Abstract
Frequent infections contribute to childhood stunting in developing countries but the causal pathways are uncertain. We tested the hypothesis that intercurrent illnesses suppress the growth hormone axis through reductions in insulin-like growth factor 1 (IGF-1). In a birth cohort of 202 HIV-unexposed Zimbabwean infants, we analyzed data on 7-day illness recall and measured plasma interleukin-6, C-reactive protein, alpha-1-acid glycoprotein, and IGF-1 by enzyme-linked immunosorbent assay, at age 6 weeks, and then 3, 6, 12, and 18 months. Children with recent acute illness had lower IGF-1 concentrations than healthy children and IGF-1 correlated inversely (P < 0.05) with inflammatory biomarkers at most time points between 3 and 18 months. Using path analysis, we showed that cough and fever had a predominantly indirect effect on suppressing IGF-1, through the acute-phase response, whereas diarrhea had a predominantly direct effect on IGF-1. Acute illness may therefore impact the growth hormone axis through both direct and indirect pathways.
Introduction
Linear growth faltering in children, or child stunting, remains a major public health problem in low-income countries worldwide. Growth faltering begins in utero and continues throughout the first 2 years of life, with the greatest decline occurring during the period between 6 and 24 mo of age.1 Poor linear growth is associated with increased morbidity and mortality, together with poor cognitive development, low educational performance, reduced lifetime earnings, and an increased risk of chronic disease in adulthood.2,3 Although suboptimal complementary feeding practices undoubtedly contribute to child stunting,4 frequent intercurrent infections, such as pneumonia and diarrhea, may also adversely impact child growth.
Research in Guatemala nearly 40 years ago showed acute illnesses, such as diarrhea, pneumonia, upper respiratory tract infections, and skin infections, were associated with short-term growth deficits.5 Subsequent studies in various settings have confirmed this observation,6–9 particularly for diarrhea, which is one of the most common illnesses in early life. More recent studies have shown that child height deficits are proportional to the cumulative burden of diarrhea during childhood, and that diarrhea during the first 6 months of life may have particularly adverse consequences on growth.10 Although there is evidence that children can experience catch-up growth after diarrheal episodes,11–13 it is well established that acute diarrhea has at least short-term impacts on child growth.
Infections may affect child growth through several mechanisms including decreased food intake caused by anorexia, increased energy demands, malabsorption, increased catabolism, and loss or sequestration of nutrients required for tissue synthesis and growth.14 Growth may also be impaired during infections as a result of the acute-phase response, which may decrease transport of nutrients to target tissues and affect bone remodeling by inflammatory pathways.15 The suppression of insulin-like growth factor 1 (IGF-1) may be a primary mechanism through which infection-induced inflammation affects growth. IGF-1 is a circulating hormone synthesized by the liver that mediates the effects of growth hormone (GH) by stimulating proliferation of chondrocytes at the epiphyseal growth plate; it is essential for healthy bone growth.16 Individuals with chronic inflammatory conditions (e.g., juvenile idiopathic arthritis and Crohn's disease) commonly have suppressed concentrations of IGF-1,17,18 which are inversely related to the level of circulating pro-inflammatory cytokines.
We have previously shown that chronic low-grade inflammation is associated with reduced concentrations of IGF-1 and poor linear growth in a cohort of Zimbabwean infants.19 However, the impact of acute infection on the hormonal regulation of growth has not been well characterized among infants in developing countries.20 Therefore, in this cohort of Zimbabwean infants, we aimed to determine: 1) the association between recent acute illness and IGF-1 concentrations, and 2) the relative strength of the direct and indirect pathways mediating the association between recent acute illness and IGF-1 (i.e., direct pathway: recent acute illness is directly associated with IGF-1; indirect pathway: the association between illness and IGF-1 is mediated by the acute phase inflammatory response). We hypothesized that recent acute illness would be associated with suppression of IGF-1, predominantly through activation of the inflammatory response (i.e., the indirect pathway).
Materials and Methods
Study design and sampling.
The data used in this current study were collected during the ZVITAMBO trial, conducted between 1997 and 2001 in Zimbabwe.21 The ZVITAMBO was a randomized clinical trial of high-dose vitamin A supplementation in 14,110 mother–infant pairs, enrolled within 96 hours of delivery from peri-urban clinics in Harare. Mothers were interviewed upon enrollment, providing data on household demographic and socioeconomic status. Follow-up visits were conducted at 6 weeks postpartum, and at 3, 6, 9, 12, 15, 18, 21, and 24 months. At each visit, a maternal interview was undertaken, and blood and anthropometry data were collected for infants.
We selected infants who were stunted (length-for-age Z-score (LAZ) < −2.0) or non-stunted (LAZ > −0.5) at 18 months of age, to investigate differences in biomarkers of inflammation, gut damage and the GH axis.19 Eligible children were born to mothers who remained HIV-negative throughout follow-up, and who had anthropometric data and archived plasma (> 0.2 mL) available from at least four follow-up time points. From the 14,110 enrolled mother–infant pairs, 101 non-stunted infants fulfilled these selection criteria and were included in the study. Of the 132 eligible stunted children identified, 101 were randomly selected for inclusion.
Measurement of recent acute illness in children.
Data on recent acute illness in infants were collected by maternal interview at each follow-up visit. Mothers were asked to recall whether the child had diarrhea, cough, or fever in the previous 7 days and, for diarrhea and cough, to recall the number of days that the illness persisted. Morbidity data were then assessed over a 7-day recall period at five time points (i.e., 6 weeks, 3, 6, 12, and 18 months of age), to align with the available biomarker data.
Plasma collection and measurement of biomarkers.
Plasma samples were collected from all enrolled mothers and infants at baseline, and from a representative subsample (52% of infants) at all follow-up visits. Archived plasma was available for all 202 children at 18 months of age and for 75%, 85%, 91%, and 97% at 6 weeks, 3, 6, and 12 months of age, respectively. Samples were centrifuged and plasma removed within 2 hours of blood collection for storage in −80 C freezers, with generator back-up.
We used commercially available enzyme-linked immunosorbent assay (ELISA) kits to measure a range of biomarkers at each time point for which archived infant plasma samples were available. We selected markers of immune activation, including the pro-inflammatory cytokine interleukin-6 (IL-6; R&D Systems Inc., Minneapolis, MN) and soluble CD14 (sCD14), a marker of monocyte/macrophage activation that is released predominantly after exposure to lipopolysaccharide, a component of the gram-negative bacterial membrane. We measured two acute-phase proteins, alpha-1-acid glycoprotein (AGP; R&D Systems), and C-reactive protein (CRP; R&D Systems), both of which are synthesized by the liver during an acute-phase inflammatory response; AGP rises more slowly than CRP during an acute-phase response and remains elevated longer.22 We measured IGF-1 (R&D Systems), which is synthesized by the liver in response to GH and acts on peripheral organs such as bone and muscle, to assess activity of the GH axis. We chose intestinal fatty acid binding protein (I-FABP; Hycult Biotechnology, Uden, The Netherlands) as a plasma marker of small intestinal damage; I-FABP is a cytoplasmic protein that is rapidly released into the bloodstream after damage to enterocytes.23
Child anthropometry.
Child weights and heights were measured using an electronic scale (Seca model 727, Hanover, MD) and length board (ShorrBoard, Olney, MD), respectively, at each visit based on standard methods.24 Length-for-age Z-scores were calculated based on the World Health Organization (WHO) 2010 reference standards using WHO Anthro version 3.0.1 (http://www.who.int/childgrowth/en). Growth velocity was defined as height change per day from the previous follow-up visit to the current visit.
Statistical analysis.
All statistical analyses were carried out in Stata v. 12.1 (College Station, TX). Because the proportion of children with recent acute illness did not differ between stunted and non-stunted children at any time point, data for all children were combined for primary analyses. However, in sensitivity analyses, we used linear regression analyses to examine potential differences in the association of recent illness with IGF-1 concentrations among stunted and non-stunted children. We calculated the proportion of children with recent acute illness at each follow-up visit. We used analysis of variance to assess differences in mean biomarker concentrations at each follow-up visit between children with and without reports of recent acute illness. Spearman correlation coefficients are reported for the statistical significance of the associations between recent acute illness, inflammatory markers, and IGF-1, and for the relationship between inflammatory markers and IGF-1. We constructed bivariate and multiple logistic regression models to calculate the odds of stunting at 18 months of age and to examine differences in the continuous response variables, attained LAZ and growth velocity, by report of recent acute illness. We included child sex, maternal height, household income, and infant birth length in multiple logistic regression models to control for factors that could potentially confound the relationship between recent acute illness and child linear growth.
We conducted a path analysis to assess the potential mediating effect of inflammation on the relationship between recent acute illness and IGF-1. Path analysis allows the simultaneous estimation of all regression equations identified in a model. Such an analytic approach is especially useful for discerning the relative importance of pathways in hypothesized causal models by estimating direct and indirect (i.e., mediated) effects.25 This approach allows for a more rigorous assessment of mediation (i.e., as compared with the causal steps approach) by directly estimating path coefficients and independent error terms using maximum likelihood estimation.26,27 Given our objective of determining the potential mediation of the association between recent acute illness and IGF-1 by activation of the inflammatory response, path analysis is an ideally suited analytic approach.
We used the sem (structural equation modeling) command in Stata to calculate standardized path coefficients using maximum-likelihood estimation for the direct, indirect, and total effects of recent acute illness on IGF-1 concentrations. Standard errors robust to heteroskedasticity were calculated using the Huber-White sandwich estimator. These path coefficients are regression coefficients converted to standardized Z-scores for measuring the relative strength and sign of effects in the path diagram (Figure 1). For the direct effect (DE), the coefficient is the partial regression coefficient of the association between recent acute illness and IGF-1 (i.e., regressing IGF-1 concentration on recent acute illness), controlling for the effect of inflammation as measured by plasma AGP concentration. We chose AGP as the marker of inflammation because of its longer time course during an acute-phase response. The magnitude of the indirect effect (IE) of recent acute illness on IGF-1 as mediated by inflammation was calculated by taking the product of the two path coefficients: 1) between recent acute illness and inflammation, and 2) between inflammation and IGF-1. The total effect is the sum of the direct and indirect effects.
Figure 1.
Path diagram showing hypothesized direct and indirect effects of recent acute illness on the growth hormone axis with mediation by inflammation. Path coefficients are shown to illustrate the relationships between the variables in the path model. The coefficients are for the relationships between recent fever, alpha-1-acid glycoprotein (AGP) and insulin-like growth factor 1 (IGF-1) at 3 mo (Table 2).
Ethical approval.
The ZVITAMBO trial and the current study were approved by the Medical Research Council of Zimbabwe, Johns Hopkins Bloomberg School of Public Health Committee on Human Research, and the Montreal General Hospital Ethics Committee. Written informed consent was provided by mothers for themselves and their infants.
Results
Recent acute illness in children.
Nearly two-thirds of children who were stunted (63%) and non-stunted (64%) at 18 months of age were reported to have been ill with recent diarrhea at one or more follow-up visits (P = 0.88). Most children were reported to have had cough or fever during the previous 7 days at one or more follow-up visits (i.e., cough: stunted: 88%; non-stunted: 88%; P = 1.00; fever: stunted: 72%; non-stunted: 68%; P = 0.54). The proportion of children with reported illnesses at each visit increased from 6 weeks through 6 months of age (diarrhea: 4.6%, 8.7%, and 20%; cough: 23%, 42%, 50%; fever: 5.7%, 22%, 28%; at 6 weeks, 3 months, and 6 months, respectively) (Table 1). Thereafter, proportions declined moderately for cough (42% at 18 months), but stayed relatively constant for diarrhea and fever (18% and 27%, respectively, at 18 months). The proportion of caregivers reporting cough was higher at all follow-up visits than the proportion reporting diarrhea or fever.
Table 1.
Prevalence of recent acute illness reported at each follow-up visit
| 6 weeks | 3 months | 6 months | 12 months | 18 months | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n* | N† | % | n | N | % | n | N | % | n | N | % | n | N | % | |
| Diarrhea | 8 | 173 | 4.6 | 17 | 196 | 8.7 | 39 | 195 | 20 | 44 | 201 | 22 | 37 | 202 | 18 |
| Cough | 41 | 175 | 23 | 81 | 194 | 42 | 99 | 197 | 50 | 82 | 201 | 41 | 85 | 201 | 42 |
| Fever | 10 | 176 | 5.7 | 42 | 192 | 22 | 55 | 197 | 28 | 44 | 202 | 22 | 54 | 202 | 27 |
n refers to the number of reported cases of the illness during the prior 7 days at each follow-up visit.
N refers to the total sample size for which illness data were available at each follow-up visit.
There were no differences in the proportion of children with recent acute illness between stunted and non-stunted children at any time point.
Because there were no differences in illness episodes between stunted and non-stunted children, groups were combined for all analyses. Child and maternal anthropometric differences between stunted and non-stunted children in this cohort have been reported previously.16 For example, children who were stunted at 18 months of age had lower mean birth weight (kg) (mean [SD]: 2.85 [0.45]) and birth length (cm) (47.9 [2.3]) compared with children who were not stunted at 18 months (weight: 3.17 [0.44]; length: 49.4 [2.5]) (P < 0.001), and mothers of stunted children had lower mean weight (kg) (58.8 [8.8]) and height (cm) (158.6 [6.1]) compared with mothers of non-stunted children (weight: 65.3 [12.4]; height: 162.8 [6.8]) (P ≤ 0.001).
Association of recent acute illness with inflammation.
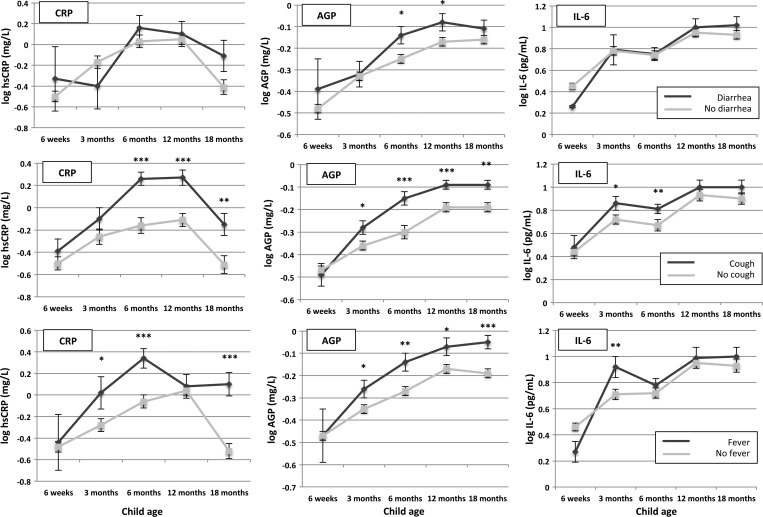
As anticipated, reports of recent acute illness, especially cough and fever, were strongly associated with elevated markers of inflammation (Figure 2). The AGP was significantly higher in children with recent cough and fever compared with children without these symptoms at all time points from 3 months of age; CRP was significantly higher at all time points from 6 months of age after report of recent cough, and at 3 months (P = 0.045), 6 months (P < 0.001), and 18 months (P < 0.001) after report of recent fever. The IL-6 showed a less consistent relationship, but was elevated at 3 months (P = 0.04) and 6 months (P = 0.001) for children with recent cough and at 3 months for children with recent fever (P = 0.004). Reports of recent diarrhea were less consistently associated with elevated inflammatory markers, with no associations seen at any time point for CRP and IL-6; AGP concentrations were higher only at 6 months (P = 0.04) and 12 months (P = 0.03) in those with diarrhea.
Figure 2.
Relationships between report of child recent acute illness and inflammatory markers. Mean estimates at each time point are shown together with error bars representing standard errors (SE). The top, middle, and lower panels show data for report of diarrhea, cough, and fever, respectively. P values are for differences in biomarker concentrations at each time point between children with reported recent illness versus no reported recent illness: ***P < 0.001, **P < 0.01, *P < 0.05.
Association of recent acute illness with IGF-1 concentrations.
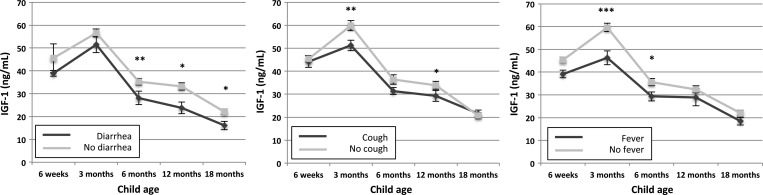
Mean IGF-1 concentrations were consistently lower in children with recent diarrhea, compared with children without diarrhea, with differences especially strong from 6 mo of age onwards (Figure 3). Mean IGF-1 concentrations were similarly lower in children with recent cough and fever at nearly all time points, with pronounced differences at 3 months (P = 0.004) and 12 months (P = 0.03) for children with recent cough and at 3 months (P < 0.001) and 6 months (P = 0.03) for children with recent fever (Figure 3). The association between recent illness and IGF-1 was not statistically significantly different at any time point between children who were stunted and non-stunted at 18 months (P > 0.1 at all time points).
Figure 3.
Relationships between report of recent acute illness and plasma insulin-like growth factor 1. Mean estimates at each time point are shown together with error bars representing standard errors (SE). The left, middle, and right panels show data for report of diarrhea, cough, and fever, respectively. P values are for differences in biomarker concentrations at each time point between children with reported recent illness versus no reported recent illness: ***P < 0.001, **P < 0.01, *P < 0.05.
Association of inflammation with the IGF-1 concentrations.
To determine whether differences in inflammatory markers and suppression of the GH axis were associated, we next looked at correlations between each inflammatory or acute-phase biomarker and IGF-1 concentrations. At nearly all time points from 3 months to 18 months in children for whom recent acute illness was reported (i.e., 33 of 36 associations), plasma IGF-1 showed a negative relationship with each marker of inflammation, although the strength of these associations varied by follow-up time point and inflammatory marker (Table 2).
Table 2.
Associations between IGF-1 and inflammatory biomarkers in children with report of recent acute illness
| Child age | IGF-1 (ng/mL) | ||||
|---|---|---|---|---|---|
| 6 weeks | 3 months | 6 months | 12 months | 18 months | |
| Children with recent diarrhea | |||||
| log AGP (g/L) | −0.83* (N = 6) | −0.62* (N = 14) | −0.29 (N = 27) | −0.38 (N = 19) | −0.57*** (N = 32) |
| log CRP (mg/L) | −0.54 (N = 6) | −0.47 (N = 15) | −0.11 (N = 35) | −0.58* (N = 18) | −0.12 (N = 33) |
| log IL-6 (pg/mL) | 1.00*** (N = 3) | −0.52* (N = 15) | −0.20 (N = 35) | −0.65** (N = 18) | 0.24 (N = 36) |
| Children with recent cough | |||||
| log AGP (g/L) | −0.42* (N = 29) | −0.57*** (N = 66) | −0.19 (N = 64) | −0.40** (N = 53) | −0.13 (N = 76) |
| log CRP (mg/L) | −0.40* (N = 28) | −0.54*** (N = 65) | −0.32** (N = 85) | −0.29* (N = 52) | −0.11 (N = 80) |
| log IL-6 (pg/mL) | −0.09 (N = 18) | −0.49*** (N = 70) | 0.01 (N = 83) | −0.34* (N = 55) | −0.19 (N = 85) |
| Children with recent fever | |||||
| log AGP (g/L) | 0.70 (N = 5) | −0.35* (N = 38) | −0.36* (N = 37) | −0.48* (N = 21) | −0.21 (N = 52) |
| log CRP (mg/L) | 0.40 (N = 5) | −0.45** (N = 35) | −0.37* (N = 47) | −0.27 (N = 21) | 0.11 (N = 50) |
| log IL-6 (pg/mL) | 0.80 (N = 4) | −0.30 (N = 40) | −0.17 (N = 47) | −0.55** (N = 24) | −0.13 (N = 54) |
Spearman correlation coefficients (r) are shown in addition to the sample size for each correlation, in parentheses.
Asterisks indicate that the correlation was statistically significant at the following levels of significance:
P < 0.001,
P < 0.01,
P < 0.05.
IGF-1 = insulin-like growth factor 1; AGP = alpha-1-acid glycoprotein; CRP = C-reactive protein; IL-6 = interleukin-6.
Given the observed negative relationships between inflammatory markers and IGF-1 concentrations in children with recent acute illness, we next examined the potential mediating effect of inflammation on the relationship between recent acute illness and the GH axis using path analysis to determine the direct, indirect, and total effects of acute illness on IGF-1 concentrations (Figure 1). Consistent with the bivariate models, there was a negative association between recent acute illness and IGF-1 concentrations in path analyses at nearly all time points for each reported illness (Table 3). The negative indirect effects along the inflammatory mediation pathway, particularly early in infancy (coefficient [P value]: for cough: −3.37 [0.042] at 3 months; −3.07 [0.002] at 6 months; for fever: −3.96 [0.021] at 3 months; −2.36 [0.018] at 6 months), contributed most strongly to the total effects of cough and fever on IGF-1. In contrast, negative direct effects, particularly late in infancy (coefficient [P value]: −6.27 [0.022] at 12 months; −5.44 [0.008] at 18 months), contributed most strongly to the total effects of recent diarrheal illness on IGF-1.
Table 3.
Direct, indirect, and total effects of recent acute illness on plasma IGF-1 concentrations*
| Recent diarrhea | Recent cough | Recent fever | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coeff. | SE | P value | Coeff. | SE | P value | Coeff. | SE | P value | |
| 6 weeks | |||||||||
| DE | −4.82 | 4.23 | 0.255 | −1.62 | 2.53 | 0.522 | −6.39 | 3.86 | 0.098 |
| IE | −1.94 | 2.87 | 0.500 | 0.31 | 1.16 | 0.787 | −0.11 | 2.38 | 0.962 |
| TE | −6.75 | 6.08 | 0.266 | −1.31 | 2.91 | 0.653 | −6.50 | 2.05 | 0.002 |
| 3 months | |||||||||
| DE | −4.93 | 3.34 | 0.140 | −5.25 | 2.92 | 0.072§ | −9.51 | 3.28 | 0.004 |
| IE | −0.24 | 3.08 | 0.939 | −3.37 | 1.66 | 0.042 | −3.96 | 1.72 | 0.021 |
| TE | −5.16 | 3.83 | 0.177 | −8.62 | 3.16 | 0.006 | −13.5 | 3.56 | <0.001 |
| 6 months | |||||||||
| DE | −4.43 | 3.28 | 0.177 | −1.85 | 2.51 | 0.462 | −3.87 | 2.52 | 0.125 |
| IE | −2.56 | 1.22 | 0.036 | −3.07 | 0.98 | 0.002 | −2.36 | 1.00 | 0.018 |
| TE | −6.99 | 3.18 | 0.028 | −4.92 | 2.56 | 0.054 | −6.23 | 2.54 | 0.014 |
| 12 months | |||||||||
| DE | −6.27 | 2.74 | 0.022 | −1.08 | 2.86 | 0.706 | 1.27 | 3.77 | 0.737 |
| IE | −2.60 | 1.36 | 0.056 | −3.32 | 1.17 | 0.005 | −3.12 | 1.65 | 0.059 |
| TE | −8.87 | 2.83 | 0.002 | −4.40 | 2.87 | 0.126 | −1.85 | 3.89 | 0.634 |
| 18 months | |||||||||
| DE | −5.44 | 2.05 | 0.008 | 2.32 | 2.04 | 0.254 | −2.02 | 2.14 | 0.346 |
| IE | −0.57 | 0.53 | 0.288 | −1.39 | 0.62 | 0.025 | −1.50 | 0.79 | 0.057 |
| TE | −6.00 | 2.16 | 0.005 | 0.93 | 2.03 | 0.647 | −3.52 | 2.07 | 0.089 |
DE = direct effect of recent acute illness on plasma insulin-like growth factor 1 (IGF-1) concentrations (ng/mL) adjusted for plasma alpha-1-acid glycoprotein (AGP) concentrations (log g/L) in the model; IE = indirect effect of recent acute illness on IGF-1 as mediated by AGP; TE = total effect, that is, the combined direct and indirect effects of recent acute illness on IGF-1.
Coefficients shown are standardized path coefficients. For the DE, the coefficient is the partial regression coefficient of recent acute illness on IGF-1, controlling for AGP.
The coefficient for the DE of recent fever on IGF-1 at 3 mo is depicted in Figure 1. The IE shown here may be calculated from the product of the path coefficients in Figure 1 between recent fever and AGP (depicted as “inflammation” in Figure 1), and AGP and IGF-1 (depicted as “growth hormone axis” in Figure 1). The TE effect shown here may be calculated from the sum of the DE and IE effects.
Association of recent diarrhea with small intestinal damage and microbial translocation.
We hypothesized that diarrhea may contribute to small intestinal damage and that children with reports of recent diarrheal morbidity would have a greater degree of microbial translocation after insult to the gut barrier. We therefore next examined the association between recent diarrhea and plasma concentrations of I-FABP and soluble CD14. However, children with reports of recent diarrhea did not show higher concentrations of I-FABP or sCD14 at any follow-up time point, compared with children with no recent report of diarrhea (data not shown).
Association of recent acute illness with child growth.
Stunting at 18 months of age was not associated with the cumulative number of diarrheal episodes reported, nor with the total duration of diarrhea reported by caregivers (Supplemental Figure 1). Similarly, children with recent diarrhea did not show differences in attained LAZ or growth velocity at any follow-up time point, compared with children without reports of recent diarrhea (data not shown). We examined these relationships in bivariate models and in multiple logistic regression models controlling for child sex, maternal height, household income and birth length, and found similar results in all models. Likewise, we found no strong associations between recent cough or fever and child growth.
Discussion
In this longitudinal cohort of Zimbabwean infants, followed from birth through 18 months of age, we showed that recent acute illness is associated with suppression of the growth hormone axis. Depending on infant age and type of intercurrent illness, we found evidence of both direct suppression of IGF-1, and indirect suppression mediated by an acute-phase inflammatory response. Using path analysis, we showed that indirect effects, mediated through inflammation, were the predominant mechanism through which recent cough and fever were associated with depressed IGF-1 concentrations, particularly in the first 6 months of life, whereas diarrhea had predominantly direct effects on IGF-1 concentrations, particularly in the second half of infancy.
Cough and fever were associated with reduced IGF-1 concentrations, mediated predominantly through raised pro-inflammatory markers. Cough and fever are common presentations of bacterial pneumonia, which is usually associated with a robust acute-phase response.28 Associations between inflammatory markers and reduced IGF-1 concentrations have been seen in other settings. For example, children with chronic inflammatory diseases, such as Crohn's disease and juvenile idiopathic arthritis, have reduced circulating IGF-1 concentrations caused by elevated pro-inflammatory cytokines.17,18 Although chronic inflammatory conditions such as Crohn's disease are characterized by highly elevated inflammatory markers over long periods, leading to severe growth failure, they provide a proof of concept of the association between inflammation and reduced IGF-1; data from transgenic mice confirm that these associations are causal.29 It therefore seems plausible that even a short illness associated with an acute-phase response, such as pneumonia, temporarily suppresses the GH-IGF-1 axis, and that recurrent infections may have a cumulative effect on growth through frequent, short-term reductions in IGF-1. We have previously shown in this same cohort that low-grade chronic inflammation is similarly associated with reduced IGF-1 concentrations in infancy.19 Acute infections may transiently exacerbate the chronic inflammatory milieu that characterizes stunting19 and further suppress IGF-1 concentrations.
Diarrhea had profound, predominantly direct effects on IGF-1 concentrations, particularly from 6 mo of age onwards. Children with diarrhea showed only a modest acute-phase response; although we had no information on etiology of diarrhea in this cohort, it is plausible that common enteric pathogens that do not typically cause a pronounced acute-phase response (e.g., rotavirus and cryptosporidium) were responsible for much of the diarrhea reported.30 The mechanisms through which diarrhea may directly influence IGF-1 are uncertain. Dietary protein restriction has been shown to reduce circulating serum IGF-1 concentrations31; diarrheal illness may induce moderate protein deficiency through decreased dietary intake, malabsorption, or catabolic loss. Zinc deficiency is also associated with low IGF-1 concentrations32; diarrhea may induce or exacerbate zinc deficiency through excess fecal losses.32 Moreover, zinc supplementation in young children has been shown to increase plasma concentrations of IGF-1 while also reducing the risk of diarrhea episodes and increasing linear growth velocity.33 Alternatively, zinc deficiency may cause reduced concentrations of serum IGF-1 while also increasing risk of diarrhea. Unfortunately, we did not have data on zinc or IGF-1 status in children before their acute illness so cannot establish the extent or direction through which this mechanism may have contributed to the observed associations. ZVITAMBO was conducted before the introduction of WHO guidelines on zinc supplementation for children during acute diarrhea34; therefore, we do not know whether supplementary zinc may have changed the observed associations.
Several prior studies investigating IGF-1 concentrations in children with intercurrent infections support our findings. Bhutta and others described severely depressed IGF-1 concentrations in young children hospitalized for persistent diarrhea in Pakistan, but rapid increases after nutritional recovery, with increments in IGF-1 correlating well with weight gain.33 Low concentrations of IGF-1 have previously been described in children with acute shigellosis34,35 and severe acute malnutrition,36,37 with increases in IGF-1 seen upon nutritional recovery.37,38 IGF-1 and IGF-2 were also found to be lower in patients with viral infections compared with healthy controls in a cohort of European adults 20–75 years of age.39
This study had several strengths and some limitations. We studied a well-characterized infant birth cohort that had suitable data and samples to test our hypotheses. In particular, our examination of blood biomarker data collected in concert with recent morbidity data allowed us to rigorously examine acute IGF-1 suppression among children with and without recent illness. Though we assessed the association between child illness and growth, it was not a primary objective of this research. The original ZVITAMBO trial was not explicitly designed to explore this relationship and, therefore, daily morbidity data were not collected as part of the trial. We therefore had data available on 7-day illness recall from five visits conducted between 6 weeks and 18 months of age. These data did not allow us to examine associations between cumulative morbidity and growth. Although maternal report of recent (7-day) illness is a reliable and valid instrument for assessing morbidity,40 we do not know whether intercurrent infections before the 7-day recall period would continue to have an effect on IGF-1 concentrations; if anything, this would mean we underestimated the impact of acute illness on IGF-1 concentrations, because children classified as healthy may in fact have had a recent illness associated with reduced IGF-1. The lack of daily morbidity data also meant that we were unable to assess the cumulative burden of illness or catch-up growth that may have occurred between bouts of illness. This may have contributed to the null association we observed between recent acute illness and child growth. As described earlier, the relationship between child illness and linear growth faltering has been well documented in many contexts.10,41 However, the impact of acute infection on the hormonal regulation of growth is not well understood, particularly among infants in low-income countries. Therefore, our specific research objectives were aimed at determining the association of acute infection on the growth hormone axis. IGF-1 is the circulating mediator of GH activity and maintaining adequate concentrations of IGF-1 during infancy is crucial for promoting linear growth, as we have previously shown in this cohort.19 It is therefore likely that the strong negative associations we observed between recent acute illness and IGF-1 concentrations have important implications for child growth in the context of frequent recurrent infections. Pooling data from stunted and non-stunted infants for this analysis is another potential limitation. Although infants were categorized on the basis of anthropometry undertaken at 18 months of age, differences in weight and length were evident from birth, suggesting that stunting may originate in utero. Whether fetal programming influences the postnatal inflammatory response to illness is unknown, but it is plausible that stunted and non-stunted infants respond differently to acute infections. However, neither the proportion of children with recent acute illness nor the association between recent illness and IGF-1 concentrations differed between the two groups at any time point. This suggests that prior growth parameters did not have a major influence on the pattern of morbidity or acute-phase response.
In summary, we show that recent acute illness in a cohort of Zimbabwean infants is associated with suppression of the GH axis. We found that cough and fever are associated with indirect suppression of IGF-1 concentrations through an acute-phase inflammatory response, particularly early in infancy, whereas diarrhea is associated with direct suppression of IGF-1 concentrations, particularly later in infancy. Our findings plausibly show distinct pathways through which even mild infections, not severe enough to cause malabsorption or prolonged anorexia, may temporarily suppress IGF-1 concentrations. Prevention of infections through exclusive breastfeeding and vaccination, and prompt treatment of illnesses to reduce their duration, may limit suppression of IGF-1, which in turn could positively contribute to healthy growth in early childhood.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Larry Moulton for his helpful feedback on earlier drafts of the manuscript.
Disclaimer: The authors have no conflicts of interest to declare.
Footnotes
Financial support: This study was funded by the Wellcome Trust (093768/Z/10/Z).
Authors' addresses: Andrew D. Jones, Human Nutrition Program, Department of Environmental Health Sciences, University of Michigan, Ann Arbor, MI, E-mail: jonesand@umich.edu. Sandra Rukobo, Bernard Chasekwa, Kuda Mutasa, Robert Ntozini, Mduduzi N. N. Mbuya, and Jean H. Humphrey, Zvitambo Institute for Maternal and Child Health Research, Harare, Zimbabwe, E-mails: srukobo@zvitambo.co.zw, bchasekwa@zvitambo.co.zw, kmutasa@zvitambo.co.zw, rntozini@zvitambo.co.zw, mmbuya@zvitambo.co.zw, and jhumphrey@zvitambo.co.zw. Rebecca J. Stoltzfus, Division of Nutritional Sciences, Cornell University, Ithaca, NY, E-mail: rjs62@cornell.edu. Andrew J. Prendergast, Centre for Paediatrics, Blizard Institute, Queen Mary University of London, London, UK, E-mail: a.prendergast@qmul.ac.uk.
References
- 1.Victora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125:e473–e480. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- 2.Hoddinott J, Behrman JR, Maluccio JA, Melgar P, Quisumbing AR, Ramirez-Zea M, Stein AD, Yount KM, Martorell R. Adult consequences of growth failure in early childhood. Am J Clin Nutr. 2013;98:1170–1178. doi: 10.3945/ajcn.113.064584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Victora CG. Nutrition in early life: a global priority. Lancet. 2009;374:1123–1125. doi: 10.1016/S0140-6736(09)61725-6. [DOI] [PubMed] [Google Scholar]
- 4.Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4((Suppl 1)):24–85. doi: 10.1111/j.1740-8709.2007.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mata LJ, Kronmal RA, Garcia B, Butler W, Urrutia JJ, Murillo S. Breast-feeding, weaning and the diarrheal syndrome in a Guatemalan Indian village. Ciba Found Symp. 1976;42:311–338. doi: 10.1002/9780470720240.ch17. [DOI] [PubMed] [Google Scholar]
- 6.Black RE, Brown KH, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. 1984;73:799–805. [PubMed] [Google Scholar]
- 7.Guerrant RL, Kirchhoff LV, Shields DS, Nations MK, Leslie J, de Sousa MA, Araujo JG, Correia LL, Sauer KT, McClelland KE, Trowbridge FL, Hughes JM. Prospective study of diarrheal illnesses in northeastern Brazil: patterns of disease, nutritional impact, etiologies, and risk factors. J Infect Dis. 1983;148:986–997. doi: 10.1093/infdis/148.6.986. [DOI] [PubMed] [Google Scholar]
- 8.Lima AA, Moore SR, Barboza MS, Soares AM, Schleupner MA, Newman RD, Sears CL, Nataro JP, Fedorko DP, Wuhib T, Schorling JB, Guerrant RL. Persistent diarrhea signals a critical period of increased diarrhea burdens and nutritional shortfalls: a prospective cohort study among children in northeastern Brazil. J Infect Dis. 2000;181:1643–1651. doi: 10.1086/315423. [DOI] [PubMed] [Google Scholar]
- 9.Moore SR, Lima AA, Conaway MR, Schorling JB, Soares AM, Guerrant RL. Early childhood diarrhea and helminthiases associate with long-term linear growth faltering. Int J Epidemiol. 2001;30:1457–1464. doi: 10.1093/ije/30.6.1457. [DOI] [PubMed] [Google Scholar]
- 10.Checkley W, Buckley G, Gilman RH, Assis A, Guerrant RL, Morris SS, Molbak K, Valentiner-Branth P, Lanata CF, Black RE. Childhood Malnutrition and Infection Network Multi-country analysis of the effects of diarrhea on childhood stunting. Int J Epidemiol. 2008;37:816–830. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry FJ, Alam N, Aziz KM, Rahaman MM. Dysentery, not watery diarrhea, is associated with stunting in Bangladeshi children. Hum Nutr Clin Nutr. 1987;41:243–249. [PubMed] [Google Scholar]
- 12.Briend A, Hasan KZ, Aziz KM, Hoque BA. Are diarrhea control programmes likely to reduce childhood malnutrition? Observations from rural Bangladesh. Lancet. 1989;2:319–322. doi: 10.1016/s0140-6736(89)90498-4. [DOI] [PubMed] [Google Scholar]
- 13.Moy RJ, de C Marshall TF, Choto RG, McNeish AS, Booth IW. Diarrhea and growth faltering in rural Zimbabwe. Eur J Clin Nutr. 1994;48:810–821. [PubMed] [Google Scholar]
- 14.Brown KH. Diarrhea and malnutrition. J Nutr. 2003;133:328S–332S. doi: 10.1093/jn/133.1.328S. [DOI] [PubMed] [Google Scholar]
- 15.Stephensen CB. Burden of infection on growth failure. J Nutr. 1999;129:534–538. doi: 10.1093/jn/129.2.534S. [DOI] [PubMed] [Google Scholar]
- 16.Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katsanos KH, Tsatsoulis A, Christodoulou D, Challa A, Katsaraki A, Tsianos EV. Reduced serum insulin-like growth factor-1 (IGF-1) and IGF-binding protein-3 levels in adults with inflammatory bowel disease. Growth Horm IGF Res. 2001;11:364–367. doi: 10.1054/ghir.2001.0248. [DOI] [PubMed] [Google Scholar]
- 18.Walters TD, Griffiths AM. Mechanisms of growth impairment in pediatric Crohn's disease. Nat Rev Gastroenterol Hepatol. 2009;6:513–523. doi: 10.1038/nrgastro.2009.124. [DOI] [PubMed] [Google Scholar]
- 19.Prendergast AJ, Rukobo S, Chasekwa B, Mutasa K, Ntozini R, Mbuya MN, Jones AD, Stoltzfus RJ, Humphrey JH. Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS ONE. 2014 doi: 10.1371/journal.pone.0086928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prentice AM, Moore SE, Fulford AJ. Growth faltering in low-income countries. World Rev Nutr Diet. 2013;106:90–99. doi: 10.1159/000342563. [DOI] [PubMed] [Google Scholar]
- 21.Humphrey JH, Iliff PJ, Marinda ET, Mutasa K, Moulton LH, Chidawanyika H, Ward BJ, Nathoo KJ, Malaba LC, Zijenah LS, Zvandasara P, Ntozini R, Mzengeza F, Mahomva AI, Ruff AJ, Mbizvo MT, Zunguza CD. ZVITAMBO Study Group Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis. 2006;193:860–871. doi: 10.1086/500366. [DOI] [PubMed] [Google Scholar]
- 22.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman JM, Sacchettini J, Marks C, Marks WH. Human intestinal fatty acid binding protein: report of an assay with studies in normal volunteers and intestinal ischemia. Surgery. 1997;121:335–342. doi: 10.1016/s0039-6060(97)90363-9. [DOI] [PubMed] [Google Scholar]
- 24.Gibson RS. Principles of Nutritional Assessment. New York, NY: Oxford University Press; 1990. [Google Scholar]
- 25.Olney DK, Kariger PK, Stoltzfus RJ, Khalfan SS, Ali NS, Tielsch JM, Sazawal S, Black R, Allen LH, Pollitt E. Development of nutritionally at-risk young children is predicted by malaria, anemia, and stunting in Pemba, Zanzibar. J Nutr. 2009;139:763–772. doi: 10.3945/jn.107.086231. [DOI] [PubMed] [Google Scholar]
- 26.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 28.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 29.De Benedetti F, Alonzi T, Moretta A, Lazzaro D, Costa P, Poli V, Martini A, Ciliberto G, Fattori E. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammation. J Clin Invest. 1997;99:643–650. doi: 10.1172/JCI119207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotloff KL, Blackwelder WC, Nasrin D, Nataro JP, Farag TH, van Eijk A, Adegbola RA, Alonso PL, Breiman RF, Faruque AS, Saha D, Sow SO, Sur D, Zaidi AK, Biswas K, Panchalingam S, Clemens JD, Cohen D, Glass RI, Mintz ED, Sommerfelt H, Levine MM. Burden and aetiology of diarrheal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 31.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cossack ZT. Decline in somatomedin-C (insulin-like growth factor-1) with experimentally induced zinc deficiency in human subjects. Clin Nutr. 1991;10:284–291. doi: 10.1016/0261-5614(91)90008-z. [DOI] [PubMed] [Google Scholar]
- 33.Bhutta ZA, Bang P, Karlsson E, Hagenäs L, Nizami SQ, Söder O. Insulin-like growth factor I response during nutritional rehabilitation of persistent diarrhea. Arch Dis Child. 1999;80:438–442. doi: 10.1136/adc.80.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pucilowska JB, Davenport ML, Kabir I, Clemmons DR, Thissen JP, Butler T, Underwood LE. The effect of dietary protein supplementation on insulin-like growth factors (IGFs) and IGF-binding proteins in children with shigellosis. J Clin Endocrinol Metab. 1993;77:1516–1521. doi: 10.1210/jcem.77.6.7505287. [DOI] [PubMed] [Google Scholar]
- 35.Kabir I, Butler T, Underwood LE, Rahman MM. Effects of a protein-rich diet during convalescence from shigellosis on catch-up growth, serum proteins, and insulin-like growth factor-I. Pediatr Res. 1992;32:689–692. doi: 10.1203/00006450-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Hintz RL, Suskind R, Amatayakul K, Thanangkul O, Olson R. Plasma somatomedin and growth hormone values in children with protein-calorie malnutrition. J Pediatr. 1978;92:153–156. doi: 10.1016/s0022-3476(78)80099-7. [DOI] [PubMed] [Google Scholar]
- 37.Donahue SP, Phillips LS. Response of IGF-1 to nutritional support in malnourished hospital patients: a possible indicator of short-term changes in nutritional status. Am J Clin Nutr. 1989;50:962–969. doi: 10.1093/ajcn/50.5.962. [DOI] [PubMed] [Google Scholar]
- 38.Soliman AT, Hassan AE, Aref MK, Hintz RL, Rosenfeld RG, Rogol AD. Serum insulin-like growth factors I and II concentrations and growth hormone and insulin responses to arginine infusion in children with protein-energy malnutrition before and after nutritional rehabilitation. Pediatr Res. 1986;20:1122–1130. doi: 10.1203/00006450-198611000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Baricevic I, Nedic O, Nikolic JA, Bojic B, Jojic N. Circulating insulin-like growth factors in patients infected with Helicobacter pylori. Clin Biochem. 2004;37:997–1001. doi: 10.1016/j.clinbiochem.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Arnold BF, Galiani S, Ram PK, Hubbard AE, Briceno B, Gertler PJ, Colford JM. Optimal recall period for caregiver-reported illness in risk factor and intervention studies: a multicountry study. Am J Epidemiol. 2013;177:361–370. doi: 10.1093/aje/kws281. [DOI] [PubMed] [Google Scholar]
- 41.Martorell R, Habicht J, Yarbrough C, Lechtig A, Klein R, Western K. Acute morbidity and physical growth in rural Guatemalan children. Am J Dis Child. 1975;129:1206–1301. doi: 10.1001/archpedi.1975.02120480022007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.