Abstract
The elimination of domestic triatomines is the foundation of Chagas disease control. Regional initiatives are eliminating introduced triatomine species. In this scenario, endemic triatomines can occupy the ecological niches left open and become a threat to long-term Chagas disease control efforts. This study determined the abundance, colonization, and Trypanosoma cruzi infection rate of the endemic Panstrongylus howardi in 10 rural communities located in Ecuador's Manabí Province. In total, 518 individuals of P. howardi were collected. Infestation indices of 1.4% and 6.6% were found in the domestic and peridomestic environments, respectively. We determined a T. cruzi infection rate of 53.2% (N = 47) in this species. P. howardi has a high capacity to adapt to different habitats, especially in the peridomicile. This implies a considerable risk of transmission because of the frequency of intradomicile invasion. Therefore, this species needs to be taken into account in Chagas control and surveillance efforts in the region.
Introduction
Chagas disease is caused by the parasite Trypanosoma cruzi and constitutes a complex public health problem in Latin America, where an estimated 8 million people are infected.1,2 In Ecuador, approximately 230,000 people are infected, and 6.2 million people are at risk of infection.1 Chagas is transmitted to humans and other mammals by Reduviidae bugs that belong to the subfamily Triatominae. This subfamily is currently composed of 140 species distributed among six tribes and 19 genera.3 Three of the most abundant genera within the subfamily triatomine are Panstrongylus Berg 1879, Triatoma Laporte 1832, and Rhodnius Stal 1859; all of them are important in Chagas transmission.4 The genus Panstrongylus comprises 13 species: P. chinai Del Ponte 1929, P. diasi Pinto and Lent 1946, P. geniculatus Latreille 1811, P. guentheri Berg 1879, P. herreri Wygodzinsky 1948, P. howardi Neiva 1911, P. humeralis Usinger 1939, P. lenti Galvão and Palma 1968, P. lignarius Walker 1873, P. lutzi Neiva and Pinto 1923, P. megistus Burmeister 1835, P. rufotuberculatus Champion 1899, and P. tupynambai Lent 1942.3,5,6 These species are distributed from Argentina to Nicaragua4,7–9 and widespread in sylvatic, peridomestic, and domestic habitats, especially those associated with mammals and birds.6
The epidemiological importance of some species of Panstrongylus has increased in the last few years, because species that were previously restricted to sylvatic environments have now invaded and colonized human dwellings.6,10 In addition to that, some species of this genus have a high capacity as Chagas vectors because of their longevity, rapid response to the presence of a host, large volume of blood ingested, and frequent defecation during the feeding process.10
In Ecuador, 16 species of triatomines have been reported, including 6 species of Panstrongylus (P. chinai, P. geniculatus, P. howardi, P. lignarius, P. herreri, and P. rufotuberculatus).3,11–13 P. howardi is endemic to Ecuador9,11,14 and considered a sylvatic species, with occasional records of specimens found in human dwellings.10 Its distribution seems to be limited to Manabí Province in the central coastal region of Ecuador.11
Diverse habitats, temperature, and humidity conditions, such as housing characteristics, are factors that influence the distribution of different species of triatomines. For example, traditional house construction in Manabí Province consists of walls made with bamboo cane, locally known as guadua cane (Guadua angustifolia [Kunth, 1822]), and roofs constructed with leaves of the Phytelephas aequatorialis (Spruce 1869) palm, locally known as cade or tagua palm, the nuts of which are used to manufacture handcrafts and buttons as an income source.15 Previous studies have suggested that, although permeable to bugs, guadua cane walls do not offer good triatomine hiding places, possibly preventing colonization.16 However, the environmental heterogeneity of the peridomestic habitat increases the probability of the presence of different species of triatomines.17,18 The presence of sylvatic triatomines in this region13 that can readily invade treated houses when residual effect of the insecticide declines constitutes a major obstacle for long-term control of Chagas disease.16
Although little is known about its ecology and habitats, P. howardi has been shown to have strong synanthropic tendencies and should be considered as an important secondary vector of T. cruzi, especially in areas where the populations of primary vectors (e.g., T. dimidiata) are being eliminated from domestic and peridomestic environments.
The aims of this study are to (1) describe the abundance and colonization of P. howardi in domestic and peridomestic habitats in the coastal Ecuadorian province of Manabí, (2) determine the infection by trypanosomes in the bugs and identify the blood source used for feeding, and (3) determine the habitat overlap with other epidemiologically important triatomine species—such as T. dimidiata and R. ecuadoriensis—that are currently being targeted by active vector control campaigns and its potential to replace them.
Materials and Methods
Study area.
This study was carried out in 10 rural communities in Portoviejo County, Manabí Province, Ecuador (Figure 1 and Table 1) during 2004, 2007, and 2008. Manabí Province is located along the central coast of Ecuador and receives an average annual rainfall of 563 mm per year.19 The main economic activity is agriculture, predominantly sugar cane, oranges, bananas, yucca, corn (or maize), and rice. In addition, some palms, such as cade or tagua palm (P. aequatorialis) and coconut (Cocos nucifera L.),15,20 are cultivated between 0 and 1,500 m in this region.
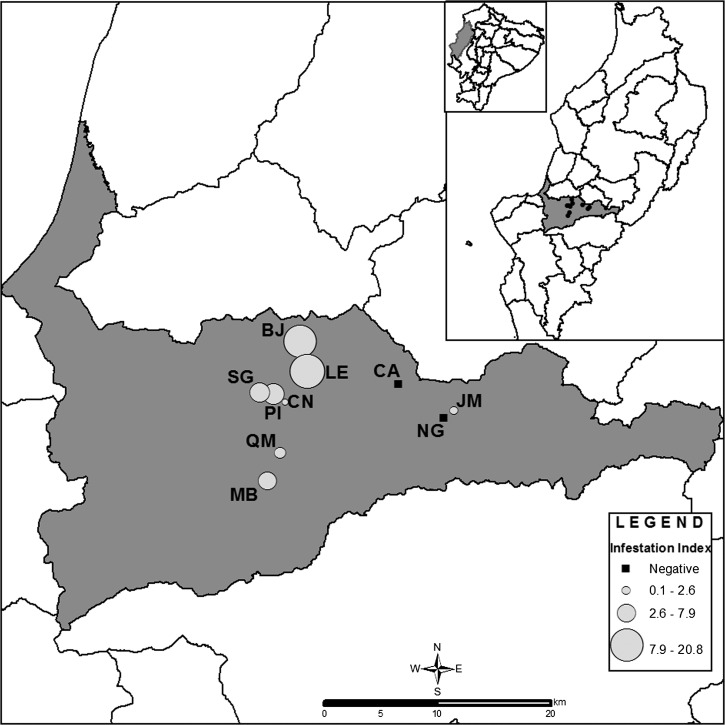
Figure 1.
Map of Portoviejo County, Manabí Province indicating the location and P. howardi infestation rates of 10 rural communities studied on the central coast of Ecuador. Lines indicate county boundaries. Inset shows Ecuador's political division and study area. BJ, Bejuco; CA, Cruz Alta de Miguelillo; CN, La Ciénega; JM, Jesús María; LE, La Encantada; MB, Maconta Abajo; NG, San Gregorio; PI, Pimpiguasí; QM, Quebrada de Maconta; SG, San Gabriel.
Table 1.
Entomological indices of P. howardi in rural communities in the central coastal region of Ecuador
| Communities | Altitude (m) | DUs | DUs searched | Number of bugs collected | Infestation index (%) | Density | Crowding | Colonization index (%) |
|---|---|---|---|---|---|---|---|---|
| Pimpiguasí | 24–70 | 117 | 91 | 93 | 8.8 | 1.0 | 11.6 | 25.0 |
| La Encantada | 19–290 | 124 | 98 | 109 | 21.4 | 1.1 | 5.2 | 23.8 |
| Cruz Alta de Miguelillo | 105–150 | 132 | 102 | – | – | – | – | – |
| San Gabriel | 56 | 113 | 94 | 126 | 8.5 | 1.3 | 15.8 | 50.0 |
| La Ciénega | 52 | 84 | 84 | 99 | 1.2 | 1.2 | 99.0 | 100.0 |
| Quebrada de Maconta | 92 | 162 | 123 | 30 | 4.1 | 0.2 | 6.0 | 60.0 |
| Bejuco | 65–400 | 106 | 72 | 50 | 20.8 | 0.7 | 3.3 | 60.0 |
| Maconta Abajo | 68–144 | 53 | 38 | 4 | 7.9 | 0.1 | 1.3 | – |
| Jesús María | 65–400 | 50 | 39 | 7 | 2.6 | 0.2 | 7.0 | 100.0 |
| San Gregorio | 74–220 | 50 | 34 | – | – | – | – | – |
| Total | 991 | 775 | 518 | 8.0 | 0.7 | 8.4 | 40.3 |
Triatomine searches and entomological indices.
Teams of two trained field workers from the National Chagas Disease Control Program from the Ecuadorian Ministry of Health searched for triatomines in each domestic unit (DU), which included domestic and peridomestic habitats. Pontifical Catholic University of Ecuador and Ohio University personnel closely monitored all field activities. The teams conducted manual searches using the 1 man-hour method as previously described.12 Signed informed consent was obtained from the head of each DU according to a protocol approved by the Ohio University and the Catholic University of Ecuador Institutional Review Boards (IRBs).
The following entomological indices for domestic and peridomestic infestations were calculated: infestation index (100 × number of houses infested/number of houses searched), density (number of triatomines captured/number of houses searched), crowding (number of triatomines captured/number of houses infested), and colonization index (100 × number of houses with nymphs/number of houses infested).21
Natural infection with trypanosomes and meal source analysis.
We determined natural infection with trypanosomes by direct microscopy. Differential identification of T. cruzi and T. rangeli infection was carried out by polymerase chain reaction (PCR) of the kinetoplast DNA from intestinal contents samples with the S35/S36 primer set as previously described.22 One band of 330 bp was expected for samples infected with T. cruzi, and a band of 740 bp, together with a series of bands among 300–400 pb, was expected for T. rangeli infections. A mixed infection can be identified when bands of 330 and 740 bp are present. All of the samples determined as T. rangeli or mixed infection were analyzed with an additional pair of primers that targets the D7a divergent domain of the large subunit ribosomal RNA gene (D75/D76),23 which differentiates the species of Trypanosoma because of the expected band size of 250 or 265 bp for T. cruzi and 210 bp for T. rangeli. The infection index (100 × number of triatomines with Trypanosoma spp./number of studied triatomines)21 was calculated for infection with T. cruzi, T. rangeli, and mixed infections.
The triatomines' intestinal contents were analyzed using primers for cytochrome b as described previously24 to determine the origin of their blood meal (mammal and/or avian). Additional analysis with cytochrome b of amphibians was carried out in a sample isolated from a triatomine found in association with frogs using the protocol as previously described.25 Sequencing of cytochrome b of seven intestinal content samples from triatomines was carried out as previously described.26 Sequences were visualized and edited using BioEdit software (Tom Hall Ibis Biosciences, Carlsbad, CA), and the identification of the blood meal source was done comparing the homology of the sequences obtained with the sequences in the nucleotide BLAST database (http://blast.ncbi.nlm.nih.gov/).
Results
Entomological indices.
In total, 775 DUs were enrolled in 2004, 2007, and 2008. Sixty-two houses (infestation index = 8%) in 8 of 10 communities visited were found to be infested with P. howardi. The infestation index was higher in La Encantada (21.4%) and El Bejuco (20.8%). In total, 518 bugs were collected (density = 0.7 bugs/DUs searched, crowding = 8.4 bugs/infested DUs, colonization index = 40.3% DUs with nymphs/infested DUs) (Figure 1 and Table 1).
Domiciliary infestation.
In total, 21 bugs were collected inside 11 DUs (infestation index = 1.4%, density = 0.03, crowding = 1.9). All of the individuals were found in bedrooms under the beds. Only 4 of 11 houses harbored nymphs inside of the dwelling (colonization index = 36.4%) (Table 2).
Table 2.
Peridomestic and domestic entomological indices of P. howardi in rural communities in central coastal Ecuador
| Communities | Infestation index (%) | Density | Crowding | Colonization index (%) | ||||
|---|---|---|---|---|---|---|---|---|
| P | D | P | D | P | D | P | D | |
| Bejuco | 18.1 | 2.8 | 0.7 | 0.04 | 3.6 | 1.5 | 61.5 | 50.0 |
| Pimpiguasí | 7.7 | 1.0 | 1.0 | 0.01 | 13.1 | 1.0 | 28.6 | – |
| Cruz Alta | – | – | – | – | – | – | – | – |
| San Gabriel | 7.5 | 1.1 | 1.3 | 0.01 | 17.9 | 1.0 | 42.9 | 100.0 |
| La Encantada | 15.3 | 6.1 | 1.0. | 0.2 | 6.3 | 2.5 | 20.0 | 33.3 |
| Macota Abajo | 7.9 | – | 0.1 | – | 1.3 | – | – | – |
| Jesús María | 2.6 | – | 0.2 | – | 7.0 | – | 100.0 | – |
| San Gregorio | – | – | – | – | – | – | – | – |
| La Ciénega | 1.2 | – | 1.2 | – | 99.0 | – | 100.0 | – |
| Quebrada de Maconta | 3.3 | 0.8 | 0.2 | 0.01 | 7.3 | 1.0 | 75.0 | – |
| Total | 6.6 | 1.4 | 0.6 | 0.03 | 9.8 | 1.9 | 41.2 | 36.4 |
D = domestic; P = peridomestic.
Peridomiciliary infestation.
In total, 474 bugs were collected in the peridomestic habitats in 50 DUs (infestation index = 6.6%, density = 0.6, crowding = 9.8). Colonization was found in the peridomicile of 41.2% of the infested DUs (Table 2).
Microhabitat preferences in the peridomicile.
In total, specimens of P. howardi were found in 50 infested DUs occupying a variety of microhabitats that included rodent nests under bricks (71.1% of bugs collected), wood (11.8%), rock piles (1.7%), and within leafs of the thorny piñuela plant (Aechmea magdalenae [André ex Baker], Bromeliaceae; 15.4%). Of those 474 specimens, 13 individuals (7 individuals in bricks, 5 individuals in wood, and 1 individual within piñuelas) were found sharing microhabitats with R. ecuadoriensis. In El Bejuco, nine individuals (one nymph I [NI], four NII, three NIII, and one male adult) of P. howardi were found near two bullfrogs (cf. Rana catesbeiana) that were hiding under a pile of bricks.
Natural infection with trypanosomes and meal source analysis.
In total, 47 individuals of P. howardi from 5 (La Ciénega, El Bejuco, La Encantada, Pimpiguasí, and San Gabriel) of 10 communities (entomological searches) were assessed for infection by trypanosomes. Trypanosoma-like parasites were detected in 29.8% of the samples analyzed by microscopy. By PCR, infection was detected in 61.7% of the samples; from these samples, 57.5% were infected only with T. cruzi, and 8.6% were infected with T. rangeli, including mixed infections (Table 3). Triatomines infected with T. cruzi were collected in the peridomestic environment, brick and rock piles, leaves of piñuelas, and association with rat and opossum nests. One infected triatomine was collected under a pile of bricks near cf. R. catesbiana. All of the intestinal content samples were also analyzed to identify the source of blood meals. Mammalian blood was found in 14 samples (29.8%), and avian blood was found in 3 samples (6.4%). PCR of the remaining 30 triatomine samples did not produce any product. The sample from the triatomine found associated with frogs did not amplify for amphibian cytochrome b. Specific determination of source blood meal conducted by DNA sequencing of the cytochrome b was possible in three samples. The blood meal sources included pigeon (Columbidae), a spiny rat of the genus Proechimys, and black rat (Rattus rattus). It was not possible to get analyzable sequences from the other samples.
Table 3.
Infection index with T. cruzi and/or T. rangeli in intestinal content samples from P. howardi collected in the peridomestic environment from five communities in central coastal Ecuador
| Community (n)* | T. cruzi (%) | T. rangeli (%) | Mixed infection T. cruzi/T. rangeli (%) |
|---|---|---|---|
| El Bejuco (14) | 78.6 | –† | 7.1 |
| La Ciénega (15) | 66.7 | 6.7 | –† |
| La Encantada (6) | –† | –† | –† |
| Pimpiguasí (1) | –† | –† | –† |
| San Gabriel (11) | 36.4 | 9.1 | 9.1 |
| Total (47) | 53.2 | 4.3 | 4.3 |
Number of triatomines analyzed from each community.
Represents 0.00% of infection of trypanosomes.
Discussion
We found a high infestation of P. howardi in DUs located in the Portoviejo River Valley, especially in peridomestic habitats. The collection of individuals of this species in the domestic environment in addition to reports from community members describing adults of P. howardi invading DUs when attracted to lights suggest the potential of this species and the genus10,11 to invade the domicile environment, with the increased chance of feeding from synanthropic animals and human beings. The results of this study indicate that P. howardi can colonize multiple microhabitats in the peridomicile. The main peridomestic microhabitats were associated with rodent nests located between brick piles. It is possible that the color of the bricks, which blends well with the orange dorsal coloration of P. howardi, provides camouflage that protects them from predators living or roaming in the spaces between the bricks. La Encantada and Bejuco were the communities with the highest infestation index, but more individuals were found in San Gabriel. The characteristics of all of the study communities are relatively similar. However, our data indicate that P. howardi has a preference for rat nests found within bricks piles, which could suggest that the presence of leftover bricks from house improvement projects could attract this species to colonize the peridomicile. This hypothesis should be further explored. Although less abundant, colonies of this species were also found in wood piles and piñuelas and in association with nesting places of rodents and marsupials. Moreover, it is important to consider that an increased risk of invasion from the peridomicile to the domicile has been pointed out in previous reports of the presence of adults and nymphs of P. howardi in the peridomestic habitats of the houses nearby sylvatic environment13 and the stable presence of peridomestic populations, despite periodic insecticide-based control.16 However, to date, only one individual of this endemic species has been found in a sylvatic habitat associated with mouse nests in a palm of Aiphanes eggersi.13
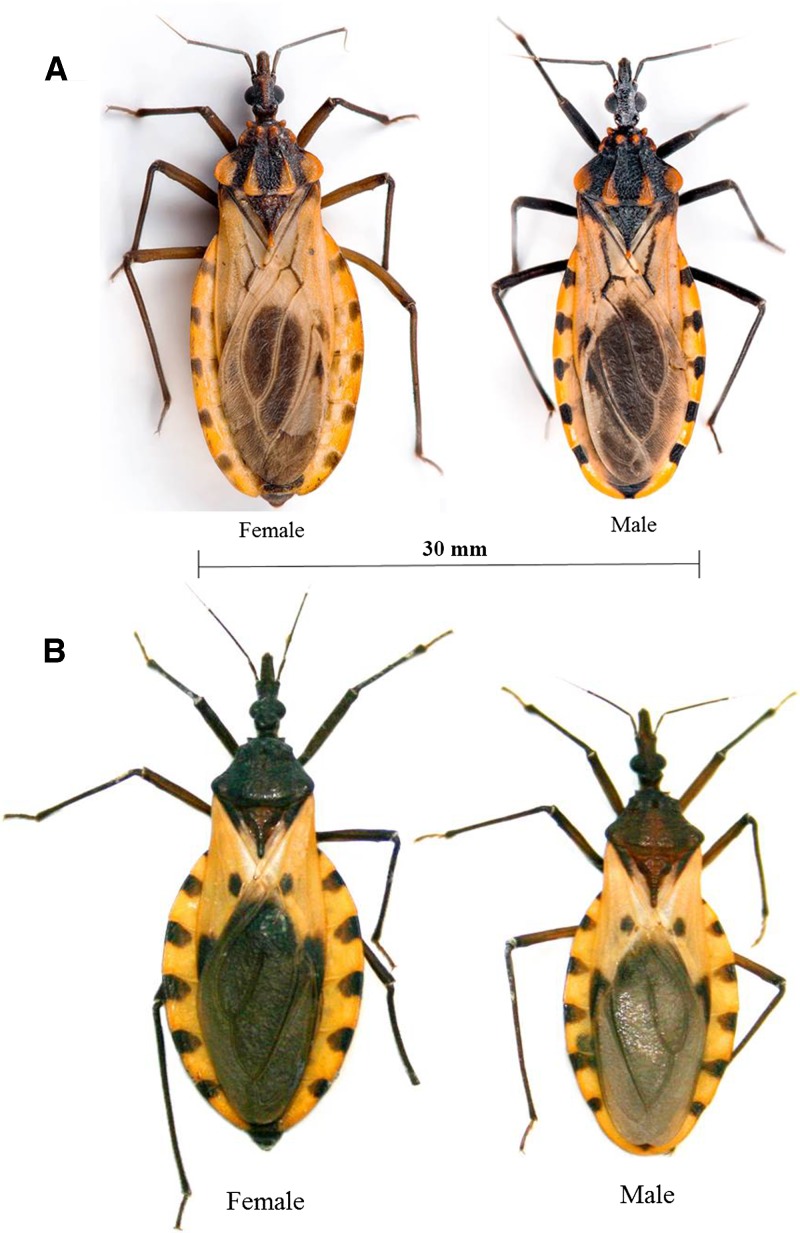
An important aspect to discuss is the overlapping distribution of P. howardi and T. dimidiata. The latter species has been considered for years the primary vector of Chagas disease in the coastal region of Ecuador.11 Moreover, the local Malaria Control Service (SNEM) has multiple records of T. dimidiata found in Manabí Province, including the studied area (Peñaherrera J and SNEM, personal communication). Interestingly, the extensive searches conducted by our group since 2001 have not found any T. dimidiata in rural areas of Manabí. This raises two possible scenarios. First, it is possible that the remarkable dorsal chromatic convergence shared by P. howardi and T. dimidiata3,9 and the lack of close examination of the antenniferous tube and pronotum could have caused misidentification of P. howardi as T. dimidiata (Figure 2) by field personnel from SNEM. Unfortunately, no specimens have been maintained by SNEM that would allow for taxonomical confirmation. Second, earlier spraying efforts to control mosquitoes and triatomine populations (especially for T. dimidiata in Ecuador as one of the main goals within the Andean Countries Initiative) might have decreased or eliminated T. dimidiata from this area, leaving an open ecological niche that could be occupied by P. howardi, which was previously suggested.27
Figure 2.
(A) Female and male P. howardi (endemic species from Manabí Province, Ecuador). (B) Female and male T. dimidiata (introduced species in Manabí and Guayas Provinces in Ecuador). Notice the similarities on dorsal coloration patterns, which could lead to misidentification.
To address a likely issue of misidentification of species, since 2004, the Center for Infectious Disease Research at the Catholic University of Ecuador has provided periodic training to field personnel from SNEM and the National Chagas Disease Control program. This training has included taxonomical identification and technical support to conduct entomological surveillance. However, to our knowledge, no systematic triatomine control work has been done during the last 5 years in these communities. Recent province-wide entomological surveys indicate that the infestation levels with P. howardi in this area remain unchanged (Grijalva MJ, unpublished data).
In relation to the presence of Trypanosoma parasite populations in the study area, previous studies in reservoirs from Manabí Province have determined the presence of T. cruzi and T. rangeli circulating in the peridomestic environment among rodents and opossums,28 which confirms a continuous flow of parasite populations among mammalian hosts. However, the high T. cruzi infection rate of P. howardi collected in peridomestic habitats suggests the existence of active transmission between this vector species and its associated vertebrate hosts. The circulation of Trypanosoma populations in peridomestic hosts constitutes an important risk factor for transmission of Chagas disease to humans. Moreover, the fact that adult P. howardi reach houses when attracted by lights increases the potential risk for T. cruzi transmission.
In general, we have found a low infection rate with T. rangeli in triatomines collected in domestic/peridomestic habitats (Grijalva MJ, unpublished data). Furthermore, T. rangeli has been associated mainly with bugs from the genus Rhodnius.29 The finding of this parasite in intestinal contents of P. howardi opens an interesting topic for research on the vectorial capacity of these bugs and their role in the dispersal of T. rangeli in the study area, and additional studies that include the analysis of salivary glands need to be carried out.
The meal source of the triatomines could not be assessed in most of the cases; however, the results showed presence of DNA from vertebrate hosts (mammals and birds) in P. howardi collected in the peridomestic environment. Interestingly, finding species such as Proechymis spp. and R. rattus, which are considered synanthropic species because of their capacity to circulate in the sylvatic environment as well as the peridomestic environment, is an important piece of information for a better understanding of the flow of T. cruzi among the sylvatic, peridomestic, and sylvatic transmission cycles. These synanthopic species can be the link for the parasite populations from sylvatic to domestic/peridomestic areas, which implies a source of infection for humans. These results, together with previous studies in Ecuador,28 remark on the importance of considering the reservoirs when triatomine control strategies are designed. Additionally, the association of triatomines with other vertebrates, such as amphibians, needs to be further addressed. Although we found several P. howardi individuals in close association with bullfrogs, our results did not provide any evidence that the triatomines were feeding on them. However, amphibian–triatomine association has been previously reported in palm trees in the Amazon.30
Additional research on P. howardi is needed to describe its feeding and defecation habits, lifecycle, geographical distribution, and phylogenetic associations with related species. Nevertheless, the ecological preferences of P. howardi, the high infestation index, the high levels of T. cruzi infection, the efficient prandial and post-prandial defecation habits (Villacis AG, unpublished data), and the high adaptive ability observed all contribute to the importance of P. howardi as an emerging vector for Chagas disease transmission in the central coastal region of Ecuador and should be considered in the implementation of effective short- and long-term control strategies.
ACKNOWLEDGMENTS
Special thanks to the inhabitants of the communities where this study was conducted. Technical assistance was provided by Sofía Muñoz, Alejandra Zurita, and Josselyn García from the Center for Infectious Disease Research at Catholic University of Ecuador; the personnel from the National Chagas Control Program–National Vector Borne-Disease Control Service, Ecuadorian Ministry of Health; and Melissa Bell from the London School of Hygiene and Tropical Medicine. This research received technical assistance from international collaboration through the European Community - Latin American Network for Research on the Biology and Control of Triatominae (ECLAT) network. Training in insecticide application was provided to field workers by Manuel Lluberas from H. D. Hudson Manufacturing Company. Assistance on data analyses and manuscript preparation were provided by Mario Chen and Suzanne Fischer from Family Health International and the International Clinical Sciences Support Center under contract to the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Financial support: Financial support was received from The United Nations Children's Fund (UNICEF) - United Nations Development Programme (UNDP)/World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases Research Capability Strengthening Group, Ohio University Heritage College of Osteopathic Medicine, Pontifical Catholic University of Ecuador, Children's Heartlink, and Plan International Ecuador.
Authors' addresses: Anita G. Villacís, Sofia Ocaña-Mayorga, César A. Yumiseva, and Esteban G. Baus, Center for Infectious Disease Research, School of Biological Sciences, Catholic University, Quito, Ecuador, E-mails: agvillacis@puce.edu.ec, sbocana@puce.edu.ec, cayumiseva@puce.edu.ec, and egbaus@puce.edu.ec. Mauricio S. Lascano, Center for Global Health, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: SLascano@cdc.gov. Mario J. Grijalva, Tropical Disease Institute, Biomedical Sciences Department, Heritage College of Osteopathic Medicine, Ohio University, Athens, OH, E-mail: grijalva@ohio.edu.
References
- 1.PAHO . Technical Report: Quantitative Chagas Disease Estimates. Montevideo, Uruguay: Pan American Health Organization; 2006. [Google Scholar]
- 2.Schofield CJ, Jannin J, Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Schofield CJ, Galvao C. Classification, evolution, and species groups within the Triatominae. Acta Trop. 2009;110:88–100. doi: 10.1016/j.actatropica.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Marcilla A, Bargues MD, Abad-Franch F, Panzera F, Carcavallo RU, Noireau F, Galvao C, Jurberg J, Miles MA, Dujardin JP, Mas-Coma S. Nuclear rDNA ITS-2 sequences reveal polyphyly of Panstrongylus species (Hemiptera: Reduviidae: Triatominae), vectors of Trypanosoma cruzi. Infect Genet Evol. 2002;1:225–235. doi: 10.1016/s1567-1348(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 5.Galvao CCR, Rocha DS, Jurberg JA. A checklist of the current valid species of the subfamily Triatominae Jeannel, 1919 (Hemiptera, Reduviidae) and their geographical distribution, with nomenclatural and taxonomic notes. Zootaxa. 2003;202:1–36. [Google Scholar]
- 6.dos Santos CM, Jurberg J, Galvao C, Rocha Dda S, Fernandez JI. Morphometric study of the genus Panstrongylus Berg, 1879 (Hemiptera, Reduviidae, Triatominae) Mem Inst Oswaldo Cruz. 2003;98:939–944. doi: 10.1590/s0074-02762003000700014. [DOI] [PubMed] [Google Scholar]
- 7.Jurberg J, Carcavallo RU, Lent H. Panstrongylus sherlocki sp.n. do estado da Bahia, Brasil (Hemiptera, Reduviidae, Triatominae) Entomol Vect. 2001;8:261–274. [Google Scholar]
- 8.Jurberg J, Galvão C. Biology, ecology, and systematics of Triatominae (Heteroptera, Reduviidae), vectors of Chagas disease, and implications for human health. Festschrift zum 70. Geburtstag von Ernst Heiss. Denisia 19, zugleich Katalogue der OÖ. Landesmuseen Neue Serie 50 Linz. 2006;19:1095–1116. [Google Scholar]
- 9.Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera: Reduviidae), and their significance as vectors of Chagas´ disease. Bull Amer Mus Nat Hist. 1979;163:123–520. [Google Scholar]
- 10.Patterson JS, Barbosa SE, Feliciangeli MD. On the genus Panstrongylus Berg 1879: evolution, ecology and epidemiological significance. Acta Trop. 2009;110:187–199. doi: 10.1016/j.actatropica.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Abad-Franch F, Paucar A, Carpio C, Cuba CA, Aguilar HM, Miles MA. Biogeography of Triatominae (Hemiptera: Reduviidae) in Ecuador: implications for the design of control strategies. Mem Inst Oswaldo Cruz. 2001;96:611–620. doi: 10.1590/s0074-02762001000500004. [DOI] [PubMed] [Google Scholar]
- 12.Grijalva MJ, Palomeque-Rodriguez FS, Costales JA, Davila S, Arcos-Teran L. High household infestation rates by synanthropic vectors of Chagas disease in southern Ecuador. J Med Entomol. 2005;42:68–74. doi: 10.1093/jmedent/42.1.68. [DOI] [PubMed] [Google Scholar]
- 13.Suarez-Davalos V, Dangles O, Villacis AG, Grijalva MJ. Microdistribution of sylvatic triatomine populations in central-coastal Ecuador. J Med Entomol. 2010;47:80–88. doi: 10.1603/033.047.0111. [DOI] [PubMed] [Google Scholar]
- 14.Marcilla A, Bargues MD, Abad-Franch F, Panzera F, Carcavallo RU, Noireau F, Galvao C, Jurberg J, Miles MA, Dujardin JP, Mas-Coma S. Nuclear rDNA ITS-2 sequences reveal polyphyly of Panstrongylus species (Hemiptera: Reduviidae: Triatominae), vectors of Trypanosoma cruzi. Infect Genet Evol. 2002;1:225–235. doi: 10.1016/s1567-1348(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 15.Pintaud JC, Galeano G, Balslev H, Bernal R, Borchsenius F, Ferreira E, de Granville JJ, Mejía K, Milán B, Moraes M, Noblick L, Stauffer FW, Kahn F. Las palmeras de América del Sur: diversidad, distribuci?n e historia evolutiva. Rev peru biol. 2008;15:7–29. [Google Scholar]
- 16.Grijalva MJ, Villacis AG, Ocana-Mayorga S, Yumiseva CA, Baus EG. Limitations of selective deltamethrin application for triatomine control in central coastal Ecuador. Parasit Vectors. 2011;4:20. doi: 10.1186/1756-3305-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gürtler RE. Control campaigns against Triatoma infestans in a rural community of northwestern Argentina. Medicina (B Aires) 1999;59((Suppl 2)):47–54. [PubMed] [Google Scholar]
- 18.Abad-Franch F, Monteiro FA. Biogeography and evolution of Amazonian triatomines (Heteroptera: Reduviidae): implications for Chagas disease surveillance in humid forest ecoregions. Mem Inst Oswaldo Cruz. 2007;102((Suppl 1)):57–70. doi: 10.1590/s0074-02762007005000108. [DOI] [PubMed] [Google Scholar]
- 19.INAMHI Manabí: Climatología: características generales del clima en la provincia de Manabí-Ecuador. 2009. http://www.inamhi.gov.ec Available at. Accessed November 12, 2009.
- 20.Abad-Franch F, Palomeque FS, Aguilar HM, Miles MA. Field ecology of sylvatic Rhodnius populations (Heteroptera, Triatominae): risk factors for palm tree infestation in western Ecuador. Trop Med Int Health. 2005;10:1258–1266. doi: 10.1111/j.1365-3156.2005.01511.x. [DOI] [PubMed] [Google Scholar]
- 21.WHO . Control of Chagas Disease. Geneva: World Health Organization; 2002. [Google Scholar]
- 22.Vallejo GA, Guhl F, Chiari E, Macedo AM. Species specific detection of Trypanosoma cruzi and Trypanosoma rangeli in vector and mammalian hosts by polymerase chain reaction amplification of kinetoplast minicircle DNA. Acta Trop. 1999;72:203–212. doi: 10.1016/s0001-706x(98)00085-0. [DOI] [PubMed] [Google Scholar]
- 23.Souto RP, Vargas N, Zingales B. Trypanosoma rangeli: discrimination from Trypanosoma cruzi based on a variable domain from the large subunit ribosomal RNA gene. Exp Parasitol. 1999;91:306–314. doi: 10.1006/expr.1998.4380. [DOI] [PubMed] [Google Scholar]
- 24.Ngo KA, Kramer LD. Identification of mosquito bloodmeals using polymerase chain reaction (PCR) with order-specific primers. J Med Entomol. 2003;40:215–222. doi: 10.1603/0022-2585-40.2.215. [DOI] [PubMed] [Google Scholar]
- 25.Goebel AM, Donnelly JM, Atz ME. PCR primers and amplification methods for 12S ribosomal DNA, the control region, cytochrome oxidase I, and cytochrome b in bufonids and other frogs, and an overview of PCR primers which have amplified DNA in amphibians successfully. Mol Phylogenet Evol. 1999;11:163–199. doi: 10.1006/mpev.1998.0538. [DOI] [PubMed] [Google Scholar]
- 26.Svobodova M, Alten B, Zidkova L, Dvorak V, Hlavackova J, Myskova J, Seblova V, Kasap OE, Belen A, Votypka J, Volf P. Cutaneous leishmaniasis caused by Leishmania infantum transmitted by Phlebotomus tobbi. Int J Parasitol. 2009;39:251–256. doi: 10.1016/j.ijpara.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Abad-Franch F, Aguilar VH, Paucar CA, Lorosa ES, Noireau F. Observations on the domestic ecology of Rhodnius ecuadoriensis (Triatominae) Mem Inst Oswaldo Cruz. 2002;97:199–202. doi: 10.1590/s0074-02762002000200010. [DOI] [PubMed] [Google Scholar]
- 28.Pinto CM, Ocana-Mayorga S, Lascano MS, Grijalva MJ. Infection by trypanosomes in marsupials and rodents associated with human dwellings in Ecuador. J Parasitol. 2006;92:1251–1255. doi: 10.1645/GE-886R.1. [DOI] [PubMed] [Google Scholar]
- 29.Vallejo GA, Guhl F, Schaub GA. Triatominae-Trypanosoma cruzi/T. rangeli: vector-parasite interactions. Acta Trop. 2009;110:137–147. doi: 10.1016/j.actatropica.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Teixeira AR, Monteiro PS, Rebelo JM, Arganaraz ER, Vieira D, Lauria-Pires L, Nascimento R, Vexenat CA, Silva AR, Ault SK, Costa JM. Emerging Chagas disease: trophic network and cycle of transmission of Trypanosoma cruzi from palm trees in the Amazon. Emerg Infect Dis. 2001;7:100–112. doi: 10.3201/eid0701.700100. [DOI] [PMC free article] [PubMed] [Google Scholar]