Abstract
Colorectal (CRC) and pancreatic cancers are two very significant contributors to cancer-related deaths. Chronic alcohol consumption is an important risk factor for these cancers. Ethanol is oxidized primarily by alcohol dehydrogenases to acetaldehyde, an agent capable of initiating tumors by forming adducts with proteins and DNA. Acetaldehyde is metabolized by ALDH2, ALDH1B1 and ALDH1A1 to acetate. Retinoic acid (RA) is required for cellular differentiation and is known to arrest tumor development. RA is synthesized from retinaldehyde by the retinaldehyde dehydrogenases, specifically ALDH1A1, ALDH1A2, ALDH1A3 and ALDH8A1. By eliminating acetaldehyde and generating RA, ALDHs can play a crucial regulatory role in the initiation and progression of cancers. ALDH1 catalytic activity has been used as a biomarker to identify and isolate normal and cancer stem cells; its presence in a tumor is associated with poor prognosis in colon and pancreatic cancer. In summary, these ALDHs are not only biomarkers for CRC and pancreatic cancer but also play important mechanistic role in cancer initiation, progression and eventual prognosis.
Keywords: Acetaldehyde, ALDH, Biomarker, Colorectal cancer, Pancreatic cancer, Retinaldehyde, Stem cells
Introduction
Colorectal cancer (CRC) and pancreatic cancer represent serious health concerns because of their very high morbidity and mortality. Each year, more than one million new CRC cases are diagnosed and over 500,000 deaths are associated with this condition worldwide [1]. In the USA, CRC is the fourth most commonly diagnosed cancer and second leading cause of cancer-related death. Pancreatic cancer ranks tenth in incidence but is disproportionately fatal in being the fourth largest cause of cancer-related deaths in the USA. The American Cancer Society estimated diagnosis of 142,820 new cases of CRC in the USA during 2013; of these, approximately 50,830 people are expected to die. The estimated incidence of pancreatic cancer is 45,220 with 38,460 deaths [2]. Although the exact mechanisms that promote CRC remain obscure, there is increasing evidence suggesting the involvement of lifestyle-related factors in addition to genetic predisposition. These factors include waist circumference, folate and multivitamins in the diet, high fat and high energy diet, physical exercise, tobacco smoking and alcohol consumption [3, 4].
According to dose-response meta-analysis and pooled results from cohort studies, chronic daily consumption of approximately 50 g alcohol increases the relative risk for colon cancer by 40 per cent [5, 6]. Alcohol and its primary metabolite, acetaldehyde, have also been linked with pancreatic cancer [7]. Various theories have been advanced regarding the mechanism by which alcohol induces cancer. For example, ethanol may enhance mucosal penetration of a carcinogen by serving as a solvent. In addition, ethanol induces cytochrome P4502E1 (CYP2E1), an enzyme capable of generating reactive oxygen species (Fig. 1). However, the most well accepted theory regarding ethanol-induced cancer involves acetaldehyde acting as a carcinogen [8, 9]. Ethanol is metabolized to acetaldehyde by alcohol dehydrogenase (ADH), CYP2E1 and catalase [10, 11] (Fig. 1). Acetaldehyde is a molecule capable of forming adducts with DNA which is considered an initial step in carcinogenesis [12]. In 2009, the International Agency for Research on Cancer (IARC) designated acetaldehyde (as associated with alcohol consumption) to be a group I human carcinogen [13]. Acetaldehyde is metabolized to acetate, a process catalyzed by aldehyde dehydrogenase (ALDH) 2, ALDH1B1 and ALDH1A1 (Fig. 1) [14]. The ability of these ALDHs to repress cellular acetaldehyde levels is consistent with a role for ALDHs in colon and pancreatic cancers and is supported strongly by the association of ALDH2 deficiency with high incidence of CRC and pancreatic cancer in heavy ethanol drinkers [15, 16]. In addition to metabolizing acetaldehyde, ALDH1 isozymes are the primary enzymes involved in the metabolism of retinaldehyde to retinoic acid (RA), a signaling molecule that plays a crucial role in cellular proliferation and differentiation [11]. Given their ability to affect cellular RA levels, it is likely that RA-generating ALDHs have a role in modulating carcinogenesis. Several other observations lend support to the notion that ALDHs are implicated in cancer. First, ALDH activity has been used to identify and isolate normal and cancer stem cells of various lineages [17–19] (Table 1). Second, high ALDH expression has been found to be associated with poor clinical outcome in leukemia [20], ovarian [21–23], prostate [24, 25], breast [26–28], colorectal [29] and pancreatic cancer [15, 30]. Third, ALDH+ cells (cells with very high ALDH expression) exhibit a greater tumorigenic capacity, as reflected in colony-forming capability in vitro and in xenograft-induced tumor formation in vivo [31]. We have found very strong up-regulation of ALDH1B1 expression in an animal model of colon polyps, specifically adenomatous polyposis coli multiple intestinal neoplasia (Apc(Min)/+) mice (our unpublished data). These mice have point mutation in Apc, a tumor suppressor gene which when mutated leads to dysregulation of the Wnt-signaling pathway and results in up-regulation of oncogenes like c-Myc [32]. Overexpression of ALDH1B1 in polyps from these mice is suggestive of a possible relationship between Wnt-signaling and ALDH1B1 expression, a consideration that warrants further study.
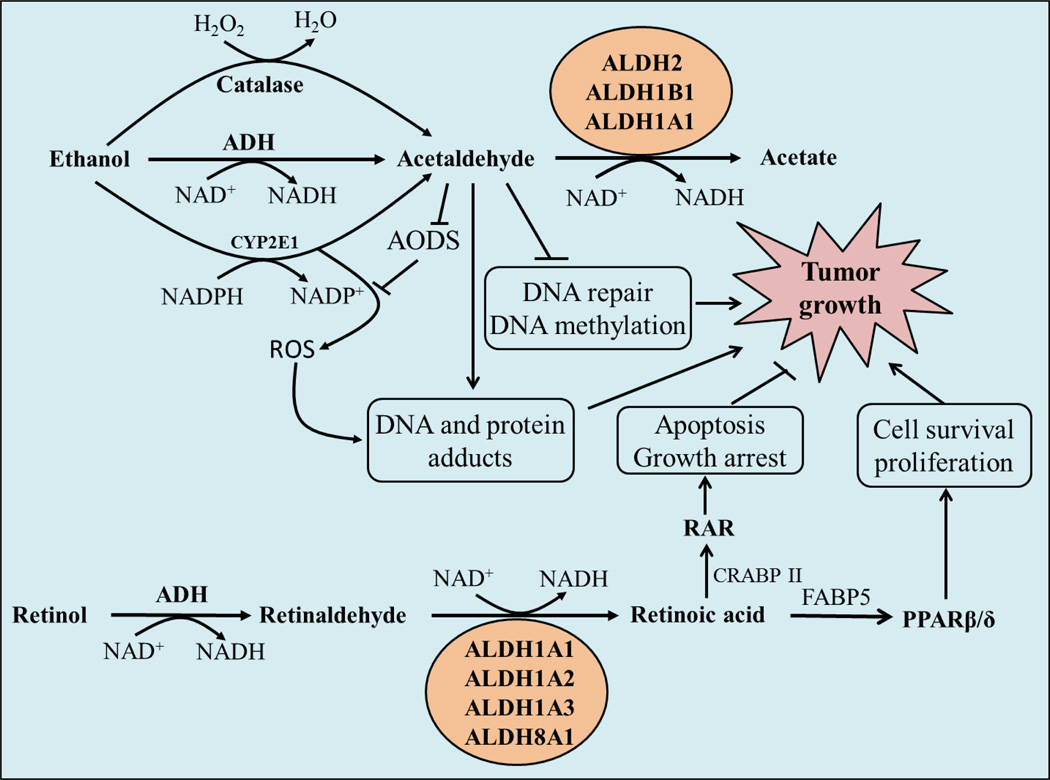
Fig. 1. ALDHs modulate carcinogenesis by metabolizing acetaldehyde and retinaldehyde.
Ethanol is metabolized by alcohol dehydrogenase (ADH), catalase and CYP2E1 to acetaldehyde. Acetaldehyde can interfere with anti-oxidative defense systems (AODS) and generate reactive oxygen species (ROS); inhibits DNA repair and methylation; and forms DNA and protein adducts to promote tumor growth. Acetaldehyde is metabolized to acetate primarily by ALDH2, ALDH1B1 and ALDH1A1. Retinaldehyde, formed from retinol by ADH, is converted to retinoic acid (RA) by retinaldehyde-metabolizing ALDHs. RA exerts anti-carcinogenic activity by binding to cellular retinoic acid binding proteins (CRBPII) and activating the RA receptor (RAR). When RA binds to fatty acid binding protein 5 (FABP5), it activates orphan nuclear receptor peroxisome proliferator-activated receptor (PPAR)β/δ and acts as procarcinogenic agent. ALDH, aldehyde dehydrogenase; NAD+, NAD(P), nicotinamide adenine dinucleotide (phosphate); H2O2, hydrogen peroxide.
Table 1.
ALDH expression in various progenitor, stem and cancer cell types.
| S.No. | Cell or tumor type | ALDH isozyme(s) a | Reference |
|---|---|---|---|
| 1 | Hematopoietic progenitor | ALDH, ALDH1A3 | [17–19, 74, 75] |
| 2 | Mesenchymal progenitors | ALDH | [74] |
| 3 | Endothelial progenitors | ALDH | [74] |
| 4 | Neural stem cells | ALDH, ALDH1L1 | [76, 77] |
| 5 | Normal mammary stem cells | ALDH1A1 | [26] |
| 6 | Breast cancer stem cells | ALDH1A1, ALDH1A3, ALDH2, ALDH6A1, | [26, 28, 75, 78, 79] |
| 7 | Prostate cancer | ALDH, ALDH7A1 | [24, 25, 80] |
| 8 | Ovarian cancer stem cells | ALDH, ALDH1A1 | [21–23, 81] |
| 9 | Ovarian cancer cells | ALDH1A1, ALDH1A3, ALDH3A2, ALDH7A1 | [82] |
| 10 | Colon stem cells | ALDH1A1, ALDH1B1 | [64, 70] |
| 11 | Colon cancer stem cells | ALDH1A1, ALDH1B1 | [21, 29, 31, 64, 70, 83] |
| 12 | Leukemia stem cells | ALDH | [20] |
| 13 | Human lung cancer cells | ALDH1A1 | [21, 84, 85] |
| 14 | Head and neck cancer stem cells | ALDH1A1 | [86] |
| 15 | Pancreatic cancer | ALDH, ALDH1A1, ALDH1A3 | [30, 75, 87] |
| 16 | Liver cancer stem cells | ALDH, ALDH1A1 | [88, 89] |
ALDH is designated for studies in which ALDH+ cells were identified and isolated using the ALDEFLUOR™ assay.
A causal relationship exists between alcohol consumption and CRC or pancreatic cancer and this may be mediated, at least in part, by acetaldehyde [12, 15]. The significance of retinaldehyde and acetaldehyde in tumor formation, and very high expression of the ALDHs in colorectal and pancreatic cancer are suggestive of a crucial role for acetaldehyde-and retinaldehyde-metabolizing ALDHs in these cancers. Lack of ALDH2 activity and resultant high acetaldehyde levels are linked with colon cancer initiation. By contrast high ALDH1 activity (primarily ALDH1A1 and ALDH1B1) is required for the stemness and tumorigenic potential of cancer stem cells.
Acetaldehyde: A carcinogen
Acetaldehyde is categorized as ‘carcinogenic to humans’ and ‘reasonably anticipated to be a human carcinogen’ according to IARC regulations and United States National Toxicology Program (NTP), respectively [13, 33]. Acetaldehyde has been shown to be a highly toxic, mutagenic and carcinogenic compound in a variety of in vitro and in vivo studies. Its effects range from damaging antioxidant defenses [11] to interfering with DNA methylation and repair mechanisms through formation of adducts with DNA and proteins (Fig. 1) [10, 12]. In the colon, acetaldehyde is primarily produced from ethanol by resident bacteria and, to a lesser extent, by mucosal ADHs. As a result of metabolism by intra-colonic microbes, large quantities (nine-fold higher than normal) of acetaldehyde accumulate in the rat colon 2 hrs after intra-peritoneal injection of ethanol [34]. Human colon mucosal cells harbor ADH1, ADH3 and ADH5, with the ADH1 and ADH3 isozymes being most active [35]. In an in vitro experiment, human colon contents were able to generate 60 to 250 µM acetaldehyde when incubated with concentration of ethanol (10–100 mg%), which is known to be attained during normal ethanol drinking [36]. The high levels of acetaldehyde attained in the colon after drinking ethanol likely underlies the correlation between chronic, heavy ethanol consumption and CRC in humans. In ethanol-treated rats, a high concentration of acetaldehyde (50 to 350 µM) in the colon mucosa has been shown to correlate positively with hyper-proliferation of the colon crypt cells. Such a phenomenon would be anticipated to favor the development of CRC [37, 38].
Acetaldehyde is metabolized primarily by mitochondrial ALDH2 and ALDH1B1 and, to lesser extent, by cytosolic ALDH1A1 (Table 2) [14]. The most convincing evidence for a role of acetaldehyde in CRC initiation emanates from studies involving Asians who possess a polymorphism in their ALDH2 enzyme known as ALDH2*2. These subjects possess a single nucleotide polymorphism (SNP) that leads to a lysine to glutamate substitution at residue 504 that renders the enzyme functionally-inactive [39]. Approximately 40 per cent of the Asian population carry an ALDH2*2 allele; this compromises their ability to metabolize acetaldehyde and increases their colon cancer risk 3.4 times [16].
Table 2.
Affinity of ALDHs for acetaldehyde and retinaldehyde
| S.No. | ALDH isozyme(s) | Substrate | Km | Reference |
|---|---|---|---|---|
| 1 | ALDH1A1 | Acetaldehyde | 180 µM | [14] |
| All-trans Retinaldehyde 9-cis Retinaldehyde |
11.6–26.8 µM 3.59 µM |
[90], Jackson et al, under preparation. | ||
| 2 | ALDH1A2 | All-trans Retinaldehyde 9-cis Retinaldehyde |
0.66 µM 0.62 µM |
[91] |
| 3 | ALDH1A3 | All-trans Retinaldehyde | 0.2 µM | [92] |
| 4 | ALDH1B1 | Acetaldehyde | 55 µM | [14] |
| Retinaldehyde | 24.9 µM | Jackson et al., under preparation | ||
| 5 | ALDH2 | Acetaldehyde | 3.2 µM | [14] |
| 6 | ALDH8A1 | 9-cis Retinaldehyde | 3.15 µM | [45] |
Opposing effects of retinoic acid on cancer cell proliferation
Retinoids exert many physiologically-important and diverse functions in relation to cellular proliferation and differentiation of normal and cancer cells. For example, retinoids are crucial for embryonic development and adult tissue remodeling. The retinoids comprise all of the derivatives of retinol, including all-trans-, 9-cis- and 13-cis-retinoic acid (RA). Retinol is oxidized to retinaldehyde by retinol dehydrogenases. The resultant retinaldehydes are further metabolized to their corresponding RA by retinaldehyde dehydrogenases which include RALDH1 (ALDH1A1), RALDH2 (ALDH1A2), RALDH3 (ALDH1A3) and RALDH4 (ALDH8A1) (Table 2) [40–45]. Among the RAs, all-trans-RA (ATRA) is the most biologically potent retinoid. Abnormally low levels of ALDH1A2 have been observed in breast and prostate cancers [46, 47]. Impaired RA formation and high levels of CYP26A1 (a RA-metabolizing enzyme) in human breast cancer are consistent with a protective role for RA in this cancer [46–48]. The physiological actions of the retinoids are mediated through binding of the RA receptor (RAR) and retinoid X receptor (RXR) heterodimer to the regulatory region of retinoid-responsive genes, known as RA response elements [49]. RARs and RXRs are ligand-dependent transcription factors and exist as α, β or γ isoforms. RAR isoforms interact with both ATRA and 9-cis RA, whereas RXR isoforms interacts only with 9-cis RA [50, 51]. The binding of RA with the RAR/RXR dimer recruits co-activator proteins and initiates transcriptional activation of the retinoid-responsive genes [49]. Retinoids have been found to be effective for the treatment of acute promyelocytic leukemia and prevention of liver, lung, breast, prostate, skin and colon cancers [52–54]. In vivo studies involving rats have revealed that retinoids added to the diet reduced colon cancer cell proliferation and prevented azoxymethane-induced aberrant crypt foci (putative precancerous lesions in colon) and colon tumor formation [54, 55]. A RXR-selective retinoid, AGN194204, has been found to inhibit the proliferation of human pancreatic cancer cells, an effect that can be reversed by a RXR-selective antagonist [56]. In addition to inhibiting the growth of pancreatic cancer cells, RA increases the sensitivity of pancreatic adenocarcinoma cells to the antineoplastic drugs gemcitabine and cisplatin [57].
In contrast to the anti-proliferative and anti-survival role of RA in cancer cells, dietary ATRA has been shown to enhance initiation and growth of intestinal tumors in the Apc (Min)/+ mouse model in vivo [58]. RA can promote cell survival and hyperplasia in cells expressing high levels of fatty acid-binding protein 5 (FABP5) by activating an orphan nuclear receptor, peroxisome proliferator-activated receptor (PPAR)β/δ [59]. PPARβ/δ mediates antiapoptotic properties partly by inducing the PDK1/Akt survival pathway [60]. RA binds to intracellular lipid binding proteins (iLBPs), including cellular retinoic acid-binding proteins (CARBPII) and FABP5. CARBPII and FABP5 are selective for nuclear receptors RARα and PPARβ/δ, respectively [59]. Hence, RA induces CARBPII- or FABP5-mediated activation of RAR or PPARβ/δ (respectively), depending on the ratio of FABP5/ CRBPII in the cells [59]. Human colorectal cancer cell lines (specifically, T84, COLO205, SW620, SW480, HCT116 and DLD-1) express ~30-fold higher levels of FABP5 relative to normal colorectal cells (CCD18-Co), suggesting the possibility of pro-proliferative and anti-apoptotic roles for RA in these cells [59, 61]. However, the expression levels of PPARβ/δ in colorectal cancer cells and its role in tumorigenesis are unresolved in various cancers, including CRC [62].
RA inhibits the proliferation and increases chemosensitivity of pancreatic cancer cells. However, the involvement of RA in CRC is less clear, with opposing findings suggesting pro- or anti-proliferative roles.
ALDH and cancer stem cells
In the gastrointestinal (GI) tract, tissue-specific stem cells are at the top of the cellular hierarchy and play a critical role in regulating tissue homeostasis. These specialized epithelial cells are characterized by their ability to self-renew and differentiate into a variety of cellular populations that perform specific functions within the GI tract. Currently, it is believed that these tissue-specific stem cells (or progenitor cells), when oncogenically transformed, become cancer stem cells (CSCs) or tumor initiating-cells (TICs) since they functionally possess the capacity to form tumors and maintain tumor growth. Accumulating evidence also suggests that CSCs are responsible for chemotherapeutic/radiation resistance and tumor recurrence (Fig. 2). ALDH catalytic activity has been identified in many human cancers [28] and, as such, is used as a marker of CSCs, including colorectal and pancreatic cancer. The pathophysiological function of ALDH in CSCs remains unresolved. Intense research of ALDH enzymes is underway in order to elucidate the role of these proteins in the development and progression of cancer as well as drug resistance.
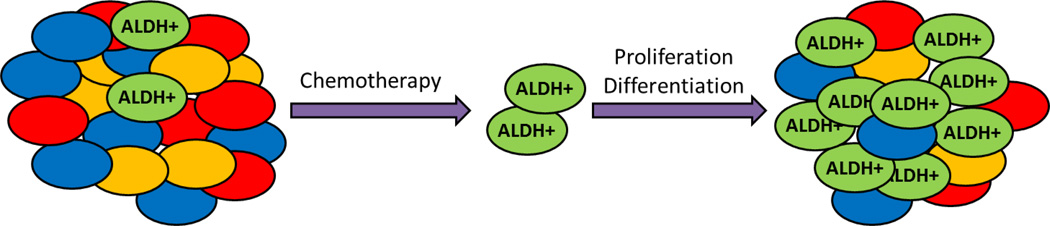
Fig. 2. ALDH-expressing cells are responsible for chemoresistance and relapse of many tumors after chemotherapy.
Most current chemotherapy drugs are effective against the bulk of the tumor cells. However, the high ALDH-expressing (ALDH+) cancer stem cells are resistant to these treatments. As a result, during chemotherapy, the ALDH+ cells proliferate and promote tumor growth. The resultant tumors contain an increased proportion of ALDH+ cells, making them more resistant to chemotherapy than the original tumor.
Colorectal cancer
Although earlier stages of CRC are highly curable, therapeutic interventions in advanced disease have proven to be poorly effective at increasing the 5-year survival rate. Recent drug development has focused on targeting the CSC population as a potential therapy. In normal colon, CSC’s reside at the bottom of the crypt and generate upward, migrating and differentiating transit amplifying cells (in the middle of the crypt) which become terminally differentiated cells as they move upward and eventually shed into the lumen (Fig. 3A) [63]. In CRC, several different molecules, including the cell surface markers CD133 and CD44 as well as ALDH activity, have been proposed as biomarkers for identification and isolation of the CSC population [31, 64–67]. CD133+ colon cancer cells were initially shown to be tumorigenic [66, 67]. However, subsequent studies identified that both CD133+ and CD133-cells possess tumorigenic potential [68]. CD44+ (either with or without epithelial-specific antigen (ESA+)) was demonstrated to be a marker in colon CSCs [68]. However, additional studies showed that CD44+ cells reside throughout the entire crypt, including the proliferative compartment, suggesting that the CD44+ colon cells are not necessarily stem-like [64]. We have examined CD44 and ALDH together in one of our CRC patient-derived tumor xenograft (PDTX) models to determine if CD44+ cells had tumorigenic properties [69]. Despite ALDH+/CD44+ cells showing some tumorigenic growth, ALDH+/CD44-cells exhibited a higher incidence and faster growing tumors. In this same PDTX model, isolation and injection of ALDH+ and ALDH-cells in mice showed a significant difference with respect to tumor growth [69]. ALDH+ cells produced fast growing and large tumors when compared to ALDH-cells that either produced very small tumors or no tumors in five separate PDTX models. Importantly, all ALDH+ tumors looked morphologically the same as the original tumor. Several other studies have shown that injection of ALDH+ cells from colitis and colon cancer patients facilitated spheroid formation (in vitro 3-dimensional spheroid cell culture that more closely resembles the in vivo environment) and tumor growth in a xenograft model, while ALDH-cells were incapable of tumor growth [31, 64]. These studies demonstrate that ALDH catalytic activity appears to be a robust marker of CSCs in CRC.
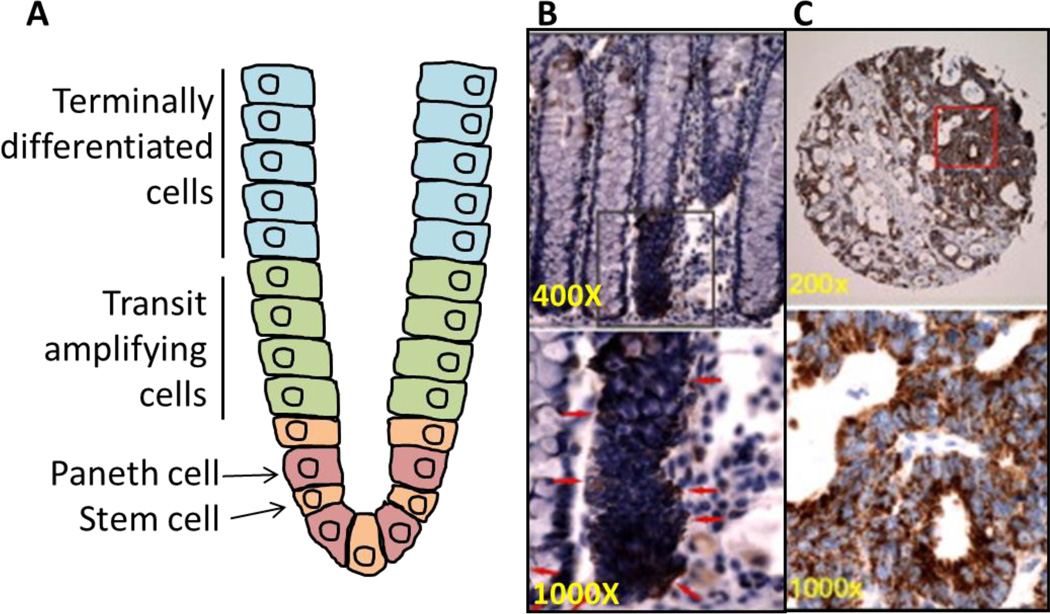
Fig. 3. ALDH1B1 expression pattern in normal colon and colon adenocarcinoma.
Location of various cell types in normal colon (A). ALDH1B1 expression (red arrows) is strictly localized to stem-like cells at the base of crypts in the normal human colon (B). ALDH1B1 is expressed at extremely high levels throughout all cells of human colon adenocarcinomas (C). In figures B and C (reproduced from Chen et al., 2011 [70]), lower panels are higher magnification of areas identified by squares in the upper panel.
Given the apparent promise of ALDH activity as a potential biomarker of CSCs, many investigations are currently exploring the role of ALDH in CSC function. In particular, a great deal of focus is being placed on which ALDH isoform(s) mediate the catalytic activity in the CSCs. In normal colon stem cells, ALDH1 has been demonstrated to be primarily expressed at the bottom of the crypt compartment in the colon (where colon-specific stem cells are located) and ALDH1 levels are significantly elevated in the development and progression of CRC [64]. Interestingly, ALDH1 protein levels are elevated in the colon of patients with ulcerative colitis (a risk factor for colon cancer) compared to normal colon cells; such expression may be important in the transformation from colitis to colon cancer [31]. We have shown that ALDH1B1 protein is 5.6-fold higher when compared to ALDH1A1 in CRC patients and may be a potential biomarker in CRC (Fig. 3B–C) [70]. Similarly, very high expression of ALDH1B1 was found in the colon polyps of Apc (Min)/+ mice (our unpublished data). While these studies indicate elevations in individual ALDH isoforms in CRC, the contribution of these enzymes to the progression of CSCs and CRC remain to be clarified.
A common problem associated with standard chemotherapeutic regimens in CRC is treatment resistance. Although chemotherapy is effective at reducing tumor burden, many CRC patients will experience disease recurrence and ultimately succumb to their disease. CSCs are thought to be responsible for chemotherapy-resistance and disease recurrence [71]. Therefore, therapeutic elimination of this population would be predicted to reduce tumor recurrence and ultimately improve survival. In our CRC PDTX model, the effects of an inhibitor of the Notch pathway (considered to be important for self-renewal of colon stem cells) in combination with irinotecan was investigated on the ALDH+ cell population [69]. The combination therapy was effective at reducing the number of ALDH+ cells as well as tumor recurrence, even after treatment was discontinued when compared to single agent Notch pathway inhibition and irinotecan. Administration of the combination therapy for 28 days prevented tumor growth in the ALDH+ cell xenograft model; this protection continued for 3 months after combination treatment was completed [69]. These data indicate that the ALDH+ population has the ability to self-renew, and significantly reducing this population of cells delays tumor recurrence (Fig. 2). Whether specific ALDH isozymes contribute to chemotherapy resistance remains to be determined.
Pancreatic cancer
Despite considerable research, the 5-year survival rate for pancreatic cancer still remains extremely poor. A concerted effort is underway to delineate pathways that are dysregulated in the CSC population of this disease and thereby identify novel potential therapeutic targets.
In pancreatic cancer, ALDH+ cells have been shown to possess stem cell features, as evidenced by enhanced clonogenicity in vitro and tumorigenic growth in mice [72]. These cells also have greater tumorigenic potential than CD133+ cells [72]. Interestingly, ALDH+ cells from pancreatic cancers have been demonstrated to: i) express many genes of the mesenchymal phenotype, ii) have an increased capacity to migrate and invade, and iii) be more numerous in metastatic lesions [30]. Furthermore, in pancreatic cancer patients, expression of ALDH in tumors is associated with a worse survival rate than those tumors that do not express ALDH [30]. ALDH1A1 expression has been linked to resistance to chemotherapy in a pancreas PDTX model. In this context, treatment with gemcitabine was shown to enhance gene and protein expression of ALDH. Inclusion of an inhibitor of hedgehog (a pathway important for stem cell regulation in the pancreas) with gemcitabine resulted in decreased expression of ALDH [73]. These studies suggest that ALDH+ cells are stem-like cells in pancreas cancer and may be important contributors in disease progression and chemoresistance; therefore contribute to the negative outcomes in patients with pancreatic cancer.
Summary
There is accumulating evidence that supports a role for ALDHs in cancer development and progression. The exact mechanisms by which ALDHs influence tumorigenesis remain to be defined. Certainly, metabolism of acetaldehyde and/or the generation of retinoic acid represent modalities by which ALDHs could influence CRC and pancreatic cancer. ALDH catalytic activity appears to be an excellent biomarker that can be utilized for the isolation and characterization of the CSC population in tumors obtained from patients with CRC or pancreatic cancer. It is becoming apparent that the various ALDH isozymes may have different roles in tumorigenesis (from metabolism of the carcinogen to modulation of the proliferation-regulating retinoids) and that the timing and cellular localization of isozyme expression may be critical factors that influence how ALDHs modulate cancer development and progression. Further studies are needed that identify (i) the importance of ALDH catalytic activity in modulation of tumorigenesis, (ii) the specific ALDH isozymes involved (and that regulate CSCs), and (iii) the signaling pathways that regulate tumor-associated ALDH expression. The results obtained from such studies should lead to the development of novel therapies that may more effectively treat these devastating diseases.
Acknowledgments
We would like to thank our colleagues for critically reviewing this manuscript. This work was supported, in part, by the following NIH grants; AA022057 and EY11490.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Wei EK, Wolin KY, Colditz GA. Time course of risk factors in cancer etiology and progression. J Clin Oncol. 2010;28:4052–4057. doi: 10.1200/JCO.2009.26.9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaneko R, Sato Y, An Y, et al. Clinico-epidemiologic study of the metabolic syndrome and lifestyle factors associated with the risk of colon adenoma and adenocarcinoma. Asian Pac J Cancer Prev. 2010;11:975–983. [PubMed] [Google Scholar]
- 5.Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140:603–613. doi: 10.7326/0003-4819-140-8-200404200-00007. [DOI] [PubMed] [Google Scholar]
- 6.Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22:1958–1972. doi: 10.1093/annonc/mdq653. [DOI] [PubMed] [Google Scholar]
- 7.Wilson JS, Apte MV. Role of alcohol metabolism in alcoholic pancreatitis. Pancreas. 2003;27:311–315. doi: 10.1097/00006676-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Brennan P, Boffetta P. Mechanistic considerations in the molecular epidemiology of head and neck cancer. IARC Sci Publ. 2004:393–414. [PubMed] [Google Scholar]
- 9.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 10.Seitz HK, Stickel F. Acetaldehyde as an underestimated risk factor for cancer development: role of genetics in ethanol metabolism. Genes Nutr. 2010;5:121–128. doi: 10.1007/s12263-009-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh S, Brocker C, Koppaka V, et al. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radic Biol Med. 2013;56:89–101. doi: 10.1016/j.freeradbiomed.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu HS, Oyama T, Isse T, et al. Formation of acetaldehyde-derived DNA adducts due to alcohol exposure. Chem Biol Interact. 2010;188:367–375. doi: 10.1016/j.cbi.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Secretan B, Straif K, Baan R, et al. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 14.Stagos D, Chen Y, Brocker C, et al. Aldehyde dehydrogenase 1B1: molecular cloning and characterization of a novel mitochondrial acetaldehyde-metabolizing enzyme. Drug Metab Dispos. 2010;38:1679–1687. doi: 10.1124/dmd.110.034678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanda J, Matsuo K, Suzuki T, et al. Impact of alcohol consumption with polymorphisms in alcohol-metabolizing enzymes on pancreatic cancer risk in Japanese. Cancer Sci. 2009;100:296–302. doi: 10.1111/j.1349-7006.2008.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama A, Muramatsu T, Ohmori T, et al. Alcohol-related cancers and aldehyde dehydrogenase-2 in Japanese alcoholics. Carcinogenesis. 1998;19:1383–1387. doi: 10.1093/carcin/19.8.1383. [DOI] [PubMed] [Google Scholar]
- 17.Storms RW, Trujillo AP, Springer JB, et al. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci U S A. 1999;96:9118–9123. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess DA, Meyerrose TE, Wirthlin L, et al. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong L, Stojkovic M, Dimmick I, et al. Phenotypic characterization of murine primitive hematopoietic progenitor cells isolated on basis of aldehyde dehydrogenase activity. Stem Cells. 2004;22:1142–1151. doi: 10.1634/stemcells.2004-0170. [DOI] [PubMed] [Google Scholar]
- 20.Ran D, Schubert M, Pietsch L, et al. Aldehyde dehydrogenase activity among primary leukemia cells is associated with stem cell features and correlates with adverse clinical outcomes. Exp Hematol. 2009;37:1423–1434. doi: 10.1016/j.exphem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Deng S, Yang X, Lassus H, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 2010;5:e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva IA, Bai S, McLean K, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YC, Yo YT, Lee HY, et al. ALDH1-bright epithelial ovarian cancer cells are associated with CD44 expression, drug resistance, and poor clinical outcome. Am J Pathol. 2012;180:1159–1169. doi: 10.1016/j.ajpath.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 24.van den Hoogen C, van der Horst G, Cheung H, et al. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 2010;70:5163–5173. doi: 10.1158/0008-5472.CAN-09-3806. [DOI] [PubMed] [Google Scholar]
- 25.van den Hoogen C, van der Horst G, Cheung H, et al. The aldehyde dehydrogenase enzyme 7A1 is functionally involved in prostate cancer bone metastasis. Clin Exp Metastasis. 2011;28:615–625. doi: 10.1007/s10585-011-9395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charafe-Jauffret E, Ginestier C, Iovino F, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcato P, Dean CA, Pan D, et al. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells. 2011;29:32–45. doi: 10.1002/stem.563. [DOI] [PubMed] [Google Scholar]
- 29.Langan RC, Mullinax JE, Ray S, et al. A Pilot Study Assessing the Potential Role of non-CD133 Colorectal Cancer Stem Cells as Biomarkers. J Cancer. 2012;3:231–240. doi: 10.7150/jca.4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasheed ZA, Yang J, Wang Q, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpentino JE, Hynes MJ, Appelman HD, et al. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69:8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCart AE, Vickaryous NK, Silver A. Apc mice: models, modifiers and mutants. Pathol Res Pract. 2008;204:479–490. doi: 10.1016/j.prp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Zakhari S, Vasiliou V, Guo QM, editors. Alcohol and Cancer. 1 ed. Springer; 2011. [Google Scholar]
- 34.Visapaa JP, Jokelainen K, Nosova T, Salaspuro M. Inhibition of intracolonic acetaldehyde production and alcoholic fermentation in rats by ciprofloxacin. Alcohol Clin Exp Res. 1998;22:1161–1164. [PubMed] [Google Scholar]
- 35.Jelski W, Zalewski B, Chrostek L, Szmitkowski M. The activity of class I, II, III, and IV alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in colorectal cancer. Dig Dis Sci. 2004;49:977–981. doi: 10.1023/b:ddas.0000034557.23322.e0. [DOI] [PubMed] [Google Scholar]
- 36.Salaspuro M. Microbial metabolism of ethanol and acetaldehyde and clinical consequences. Addiction Biology. 1997;2:35–46. doi: 10.1080/13556219772840. [DOI] [PubMed] [Google Scholar]
- 37.Seitz HK, Simanowski UA, Garzon FT, et al. Possible role of acetaldehyde in ethanol-related rectal cocarcinogenesis in the rat. Gastroenterology. 1990;98:406–413. doi: 10.1016/0016-5085(90)90832-l. [DOI] [PubMed] [Google Scholar]
- 38.Simanowski UA, Suter P, Russell RM, et al. Enhancement of ethanol induced rectal mucosal hyper regeneration with age in F344 rats. Gut. 1994;35:1102–1106. doi: 10.1136/gut.35.8.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Zhang D, Jin W, et al. Mitochondrial aldehyde dehydrogenase-2 (ALDH2) Glu504Lys polymorphism contributes to the variation in efficacy of sublingual nitroglycerin. J Clin Invest. 2006;116:506–511. doi: 10.1172/JCI26564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur J Biochem. 2000;267:4315–4324. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida A, Hsu LC, Dave V. Retinal oxidation activity and biological role of human cytosolic aldehyde dehydrogenase. Enzyme. 1992;46:239–244. doi: 10.1159/000468794. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki R, Shintani T, Sakuta H, et al. Identification of RALDH-3, a novel retinaldehyde dehydrogenase, expressed in the ventral region of the retina. Mech Dev. 2000;98:37–50. doi: 10.1016/s0925-4773(00)00450-0. [DOI] [PubMed] [Google Scholar]
- 43.Niederreither K, Fraulob V, Garnier JM, et al. Differential expression of retinoic acid-synthesizing (RALDH) enzymes during fetal development and organ differentiation in the mouse. Mech Dev. 2002;110:165–171. doi: 10.1016/s0925-4773(01)00561-5. [DOI] [PubMed] [Google Scholar]
- 44.Sima A, Parisotto M, Mader S, Bhat PV. Kinetic characterization of recombinant mouse retinal dehydrogenase types 3 and 4 for retinal substrates. Biochim Biophys Acta. 2009;1790:1660–1664. doi: 10.1016/j.bbagen.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Lin M, Napoli JL. cDNA cloning and expression of a human aldehyde dehydrogenase (ALDH) active with 9-cis-retinal and identification of a rat ortholog, ALDH12. J Biol Chem. 2000;275:40106–40112. doi: 10.1074/jbc.M008027200. [DOI] [PubMed] [Google Scholar]
- 46.Kim H, Lapointe J, Kaygusuz G, et al. The retinoic acid synthesis gene ALDH1a2 is a candidate tumor suppressor in prostate cancer. Cancer Res. 2005;65:8118–8124. doi: 10.1158/0008-5472.CAN-04-4562. [DOI] [PubMed] [Google Scholar]
- 47.Mira YLR, Zheng WL, Kuppumbatti YS, et al. Retinol conversion to retinoic acid is impaired in breast cancer cell lines relative to normal cells. J Cell Physiol. 2000;185:302–309. doi: 10.1002/1097-4652(200011)185:2<302::AID-JCP15>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 48.Touma SE, Perner S, Rubin MA, et al. Retinoid metabolism and ALDH1A2 (RALDH2) expression are altered in the transgenic adenocarcinoma mouse prostate model. Biochem Pharmacol. 2009;78:1127–1138. doi: 10.1016/j.bcp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol. 2011;6:345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- 50.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 51.Shimizu M, Takai K, Moriwaki H. Strategy and mechanism for the prevention of hepatocellular carcinoma: phosphorylated retinoid X receptor alpha is a critical target for hepatocellular carcinoma chemoprevention. Cancer Sci. 2009;100:369–374. doi: 10.1111/j.1349-7006.2008.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boone CW, Kelloff GJ, Malone WE. Identification of candidate cancer chemopreventive agents and their evaluation in animal models and human clinical trials: a review. Cancer Res. 1990;50:2–9. [PubMed] [Google Scholar]
- 53.Warrell RP, Jr, Frankel SR, Miller WH, Jr, et al. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid) N Engl J Med. 1991;324:1385–1393. doi: 10.1056/NEJM199105163242002. [DOI] [PubMed] [Google Scholar]
- 54.Zheng Y, Kramer PM, Lubet RA, et al. Effect of retinoids on AOM-induced colon cancer in rats: modulation of cell proliferation, apoptosis and aberrant crypt foci. Carcinogenesis. 1999;20:255–260. doi: 10.1093/carcin/20.2.255. [DOI] [PubMed] [Google Scholar]
- 55.Wargovich MJ, Jimenez A, McKee K, et al. Efficacy of potential chemopreventive agents on rat colon aberrant crypt formation and progression. Carcinogenesis. 2000;21:1149–1155. [PubMed] [Google Scholar]
- 56.Balasubramanian S, Chandraratna RA, Eckert RL. Suppression of human pancreatic cancer cell proliferation by AGN194204, an RXR-selective retinoid. Carcinogenesis. 2004;25:1377–1385. doi: 10.1093/carcin/bgh122. [DOI] [PubMed] [Google Scholar]
- 57.Pettersson F, Colston KW, Dalgleish AG. Retinoic acid enhances the cytotoxic effects of gemcitabine and cisplatin in pancreatic adenocarcinoma cells. Pancreas. 2001;23:273–279. doi: 10.1097/00006676-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Mollersen L, Paulsen JE, Olstorn HB, et al. Dietary retinoic acid supplementation stimulates intestinal tumour formation and growth in multiple intestinal neoplasia (Min)/+ mice. Carcinogenesis. 2004;25:149–153. doi: 10.1093/carcin/bgg176. [DOI] [PubMed] [Google Scholar]
- 59.Schug TT, Berry DC, Shaw NS, et al. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di-Poi N, Tan NS, Michalik L, et al. Antiapoptotic role of PPARbeta in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol Cell. 2002;10:721–733. doi: 10.1016/s1097-2765(02)00646-9. [DOI] [PubMed] [Google Scholar]
- 61.Koshiyama A, Ichibangase T, Imai K. Comprehensive fluorogenic derivatization-liquid chromatography/tandem mass spectrometry proteomic analysis of colorectal cancer cell to identify biomarker candidate. Biomed Chromatogr. 2013;27:440–450. doi: 10.1002/bmc.2811. [DOI] [PubMed] [Google Scholar]
- 62.Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer. 2012;12:181–195. doi: 10.1038/nrc3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McDonald SA, Preston SL, Lovell MJ, et al. Mechanisms of disease: from stem cells to colorectal cancer. Nat Clin Pract Gastroenterol Hepatol. 2006;3:267–274. doi: 10.1038/ncpgasthep0473. [DOI] [PubMed] [Google Scholar]
- 64.Huang EH, Hynes MJ, Zhang T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 67.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 68.Shmelkov SV, Butler JM, Hooper AT, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arcaroli JJ, Powell RW, Varella-Garcia M, et al. ALDH+ tumor-initiating cells exhibiting gain in NOTCH1 gene copy number have enhanced regrowth sensitivity to a gamma-secretase inhibitor and irinotecan in colorectal cancer. Mol Oncol. 2012;6:370–381. doi: 10.1016/j.molonc.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y, Orlicky DJ, Matsumoto A, et al. Aldehyde dehydrogenase 1B1 (ALDH1B1) is a potential biomarker for human colon cancer. Biochem Biophys Res Commun. 2011;405:173–179. doi: 10.1016/j.bbrc.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 72.Kim MP, Fleming JB, Wang H, et al. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One. 2011;6:e20636. doi: 10.1371/journal.pone.0020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jimeno A, Feldmann G, Suarez-Gauthier A, et al. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther. 2009;8:310–314. doi: 10.1158/1535-7163.MCT-08-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gentry T, Foster S, Winstead L, et al. Simultaneous isolation of human BM hematopoietic, endothelial and mesenchymal progenitor cells by flow sorting based on aldehyde dehydrogenase activity: implications for cell therapy. Cytotherapy. 2007;9:259–274. doi: 10.1080/14653240701218516. [DOI] [PubMed] [Google Scholar]
- 75.Marcato P, Dean CA, Giacomantonio CA, Lee PW. Aldehyde dehydrogenase: its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle. 2011;10:1378–1384. doi: 10.4161/cc.10.9.15486. [DOI] [PubMed] [Google Scholar]
- 76.Corti S, Locatelli F, Papadimitriou D, et al. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 2006;24:975–985. doi: 10.1634/stemcells.2005-0217. [DOI] [PubMed] [Google Scholar]
- 77.Foo LC, Dougherty JD. Aldh1L1 is expressed by postnatal neural stem cells in vivo. Glia. 2013;61:1533–1541. doi: 10.1002/glia.22539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanei T, Morimoto K, Shimazu K, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15:4234–4241. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 79.Charafe-Jauffret E, Ginestier C, Iovino F, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Le Magnen C, Bubendorf L, Rentsch CA, et al. Characterization and Clinical Relevance of ALDHbright Populations in Prostate Cancer. Clin Cancer Res. 2013;19:5361–5371. doi: 10.1158/1078-0432.CCR-12-2857. [DOI] [PubMed] [Google Scholar]
- 81.Landen CN, Jr, Goodman B, Katre AA, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9:3186–3199. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saw YT, Yang J, Ng SK, et al. Characterization of aldehyde dehydrogenase isozymes in ovarian cancer tissues and sphere cultures. BMC Cancer. 2012;12:329. doi: 10.1186/1471-2407-12-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dylla SJ, Beviglia L, Park IK, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang F, Qiu Q, Khanna A, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Q, Taguchi A, Schliekelman M, et al. Comprehensive proteomic profiling of aldehyde dehydrogenases in lung adenocarcinoma cell lines. Int J Proteomics. 2011;2011:145010. doi: 10.1155/2011/145010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen YC, Chen YW, Hsu HS, et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385:307–313. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 87.Kim SK, Kim H, Lee DH, et al. Reversing the Intractable Nature of Pancreatic Cancer by Selectively Targeting ALDH-High, Therapy-Resistant Cancer Cells. PLoS One. 2013;8:e78130. doi: 10.1371/journal.pone.0078130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma S, Chan KW, Lee TK, et al. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 89.Colombo F, Baldan F, Mazzucchelli S, et al. Evidence of distinct tumour-propagating cell populations with different properties in primary human hepatocellular carcinoma. PLoS One. 2011;6:e21369. doi: 10.1371/journal.pone.0021369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gagnon I, Duester G, Bhat PV. Enzymatic characterization of recombinant mouse retinal dehydrogenase type 1. Biochem Pharmacol. 2003;65:1685–1690. doi: 10.1016/s0006-2952(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 91.Gagnon I, Duester G, Bhat PV. Kinetic analysis of mouse retinal dehydrogenase type-2 (RALDH2) for retinal substrates. Biochim Biophys Acta. 2002;1596:156–162. doi: 10.1016/s0167-4838(02)00213-3. [DOI] [PubMed] [Google Scholar]
- 92.Graham CE, Brocklehurst K, Pickersgill RW, Warren MJ. Characterization of retinaldehyde dehydrogenase 3. Biochem J. 2006;394:67–75. doi: 10.1042/BJ20050918. [DOI] [PMC free article] [PubMed] [Google Scholar]