Abstract
In Colombia, the main vectors of Trypanosoma cruzi, the causative agent of Chagas disease, are Rhodnius prolixus and Triatoma dimidiata. T. dimidiata is present in the east region of Colombia as domestic, peridomestic, and sylvatic populations, resulting in difficulties for its control. A cost-effective way to prioritize houses for treatment is to stratify houses based on risk factors. In this study, risk factors were evaluated for potential associations with domicile infestation of T. dimidiata. There was an increased likelihood of domestic infestation associated with the presence of mixed roofs (odds ratio [OR] = 36.14, 95% confidence interval [95% CI] = 12.21–106.97), cats (OR = 3.94, 95% CI = 1.36–11.38), rock piles (OR = 5.28, 95% CI = 1.64–16.98), and bushes with height above 10 m (OR = 11.21, 95% CI = 2.08–60.45). These factors could be used to target surveillance and control of T. dimidiata to houses with an increased risk of being infested.
Introduction
Chagas disease, caused by Trypanosoma cruzi, is one of Latin America's highest burden diseases; almost all of the 8–9 million cases occur in poor rural and, increasingly, new urban and periurban areas of Latin America, where the burden of disease is between 5 and 10 times greater than malaria.1,2 The most updated statistics in Colombia show that 436,000 people are infected (prevalence rate = 0.96%), 131,474 persons have cardiopaty, 5,250 cases caused by vectorial transmission are found each year, 1,000 new cases of congenital Chagas disease are also found each year, 107,800 women with ages ranging between 15 to 44 years old are infected, and finally, 4,792,000 people are at risk.3
In Colombia, there are 26 recorded triatomine species, of which Rhodnius prolixus, Triatoma dimidiata, T. maculata, and T. venosa have been registered in domiciles and peridomiciles.4 R. prolixus is the main vector, but T. dimidiata is also important in the rural transmission of T. cruzi and considered the secondary vector in the country.5,6 The Chagas Disease Control Program (CDCP) carried out in 1998–1999 in 79 municipalities and 205 villages showed that T. dimidiata was widespread in 13 departments that were both endemic and non-endemic for Chagas disease.7
The species has been found in dry and humid forests from sea level to about 2,000 m above sea level.8,9 Its natural microenvironments include caves, palm trees, rock piles, hollow trees, and opossum nests.9,10 Regarding its distribution in the country, T. dimidiata is found in domestic, peridomestic, and sylvatic environments. Populations from the northwest of the country (Caribbean plains) are restricted to palm tree habitats, and domestic involvement is limited to sporadic visits because of attraction by light. However, populations from the east region of the country (Boyacá and Santander states) present a complex epidemiological distribution, including sylvatic, peridomestic, and domiciliated ecotopes. For this reason, T. dimidiata is considered native and widespread throughout the country in extradomestic habitats, and therefore, elimination is almost impossible. Continuous monitoring and cost-effective targeting of control will be necessary to maintain domiciles free of infestation (Parra-Henao G and others, unpublished data).
Previous studies, mainly in Central America, have shown the importance of housing characteristics for T. dimidiata infestation, with houses with poor sanitary conditions, dirt floors, and tile roofs being more commonly infested.8,11–17 A study carried out in Colombia to evaluate the house-level risk factors for triatomine infestation showed that domestic infestation is associated with over seven inhabitants (odds ratio [OR] = 1.24, 95% confidence interval [95% CI] = 1.11–1.39), overhead storage space (OR = 1.16, 95% CI = 1.03–1.32), a grain shed (OR = 1.25, 95% CI = 1.02–1.52), and the presence of cats (OR = 1.27, 95% CI = 1.14–1.42).18 In Colombia, T. dimidiata has a great capacity to adapt to different environments, including sylvatic ecotypes, such as caves, rocks, tree holes, palms, and vertebrates' nests, in wet and dry zones and over a wide altitudinal range. It tends to inhabit urban and periurban areas because of its attraction to lights.6 In areas of Colombia where Chagas disease is endemic—mainly the eastern departments of Boyacá, Santander, Casanare, and Cundinamarca—T. dimidiata can generally be found in rural houses and peridomestic structures, establishing colonies hidden in adobe walls, under loose plaster, behind furniture, behind pictures, and in various peridomestic structures, such as chicken coops, caneys (peridomestic structures for drying tobacco leaves), and rock piles (usually accumulated in the course of land improvement).
House-level risk factors for triatomine infestation in Colombia have concentrated on R. prolixus.18,19 Although T. dimidiata is considered the secondary Chagas disease vector in Colombia, information about risk factors for domestic and peridomestic infestation is scarce and was obtained from the CDCP directed to six triatominae species; more accurate information regarding its behavior and risk factors for infestation is needed. Risk factors may vary spatially between regions because of variation in human and vector behavior, ecology, and environmental factors. This local knowledge can then be used to more effectively target houses for vector control and also, determine house characteristics that could be prioritized in house improvement programs.18 To better understand the risk of house infestation by T. dimidiata in Colombia, a large entomologic survey was done in 8 of 32 departments of the country, a total of 18 municipalities, and 44 villages. The identification of risk factors will be fundamental to the development of vector control interventions.
Materials and methods
Study area.
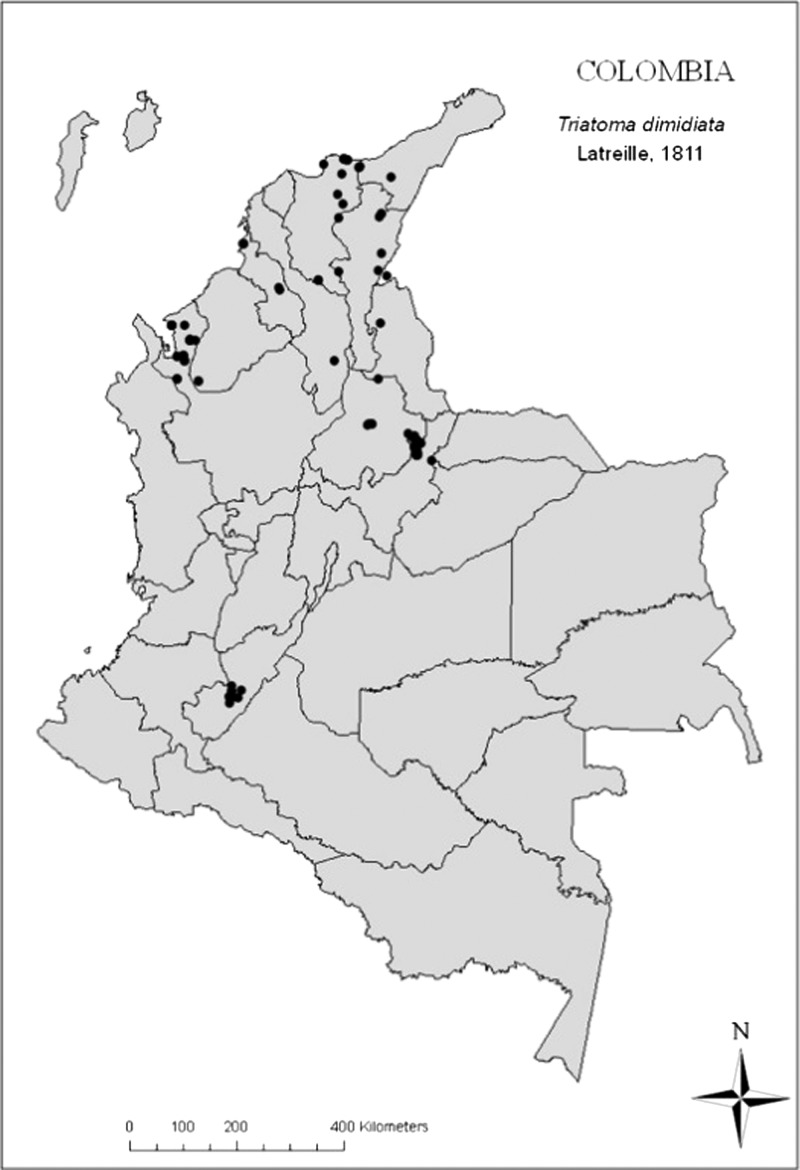
Field work was carried out from October of 2006 to June of 2008 in a broad area of the country that is located between latitudes 72° and 76° W and longitudes 2° and 11° N. The area includes the Colombian departments of Antioquia, Bolívar, Boyacá, Cesar, Magdalena, Santander, Sucre, and Huila (Figure 1). The departments were selected according to the known distribution of T. dimidiata in the country. We selected these departments, because they represent the main ecosystems in which T. dimidiata was found and fit our budget. The predominant Holdridge life zones are tropical wet forest in Antioquia, Santander, and Magdalena departments (western and central regions of the country), tropical dry forest in Sucre and Bolivar departments (western region of the country), lower montane wet forest and tropical dry forest in Boyacá and Santander departments (eastern region of the country), and finally, tropical wet forest and tropical dry forest in the southern region of the country (Huila department).20 The localities included are rural settlements where people cleared forested areas for raising crops and cattle. The peridomestic areas are characterized by chicken coops, cow sheds, and caneys.
Figure 1.
Geographic distribution of sampling sites.
Sample selection.
A cross-sectional survey was carried out at house level to investigate the role of potential risk factors for house infestation (indoor and outdoor) by T. dimidiata in a sample of the houses in the municipalities and villages where T. dimidiata has been reported. The dwellings were randomly selected from a pre-existing list of houses obtained from the health office of each municipality. T. dimidiata in Colombia is distributed in nine biogeographic zones; required sample sizes for houses in each zone were calculated assuming 5% infestation indices (error = 5%; confidence = 95%) determined by previous surveys.18 Overall, 500 houses in nine zones were selected, and 63 houses were sampled for each department.
Collection of data for risk factor analysis.
The domiciliary unit was divided into two areas: indoor (bedrooms, kitchen, and living area) and peridomestic (all outbuildings present around the house). Each locality was visited initially to inform residents about the objectives and activities of the study. Each household received educational leaflets describing Chagas disease signs and symptoms, transmission patterns, and the role of vectors. To identify risk factors for T. dimidiata infestation, a questionnaire was completed by interviewing an adult resident, and direct observation was made of variables related to housing materials (walls, roof, and floor), domestic animals (presence of dogs, cats, chickens, etc.), outbuildings (overhead storage, chicken coop, and grain shed), presence of vegetation (bushes, trees, and palm trees) around the houses, and domestic use of insecticides.
Triatomine collections.
Presence or absence of bugs in each residence was assessed through systematic searches for adult or nymph triatomines. We used standard methods for sampling bugs in each type of habitat intradomicile and peridomicile and calculated the infestation, colonization, and infection and dispersion indices.5 During each house survey visit performed by a pair of collectors, indoor and outdoor niches were searched for 30 minutes each. We recorded the presence and site of bugs (inside or outside the house), time of collection, and movement of furniture or outdoor materials for bug searching. A flashlight was used to help see into cracks and crevices throughout the fabric of buildings, behind pictures on the walls, behind furniture, in closets, and especially, under bedding material.
From each house, all of the bugs collected alive and dead (adults and nymphs) were placed in plastic tubes (separately for intradomicile and peridomicile samples) numbered with the house code and collection site. Bugs were transported to the laboratory, registered, and identified using taxonomic keys.21 The numbers, species, and stages of bugs obtained from each house were recorded on laboratory forms.
Detection of infection by T. cruzi in wild triatomines.
Collected insects were examined for T. cruzi infection. Fecal material was obtained through abdominal pressure or after meals. Feces from each insect were diluted in 500 μL sterile phosphate buffered saline (PBS) (pH 7.2). One aliquot was examined through direct observation with an optical microscope; the remaining fecal sample was conserved for posterior verification of T. cruzi infection when the other tests were positive. Blood parasites were diagnosed as T. cruzi by polymerase chain reaction (PCR) analysis. DNA was isolated from feces by the DNAzol® Reagent (Invitrogen, Carlsbad, CA) method following the manufacturer's instructions. T. cruzi was identified by amplification of kinetoplast DNA (kDNA) minicircles and intergenic spacer of miniexon gene using the S35 and S36 primers.22,23
Calculation of entomological indices.
The domiciliary infestation index was calculated by the quotient between the numbers of infested houses and the total numbers of examined houses. The colonization index was calculated as the result of dividing the number of houses with nymphs by the total number of houses positive for triatomines. The infection index was calculated by the quotient between the numbers of triatomines infected with T. cruzi and the total numbers of triatomines evaluated. The dispersion index was calculated as the quotient of the number of localities infested with triatomines by the whole of localities examined.
Data analysis.
To identify risk factors for house infestation by T. dimidiate, we performed bivariate analyses: χ2 or Fisher's exact tests for nominal variables and Student's t tests for continuous variables. ORs were also calculated to estimate the magnitude of the association with house infestation. Statistically significant (P < 0.05) variables were then fitted in logistic regression models for multivariable analysis of risk factors. Variables with P values of less than 0.1 were retained in the final multivariable model. Statistical analyses were performed using STATA, version 10 (StataCorp, College Station, TX).
Ethical considerations.
The ethical committee of the Colombian Institute of Tropical Medicine approved the research protocol through Resolution 31. Informed consent was obtained from each head of household.
Results
In total, 525 houses (5% more than the calculated sample size) in the eight selected departments were surveyed for T. dimidiata (Figure 1). Here, we describe only the findings for T. dimidiata. Infestation was detected in four departments, 10 municipalities, and 17 villages. The proportion of indoor infested house was 8.8% (46 of 525), and the proportion of infested peridomiciles was 6.3% (33 of 525). From the collected T. dimidiata, 33% were infected with T. cruzi. The highest entomological indices were found in the east region of the country (Boyacá and Santander departments), which shown in Table 1, and the lowest indices were found in the northwest region of the country (Antioquia, Sucre, and Magdalena departments). House walls were generally of wood (25.1%), cement blocks (25%), adobe (8.6%), or bahareque (earth or mud applied to a layer of wood or cane; 23.6%); they were fully plastered in 23.6% of houses, unplastered in 29.1% of houses, and partially plastered in 16.6% of houses. Roofs were generally corrugated metal sheets (54.9%), palm leaves (23.8%), or a combination (14.9%). House floors were made of concrete (51.8%), earth (40.2%), or a combination (5.9%).
Table 1.
Entomological indices for T. dimidiata in Colombia
| Department, municipality, and village | Infestation (%) | Colonization (%) | Natural infection (%) | Dispersion (%) |
|---|---|---|---|---|
| Santander | ||||
| Capitanejo | ||||
| Chorreras | 68.7 | 12.5 | 20.6 | 100 |
| Macaravita | ||||
| Buraga | 9.8 | 9.8 | 50 | 100 |
| San Vicente | ||||
| El Peltrecho | 4.4 | 0 | 0 | 75 |
| Granada | 13.5 | 0 | 25 | |
| Pradera | 33 | 0 | 0 | |
| El Carmen | ||||
| Cirales | 7.7 | 0 | 100 | 16 |
| Boyacá | ||||
| Soatá | ||||
| El Espinal | 60 | 20 | 10 | 75 |
| El Hatillo | 22 | 22 | 0 | |
| La Costa | 12.5 | 12.5 | 15.4 | |
| Tipacoque | ||||
| Nogal | 33 | 0 | 0 | 75 |
| Bavatá | 25 | 25 | 20 | |
| Ovachía | 33 | 100 | 0 | |
| Huila | ||||
| El Agrado | ||||
| Remolinos | 10 | 0 | 0 | 100 |
| Pital | ||||
| San Joaquín | 9.09 | 0 | 0 | 100 |
| Arrayán | 54.5 | 0 | 0 | |
| Gigante | ||||
| Río Loro | 40 | 0 | 0 | 50 |
| Cesar | ||||
| Valledupar | ||||
| Seynimen | 11.3 | 7.5 | 4 | 100 |
Infestation is the proportion of infested houses with triatomines. Colonization is the proportion of houses with triatomine nymph or eggs. Natural infection is the proportion of T. cruzi-infected triatomines. Dispersion is the proportion of villages with triatomines.
Domestic animals were found in 86.1% of houses, with the most common being chickens (81.1%), dogs (78.9%), and cats (52.2%). Wild animals, such as rats, were found indoors and outdoors (28.4% and 26.1%, respectively), and 26.8% of the houses had palm trees, mainly within a 10-m radius. Bushes of height less than 10 m were observed near 69.6% of the houses, and this vegetation predominates within a radius of 10 m. Trees higher than 10 m were found near 67.9% of the houses, mainly at a distance of 10–30 m.
Entomological indices.
The highest indices of domiciliary infestation, colonization, infection, and dispersion by T. dimidiata were found in the eastern region of Colombia (Santander and Boyacá departments), the eastern slope of the Sierra Nevada of Santa Marta (Seynimen village), and the upper Magdalena (Huila department) (Table 1). In the eastern region of the country (Boyacá and Santander departments), domestic infestation indices vary from 9.8% to 68.7%, the colonization index of houses varies from 9.8% to 22%, the dispersion index varies from 75% to 100%, and the infection index of T. dimidiata varies from 20% to 100%. In Seynimen, we found that those indices were lower. To the south of the country, in Huila department, the index of domiciliary infestation fluctuated between 9% and 54%, but the colonization index in all of the villages was zero, showing that insects visit peridomicles and extradomiciles but do not establish colonies inside houses. In those regions, the predominant life zones were humid premontane forest and dry tropical forest with altitudes from 300 to 2,300 m above sea level. The main habitats where T. dimidiata was found were houses followed by rock piles around the houses and rock caves in the extradomicile.
Intradomiciliary infestation.
Among variables associated with the intradomiciliary presence of T. dimidiata by bivariable analysis, walls of adobe or bahareque and mixed roofs were associated with a 7- to 27-fold increase in risk, and domestic animals were associated with an 8-fold increase in risk.
In terms of intradomicilary presence of T. dimidiata, houses with bahareque walls were found to have 13.2 times the infestation risk of those with block or brick walls (95% CI = 3.9–44.3) (Table 2). The corresponding OR for adobe was 6.6 (95% CI = 1.6–24.4). Houses with mixed-material roofs had 27 times the infestation risk of those with zinc roofs (95% CI = 12.0–59.1). No statistically significant differences between floor types were found (Table 2). The presence of domestic animals was associated with an eightfold increased risk of intradomiciliary T. dimidiata (95% CI = 1.1–58.7). More specifically, cats were associated with an OR of 2.2 (95% CI = 1.2–4.3), chickens were associated with an OR of 5.6 (95% CI = 1.1–58.7), and dogs were associated with an OR of 13.4 (95% CI = 1.8–98.4) (Table 3). With respect to annexes, the rock piles did not have statistical significance.
Table 2.
Selected results of bivariate analyses of associations between infestation and exposure variables in intradomicile
| Variable | N | With triatomines (%) | OR (95% CI) | χ2 (P value) |
|---|---|---|---|---|
| Wall type | 61.16 (< 0.0001) | |||
| Adobe | 45 | 6 (13.3) | 6.56 (1.57–27.47) | |
| Bahareque | 124 | 29 (23.4) | 13.2 (3.85–44.30) | |
| Brick/block* | 131 | 3 (2.3) | 1 | |
| Wood | 132 | 0 (0.0) | 0 | |
| Combination | 48 | 8 (16.7) | 8.53 (2.16–33.70) | |
| Plastering | ||||
| Full* | 124 | 19 (15.3) | 1 | 5.99 |
| Partial | 87 | 14 (16.1) | 1.06 (0.50–2.25) | 0.05 |
| No plaster | 153 | 11 (7.2) | 0.43 (0.20–0.94) | |
| Roof | ||||
| Zinc* | 288 | 9 (3.1) | 1 | 164.48 (< 0.0001) |
| Palm | 125 | 0 (0.0) | 0 | |
| Tiles | 9 | 0 (0.0) | 0 | |
| Combination | 78 | 36 (46.2) | 26.57 (11.95–59.09) | |
| Floor | 28.09 (< 0.001) | |||
| Mud | 211 | 2 (0.9) | 0.06 (0.01–0.25) | |
| Cement* | 272 | 38 (13.9) | 1 | |
| Other | 5 | 1 (20.0) | 1.54 (0.17–14.15) | |
| Combination | 31 | 5 (16.1) | 1.18 (0.43–3.27) | |
| Wood | 1 | 0 (0.0) | 0 | |
| Number of bushes with height < 10 m | 43.8 (< 0.0001) | |||
| Absence* | 160 | 14 (8.8) | 1 | |
| 1–10 | 193 | 5 (2.6) | 0.28 (0.10–0.79) | |
| 10–30 | 96 | 6 (6.3) | 0.7 (0.26–1.87) | |
| > 30 | 76 | 21 (27.6) | 3.98 (1.89–8.38) | |
| Number of bushes with height > 10 m | 54.5 (< 0.0001) | |||
| Absence* | 168 | 8 (4.8) | 1 | |
| 1–10 | 126 | 2 (1.6) | 0.32 (0.07–1.55) | |
| 10–30 | 132 | 9 (6.8) | 1.46 (0.55–3.9) | |
| > 30 | 99 | 27 (27.3) | 7.5 (3.25–17.31) | |
Reference category.
Table 3.
ORs and 95% CIs for the presence of triatomines in intradomicile according to the presence of domestic animals
| Variable | N | With triatomines (%) | OR (95% CI) | χ2 (P value) |
|---|---|---|---|---|
| Presence of domestic animals in houses | 5.8 (0.016) | |||
| Yes | 452 | 45 (9.9) | 7.96 (1.08–58.67) | |
| No* | 73 | 1 (1.4) | 1 | |
| Chickens | 6.94 (0.008) | |||
| Yes | 426 | 44 (10.3) | 5.59 (1.33–23.45) | |
| No* | 99 | 2 (2.0) | 1 | |
| Dogs | 10.88 (0.001) | |||
| Yes | 414 | 45 (10.9) | 13.41 (1.83–98.43) | |
| No* | 111 | 1 (0.9) | 1 | |
| Cats | 6.1 (0.014) | |||
| Yes | 274 | 32 (11.7) | 2.24 (1.16–4.30) | |
| No* | 251 | 14 (5.6) | 1 | |
| Pigs | 9.8 (0.002) | |||
| Yes | 120 | 2 (1.7) | 0.14 (0.03–0.58) | |
| No* | 405 | 44 (10.9) | 1 | |
| Goats | 0.002 (0.968) | |||
| Yes | 58 | 5 (8.6) | 0.98 (0.37–2.59) | |
| No* | 467 | 41 (8.8) | 1 | |
| Cattle | 1 (0.317) | |||
| Yes | 110 | 7 (6.4) | 0.66 (0.28–1.51) | |
| No* | 415 | 39 (9.4) | 1 | |
| Horses | ||||
| Yes | 53 | 6 (11.3) | 1.38 (0.56–3.42) | † (0.446) |
| No* | 472 | 40 (8.5) | 1 |
Reference category.
Fisher exact test.
In multivariable analysis, we found that, for every 1 house infested with T. dimidiate with a roof of zinc, there were 36 houses with a mixed roof infested with T. dimidiata (OR = 36.14, 95% CI = 12.21–106.97); for every 1 house without cats infested by T. dimidiata, there were 3.94 houses with cats infested by T. dimidiata (95% CI = 1.36–11.38) (Table 4).
Table 4.
ORs (95% CIs) of risk factors associated with intradomiciliary infestation in multivariable analysis
| Variable | OR (95% CI) adjusted |
|---|---|
| Roof type | |
| Zinc | 1 |
| Palm | 0 |
| Tiles | 0 |
| Combination | 36.14 (12.21–63) |
| Domestic animals | |
| Cats | 3.94 (1.36–11.38) |
| Number of bushes with height < 10 m | |
| Absence | 1 |
| 1–10 | 0.08 (0.01–0.54) |
| 10–30 | 0.12 (0.02–0.68) |
| > 30 | 0.4 (0.07–2.19) |
| Number of bushes with height > 10 m | |
| Absence | 1 |
| 1–10 | 1.36 (0.14–13.01) |
| 10–30 | 7.44 (1.12–49.53) |
| > 30 | 11.21 (2.08–60.45) |
Peridomiciliary infestation.
The presence of T. dimidiata in the peridomiciliary area was examined in relation to characteristics, such as presence of domestic and wild animals, outhouses, vegetation, and building construction materials. It was found to be associated with presence of dogs (OR = 4.4, 95% CI = 1.04–18.7), cats (OR = 3.1, 95% CI = 1.4–6.9), goats (OR = 2.8, 95% CI = 1.2–6.6), and cows (OR = 2.3, 95% CI = 1.4–6.9). There was no association with rats (P = 0.57). In terms of outhouses, the presence of caneys was associated with an OR of 5.0 (95% CI = 1.9–13.4). With respect to building construction materials, adobe (OR = 13.8, 95% CI = 3.7–52.2) and bahareque (OR = 3.7, 95% CI = 1.01–13.9) walls and mixed roofs (OR = 2.7, 95% CI = 1.3–5.9) were all associated. No statistically significant associations were found with other kinds of outhouses or the presence of palm trees or bushes.
For peridomiciliary infestation, we found that barn, caneys, trapiches (cane mills), and rock piles increased the risk by 11- to 32-fold (Table 5). In the multivariable model, wall material, annexes, numbers of palm trees, and rock piles showed independent associations with infestation. We found that, for every 1 house with block walls and the presence of T. dimidiate, there were 52 houses with bahareque walls infested by this species (OR = 51.69, 95% CI = 6.44–414.96), 42 houses with adobe walls infested by this species (OR = 42.4, 95% CI = 5.8–50.7), and 39 houses with mud walls infested with this species (OR = 39.09, 95% CI = 2.66–573.74). For every one house without caneys as annexes infested by T. dimidiate, we found nine houses with this type of annex infested by T. dimidiata (95% CI = 1.36–54.52). For rock piles, we found that, for every 1 house without rock piles infested by T. dimidiata, there were 28 houses with rock piles infested by T. dimidiata (95% CI = 6.52–119.69) (Table 6).
Table 5.
ORs and 95% CIs for the peridomestic presence of triatomines according to presence of annexes
| Variable | With triatomines | OR (95% CI) | χ2 (P value) | |
|---|---|---|---|---|
| Yes | No | |||
| Barn | † (0.011) | |||
| Yes | 2 | 1 | 31.68 (2.8–35.1) | |
| No* | 31 | 491 | 1 | |
| Caney | † (0.004) | |||
| Yes | 3 | 3 | 16.30 (3.15–22.22) | |
| No* | 30 | 489 | 1 | |
| Trapiche | † (0.034) | |||
| Yes | 2 | 3 | 10.52 (1.69–15.27) | |
| No* | 31 | 489 | 1 | |
| Rock piles | † (0.007) | |||
| Yes | 3 | 4 | 12.2 (2.61–27.0) | |
| No* | 30 | 488 | 1 | |
| Wall type | 32.21 (< 0.001) | |||
| Brick/block* | 3 | 128 | 1 | |
| Bahareque | 10 | 114 | 3.74 (1.01–13.94) | |
| Adobe | 11 | 34 | 13.8 (3.65–52.27) | |
| Wood | 4 | 128 | 1.33 (0.29–6.08) | |
| Mud | 2 | 29 | 2.94 (0.47–18.42) | |
| Combination | 3 | 45 | 2.84 (0.55–14.6) | 12.09 (0.017) |
| Floor | ||||
| Cement* | 25 | 247 | 1 | |
| Mud | 4 | 207 | 0.19 (0.07–0.56) | |
| Other | 0 | 5 | NC | |
| Combination | 3 | 28 | 1.06 (0.30–3.73) | |
| Wood | 0 | 1 | NC | |
| Reported presence of rats | 0.323 (0.57) | |||
| Yes | 10 | 127 | 1.25 (0.58–2.7) | |
| No* | 23 | 365 | 1 | |
| Palm trees number | 2.84 (0.417) | |||
| Absence* | 25 | 359 | 1 | |
| 1–10 | 5 | 106 | 0.68 (0.25–1.81) | |
| 10–30 | 1 | 17 | 0.84 (0.11–6.61) | |
| > 30 | 2 | 10 | 2.87 (0.60–13.82) | |
| Number of bushes < 10 m | 11.77 (0.008) | |||
| Absence* | 15 | 145 | 1 | |
| 1–10 | 7 | 186 | 0.36 (0.14–0.92) | |
| 10–30 | 2 | 94 | 0.21 (0.05–0.92) | |
| > 30 | 9 | 67 | 1.30 (0.54–3.12) | |
| Number of bushes > 10 m | ||||
| Absence* | 16 | 152 | 1 | 10.63 (0.014) |
| 1–10 | 1 | 25 | 0.08 (0.01–0.58) | |
| 10–30 | 11 | 121 | 0.86 (0.39–1.93) | |
| > 30 | 5 | 94 | 0.51 (0.18–1.42) | |
NC = not calculated.
Reference category.
Fisher exact test.
Table 6.
ORs and 95% CIs of risk factors associated with peridomestic infestation in multivariable analysis
| Variable | OR (95% CI) adjusted |
|---|---|
| Wall type | |
| Brick/block | 1 |
| Bahareque | 51.69 (6.44–61.96) |
| Adobe | 42.37 (5.81–50.66) |
| Wood | 7.49 (1.04–33.81) |
| Mud | 39.09 (2.66–53.74) |
| Combination | 6.12 (0.55–28.18) |
| Floor | |
| Cement | 1 |
| Mud | 0.03 (0.01–0.11) |
| Other | NC |
| Combination | 0.66 (0.09–4.68) |
| Wood | NC |
| Reported presence of rats | 0.2 (0.05–0.76) |
| Annexes | |
| Caney | 8.62 (1.36–34.52) |
| Palm trees number | |
| Absence | 1 |
| 1–10 | 1.32 (0.29–6.06) |
| 10–30 | 1.8 (0.11–30.71) |
| > 30 | 80.04 (61.92–155.58) |
| Number of bushes < 10 m | |
| Absence | 1 |
| 1–10 | 0.45 (0.05–3.82) |
| 10–30 | 0.05 (0–0.78) |
| > 30 | 1.06 (0.13–8.67) |
| Number of bushes > 10 m | |
| Absence | 1 |
| 1–10 | 0.04 (0–0.59) |
| 10–30 | 0.53 (0.07–3.83) |
| > 30 | 0.07 (0.01–0.55) |
| Rock piles | 27.93 (6.52–49.69) |
NC = not calculated.
Discussion
This geographically extensive study overlapped the distribution of the Chagas disease vector T. dimidiata in Colombia. Entomological infestation indices—colonization, dispersion, and infection—tended to be higher toward the east of the country, where there is also higher seroprevalence of T. cruzi in humans.24 Previous studies found that, in villages with an infestation rate of approximately 14.3%, natural infection index of 5.55%, and colonization index of 34.8%, there was a T. cruzi seropositivity among inhabitants of 2%.25 However, according to epidemiological context, it is possible to find contrasting results, because in the study carried out in Pedro Carbo (province of Guayas, Ecuador), an investigator found a natural infection index of 46.3% but seropositivity of 0.5%.26
This study indicates that the villages of Capitanejo and Pradera (Santander department), El Espinal, Nogal-Carrera, Bavata, and Ovachia (Boyacá department), and Arrayan and Rio Loro (Huila department) are, therefore, in urgent need of T. dimidiata control. A long-term period of surveillance would be necessary after control interventions in those departments where T. dimidiata is native and the possibility of reinvasion into the domestic environment from peridomestic and sylvatic habitats is latent.
In this study, we found statistically significant associations between the probability of infestation with T. dimidiata and some anthropogenic characteristics. The probability of infestation increased significantly with the presence of bahareque walls, mixed roofs, domestic animals, peridomiciliary annexes, and rock piles, risk factors that are consistent with previous studies. However, dirt floors had no statistical associations, despite being a recognized risk factor in other studies developed in Central America.14,27 The other risk factors identified, including the presence of uncoated bahareque walls and mixed roofs, are well-known indicators of potential triatominae infestation. T. dimidiata is known to live in cracks within walls and roof tiles.8,11,18,28,29
In the east of the country, we found a population of T. dimidiata that has continuous flux between sylvatic, peridomestic, and domestic populations and has great epidemiological importance. These findings are in agreement with studies on genetic structure of this species, and researchers have concluded that there is low genetic differentiation between these three populations. Also, they have drawn attention to the epidemiological risk of the transmission of Chagas disease by non-domiciliated populations that could colonize human dwellings.6 This study provides important information for control programs at the national and departmental levels, specifically in terms of identifying houses at risk of T. dimidiata infestation.
The main studies about risk factors of T. dimidiata infestation were done in Central America and reported that houses with poor sanitary conditions, dirt floors, tile roofs, the presence of an abandoned lot or uninhabited house next door, and piles of junk in the yard increased the infestation risk by this species.
Our results identify relationships between specific house-level characteristics and the probability of domestic and peridomestic infestation. The risk factors identified are consistent with existing knowledge of T. dimidiata ecology and both support and add to the list of risk factors identified in previous studies: presence of domestic animals (chickens, dogs, and cats), chicken sheds, caneys, mixed roofs, and rock piles.8,11–15
The results of this study have implications for maximizing surveillance efficiency (identified factors associated with an increased risk of T. dimidiata infestation that could potentially be used to prioritize houses for control), which is likely to become increasingly important if control interventions succeed in reducing infestation rates.
The peridomestic areas in our field work zone were made up of a variety of different outbuildings and structures where animals live. These outbuildings are usually made of mud, wood, and palm thatch, providing a wide range of hosts, refuges, and climatic conditions for triatomine populations to develop. The importance of peridomestic buildings in T. dimidiata infestations has already been reported from Mexico, and it is primarily associated with an abandoned lot or uninhabited house next door or piles of junk in the yard, which increased the infestation risk by three- to fourfold.15
Probably, the most important finding of this work is the demonstration that palm trees are a risk factor for domestic infestation with T. dimidiata. The effect on house infestation of a high density of palm trees (> 10) within 10 m of the house was apparent, even after adjusting for confounders (notably, the presence of a palm roof) in the multivariate analysis. Detecting high palm tree density as a risk factor for infestation is of epidemiological importance.
In conclusion, we found a high level of T. dimidiata colonization in Boyacá and Santander departments; in these areas of the country, control of this species should be prioritized. Identification of factors associated with an increased risk of T. dimidiata infestation could be used to target houses for control in a cost-effective way. This study indicates that, in Colombia, stratifying houses by walls and roofs type would enable the targeting of control interventions to those houses at increased risk.
ACKNOWLEDGMENTS
We thank the health technicians of the eight departments for support in the field work. We also thank Bibiana Castro for work with the databases.
Footnotes
Financial support: Funding for this work was provided by Colciencias Colombia Contracts 410–2005 and 380–2011. N.A. received support from the United Kingdom Medical Research Council and Department for International Development Grant MR/K012126/1.
Authors' addresses: Gabriel Parra-Henao, Red Chagas Colombia, Grupo de Epidemiología y Bioestadística, Universidad CES, Medellín, Colombia, and Instituto Colombiano de Medicina Tropical, Medellín, Colombia, E-mail: gparrahenao@gmail.com. Ángela Segura Cardona and Oscar Quirós-Gómez, Grupo de Epidemiología y Bioestadística, Universidad CES, Medellín, Colombia, E-mails: asegura@ces.edu.co and quiromez@hotmail.com. Víctor Angulo, Centro de Investigación en Enfermedades Tropicales, Universidad Industrial de Santander, Bucaramanga, Colombia, E-mail: pitorio@hotmail.com. Neal Alexander, London School of Hygiene and Tropical Medicine, London, United Kingdom, E-mail: neal.alexander@lshtm.ac.uk.
Reprint requests: Gabriel Parra-Henao, Avenue Calle 26 No 51-20, Bogotá, Colombia, E-mail: gparrahenao@gmail.com.
References
- 1.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases in Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2:1–11. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franco-Paredes C, Von A, Hidron A, Rodriguez-Morales AJ, Tellez I, Barragán M, Jones D, Náquira CG, Mendez J. Chagas disease: an impediment in achieving the millenium development goals in Latin America. BMC Int Health Hum Rights. 2007;7:1–7. doi: 10.1186/1472-698X-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organización Panamericana de la Salud . Estimación cuantitativa de la enfermedad de Chagas en las Américas. Organizaci?n Panamericana de la Salud. Washington/HDM/CD/425-06. 2006. [Google Scholar]
- 4.Guhl F, Aguilera G, Pinto N, Vergara D. Actualización de la distribución geográfica y ecoepidemiología de la fauna de triatominos (Reduviidae: Triatominae) en Colombia. Biomedica. 2007;27((Suppl 1)):143–162. [PubMed] [Google Scholar]
- 5.World Health Organization . Control of Chagas Disease. Technical Report Series 905. Geneva: World Health Organization; 2002. pp. 1–109. [PubMed] [Google Scholar]
- 6.Gómez-Palacio A, Triana O, Jaramillo-O N, Dotson EM, Marcet PL. Eco-geographical differentiation among Colombian populations of the Chagas disease vector Triatoma dimidiata (Hemiptera:Reduviidae) Infect Genet Evol. 2013;20:352–361. doi: 10.1016/j.meegid.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez C, Jaramillo C, Delgado MP, Pinto N, Aguilera G, Guhl F. Genetic structure of sylvatic, peridomestic and domestic populations of Triatoma dimidiata (Hemiptera: Reduviidae) from and endemic zone of Boyacá, Colombia. Acta Trop. 2005;93:23–29. doi: 10.1016/j.actatropica.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Bustamante DM, Monroy C, Pineda S, Rodas A, Castro X, Ayala V, Quiñones J, Moguel B, Trampe R. Risk factors for intradomiciliary infestation by the Chagas desease vector Triatoma dimidiata in Jutiapa, Guatemala. Cad Saude Publica. 2009;25:83–92. doi: 10.1590/s0102-311x2009001300008. [DOI] [PubMed] [Google Scholar]
- 9.Guhl F, Aguilera G, Pinto N, Vergara D. Updated geographical distribution and ecoepidemiology of the triatomine fauna (Reduviidae: Triatominae) in Colombia. Biomedica. 2007;27:143–162. [PubMed] [Google Scholar]
- 10.Dorn PL, Monroy C, Curtis A. Triatoma dimidiata (Latreille, 1811): a review of its diversity across its geographical range and the relationship among populations. Infect Genet Evol. 2006;7:343–352. doi: 10.1016/j.meegid.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Starr MD, Rojas JC, Zeledon R, Hird DW, Carpenter TE. Chagas' disease: risk factors for house infestation by Triatoma dimidiata, the major vector of Trypanosoma cruzi in Costa Rica. Am J Epidemiol. 1991;133:740–747. doi: 10.1093/oxfordjournals.aje.a115949. [DOI] [PubMed] [Google Scholar]
- 12.Dumonteil E, Gourbiere S, Barrera-Perez M, Rodriguez-Felix E, Ruiz-Piña H, Baños-Lopez O, Ramirez-Sierra MJ, Menu F, Rabinovich J. Geographic distribution of Triatoma dimidiata and transmission dynamics of Trypanosoma cruzi in the Yucatan península of Mexico. Am J Trop Med Hyg. 2002;67:176–183. doi: 10.4269/ajtmh.2002.67.176. [DOI] [PubMed] [Google Scholar]
- 13.Salazar-Schettino PM, Arteaga I, Cabrera M. Tres especies de triatominos y su importancia como vectores de Trypanosoma cruzi en México. Medicina (B Aires) 2005;65:63–69. [PubMed] [Google Scholar]
- 14.Zeledón R, Calvo N, Montenegro V, Seixas E, Arevalo C. A survey on Triatoma dimidiata in an urban area of the province of Heredia, Costa Rica. Mem Inst Oswaldo Cruz. 2005;100:607–612. doi: 10.1590/s0074-02762005000600002. [DOI] [PubMed] [Google Scholar]
- 15.Guzmán-Tapia Y, Ramírez-Sierra MJ, Dumonteil E. Urban infestation by Triatoma dimidiata in the city of Merida, Yucatan, Mexico. Vector-Borne Zoonotic Dis. 2007;7:597–606. doi: 10.1089/vbz.2007.0133. [DOI] [PubMed] [Google Scholar]
- 16.King R, Cordon-Rosales C, Cox J, Davies C, Kitron U. Triatoma dimidiata infestation in Chagas disease endemic regions of Guatemala: comparison of random and targeted cross-sectional surveys. PLoS Negl Trop Dis. 2011;5:e1035. doi: 10.1371/journal.pntd.0001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina-Torres I, Vásquez-Chagoyán J, Rodriguez-Vivas R, Montes de Oca-Jiménez R. Risk factors associated with triatomines and its infection with Trypanosoma cruzi in rural communities form southern region of the state of Mexico, Mexico. Am J Trop Med Hyg. 2010;82:49–54. doi: 10.4269/ajtmh.2010.08-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell-Lendrum D, Angulo VM, Esteban L, Tarazona Z, Parra GJ, Restrepo M, Restrepo BN, Guhl F, Pinto N, Aguilera G, Wilkerson P, Davies C. House-level risk factors for triatominae infestation in Colombia. Int J Epidemiol. 2007;14:1–7. doi: 10.1093/ije/dym065. [DOI] [PubMed] [Google Scholar]
- 19.Angulo VM, Muñoz G, Tarazona Z. Factores de riesgo de la vivienda para infestación domiciliaria por triatomineos. Biomedica. 1993;13((Suppl 1)):124–125. [Google Scholar]
- 20.Holdridge L. Ecología: Basada en zonas de vida. Traducido al español por E. Jiménez Saa. San José, Costa Rica: Instituto Interamericano de Cooperación Para la Agricultura; 1978. pp. 1–216. [Google Scholar]
- 21.Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas disease. Bull Am Mus Nat Hist. 1979;163:125–516. [Google Scholar]
- 22.Souto RP, Fernandes O, Macedo AM, Campbell DA, Zingales B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol Biochem Parasitol. 1996;83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]
- 23.Sturm NR, Degrave W, Morel C, Simpson L. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplast minicircle DNA sequences: use in diagnosis of Chagas' disease. Mol Biochem Parasitol. 1989;33:205–214. doi: 10.1016/0166-6851(89)90082-0. [DOI] [PubMed] [Google Scholar]
- 24.Guhl F, Restrepo M, Angulo VM, Antunes CM, Campbell-Lendrum D, Davies C. Lessons from a national survey of Chagas disease transmission risk in Colombia. Trends Parasitol. 2005;21:259–262. doi: 10.1016/j.pt.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Becerril-Flores M, Rangel-Flores E, Imbert-Palafox J, Gómez-Gómez J, Figueroa Gutiérrez A. Human infection and risk of transmission of Chagas disease in Hidalgo state, México. Am J Trop Med Hyg. 2007;76:318–323. [PubMed] [Google Scholar]
- 26.Guevara A, Garzón E, Bowen C, Córdova X, Gómez E, Ouaissi A. High infection rates of Triatoma dimidiata are associated with low levels of Trypanosoma cruzi seroprevalence in Pedro Carbo, Ecuador. Use of a tc24 gene-based PCR approach. Parasite. 2005;12:65–68. doi: 10.1051/parasite/2005121065. [DOI] [PubMed] [Google Scholar]
- 27.Zeledon R, Zuñiga A, Swartzwelder J. The camouflage of Triatoma dimidiate and the epidemiology of Chagas disease in Costa Rica. Bol Chil Parasitol. 1969;24:106–108. [PubMed] [Google Scholar]
- 28.Nakagawa J, Cordon-Rosales C, Juarez J, Itzep C, Nonami T. Impact of residual spraying on Rhodnius prolixus and Triatoma dimidiata in the department of Zacapa in Guatemala. Mem Inst Oswaldo Cruz. 2003;98:277–281. doi: 10.1590/s0074-02762003000200019. [DOI] [PubMed] [Google Scholar]
- 29.Tabaru Y, Monroy C, Rodas A, Mejía M, Rosales R. The geographical distribution of vectors of Chagas disease and population at risk of infestation in Guatemala. Med Entomol Zool. 1999;50:3–8. [Google Scholar]