injury in the absence of heart failure. We defined participants with abnormal opacities on chest radiograph as suspected pneumonia or acute lung injury.
Severe pneumonia.
The IMAI advises to suspect the clinical diagnosis of severe pneumonia if a patient has the following: fever or suspected infection, cough, respiratory rate > 30/minute, severe respiratory distress, oxygen saturation < 90%. As the severe pneumonia guidance does not contain composite “and/or” parameters to further define this syndrome, we defined severe pneumonia as fever or suspected infection and cough plus one of the following severity signs—respiratory rate > 30/minute or oxygen saturation < 90% or signs of respiratory distress. The IMAI gives the following guidance on signs of respiratory distress: very fast or very slow respiratory rates; use of accessory muscles to breathe (neck, intercostal, or abdominal muscles); inability to speak in complete sentences; cyanosis; depressed level of consciousness. Applying this guidance to the data systematically collected in our cohort study, we defined signs of respiratory distress as the presence of central cyanosis, chest recession, loss of consciousness, Glasgow Coma Score ≤ 12 for those > 13 years of age, and Blantyre Coma Score ≤ 426 or report of lethargy for those < 13 years of age.
In-hospital deaths.
In-hospital deaths were defined as participants who died at KCMC or MRH during the same hospitalization in which they were enrolled in the febrile illness cohort study.
Antimicrobial susceptibility testing and regimen effectiveness.
Antimicrobial susceptibility testing was performed according to the Clinical and Laboratory Standards Institute (CLSI) (Wayne, PA) guideline, and interpretative criteria for susceptibility to ampicillin, ceftriaxone, erythromycin, and gentamicin were based on CLSI M100-S23, January 2013.27 Effectiveness of macrolides for severe pneumonia was only undertaken for severe pneumonia cases caused by S. pneumoniae, as erythromycin susceptibility testing was performed on S. pneumoniae isolates. Susceptibility testing to azithromycin, doxycycline, and respiratory fluoroquinolones was not performed. For illnesses attributable to C. neoformans or H. capsulatum, susceptibility to amphotericin was presumed.28,29 For illnesses attributable to Leptospira spp., ampicillin and ceftriaxone susceptibility were presumed.30 Susceptibility to a tetracycline was presumed for R. conorii and C. burnetii.31–33 The potential use of the three IMAI-recommended macrolides was not assessed for severe pneumonia cases associated with R. conorii or C. burnetii because of heterogeneous or limited data on macrolide therapy for these organisms.34#x2013;37 Analysis of respiratory fluoroquinolones for participants with severe pneumonia was not undertaken as our laboratory did not perform antimicrobial susceptibility testing for these agents. In cases of S. pneumoniae infection detected by urine antigen testing, susceptibility to ceftriaxone, ampicillin, gentamicin, and erythromycin were extrapolated from antimicrobial susceptibility testing of S. pneumoniae bloodstream isolates from study participants.
An antimicrobial agent was considered to have potential utility for an etiologic pathogen based on antimicrobial susceptibility testing or presumed susceptibility as outlined previously. At times we also refer to potential utility as an etiologic pathogen that would be responsive to that agent. Empiric IMAI therapies evaluated included IMAI emergency antibacterials (ceftriaxone, or ampicillin plus gentamicin) for septic shock and SRD without shock, and ceftriaxone plus a macrolide for severe pneumonia. Potential utility of amphotericin refers to etiologic diagnoses of cryptococcosis or histoplasmosis. Potential utility of tetracylines refers to bacterial zoonoses that would not respond to ceftriaxone or ampicillin plus gentamicin and for which a tetracycline is considered first-line therapy; namely, Q fever, typhus group rickettsioses, and spotted fever group rickettsioses. Potential utility of anti-tuberculosis therapy refers to standard initial four-drug regimen of isoniazid, rifampin, ethambutol, and pyrazinamide for the treatment of tuberculosis.
Statistical analysis.
Data analysis was descriptive, mainly presented in the form of proportions. For these proportions, the denominator used was the number of participants who met the criteria for the syndrome of interest, not the number of participants within each syndrome who had a diagnostic result.
Ethics statement.
This study was approved by the KCMC Research Ethics Committee, the Tanzania National Institute for Medical Research National Research Ethics Coordinating Committee, and an Institutional Review Board of Duke University Medical Center. Informed consent was obtained from all participants or from the participant's parent/guardian. The United States Department of Health and Human Services human subjects research guidelines were followed.
Results
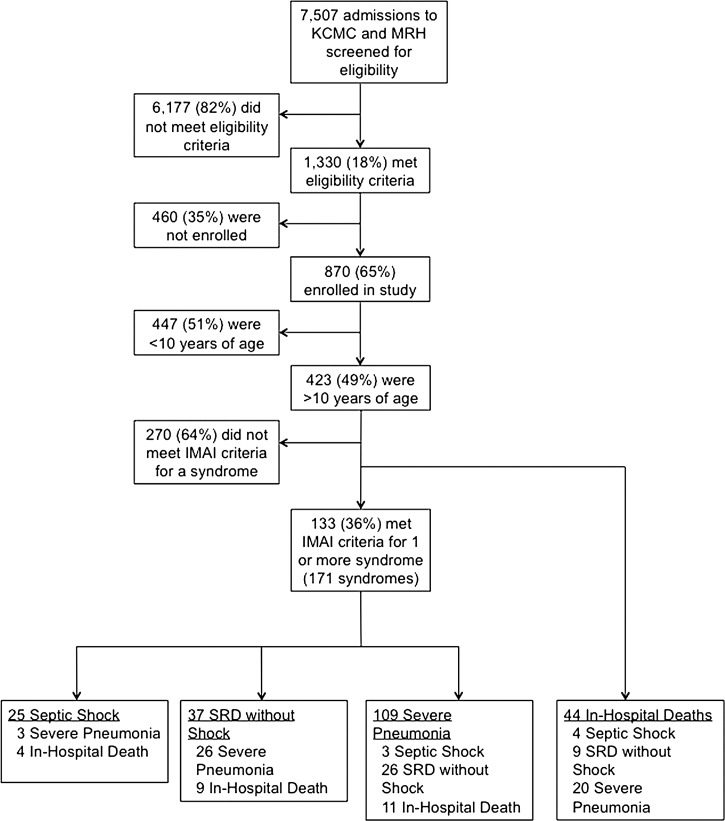
Participant screening, enrollment, and the number who met criteria for the three syndromes of interest, and the number who died in-hospital are shown in Figure 1 . Of the 423 participants >10 years of age, 133 (31%) met the IMAI criteria for ≥ 1 of the three clinical syndromes. Among these 133, the median age was 34 (range 10–95) years, 84 (55%) were female and 66 (50%) were HIV-infected, 26 (39%) of which were newly diagnosed at the time of enrollment. Thirty-eight (29%) of 133 met criteria for more than one of the three syndromes (Figure 1), for a total number of 171 syndrome cases: 25 septic shock, 37 SRD without shock, and 109 severe pneumonia cases. Forty-four (10%) of the 423 participants > 10 years of age died in-hospital. Thirty-one (70%) of the 44 deaths occurred among HIV-infected persons, and 24 of the deaths occurred in participants who met criteria for ≥ 1 of the three syndromes. Clinical characteristics of participants with these three syndromes and those who died in-hospital are presented in Table 1. Breadth of diagnostic evaluation achieved among participants in these groups is presented in Table 2.
Figure 1.
Study flow diagram for etiologies of illness among patients meeting Integrated Management of Adolescent and Adult Illness District Clinician Manual criteria for severe infections. KCMC = Kilimanjaro Christian Medical Centre; MRH = Mawenzi Regional Hospital.
Table 1.
Clinical characteristics among participants meeting Integrated Management of Adolescent and Adult Illness criteria for septic shock, severe respiratory distress without shock, severe pneumonia, and among those who died in-hospital of febrile illness, Moshi, Tanzania 2007–2008.
| Septic shock (N = 25) | Severe respiratory distress without shock (N = 37) | Severe pneumonia (N = 109) | In-hospital death (N = 44)* | |
|---|---|---|---|---|
| Age, years | 38.4 [30.7–45.7] | 35.9 [31.2–46.5] | 34.4 [25.2–44.3] | 39.0 [33.4–46.7] |
| Female (%) | 11 (44) | 18 (48) | 55 (50) | 22 (50) |
| Duration of illness, days | 7 [3–15] | 14 [7–28] | 7 [4–18] | 14 [7–38] |
| Died in-hospital (%) | 4 (16) | 9 (24) | 20 (18) | − |
| HIV-infected (%) | 14 (56) | 26 (70) | 54 (49) | 31 (70) |
| CD4+T-cells < 200 cells/μL | 9 | 20 | 35 | 28 |
| CD4+T-cells < 350 cells/μL | 13 | 22 | 44 | 29 |
| Antiretroviral therapy | 4 | 7 | 15 | 12 |
| Trimethoprim- sulfamethoxazole prophylaxis | 4 | 9 | 16 | 10 |
| Prior antimalarial (%) | 9 (36) | 16 (43) | 39 (35) | 17 (39) |
| Prior antibacterial (%) | 9 (36) | 19 (51) | 50 (45) | 24 (55) |
| Prior antifungal (%) | 2 (8) | 0 (0) | 3 (2) | 7 (16) |
| Prior anti-tubercular (%) | 1 (4) | 6 (16) | 8 (7) | 6 (14) |
| White blood cells, ×109 cell/L | 6.5 [4.9–8.4] | 5.8 [3.4–13.2] | 8 [4.5–13.8] | 8.1 [4.2–11.8] |
| Hemoglobin, g/dL | 10.5 [8.3–12.1] | 10.3 [8.1–12.5] | 11 [9.1–12.8] | 8.8 [7.9–11.7] |
| Platelets, ×109 cell/L | 173 [97–213] | 243 [163–305] | 228 [162–285] | 182 [82–316] |
Note: Among those who met criteria for severe respiratory distress without shock and those who met criteria for severe pneumonia, 31 of 37 and 74 of 109, respectively, received a clinical diagnosis of lower respiratory tract infection by the non-study staff clinical providers.
Median [inter-quartile range] unless otherwise indicated.
The in-hospital death group includes 24 deaths among participants with septic shock, severe respiratory distress without shock, and severe pneumonia, plus an additional 20 in-hospital deaths among study participants who did not meet criteria for these three IMAI syndromes.
Table 2.
Laboratory evaluations completed among participants meeting Integrated Management of Adolescent and Adult Illness criteria for septic shock, severe respiratory distress without shock, severe pneumonia, and among those who died in-hospital of febrile illness, Moshi, Tanzania 2007–2008
| Laboratory evaluations | Septic shock (N = 25) | Severe respiratory distress without shock (N = 37) | Severe pneumonia (N = 109) | In-hospital death (N = 44) |
|---|---|---|---|---|
| Microscopy | ||||
| Malaria blood film (%) | 25 (100) | 37 (100) | 108 (99) | 43 (98) |
| Nucleic acid detection | ||||
| Chikungunya PCR (%) | 22 (88) | 34 (92) | 98 (90) | 41 (93) |
| Dengue PCR (%) | 22 (88) | 34 (92) | 99 (90) | 41 (93) |
| Flaviviruses PCR (%) | 22 (88) | 34 (92) | 99 (90) | 41 (93) |
| Antigen detection | ||||
| C. neoformans (%)* | 14 (100) | 25 (96) | 53 (98) | 29 (94) |
| H. capsulatum (%) | 20 (80) | 25 (68) | 84 (77) | 30 (68) |
| L. pneumophila (%) | 20 (80) | 26 (70) | 84 (77) | 30 (68) |
| S. pneumoniae (%) | 20 (80) | 26 (70) | 72 (66) | 29 (66) |
| Acute and convalescent antibody detection | ||||
| Brucella spp. MAT (%) | 14 (56) | 16 (43) | 51 (47) | 4 |
| Leptospira spp. MAT (%) | 15 (60) | 16 (43) | 51 (47) | 4 |
| C. burnetii IFA (%) | 14 (56) | 16 (43) | 53 (49) | 4 |
| R. conorii IFA (%) | 14 (56) | 16 (43) | 53 (49) | 4 |
| R. typhi IFA (%) | 14 (56) | 16 (43) | 53 (49) | 4 |
| Blood culture isolation† | ||||
| Adequate blood culture volume (%) | 22 (88) | 31 (84) | 85 (78) | 36 (82) |
| Adequate mycobacterial blood culture volume (%) | 11 (44) | 17 (46) | 48 (44) | 21 (48) |
For C. neoformans antigen testing, percentage was derived using number of HIV-infected within each group as the denominator.
Adequate blood culture volume defined as recommended volume ± 20%.
PCR = polymerase chain reaction; MAT = microscopic agglutination test; IFA = immunofluorescent antibody.
The confirmed etiologies of febrile illness for each of the three syndromes and for in-hospital deaths are shown in Table 3. The proportion of participants without a diagnosis was 36%, 62%, 63%, and 59% for septic shock, SRD without shock, severe pneumonia, and in-hospital death, respectively. Only one participant had laboratory-confirmed malaria; this participant met criteria for severe pneumonia. No confirmed cases of dengue, flavivirus, brucellosis, typhus group rickettsioses, or legionellosis were found among participants with these three syndromes or among those who died in-hospital. Among the in-hospital deaths, six participants who had no confirmed cause of death identified met criteria for probable acute spotted fever group rickettsioses, and two met criteria for probable leptospirosis.
Table 3.
Confirmed etiologic diagnoses among participants meeting Integrated Management of Adolescent and Adult Illness criteria for septic shock, severe respiratory distress without shock, severe pneumonia, and among those who died in-hospital of febrile illness, Moshi, Tanzania 2007–2008
| Septic shock (N = 25) | Severe respiratory distress without shock (N = 37) | Severe pneumonia (N = 109) | In-hospital death (N = 44) | |
|---|---|---|---|---|
| No. participants ≥ 1 diagnosis | 16 | 14 | 40 | 18 |
| No. diagnoses | 18 | 16 | 45 | 20 |
| Malaria | 0 | 0 | 1 | 0 |
| Chikungunya | 1 | 1 | 6 | 6 |
| Dengue | 0 | 0 | 0 | 0 |
| Flaviviruses | 0 | 0 | 0 | 0 |
| Invasive cryptococcosis | 2 | 2 (1 by antigen) | 5 (4 by antigen) | 5 (3 by antigen) |
| Histoplasmosis | 0 | 3 | 3 | 0 |
| Brucellosis | 0 | 0 | 0 | 0 |
| Legionellosis | 0 | 0 | 0 | 0 |
| Leptospirosis | 2 | 1 | 2 | − |
| Q fever | 0 | 1 | 6 | − |
| Spotted fever group rickettsioses | 2 | 2 | 2 | − |
| Typhus rickettsioses | 0 | 0 | 0 | 0 |
| Invasive pneumococcus | 5 (4 by antigen) | 3 (all by antigen) | 11 (6 by antigen) | 3 |
| Bacteremic disseminated tuberculosis | 1 | 2 | 3 | 2 |
| Other bacteremia | 5 (2 E. coli, S. aureus, S. enterica non-typhoidal, Neisseria spp.) | 1 (K. pneumoniae) | 6 (3 S. enterica Typhi, 2 E. coli, K. pneumoniae) | 4 (3 E. coli, K. pneumoniae) |
Note: Two participants with septic shock had > 1 diagnosis: spotted fever group rickettsioses and invasive pneumococcus by urine pneumococcal antigen; invasive E. coli and S. aureus infections.
Two participants with severe respiratory distress had > 1 diagnosis: leptospirosis and spotted fever group rickettsioses; histoplasmosis and spotted fever group rickettsioses.
Four participants with severe pneumonia had > 1 diagnosis: invasive pneumococcus by blood culture and acute chikungunya; acute chikungunya and leptospirosis; acute chikungunya and Q fever; leptospirosis and spotted fever group rickettsioses; histoplasmosis and spotted fever group rickettsioses.
Two participants who died in-hospital had > 1 diagnosis: invasive pneumococcus by blood culture and acute chikungunya; invasive E. coli and acute chikungunya.
For participants who met criteria for the three syndromes or who died in-hospital, 29 had their etiologic diagnosis established by isolation of a bacterial, mycobacterial, or fungal isolate from blood culture (Table 3). Of the 20 bacteria isolated from the blood of these participants, all were susceptible to ceftriaxone. Six were susceptible to ampicillin, five were susceptible to gentamicin, and accordingly 11 (55%) of 20 invasive bacterial isolates were susceptible to the combined regimen of ampicillin plus gentamicin. Upon including those with a positive urine antigen for S. pneumoniae (N= 9), susceptibility to ampicillin plus gentamicin (based on extrapolation from antimicrobial susceptibilities of S. pneumoniae) was 16 (55%) of 29 invasive bacterial infections. Five (83%) of the six S. pneumoniae isolates were susceptible to erythromycin; and by extrapolation from antimicrobial susceptibilities 12 (80%) of 15 S. pneumoniae infections were susceptible to erythromycin.
Table 4 groups the confirmed etiologic diagnoses by potential utility of selected empiric therapies. The potential utility of selected empiric therapies among the subset of HIV-infected persons within each group is shown in Table 5.
Table 4.
Potential utility of antimicrobial agents for laboratory-confirmed etiologic diagnoses among participants meeting Integrated Management of Adolescent and Adult Illness criteria for septic shock, severe respiratory distress without shock, severe pneumonia, and among those who died in-hospital of febrile illness, Moshi, Tanzania 2007–2008
| Septic shock (N = 25) | Severe respiratory distress without shock (N = 37) | Severe pneumonia (N = 109) | In-hospital death (N = 44) | |
|---|---|---|---|---|
| Ceftriaxone utility (%) | 12 (48) | 5 (14) | 19 (17) | 6 (14) |
| Ampicillin + gentamicin utility (%)* | 7 (29) | 4 (11) | 11 (10) | 3 (7) |
| Tetracyclines utility (%) | 2 (8) | 3 (8) | 8 (7) | −† |
| Amphotericin utility (%) | 2 (8) | 5 (14) | 8 (7) | 5 (11) |
| Anti-tuberculosis therapy utility (%) | 1 (4) | 2 (5) | 3 (3) | 2 (5) |
Note: Denominator for percentage (%) derived from total number of patients within each group, not from total number of diagnoses.
Macrolide for severe pneumonia did not increase the number of diagnoses responsive to empiric IMAI recommendations beyond the effectiveness already achieved by ceftriaxone (i.e., the addition of a macrolide would have conferred no additional effective coverage against the diagnoses elucidated). For bacterial blood isolates from the participants who met criteria for the three syndromes or died in-hospital (enumerated in Table 3), all 20 isolates were susceptible to ceftriaxone.
Ampicillin/gentamicin utility calculation includes participants with invasive pneumococcal disease diagnosed by urine antigen and susceptibility of these cases to ampicillin is extrapolated from the study antibiogram (57% of pneumococcal blood isolates were susceptible to ampicillin).
Convalescent serology required to diagnose Q fever, spotted fever group rickettsioses, therefore no confirmed cases among the In-hospital death group.
Table 5.
Potential utility of antimicrobial agents for laboratory-confirmed etiologic diagnoses among HIV-infected participants meeting Integrated Management of Adolescent and Adult Illness criteria for septic shock, severe respiratory distress without shock, severe pneumonia, and among those who died in-hospital of febrile illness, Moshi, Tanzania 2007–2008
| Septic shock (N = 14) | Severe respiratory distress without shock (N = 26) | Severe pneumonia (N = 54) | In-hospital death (N = 31) | |
|---|---|---|---|---|
| Ceftriaxone utility (%) | 9 (64) | 3 (12) | 9 (17) | 6 (19) |
| Ampicillin + gentamicin utility (%)* | 7 (50) | 4 (15) | 7 (13) | 2 (6) |
| Tetracylines utility (%) | 0 | 2 (8) | 5 (9) | −† |
| Amphotericin utility (%) | 2 (14) | 5 (19) | 8 (15) | 5 (16) |
| Anti-tuberculosis therapy utility (%) | 1 (7) | 2 (8) | 3 (6) | 2 (7) |
NOTE: Denominator for percentage (%) derived from total number of patients within each group, not from total number of diagnoses.
Ampicillin/gentamicin utility calculation includes participants with invasive pneumococcal disease diagnosed by urine antigen and susceptibility of these cases to ampicillin is extrapolated from the study antibiogram (57% of pneumococcal blood isolates were susceptible to ampicillin).
Convalescent serology required to diagnose Q fever, spotted fever group rickettsioses, therefore no confirmed cases among the In-hospital death group.
Discussion
Among adolescents and adults enrolled in a fever etiology cohort study in northern Tanzania who met IMAI criteria for septic shock, SRD without shock, or severe pneumonia, we found that ceftriaxone would provide appropriate empiric coverage for a substantial proportion of etiologic diagnoses. Yet many of the etiologic diagnoses from our febrile illness cohort study would not be responsive to IMAI emergency antimicrobials such as ceftriaxone, but responsive to additional agents that are readily available in most resource-limited settings, such as anti-cryptococcal therapies, anti-tuberculosis therapies, and tetracyclines. Although our analysis was retrospective and our study small, to our knowledge, it is the first analysis to use patient-level data from a resource-limited setting to describe the etiologies of illness for these IMAI-defined severe infectious syndromes and assess the implications for treatment of septic shock, SRD without shock, and severe pneumonia.
For septic shock, SRD without shock, and severe pneumonia we found that 5 (20%) of 25, 10 (27%) of 37, and 19 (17%) of 109 participants, respectively, would have benefitted from additional antimicrobial therapy directed against C. neoformans, H. capsulatum, M. tuberculosis complex, and tetracycline-responsive bacterial pathogens, such as Rickettsia spp. and C. burnetii. Among those who died in-hospital, 7 (16%) of 44 would have likewise benefitted from amphotericin, anti-tuberculosis, or tetracycline therapy. The IMAI encourages providers to consider tuberculosis early in all three clinical syndromes we analyzed. Furthermore, IMAI lists doxycycline as an alternative agent to macrolide therapy in the treatment of severe pneumonia. Yet considerations for tetracycline-responsive conditions in patients with septic shock and SRD without shock are not explicitly provided in the algorithms for these two syndromes. Similarly, although detailed consideration and algorithmic approaches are given for cryptococcal disease in patients presenting with signs or symptoms of meningitis, empiric therapy for C. neoformans is not discussed for meningitis and C. neoformans, a common pathogen in the three syndromes we analyzed, is not addressed in the septic shock, SRD without shock, or severe pneumonia sections of IMAI.
Our analysis indicates that HIV-infected persons stand to benefit the most from expanded empiric therapy early in their management, and our findings support IMAI recommendations for early HIV testing as part of most management algorithms. Our results are also hypothesis-generating for potential clinical interventions that may warrant evaluation in controlled trials in resource-limited settings, specifically whether empiric tetracyclines would improve outcomes for patients admitted with these severe syndromes or whether empiric amphotericin or anti-tuberculosis therapy would impact outcomes for HIV-infected persons admitted with these severe syndromes. Our finding of a substantial proportion of severe disease caused by cryptococcosis and histoplasmosis highlights the importance of amphotericin, an agent not currently available in our setting despite being the IMAI-recommended first-line therapy for these infections.
Although expanded empiric therapy for severe infections among adults and adolescents in resource-limited settings represents an important area for further study, our results also highlight the need for accessible, high-performing diagnostics to inform early diagnosis and treatment of bacteremic disseminated tuberculosis, endemic mycoses, and bacterial zoonoses. Laboratory-confirmed diagnosis of leptospirosis, Q fever, and spotted fever group rickettsioses still relies upon acute and convalescent serologic evaluation, often performed only at regional, national, or supranational laboratories. Six participants who died in the hospital without a confirmed etiology met the definition for probable spotted fever group rickettsioses and two met the definition for probable leptospirosis, indicating the urgent need for sensitive and specific assays capable of diagnosing these infections in the acute phase of illness. An additional diagnostic consideration arising from our analysis is the high number of participants without a laboratory-confirmed diagnosis—36%, 62%, 63%, and 59% of those with septic shock, SRD without shock, severe pneumonia, and in-hospital death, respectively. Our findings add to the work of others highlighting the knowledge gap on the causes of severe infectious syndromes in resource-limited settings.38–41
Although the etiologic diagnoses elucidated in our cohort indicate a substantial proportion were pathogens that would not be responsive to ceftriaxone, among the agents we assessed for each clinical syndrome no agent would provide better empiric coverage than ceftriaxone. Additionally, as our analysis was retrospective and as none of the diagnostic tests performed is 100% sensitive, our results likely underestimate the proportion of patients with an etiologic diagnosis that was not detected but nonetheless would be responsive to ceftriaxone. In the absence of prospective studies and more complete knowledge on the microbiologic etiologies of septic shock, SRD without shock, and severe pneumonia, empiric therapy with ceftriaxone (the first-line IMAI emergency anti-bacterial) remains highly warranted. It is therefore reassuring that our antimicrobial susceptibility testing of invasive bacterial isolates showed 100% susceptibility to ceftriaxone. By contrast, ampicillin plus gentamicin combined had only 55% susceptibility, rendering this regimen a poor therapeutic choice for these three syndromes in our setting.
Our analysis has a number of limitations. The IMAI is directed to care at the level of district hospitals, while the data we present are from a cohort study conducted in regional and zonal referral hospitals. A similar febrile illness cohort study conducted at the district hospital level might yield different etiologic results and show fewer gaps in IMAI empiric therapy recommendations than our study. As the intensity of malaria transmission is low14 and HIV prevalence among adults < 5%15 in our setting, our findings may not be generalizable to settings in sub-Saharan Africa with a higher burden of malaria or HIV. Since the time of this study, the proportion of Tanzanian persons living with HIV who are receiving anti-retroviral therapy has increased 2-fold.42,43 Accordingly, a contemporary study in our two hospitals might yield different etiologic results from those elucidated in 2007–2008, as opportunistic infections and severe immunosuppression may now be less common in our setting. Although we evaluated etiologies in patients meeting IMAI criteria for severe pneumonia, this was not a pneumonia cohort, and we did not collect diagnostic respiratory specimens. Paired acute and convalescent sera for diagnosis of bacterial zoonoses were not available for all participants, especially decedents, so additional cases of bacterial zoonoses may have gone undiagnosed. We were unable to appraise use of respiratory fluoroquinolones for severe pneumonia as antimicrobial susceptibility testing for these agents was not performed as part of our study. Our analysis was retrospective and inclusion into one of the three clinical syndromes was based on vital signs at the time of enrollment such that cases of septic shock, SRD without shock, or severe pneumonia may have been present but resolved prior to enrollment or developed after enrollment in the study. A contemporary prospective study would more accurately capture and categorize patients who meet IMAI criteria for these syndromes. Finally, although our analysis has focused on specific emergency treatment algorithms in Volume 1 of the IMAI District Clinician Manual, IMAI encourages clinicians to supplement the guidance in these algorithms with more detailed and specific information for various syndromes and diseases found in Volume 2. Although our results may indicate certain gaps in IMAI guidance for the emergency treatment of these syndromes in sub-Saharan African settings, the converse argument is that a worldwide guideline such as IMAI cannot be expected to account for every severe infectious etiology in every precinct, and the target audience of clinicians should always strive to tailor these guidelines to their local epidemiology and use the IMAI guidelines as one among many tools that inform their clinical decision-making.
Despite the retrospective design and the relatively small number of cases for each group, our analysis has generated important findings that should inform future, larger and prospective investigations of IMAI district hospital guidance for these three syndromes. In conclusion, in this analysis of participants from a febrile illness cohort who met IMAI criteria for septic shock, SRD without shock, and severe pneumonia, of the agents assessed we found that empiric ceftriaxone would be the single-most effective agent for these syndromes. Yet we found a substantial proportion of infectious etiologies that would not respond to IMAI empiric antimicrobial recommendations. Prospective studies are needed to confirm our findings and to inform management guidelines for these three syndromes in resource-limited settings.
ACKNOWLEDGMENTS
We are grateful to the leadership, clinicians, and patients of KCMC and MRH for their participation in this research. The authors thank Ahaz T. Kulanga and Charles Muiruri for providing administrative support for the study; Pilli M. Chambo, Beata V. Kyara, Beatus A. Massawe, Anna D. Mtei, Godfrey S. Mushi, Lilian E. Ngowi, Flora M. Nkya, and Winfrida H. Shirima for reviewing and enrolling study participants; Evaline M. Ndosi and Enock J. Kessy for managing data entry; Gertrude I. Kessy, Janeth U. Kimaro, Edward Singo, and Bona K. Shirima for managing patient follow-up. We acknowledge the Hubert-Yeargen Center for Global Health at Duke University for providing critical infrastructure support for the Kilimanjaro Christian Medical Centre-Duke University Collaboration.
Disclaimer: The sponsors of the study had no role in the study design, data collection, data analysis, interpretation or writing of the manuscript.
Footnotes
Financial support: Funding support received for this work includes the International Studies on AIDS Associated Co-infections (ISAAC) award, a United States National Institutes of Health (NIH) funded program (U01 AI062563). Investigator support was received from NIH awards ISAAC (to J.A.C., V.P.M., J.A.B.); AIDS International Training and Research Program (D43 PA-03-018 to J.A.C., V.P.M., J.A.B.; D43 TW009595 to J.A.B); Duke Clinical Trials Unit and Clinical Research Sites (U01 AI069484 to J.A.C., V.P.M., J.A.B.); the Center for HIV/AIDS Vaccine Immunology (U01 AI067854 to J.A.C., J.A.B.); the joint NIH-NSF Ecology and Evolution of Infectious Disease program and the UK Economic and Social Research Council and Biotechnology and Biological Sciences Research Council (R01 TW009237 to J.A.C., V.P.M.); and the Fogarty International Center Global Health Fellowship Program R25TW009343 (M.P.R.). Investigator support was also received from Health Resources and Services Administration T84HA21123 (J.A.B.).
Disclosure: Presented in part at the 63rd American Society of Tropical Medicine and Hygiene annual meeting, New Orleans, LA, 2–6 November 2014, abstract 1881.
Authors' addresses: Matthew P. Rubach, Department of Pathology, University of Utah, Salt Lake City, UT, E-mail: matthew.rubach@hsc.utah.edu. Venance P. Maro, Department of Internal Medicine, Kilimanjaro Christian Medical Centre, Moshi, Tanzania, E-mail: venmaro@ymail.com. John A. Bartlett, Department of Internal Medicine, Duke University, Durham, NC, E-mail: john.bartlett@duke.edu. John A. Crump, Department of Medicine, Duke University, Durham, NC, E-mail: john.crump@duke.edu.
References
- 1.Gove S. Integrated management of childhood illness by outpatient health workers: technical basis and overview. The WHO Working Group on Guidelines for Integrated Management of the Sick Child. Bull World Health Organ. 1997;75((Suppl 1)):7–24. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Illnesses with Limited Resources. Geneva: World Health Organization; 2005. [Google Scholar]
- 3.World Health Organization . Management of the Child with a Serious Infection or Severe Malnutrition: Guidelines for Care at the First-Referral Level in Developing Countries. Geneva: World Health Organization; 2000. http://www.who.int/maternal_child_adolescent/documents/fch_cah_00_1/en/ Available at. Accessed April 16, 2014. [Google Scholar]
- 4.World Health Organization . IMAI District Clinician Manual: Hospital Care for Adolescents and Adults. Geneva: World Health Organization; 2011. http://www.who.int/hiv/pub/imai/imai2011/en/ Available at. Accessed February 11, 2013. [Google Scholar]
- 5.Bassat Q, Machevo S, O'Callaghan-Gordo C, Sigauque B, Morais L, Diez-Padrisa N, Ribo JL, Mandomando I, Nhampossa T, Ayala E, Sanz S, Weber M, Roca A, Alonso PL. Distinguishing malaria from severe pneumonia among hospitalized children who fulfilled integrated management of childhood illness criteria for both diseases: a hospital-based study in Mozambique. Am J Trop Med Hyg. 2011;85:626–634. doi: 10.4269/ajtmh.2011.11-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkley JA, Maitland K, Mwangi I, Ngetsa C, Mwarumba S, Lowe BS, Newton CR, Marsh K, Scott JA, English M. Use of clinical syndromes to target antibiotic prescribing in seriously ill children in malaria endemic area: observational study. BMJ. 2005;330:995. doi: 10.1136/bmj.38408.471991.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadjm B, Amos B, Mtove G, Ostermann J, Chonya S, Wangai H, Kimera J, Msuya W, Mtei F, Dekker D, Malahiyo R, Olomi R, Crump JA, Whitty CJ, Reyburn H. WHO guidelines for antimicrobial treatment in children admitted to hospital in an area of intense Plasmodium falciparum transmission: prospective study. BMJ. 2010;340:c1350. doi: 10.1136/bmj.c1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clifton DC, Ramadhani HO, Msuya LJ, Njau BN, Kinabo GD, Buchanan AM, Crump JA. Predicting mortality for pediatric inpatients where malaria is uncommon. Arch Dis Child. 2012;97:889–894. doi: 10.1136/archdischild-2012-301812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, Galloway RL, Ooi EE, Maro VP, Saganda W, Kinabo GD, Muiruri C, Bartlett JA. Etiology of severe non-malaria febrile illness in northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis. 2013;7:e2324. doi: 10.1371/journal.pntd.0002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crump JA, Ramadhani HO, Morrissey AB, Saganda W, Mwako MS, Yang LY, Chow SC, Morpeth SC, Reyburn H, Njau BN, Shaw AV, Diefenthal HC, Shao JF, Bartlett JA, Maro VP. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis. 2011;52:341–348. doi: 10.1093/cid/ciq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crump JA, Ramadhani HO, Morrissey AB, Msuya LJ, Yang LY, Chow SC, Morpeth SC, Reyburn H, Njau BN, Shaw AV, Diefenthal HC, Bartlett JA, Shao JF, Schimana W, Cunningham CK, Kinabo GD. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected children and infants in northern Tanzania. Trop Med Int Health. 2011;16:830–837. doi: 10.1111/j.1365-3156.2011.02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Bureau of Statistics . 2012 Population and Housing Census. Dar Es Salaam; United Republic of Tanzania: 2013. http://www.nbs.go.tz/sensa/new.html Available at. Accessed December 12, 2013. [Google Scholar]
- 13.National Bureau of Statistics . Tanzania Demographic and Health Survey 2004–2005. Dar es Salaam; United Republic of Tanzania: 2005. http://nbs.go.tz/tnada/index.php/catalog/8 Available at. Accessed December 19, 2013. [Google Scholar]
- 14.Hay SI, Guerra CA, Gething PW, Patil AP, Tatem AJ, Noor AM, Kabaria CW, Manh BH, Elyazar IR, Brooker S, Smith DL, Moyeed RA, Snow RW. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6:e1000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Bureau of Statistics . Tanzania HIV/AIDS and Malaria Indicator Survey 2007-2008. Dar es Salaam; United Republic of Tanzania: 2008. www.nbs.go.tz/tnada/index.php/catalog/9/download/16 Available at. Accessed December 27, 2013. [Google Scholar]
- 16.Prabhu M, Nicholson WL, Roche AJ, Kersh GJ, Fitzpatrick KA, Oliver LD, Massung RF, Morrissey AB, Bartlett JA, Onyango JJ, Maro VP, Kinabo GD, Saganda W, Crump JA. Q fever, spotted fever group, and typhus group rickettsioses among hospitalized febrile patients in northern Tanzania. Clin Infect Dis. 2011;53:e8–e15. doi: 10.1093/cid/cir411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biggs HM, Bui DM, Galloway RL, Stoddard RA, Shadomy SV, Morrissey AB, Bartlett JA, Onyango JJ, Maro VP, Kinabo GD, Saganda W, Crump JA. Leptospirosis among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hyg. 2011;85:275–281. doi: 10.4269/ajtmh.2011.11-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouley AJ, Biggs HM, Stoddard RA, Morrissey AB, Bartlett JA, Afwamba IA, Maro VP, Kinabo GD, Saganda W, Cleaveland S, Crump JA. Brucellosis among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hyg. 2012;87:1105–1111. doi: 10.4269/ajtmh.2012.12-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hertz JT, Munishi OM, Ooi EE, Howe S, Lim WY, Chow A, Morrissey AB, Bartlett JA, Onyango JJ, Maro VP, Kinabo GD, Saganda W, Gubler DJ, Crump JA. Chikungunya and dengue fever among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hyg. 2012;86:171–177. doi: 10.4269/ajtmh.2012.11-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lofgren SM, Kirsch EJ, Maro VP, Morrissey AB, Msuya LJ, Kinabo GD, Saganda W, Diefenthal HC, Ramadhani HO, Wheat LJ, Crump JA. Histoplasmosis among hospitalized febrile patients in northern Tanzania. Trans R Soc Trop Med Hyg. 2012;106:504–507. doi: 10.1016/j.trstmh.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldmesser M, Harris C, Reichberg S, Khan S, Casadevall A. Serum cryptococcal antigen in patients with AIDS. Clin Infect Dis. 1996;23:827–830. doi: 10.1093/clinids/23.4.827. [DOI] [PubMed] [Google Scholar]
- 22.Crump JA, Ramadhani HO, Morrissey AB, Saganda W, Mwako MS, Yang LY, Chow SC, Njau BN, Mushi GS, Maro VP, Reller LB, Bartlett JA. Bacteremic disseminated tuberculosis in sub-Saharan Africa: a prospective cohort study. Clin Infect Dis. 2012;55:242–250. doi: 10.1093/cid/cis409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention . Case Definitions: Nationally Notifiable Diseases and Conditions. Atlanta, GA: CDC; 2012. 2012. http://wwwn.cdc.gov/nndss/document/2012_Case%20Definitions.pdf Available at. Accessed December 27, 2012. [Google Scholar]
- 24.Wongsrichanalai C, Murray CK, Gray M, Miller RS, McDaniel P, Liao WJ, Pickard AL, Magill AJ. Co-infection with malaria and leptospirosis. Am J Trop Med Hyg. 2003;68:583–585. doi: 10.4269/ajtmh.2003.68.583. [DOI] [PubMed] [Google Scholar]
- 25.Russell KL, Montiel Gonzalez MA, Watts DM, Lagos-Figueroa RC, Chauca G, Ore M, Gonzalez JE, Moron C, Tesh RB, Vinetz JM. An outbreak of leptospirosis among Peruvian military recruits. Am J Trop Med Hyg. 2003;69:53–57. [PubMed] [Google Scholar]
- 26.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in pediatric cerebral malaria: a study of 131 comatose Malawian children. QJM. 1989;71:441–459. [PubMed] [Google Scholar]
- 27.Performance Standards for Antimicrobial Susceptibility Testing . 23rd Informational Supplement (M100-S23) Wayne, PA: Clinical and Laboratory Standards Institute; 2013. [Google Scholar]
- 28.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, Kauffman CA. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 30.Ressner RA, Griffith ME, Beckius ML, Pimentel G, Miller RS, Mende K, Fraser SL, Galloway RL, Hospenthal DR, Murray CK. Antimicrobial susceptibilities of geographically diverse clinical human isolates of Leptospira. Antimicrob Agents Chemother. 2008;52:2750–2754. doi: 10.1128/AAC.00044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rolain JM, Maurin M, Vestris G, Raoult D. In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob Agents Chemother. 1998;42:1537–1541. doi: 10.1128/aac.42.7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolain JM, Stuhl L, Maurin M, Raoult D. Evaluation of antibiotic susceptibilities of three rickettsial species including Rickettsia felis by a quantitative PCR DNA assay. Antimicrob Agents Chemother. 2002;46:2747–2751. doi: 10.1128/AAC.46.9.2747-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raoult D, Torres H, Drancourt M. Shell-vial assay: evaluation of a new technique for determining antibiotic susceptibility, tested in 13 isolates of Coxiella burnetii. Antimicrob Agents Chemother. 1991;35:2070–2077. doi: 10.1128/aac.35.10.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munoz-Espin T, Lopez-Pares P, Espejo-Arenas E, Font-Creus B, Martinez-Vila I, Traveria-Casanova J, Segura-Porta F, Bella-Cueto F. Erythromycin versus tetracycline for treatment of Mediterranean spotted fever. Arch Dis Child. 1986;61:1027–1029. doi: 10.1136/adc.61.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meloni G, Meloni T. Azithromycin vs. doxycycline for Mediterranean spotted fever. Pediatr Infect Dis J. 1996;15:1042–1044. doi: 10.1097/00006454-199611000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Dijkstra F, Riphagen-Dalhuisen J, Wijers N, Hak E, Van der Sande MA, Morroy G, Schneeberger PM, Schimmer B, Notermans DW, Van der Hoek W. Antibiotic therapy for acute Q fever in The Netherlands in 2007 and 2008 and its relation to hospitalization. Epidemiol Infect. 2011;139:1332–1341. doi: 10.1017/S0950268810002621. [DOI] [PubMed] [Google Scholar]
- 37.Gikas A, Kofteridis DP, Manios A, Pediaditis J, Tselentis Y. Newer macrolides as empiric treatment for acute Q fever infection. Antimicrob Agents Chemother. 2001;45:3644–3646. doi: 10.1128/AAC.45.12.3644-3646.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 39.Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, Saganda K, Shao J, Kitua A, Olomi R, Greenwood BM, Whitty CJ. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329:1212. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bassat Q, Ordi J, Vila J, Ismail MR, Carrilho C, Lacerda M, Munguambe K, Odhiambo F, Lell B, Sow S, Bhutta ZA, Rabinovich NR, Alonso PL, Menéndez C. Development of a post-mortem procedure to reduce the uncertainty regarding causes of death in developing countries. Lancet Global Health. 2013;1:e125–e126. doi: 10.1016/S2214-109X(13)70037-8. [DOI] [PubMed] [Google Scholar]
- 41.Ordi J, Ismail MR, Carrilho C, Romagosa C, Osman N, Machungo F, Bombi JA, Balasch J, Alonso PL, Menendez C. Clinico-pathological discrepancies in the diagnosis of causes of maternal death in sub-Saharan Africa: retrospective analysis. PLoS Med. 2009;6:e1000036. doi: 10.1371/journal.pmed.1000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ministry of Health and Social Welfare . National Bureau of Statistics. National AIDS Control Programme HIV/AIDS/STI Surveillance Report Number 22. Dar es Salaam; United Republic of Tanzania: 2011. http://www.nacp.go.tz/site/download Available at. Accessed July 30, 2014. [Google Scholar]
- 43.Tanzania Commission for AIDS . National HIV and AIDS Response Report 2012. Dar es Salaam: United Republic of Tanzania; 2013. http://www.tacaids.go.tz Available at. Accessed July 30, 2014. [Google Scholar]