Abstract
Calcified neurocysticercosis has been associated with hippocampal atrophy in patients with refractory epilepsy, but the relevance of this association in the population at large is unknown. We assessed calcified cysticerci and its association with hippocampal atrophy in elderly persons living in Atahualpa, an Ecuadorian village endemic for neurocysticercosis. All Atahualpa residents ≥ 60 years of age were invited to undergo computed tomography/magnetic resonance imaging for neurocysticercosis detection. Twenty-eight (11%) out of 248 enrolled persons had calcified cysticerci (case-patients) and were matched 1:1 by age, sex, and years of education to individuals without neurocysticercosis on computed tomography/magnetic resonance imaging (controls). Four case-patients and none of the controls had epilepsy (P = 0.134). Cognitive performance was similar across both groups. The Scheltens' medial temporal atrophy scale was used for hippocampal rating in case-patients and matched controls without neurocysticercosis. Mean score in the Scheltens' scale was higher in case-patients than in controls (P < 0.001). Atrophic hippocampi were noticed in 19 case-patients and five controls (P = 0.003). Atrophy was bilateral in 11 case-patients and unilateral in eight. All case-patients with unilateral hippocampal atrophy had at least one ipsilateral calcification. This study shows an association between calcified cysticerci and hippocampal atrophy and raises the possibility of an inflammation-mediated hippocampal damage as the responsible mechanism for these findings.
Introduction
Although parenchymal brain calcified cysticerci have traditionally been considered inert lesions with little clinical relevance, its contribution to the burden of neurological disease may be significant. In endemic regions, the numbers of patients with only calcified neurocysticercosis (NCC) and active epilepsy are quite large. Moreover, parenchymal calcifications can be associated with focal edema by the time of a seizure or a headache episode, likely reflecting periodical exposure of trapped antigens to the host's immune system and subsequent inflammation.1–4 An association between calcified NCC and hippocampal atrophy has also been suggested based on studies of patients with refractory epilepsy, but the actual prevalence and relevance of this association is largely unknown.5–8 Here, we report the results of a population-based and a nested case-control study aimed to assess the prevalence of calcified cysticerci and its association with hippocampal atrophy in community-dwelling elderly persons living in Atahualpa, a rural Ecuadorian village where NCC is endemic.9 The study focused on individuals ≥ 60 years of age to cover the possibility that such association exists but take a long time to develop.
Methods
The study was conducted in Atahualpa, a coastal rural community in Ecuador where previous epidemiological studies on NCC have been performed.9,10 All residents ≥ 60 years of age were invited to undergo non-enhanced brain computed tomography (CT) and magnetic resonance imaging (MRI). The institutional review board (IRB) of the Hospital-Clínica Kennedy in Guayaquil, Ecuador (FWA 00006867) approved the protocol and the informed consent.
Exams were performed with a Philips Brilliance 64 CT scanner and a Philips Intera 1.5T MRI machine (Philips Medical Systems, Eindhoven, The Netherlands) at Hospital-Clínica Kennedy, Guayaquil. The CTs were obtained in the axial plane with sagittal and coronal reconstructions; slice thickness was 3 mm with no gap between slices. The MRI included two-dimensional multi-slice turbo spin echo T1-weighted, fluid attenuated inversion recovery (FLAIR), T2-weighted, and gradient-echo sequences in the axial plane, and a T1-weighted sequence oriented in the sagittal plane. We used the pre-established brain imaging package delivered by the manufacturer to assure uniformity of the data. Slice thickness was 5 mm with 1 mm gap between slices.
A neurologist (OHD) and a neuroradiologist (JL) independently read all CTs and MRIs with attention to the presence of parenchymal brain calcifications and other abnormalities suggestive of NCC. Images corresponding to all identified lesions were sent to another expert (HHG) for further confirmation. As previously described, rounded and homogeneous non-physiological supratentorial calcifications, measuring < 1 cm in diameter and not explained by any other cause, were considered of cysticercotic origin.11,12 Inter-reader agreement was excellent (k = 0.92) and disagreements were resolved by consensus.
Persons with calcified cysticerci (case-patients) were matched 1:1 by age, sex, and years of education to individuals with no calcifications or any other evidence of NCC on CT/MRI (controls). Case-patients and controls were interviewed by one of the authors (VJD), blinded to neuroimaging findings. History taking was directed to find evidence of a seizure disorder with emphasis of age of onset and date of the last seizure, number of lifetime seizures, type of seizures, and antiepileptic drug use and efficacy; a complete neurological examination was performed and any abnormality was noticed. Cognitive performance was assessed by the use of the Spanish version of the Montreal Cognitive Assessment (MoCA) test (www.mocatest.org, Z. Nasreddine MD, version 07 November 2004) and the Leganés Cognitive Test (Prueba Cognitiva de Leganés). The MoCA evaluates major cognitive domains, including visuospatial-executive, language, short-term memory, abstraction, attention, calculation, and orientation, for a maximum score of 30 points.13 The Leganés Cognitive Test mainly evaluates orientation and memory. It is a reliable instrument (originally developed in Spanish) used in elderly persons living in poor-educated communities; the maximum score is 32 points, and a score of ≤ 22 indicates dementia.14
Case-patients and controls had an additional T1-weighted inversion recovery sequence (acquisition time 3:31 min, repetition time 2.250 msec, echo time 11 msec, and inversion time 400 msec) for hippocampal evaluation, which was oriented in the coronal plane and perpendicular to the long axis of the temporal bone; slice thickness were 4 mm with no gap between slices. These images were sent to an independent expert neuroradiologist (PS) and a neurologist (XC) blinded to other imaging findings and to case/control status. Hippocampi were rated using the Scheltens' medial temporal atrophy scale,15 which grades the width of the choroid fissure, the width of the temporal horn, and the height of the hippocampus on a five-point visual rating scale ranging from 0 (no atrophy) to 4 (severe atrophy). Up to 1 point for persons < 75 years of age, and 2 points for persons ≥ 75 years of age were considered age-related changes. Each temporal lobe was rated separately, and any asymmetry ≥ 1 point was noted.
Descriptive statistics were presented as means with standard deviations for continuous variables and as percentages with 95% confidence interval (CI) for categorical variables. Statistical significance was tested by the use of the McNemar's test for correlated proportions (matched-pair analysis). Values of P < 0.05 were considered significant.
Results
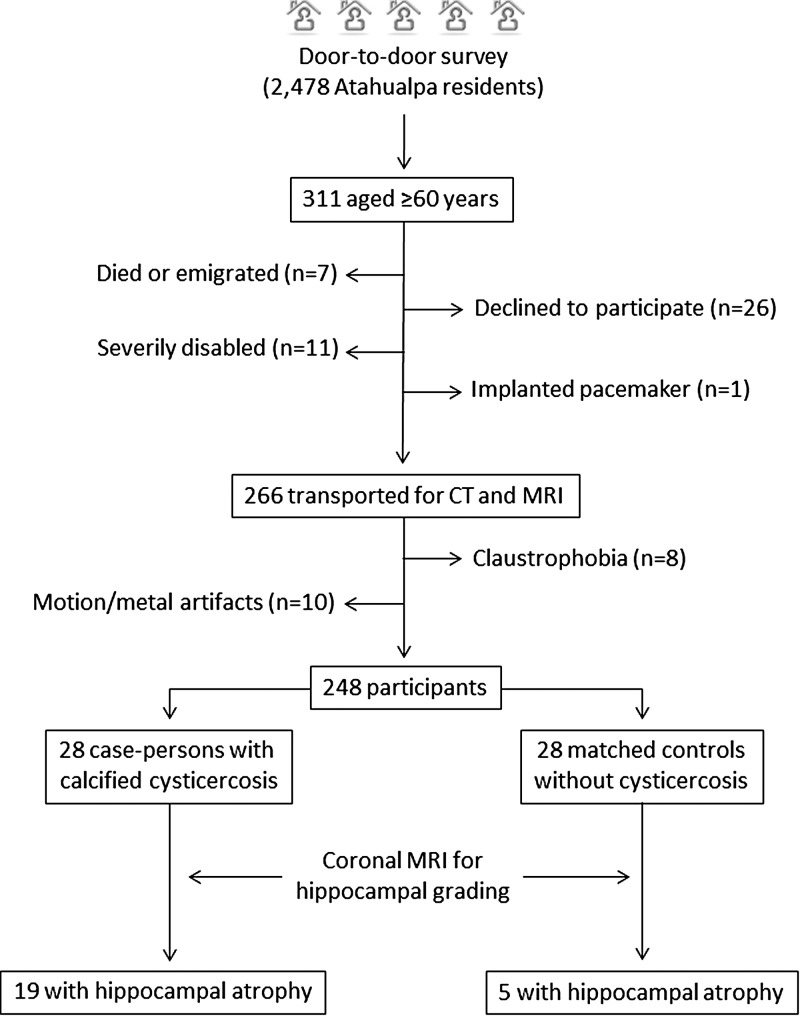
Out of 2,478 Atahualpa residents identified during a door-to-door survey, 311 were ≥ 60 years of age. Of these, 258 (83%) underwent both CT and MRI. Reasons for not performing neuroimaging included refusal to participate (N = 26), severe disability (N = 11), claustrophobia (N = 8), and implanted pacemaker (N = 1); six additional persons had died and one emigrated between the survey (October 2013) and the invitation (February 2014). Motions and metal artifacts (mostly dentures) precluded proper imaging reading in 10 of the 258 scanned persons and were not considered for analyses. Figure 1 is a flow diagram depicting the process of enrollment and the reasons for not including potentially eligible individuals.
Figure 1.
Flow diagram showing the process of enrollment and the number of participants in this study.
Mean ± SD age of the 248 participants was 70 ± 8 years, 146 (59%) were women, and 206 (83%) had only primary school education. Calcified cysticerci were found in CT in 28 individuals (11%, 95% CI: 8–16%). There was a single cerebral calcification in 22 cases, two in five cases, and the remaining patient had seven calcifications. Associated colloidal cysts were noticed in one person with a single calcification and in another with seven calcifications. The MRI detected the calcifications shown on CT in only 18 of 28 cases (65%), mostly on T2-weighted and gradient-echo sequences; the two colloidal cysts were evident on all MRI sequences and were also visible on CT.
As expected, case-patients and their 28 matched controls were similar in regards to the matched variables, including age (69 ± 6 years), sex (71% women), and education level (7 ± 3 years). Clinical interview revealed a chronic seizure disorder in four case-patients and in none of the controls (P = 0.134). Seizures were recurrent in all cases and started at ages ranging from 12 to 62 years. All these patients had > 20 lifetime seizures and three of them still had active epilepsy, which was poorly controlled by medication in two. Seizures were tonic-clonic generalized in three (one with complex partial seizures) and simple partial in one; no patient had history of status epilepticus. Mean scores in the MoCA test were 19.1 ± 4.7 for case-patients and 18.4 ± 4.8 for controls (P = 0.584). According to the Leganés Cognitive Test, only two case-patients and none of the controls had scores below the recommended cut-off level for dementia, with mean ± SD scores of 27.4 ± 2.7 for case-patients and 27.3 ± 1.8 for controls (P = 0.871).
Kappa coefficients for inter-rater agreements of findings in the T1-weighted inversion recovery MRI sequence were 0.61 for unilateral, 0.82 for bilateral, and 0.85 for any kind of hippocampal atrophy. After consensus, mean ± SD points in the Scheltens' scale for all rated hippocampi (N = 112, two per person, in 28 case-patients and 28 controls) were 3.9 ± 1.5 for case-patients and 2.8 ± 1.2 for controls (P < 0.001). A total of 19 (68%, 95% CI: 49–82%) case-patients and five (18%) controls had hippocampal atrophy (odds ratio [OR]: 5.7, 95% CI: 1.7–19.3, P = 0.003), which was bilateral in 11 case-patients and four controls, and unilateral in eight case-patients and in only one control (Figure 2). Bilateral hippocampal atrophy was not more prevalent among case-patients with a single calcification than in those with more than one calcifications. In all the eight case-patients with unilateral hippocampal atrophy there was at least one calcification in the affected side. There were no age- and sex-related differences between case-patients with normal hippocampi and those with uni- or bilateral hippocampal atrophy. Epilepsy was present in three case-patients with bilateral hippocampal atrophy and in one with normal hippocampus.
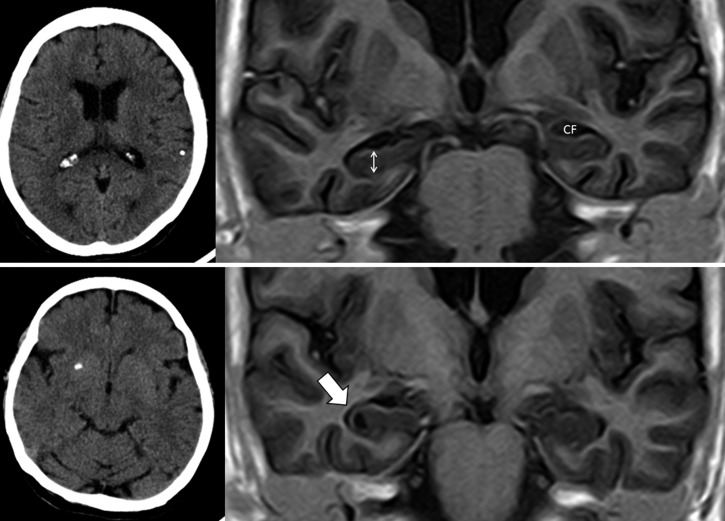
Figure 2.
Upper panel: Plain computed tomography (CT) (left) showing single calcified cysticercus in left parietal lobe and T1-weighted inversion recovery magnetic resonance imaging (MRI) sequence (right) showing bilateral hippocampal atrophy (Grade 3 in the Scheltens' medial temporal atrophy scale), characterized by increased width of the choroid fissure (CF) and decreased height of hippocampal formation (double-headed arrow). Lower panel: Plain CT (left) showing single calcified cysticercus in right basal ganglia and T1-weighted inversion recovery MRI sequence (right) showing ipsilateral hippocampal atrophy (thick arrow).
Discussion
This study shows a rather high prevalence of calcified cysticerci in elderly persons living in Atahualpa, a finding that has also been shown in other cysticercotic-endemic villages of South America.16 Of importance, a significantly higher prevalence of hippocampal atrophy (bilateral and unilateral) is noted among our participants with calcified NCC when compared with controls.
Much remains to be learned on the relationship between calcified NCC and hippocampal atrophy. Current evidence shows that these conditions often co-exist in cysticercotic-endemic areas, but to what extent this occurs just by chance remains to be determined, because this association has not been investigated in the population at large. Information derived from a series of patients with medial temporal lobe epilepsy strongly suggest a cause-and-effect relationship between calcified NCC and hippocampal atrophy, on the basis of differences in the characteristics and severity of seizures among patients with both conditions when compared with those with hippocampal atrophy alone.5–7 In this context, it has been suggested that calcified cysticerci lead to hippocampal atrophy by causing recurrent seizures or status epilepticus, and the resulting hippocampal atrophy—in turn—exacerbates the seizure disorder. Parasites may not be necessarily located within limbic circuits, suggesting a remote deleterious effect of seizures on hippocampal neurons.17–19
An alternative hypothesis proposes that, irrespective of the occurrence of seizures, calcified cysticerci might lead to variable inflammatory responses and inflammation-mediated hippocampal damage associated or not with a genetic predisposition.20,21 In this view, periodic remodeling of calcified cysticerci with exposure of trapped parasitic antigens to the host's immune system,3,4,22 might account for episodic inflammatory events, and seizures may not be a necessary trigger for hippocampal atrophy. Although this has not been shown in humans, experimental evidence showing that repeated endotoxin exposure and increased levels of pro-inflammatory cytokines correlate with hippocampal damage, support the hypothesis of inflammatory-mediated atrophy.23 In our study population only a minority of persons (3 out of 19) with both calcified NCC and hippocampal atrophy had a history of seizures, and this supports the inflammatory hypothesis. We cannot, however, rule out subclinical epileptiform activity in our patients because electroencephalogram (EEG) recordings were not obtained.
Selective hippocampal atrophy has been recognized as a marker of Alzheimer's disease and other forms of degenerative dementias on neuroimaging studies,24 and it could be argued that more persons with these disorders were inadvertently included among our case-patients. However, similar cognitive performances across case-patients and controls in this study discard this possibility. Both the MoCA and the Leganés Cognitive Test appear to be simple and reliable options to assess different cognitive domains and have previously been used and validated to evaluate cognition in Latin American underserved populations, including Atahualpa.25,26 Our results also support previous findings showing that cognition is not particularly impaired in NCC patients with hippocampal atrophy.27
Major strengths of our study include the population-based design, the unbiased characteristics of scanned persons, the method used for choosing controls, the blinded imaging rating, and the expertise (> 20 years) of involved readers. Although the lack of hippocampal volume measurements is a potential weakness of our study, the Scheltens' medial temporal atrophy scale has proved reliable for visual grading hippocampal atrophy when compared with volumetric assessment.28 Immune tests were not performed because results of either antibody or antigen detection may be erratic in patients only presenting with calcified cysticerci, leading to diagnostic misinterpretations.29
In summary, this study shows a significant association between calcified NCC and hippocampal atrophy in people ≥ 60 years of age living in an endemic community. People living in rural-endemic areas most often acquire cysticercosis during adolescence or early adulthood, therefore we are most likely dealing with chronic, old infections that were present long before hippocampal atrophy. However, a case-control study is not proof for causality. Subsequent cohort studies should scan younger populations to evaluate whether calcified cysticerci are related to the development of hippocampal atrophy in the long-term follow-up and to whether such atrophy correlates with the occurrence of temporal lobe epilepsy.
Footnotes
Financial support: This study was partially supported by Universidad Espíritu Santo – Ecuador, Guayaquil – Ecuador. HHG is supported by a Wellcome Trust International Senior Fellowship in Public Health and Tropical Medicine.
Authors' addresses: Oscar H. Del Brutto, School of Medicine, Universidad Espíritu Santo – Ecuador, Guayaquil, Ecuador, E-mail: oscardelbrutto@hotmail.com. Perla Salgado, Neuroimaging Unit, National Institute of Neurology and Neurosurgery Manuel Velasco Suarez, Mexico 22 DF, Mexico, E-mail: pmtsalgado@yahoo.com.mx. Julio Lama, Imaging Department, Hospital-Clínica Kennedy, Guayaquil, Ecuador, E-mail: julama54@hotmail.com. Victor J. Del Brutto and Mauricio Zambrano, Community Center of the Atahualpa Project, Atahualpa, Ecuador, E-mails: vjdelbrutto@gmail.com and zamaleon@hotmail.com. Xavier Campos, Outpatient Center North, Ecuadorian Institute of Social Security, Guayaquil, Ecuador, E-mail: xaviercampos63@hotmail.com. Héctor H. García, Cysticercosis Unit, Instituto Nacional de Ciencias Neurológicas, Lima, Perú, E-mail: hgarcia@jhsph.edu.
References
- 1.Bruno E, Bartoloni A, Zammarchi L, Strohmeyer M, Bartalesi F, Bustos JA, Santivañez S, Garcia HH, Nicoletti A. COHEMI Project Study Group Epilepsy and neurocysticercosis in Latin America: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7:e2480. doi: 10.1371/journal.pntd.0002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Brutto OH, Del Brutto VJ. Calcified neurocysticercosis among patients with primary headache. Cephalalgia. 2012;32:250–254. doi: 10.1177/0333102411433043. [DOI] [PubMed] [Google Scholar]
- 3.Fujita M, Mahanty S, Zoqhbi SS, Ferraris Araneta MD, Hong J, Pike VW, Innis RB, Nash TE. PET reveals inflammation around calcified Taenia solium granulomas with perilesional edema. PLoS ONE. 2013;8:e74052. doi: 10.1371/journal.pone.0074052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nash T. Edema surrounding calcified intracranial cysticerci: clinical manifestations, natural history, and treatment. Pathog Glob Health. 2012;106:275–279. doi: 10.1179/2047773212Y.0000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singla M, Singh P, Kaushal S, Bansal R, Singh G. Hippocampal sclerosis in association with neurocysticercosis. Epileptic Disord. 2007;9:1–9. doi: 10.1684/epd.2007.0122. [DOI] [PubMed] [Google Scholar]
- 6.Rathore C, Thomas B, Kesavadas C, Radhakrishnan K. Calcified neurocysticercosis lesions and hippocampal sclerosis; potential dual pathology? Epilepsia. 2012;53:e60–e62. doi: 10.1111/j.1528-1167.2011.03386.x. [DOI] [PubMed] [Google Scholar]
- 7.Bianchin MM, Velasco TR, Coimbra ER, Gargaro AC, Escorsi-Rosset SR, Wichert-Ana L, Terra VC, Alexandre V, Jr, Araujo D, Jr, dos Santos AC, Fernandes RM, Assirati JA, Jr, Carlotti CG, Jr, Leite JP, Takayanagui OM, Markowitsch HJ, Sakamoto AC. Cognitive and surgical outcome in mesial temporal lobe epilepsy associated with hippocampal sclerosis plus neurocysticercosis: a cohort study. PLoS ONE. 2013;8:e60949. doi: 10.1371/journal.pone.0060949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rathore C, Thomas B, Kesavadas C, Radhakrishnan K. Calcified neurocysticercosis lesions and antiepileptic drug-resistant epilepsy: a surgically remediable syndrome? Epilepsia. 2013;54:1815–1822. doi: 10.1111/epi.12349. [DOI] [PubMed] [Google Scholar]
- 9.Del Brutto OH, Santibañez R, Idrovo L, Rodriguez S, Díaz-Calderón E, Navas C, Gilman RH, Cuesta F, Mosquera A, Gonzalez AE, Tsang VC, Garcia HH. Epilepsy and neurocysticercosis in Atahualpa: a door-to-door survey in rural coastal Ecuador. Epilepsia. 2005;46:583–587. doi: 10.1111/j.0013-9580.2005.36504.x. [DOI] [PubMed] [Google Scholar]
- 10.Del Brutto OH, Lama J. The importance of neurocysticercosis in stroke in rural areas of a developing country. Am J Trop Med Hyg. 2013;89:374–375. doi: 10.4269/ajtmh.13-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia HH, Del Brutto OH. Imaging findings in neurocysticercosis. Acta Trop. 2003;87:71–78. doi: 10.1016/s0001-706x(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 12.Nash TE, Del Brutto OH, Butman JA, Corona T, Delgado-Escueta A, Duron RM, Evans CA, Gilman RH, Gonzalez AE, Loeb JA, Medina MT, Pietsch-Escueta S, Pretell EJ, Takayanagui OM, Theodore W, Tsang VC, Garcia HH. Calcific neurocysticercosis and epileptogenesis. Neurology. 2004;62:1934–1938. doi: 10.1212/01.wnl.0000129481.12067.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummeings JL, Chertkow H. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 14.de Yébenes MJ, Otero A, Zunzunegui MV, Rodriguez-Laso A, Sanchez-Sanchez F, Del Ser T. Validation of a short cognitive tool for the screening of dementia in elderly people with low educational level. Int J Geriatr Psychiatry. 2013;18:925–936. doi: 10.1002/gps.947. [DOI] [PubMed] [Google Scholar]
- 15.Scheltens Ph, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, Kuiper M, Steinling M, Wolters EC, Valk J. Atrophy of medical temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moyano LM, Saito M, Montano SM. Neurocysticercosis as a cause of epilepsy and seizures in two community-based studies in a cysticercosis-endemic region in Peru. PLoS Negl Trop Dis. 2014;8:e2692. doi: 10.1371/journal.pntd.0002692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi E, Guerreiro CA, Cendes F. Late onset epilepsy with MRI evidence of mesial temporal sclerosis following acute neurocysticercosis. Arq Neuropsiquiatr. 2001;59:255–258. doi: 10.1590/s0004-282x2001000200021. [DOI] [PubMed] [Google Scholar]
- 18.Chung CK, Lee SK, Chi JG. Temporal lobe epilepsy caused by intrahippocampal calcified cysticercus: a case report. J Korean Med Sci. 1998;13:445–448. doi: 10.3346/jkms.1998.13.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh G, Burneo JG, Sander W. From seizures to epilepsy and its substrates: neurocysticercosis. Epilepsia. 2013;54:783–792. doi: 10.1111/epi.12159. [DOI] [PubMed] [Google Scholar]
- 20.Bianchin MM, Velasco TR, Takayanagui OM, Sakamoto AC. Neurocysticercosis, mesial temporal lobe epilepsy, and hippocampal sclerosis: an association largely ignored. Lancet Neurol. 2006;5:20–21. doi: 10.1016/S1474-4422(05)70269-6. [DOI] [PubMed] [Google Scholar]
- 21.Bianchin MM, Velasco TR, dos Santos AC, Sakamoto AC. On the relationship between neurocysticercosis and mesial temporal lobe epilepsy associated with hippocampal sclerosis: coincidence or a pathogenic relationship? Pathog Glob Health. 2012;106:280–285. doi: 10.1179/2047773212Y.0000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nash TE, Pretell EJ, Lescano AG, Bustos JA, Gilman RH, Gonzalez AE, Garcia HH. Cysticercosis Working Group in Peru Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol. 2008;7:1099–1105. doi: 10.1016/S1474-4422(08)70243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahn MS, Kranjac D, Alonzo CA, Haase JH, Cedillos RO, McLinden KA, Boehm GW, Chumley MJ. Prolonged elevation in hippocampal Aβ and cognitive deficit following repeated endotoxin exposure in the mouse. Behav Brain Res. 2012;229:176–184. doi: 10.1016/j.bbr.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Bastos-Leite AJ, van Waesberghe JH, Oen AL, van der Flier WM, Scheltens P, Barkhof E. Hippocampal sulcus width and cavities: comparison between patients with Alzheimer disease and nondemented elderly subjects. AJNR Am J Neuroradiol. 2006;27:2141–2145. [PMC free article] [PubMed] [Google Scholar]
- 25.Gómez F, Zunzunegui MV, Lord C, Alvarado B, García A. Applicability of the MoCA-S test in populations with little education in Colombia. Int J Geriatr Psychiatry. 2013;28:813–820. doi: 10.1002/gps.3885. [DOI] [PubMed] [Google Scholar]
- 26.Del Brutto OH, Gardener H, Del Brutto VJ, Maestre GE, Zambrano M, Montenegro JE, Wright CB. Edentulism associates with worse cognitive performance in community-dwelling elders in rural Ecuador: results of the Atahualpa Project. J Community Health. 2014 doi: 10.1007/s10900-014-9857-3. doi:10.1007/s10900-014-9857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terra-Bustamante VC, Coimbra ER, Rezek KO, Escorsi-Rosset SR, Guarnieri R, Dalmagro CL, Inuzuka LM, Bianchin MM, Wichert-Ana L, Alexandre V, Takayanagui ON, Araujo D, dos Santos AC, Carlotti CG, Walz R, Markowitsch HJ, Sakamoto AC. Cognitive performance of patients with mesial temporal lobe epilepsy and incidental calcified neurocysticercosis. J Neurol Neurosurg Psychiatry. 2005;76:1080–1083. doi: 10.1136/jnnp.2004.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boutet C, Chupin M, Colliot O, Sarazin M, Mutlu G, Drier A, Pellot A, Dormont D, Lehéricy S. Alzheimer's Disease Neuroimaging Initiative Is radiological evaluation as good as computer-based volumetry to assess hippocampal atrophy in Alzheimer's disease? Neuroradiology. 2012;54:1321–1330. doi: 10.1007/s00234-012-1058-0. [DOI] [PubMed] [Google Scholar]
- 29.Garcia HH, Rodriguez S, Gilman RH, Gonzalez AE, Tsang VC. Neurocysticercosis: is serology useful in the absence of brain imaging? Trop Med Int Health. 2012;17:1014–1018. doi: 10.1111/j.1365-3156.2012.03037.x. [DOI] [PubMed] [Google Scholar]