Abstract
Rationale: Evidence linking active or passive smoking to the incidence of adult-onset asthma is inconsistent with both positive and inverse associations being reported. Most previous studies of active smoking have not accounted for passive smoke exposure, which may have introduced bias.
Objectives: To assess the separate associations of active and passive smoking to the incidence of adult-onset asthma in the U.S. Black Women’s Health Study, a prospective cohort of African American women followed since 1995 with mailed biennial questionnaires.
Methods: Active smoking status was reported at baseline and updated on all follow-up questionnaires. Passive smoke exposure during childhood, adolescence, and adulthood was ascertained in 1997. Asthma cases comprised women who reported doctor-diagnosed asthma with concurrent asthma medication use. Cox regression models were used to derive multivariable hazard ratios (HRs) and 95% confidence intervals (CIs) for former and current smoking and for passive smoking among nonsmokers compared with a reference category of never active or passive smokers.
Measurements and Main Results: Among 46,182 participants followed from 1995 to 2011, 1,523 reported incident asthma. The multivariable HRs for former active smoking, current active smoking, and passive smoking only were, respectively, 1.36 (95% CI, 1.11–1.67), 1.43 (95% CI, 1.15–1.77), and 1.21 (95% CI, 1.00–1.45), compared with never active/passive smoking.
Conclusions: In this large population with 16 years of follow-up, active smoking increased the incidence of adult-onset asthma, and passive smoke exposure increased the risk among nonsmokers. Continued efforts to reduce exposure to tobacco smoke may have a beneficial effect on the incidence of adult-onset asthma.
Keywords: asthma, cohort study, passive smoking, cigarette smoking
At a Glance Commentary
Scientific Knowledge on the Subject
Evidence from longitudinal studies about the relation of active and passive smoking with the incidence of adult-onset asthma is inconsistent.
What This Study Adds to the Field
In 15 years of follow-up in a large population of African American women, the incidence of adult-onset asthma was increased by approximately 40% among active smokers and by 20% among passive smokers compared with women with no active or passive exposure to tobacco smoke.
A large body of evidence supports a causal relationship between pre- and postnatal parental smoking and the development of asthma in children (1, 2), and two prospective studies have showed a dose–response relationship between smoking during adolescence and asthma onset before 20 years of age (3, 4). In contrast, the evidence for an association of smoking and adult-onset asthma is inconsistent (1, 5). Some studies have found a higher incidence of asthma in smokers (6–12), but others have found no association (13, 14) or have found an inverse association (15, 16). The 2014 Report of the Surgeon General on the Health Consequences of Smoking (17) deemed the overall evidence “suggestive but not sufficient to infer a causal relationship between active smoking and the incidence of asthma in adults.” Limitations of previous studies include short follow-up (7), retrospective assessment of smoking (9, 14), lack of long-term time-varying information on smoking (6, 8), and no accounting for passive smoke exposure (8, 10, 11, 14, 15). Regarding passive smoking, several studies (18–20) have found increased odds of asthma ranging from 1.5 to 3.0 among adults exposed to secondhand smoke as children or adults, but results are vulnerable to recall bias because exposure was ascertained after the diagnosis of asthma.
Inhaled tobacco smoke directly contacts the airway epithelium, a tissue that is increasingly recognized as not simply a barrier but as playing a crucial role as an immune regulator through the secretion of cytokines and chemokines, which influence the recruitment of inflammatory cells, and growth factors, which influence structural remodeling (21). Human and animal research has revealed substantial effects of tobacco smoke on the airway epithelium, including inflammation (22), increased permeability (23), and altered gene expression (24). These and other effects of smoking on the airway epithelium could predispose individuals to the development of asthma, a condition in which the airway epithelium appears to play a key role in pathogenesis (25).
The purpose of the present study was to assess the association of active and passive smoking with adult-onset asthma in a large cohort study of African American women. Exposure to secondhand smoke over the lifetime was obtained once in 1997, and information on active smoking was obtained at baseline and has been updated biennially over 16 years of follow-up.
Methods
Study Population
The Black Women’s Health Study (BWHS) is a prospective cohort study established in 1995, when 59,000 African American women 21–69 years of age enrolled by completing health questionnaires. Methods are described in detail elsewhere (26). In brief, the cohort is followed biennially by mailed questionnaire to update exposures and to ascertain incident disease. Follow-up of the original cohort through eight completed questionnaire cycles is 80%. The study protocol was approved by the Institutional Review Board of Boston University School of Medicine.
Follow-up for the present analysis occurred from 1995 through 2011 and includes 46,182 participants without prevalent asthma or lung cancer at baseline and with complete information on active and passive smoking. The average length of follow-up was 14.7 years.
Diagnosis of Asthma
On all questionnaires from 1997 through 2011, participants were asked whether they had been diagnosed with asthma and if they used “inhalers or pills” for asthma at least 3 d/wk. Incident asthma was defined as a first diagnosis of asthma with concurrent use of asthma medication.
Ascertainment of Active and Passive Smoking and Covariates
Data on smoking history were first obtained in 1995. Ever smokers were asked about the age they had started smoking, the number of cigarettes smoked, and the total number of years they had smoked; former smokers were asked when they had quit. Data on smoking status and amount smoked were updated on each follow-up questionnaire. On the 1997 questionnaire only, respondents were asked about exposure to secondhand tobacco smoke at 0 to 10 and 11 to 20 years of age at home and at home and work separately at 21 to 30 years of age, at 31 to 40 years of age, and currently; exposure was defined as being “in the same room with a smoker for at least an hour/day for 12 consecutive months or more.”
Data on body mass index (BMI; weight in kg/height2 [in meters]), alcohol consumption, and use of female hormone supplements were ascertained at baseline and updated on each subsequent follow-up questionnaire. Various follow-up questionnaires obtained information on years of education, parental asthma, household income, health insurance, if the participant had a regular doctor, and presence of sleep apnea.
Statistical Analysis
We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for asthma incidence associated with passive only, current smoking, and former smoking relative to no active or passive smoking. Participants contributed person-time from 1995 until diagnosis of asthma, death, loss to follow-up, or end of follow-up, whichever came first. Models were stratified by age in 1-year intervals and questionnaire cycle in 2-year intervals. We estimated HRs with a model accounting only for age and questionnaire cycle and a multivariable model that included covariates that have been associated with asthma incidence in BWHS or other studies: BMI (<25, 25–29, 30–34, 35–39, ≥40), duration of menopausal female hormone use (never, <5 yr, ≥5 yr), sleep apnea (yes, no), health insurance (yes, no), having a regular doctor (yes, no), years of education (≤12, 13–15, 16, ≥17), household income (≤$25,000, $25,001–$50,000, $50,001–$100,00, >$100,000), parental history of asthma (yes, no), and alcohol consumption (never, current, past).
Missing values were modeled as separate categories. All variables were time-varying with the exception of sleep apnea, income, having a regular doctor, education, and health insurance. Thus, for example, a woman who quit smoking over follow-up contributed person-time to the current-smoker category until she quit and then contributed person-time to the past-smoker category.
We conducted analyses stratified by age, obesity, and parental history of asthma and assessed interaction by using the likelihood ratio test to compare models with and without cross-product terms for smoking with the stratifying variables. We conducted sensitivity analyses using three different case definitions. First, a more stringent asthma case definition was used that included only cases where subjects reported use of a preventive asthma medication and specified the medication name (e.g., fluticasone). In a second sensitivity analysis, the case definition was expanded to include all physician-diagnosed asthma regardless of medication use. Finally, we confined the cases to those where subjects confirmed their asthma diagnoses on a supplementary questionnaire.
Results
During follow-up from 1995 through 2011, 1,523 women met the case criteria out of 593,802 person-years of follow-up. In a subset of 43 women who met the case definition and who gave permission to contact their physician, 39 (91%) were confirmed by the physician as having asthma.
Compared with the reference group of women unexposed to either passive or active smoking, passive, current, and former smokers were older, had higher BMI, had lower levels of education and income, and were more likely to be current or past drinkers (Table 1). Current and former smokers were less likely to have health insurance and a regular doctor compared with passive smokers and with the women unexposed to either active or passive smoking.
Table 1.
Baseline Characteristics by Smoking Status
| Patient Characteristics | Never Active or Passive Smoking (n = 6,279) | Passive Smoke Exposure Only (n = 21,968) | Former Smoker (n = 9,740) | Current Smoker (n = 8,195) |
|---|---|---|---|---|
| Age, yr (mean ± SD) | 34.7 ± 9.7 | 37.9 ± 10.5 | 44.6 ± 10.5 | 40.9 ± 9.6 |
| BMI (mean ± SD) | 27.0 ± 6.1 | 28.0 ± 6.6 | 28.6 ± 6.9 | 27.6 ± 6.1 |
| Education, yr | ||||
| ≤12, % | 12 | 17 | 21 | 29 |
| 13–15, % | 28 | 34 | 39 | 42 |
| 16, % | 29 | 26 | 20 | 17 |
| ≥17, % | 32 | 23 | 20 | 12 |
| Income (2003) | ||||
| ≤$25,000, % | 7 | 9 | 10 | 14 |
| $25,001–50,000, % | 21 | 24 | 23 | 26 |
| $50,001–99,999, % | 34 | 31 | 28 | 22 |
| ≥$100,000, % | 17 | 14 | 12 | 7 |
| Has health insurance (1997), % | 94 | 93 | 83 | 79 |
| Has regular doctor (1997), % | 89 | 88 | 78 | 74 |
| Parental history of asthma, % | 5 | 6 | 6 | 6 |
| Sleep apnea, % | 2 | 2 | 2 | 2 |
| Hormone therapy use | ||||
| Never, % | 84 | 83 | 81 | 81 |
| <5 yr, % | 9 | 9 | 11 | 11 |
| ≥5 yr, % | 5 | 5 | 6 | 5 |
| Alcohol consumption | ||||
| Never, % | 78 | 71 | 35 | 36 |
| Past, % | 7 | 11 | 27 | 18 |
| Current, % | 14 | 18 | 37 | 46 |
Definition of abbreviation: BMI = body mass index.
Baseline characteristics are age-adjusted in 5-yr age categories. All P values for χ2 tests or one-way analysis of variance for differences in distribution of variables across smoking categories were < 0.0001, with the exception of parental history of asthma (P = 0.066). Numbers may not add to 100% due to missing values. Data are from 1995 unless noted otherwise.
For passive smokers, the HR adjusted for age and questionnaire cycle was 1.36 (95% CI, 1.13–1.63), which was reduced to 1.21 (95% CI, 1.00–1.45) in the multivariable model (Table 2). There was little variation in the HRs for those exposed to passive smoke only before 20 years of age, only at 20 years of age or older, or in both age periods. Because secondhand smoke exposure was ascertained in 1997 only, we also calculated the HR for passive smoking confining follow-up to 1997–2011 rather than to 1995–2011; the multivariable HR for passive smoke exposure was 1.19 (95% CI, 0.97–1.48).
Table 2.
Smoking Status and Incidence of Asthma, Black Women’s Health Study, 1995–2011
| Smoking Status | Cases | Person-Years | Basic Model (Age and Questionnaire Cycle) [HR (95% CI)] | Multivariable Model [HR (95% CI)]* |
|---|---|---|---|---|
| Never active or passive | 142 | 84,071 | 1.0 | 1.0 |
| Passive only | 677 | 284,103 | 1.36 (1.13–1.63) | 1.21 (1.00–1.45) |
| Exposed before age 20 only | 225 | 105,193 | 1.26 (1.02–1.56) | 1.17 (0.94–1.45) |
| Exposed at age 20 or older only | 180 | 72,745 | 1.39 (1.11–1.74) | 1.24 (0.99–1.56) |
| Exposed before and after 20 | 272 | 106,165 | 1.43 (1.16–1.75) | 1.18 (0.96–1.46) |
| Former smoker | 423 | 139,885 | 1.71 (1.41–2.08) | 1.36 (1.11–1.67) |
| Current smoker | 281 | 85,741 | 1.72 (1.40–2.11) | 1.43 (1.15–1.77) |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio.
Adjusted for age (1-yr intervals), questionnaire cycle (2-yr increments), body mass index (<25, 25–29, 30–34, 35–39, ≥40), menopausal female hormone use (never, <5 yr, ≥5 yr), sleep apnea (yes, no), health insurance (yes, no), having a regular doctor (yes, no), years of education (≤12, 13–15, 16, ≥17), household income (≤$25,000, $25,001–$50,000, $50,001–$100,00, >$100,000), parental history of asthma (yes, no), and alcohol consumption (never, current, past).
The age- and cycle-adjusted HRs for former and current smoking were 1.71 (95% CI, 1.41–2.08) and 1.72 (95% CI, 1.40–2.11), respectively (Table 2). In the multivariable model, HRs were reduced to 1.36 (95% CI, 1.11–1.67) for former smokers and 1.43 (95% CI, 1.15–1.77) for current smokers. HRs increased with increasing number of pack-years among ever smokers, up to 1.88 (95% CI, 1.45–2.43) for ≥20 pack-years (P for trend < 0.0001) (Table 3). The HR was higher among women who started smoking before 18 years of age (HR, 1.59; 95% CI, 1.27–2.00) compared with 18 years of age and older (HR, 1.39; 95% CI, 1.12–1.73) (Table 3), although the interaction between smoking and age started smoking was not statistically significant (P = 0.20). HRs for pack-years and for age started smoking were similar among current and past smokers (data not shown). HRs increased by number of pack-years among women who began smoking before 18 years of age but not among those who started smoking at a later age (Table 3). The incidence of asthma was highest among women who started smoking before 18 years of age and accumulated ≥20 pack-years of smoking (multivariable HR, 2.34; 95% CI, 1.76–3.10).
Table 3.
Pack-Years of Smoking and Age Started Smoking in Relation to Asthma Incidence among Ever Smokers, Black Women’s Health Study, 1995–2011
| Patient Characteristics | Cases | Person-Years | Basic Model (Age and Questionnaire Cycle) [HR (95% CI)] | Multivariable Model [HR (95% CI)]* |
|---|---|---|---|---|
| Never active or passive | 142 | 84,071 | 1.0 | 1.0 |
| Pack-years† | ||||
| <10 pack years | 337 | 118,113 | 1.65 (1.34–2.01) | 1.40 (1.12–1.73) |
| 10–19 pack-years | 168 | 54,564 | 1.79 (1.41–2.26) | 1.50 (1.17–1.93) |
| ≥20 pack-years | 181 | 45,040 | 2.33 (1.83–2.97) | 1.88 (1.45–2.43) |
| P value for trend | <0.0001 | <0.0001 | ||
| Age started, yr | ||||
| <18 | 295 | 84,745 | 1.94 (1.58–2.39) | 1.59 (1.27–2.00) |
| ≥18 | 401 | 137,376 | 1.64 (1.33–2.00) | 1.39 (1.12–1.73) |
| Pack-years stratified by age started smoking | ||||
| Age started <18 | ||||
| <10 pack-years | 98 | 36,854 | 1.51 (1.16–1.96) | 1.26 (0.96–1.66) |
| 10–19 pack-years | 82 | 24,075 | 1.95 (1.47–2.58) | 1.63 (1.21–2.20) |
| ≥20 pack-years | 111 | 21,733 | 2.90 (2.22–3.78) | 2.34 (1.76–3.10) |
| Age started ≥18 | ||||
| <10 pack-years | 235 | 79,992 | 1.69 (1.37–2.10) | 1.47 (1.17–1.84) |
| 10–19 pack-years | 85 | 30,191 | 1.62 (1.22–2.15) | 1.40 (1.04–1.87) |
| ≥20 pack-years | 69 | 22,962 | 1.72 (1.27–2.35) | 1.41 (1.03–1.95) |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio.
Adjusted for age (1-yr intervals), questionnaire cycle (2-yr increments), body mass index (<25, 25–29, 30–34, 35–39, ≥40), duration of menopausal female hormone use (never, <5 yr, ≥5 yr), sleep apnea (yes, no), health insurance (yes, no), having a regular doctor (yes, no), years of education (≤12, 13–15, 16, ≥17), household income (≤$25,000, $25,001–$50,000, $50,001–$100,00, >$100,000), parental history of asthma (yes, no), and alcohol consumption (never, current, past).
Pack-years of smoking were calculated by multiplying the duration of smoking in years times the number of packs of cigarettes smoked per day. A total of 842 women (including 18 cases) were missing information on pack-years, and 266 women (including eight cases) were missing information on age started smoking.
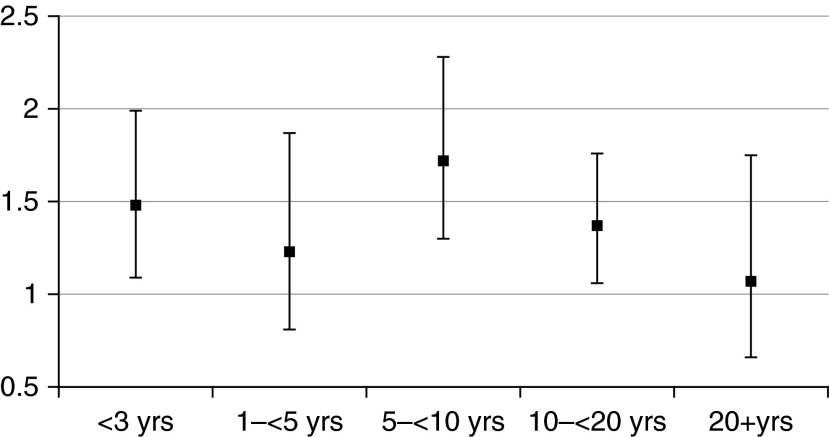
Among former smokers, multivariable HRs tended to decrease as time since quitting increased, although the pattern was not consistent (P value for trend = 0.06) (Figure 1).
Figure 1.
Multivariable hazard ratios among former smokers by time since quitting. Reference group is individuals never exposed to active or passive smoking.
In stratified analyses (Table 4), multivariable HRs for all exposure categories were higher among nonobese compared with obese women (P value for interaction = 0.08). For example, the HR for current smoking was 2.13 (95% CI, 1.56–2.92) among nonobese women and 1.01 (95% CI, 0.74–1.37) among obese women. Among obese women, the only significant increase in asthma incidence occurred among smokers with ≥20 pack-years (HR, 1.52; 95% CI, 1.08–2.14). The comparable HR among nonobese women was 2.40 (95% CI, 1.61–3.56). HRs for all exposure categories were higher among women 50 years of age and older than among younger women (P value for interaction = 0.13); for example, the HR among smokers with ≥20 pack-years was 2.81 (95% CI, 1.71–4.61) for women 50 years of age and older and 1.52 (95% CI, 1.07–2.16) for women younger than 50 years of age. HRs were similar among women who did and did not report parental history of asthma (data not shown).
Table 4.
Asthma Incidence and Smoking Status in Strata of Body Mass Index, Age, and Parental History of Asthma, Black Women’s Health Study, 1995–2011
| Smoking Status | BMI < 30 |
BMI ≥ 30 |
Age < 50
yr |
Age ≥ 50
yr |
||||
|---|---|---|---|---|---|---|---|---|
| Cases/Person-Years | Multivariable HR (95% CI)* | Cases/Person-Years | Multivariable HR (95% CI)* | Cases/Person-Years | Multivariable HR (95% CI)* | Cases/Person-Years | Multivariable HR (95% CI)* | |
| Never active/ passive | 64/58,013 | 1.0 | 75/25,429 | 1.0 | 122/66,275 | 1.0 | 20/17,796 | 1.0 |
| Passive only | 320/176,394 | 1.55 (1.18–2.04) | 347/104,660 | 0.96 (0.75–1.24) | 488/196,164 | 1.15 (0.94–1.41) | 189/87,939 | 1.62 (1.02–2.57) |
| Former smoker | 170/80,242 | 1.78 (1.31–2.42) | 247/57,862 | 1.07 (0.81–1.42) | 205/65,295 | 1.20 (0.95–1.53) | 218/74,590 | 2.02 (1.27–3.24) |
| Current smoker | 156/56,425 | 2.13 (1.56–2.92) | 123/28,340 | 1.01 (0.74–1.37) | 194/57,269 | 1.30 (1.02–1.66) | 87/28,472 | 2.14 (1.30–3.52) |
| Pack-years | ||||||||
| <10 | 162/71,815 | 1.96 (1.42–2.70) | 169/44,752 | 1.03 (0.77–1.39) | 239/76,356 | 1.31 (1.02–1.67) | 98/41,757 | 1.85 (1.13–3.04) |
| 10–19 | 79/32,933 | 2.09 (1.43–3.06) | 89/21,074 | 1.16 (0.82–1.62) | 85/27,284 | 1.26 (0.92–1.73) | 83/27,280 | 2.30 (1.39–3.82) |
| ≥20 | 75/27,120 | 2.40 (1.61–3.56) | 105/17,501 | 1.52 (1.08–2.14) | 64/15,553 | 1.52 (1.07–2.16) | 117/29,487 | 2.81 (1.71–4.61) |
| P value for trend | <0.0001 | 0.005 | 0.0400 | <0.0001 | ||||
| Age started, yr | ||||||||
| <18 | 126/50,880 | 2.06 (1.46–2.90) | 164/32,936 | 1.27 (0.93–1.73) | 185/53,027 | 1.38 (1.06–1.81) | 110/31,718 | 2.56 (1.56–4.21) |
| ≥18 | 196/83,661 | 1.99 (1.44–2.76) | 202/51,962 | 1.04 (0.78–1.40) | 209/67,962 | 1.25 (0.97–1.61) | 192/69,414 | 2.10 (1.30–3.37) |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; HR = hazard ratio.
Adjusted for age (1-yr intervals), questionnaire cycle (2-yr increments), BMI (<25, 25–29, 30–34, 35–39, ≥40), duration of menopausal female hormone use (never, <5 yr, ≥5 yr), sleep apnea (yes, no), health insurance (yes, no), having a regular doctor (yes, no), years of education (≤12, 13–15, 16, ≥17), household income (≤$25,000, $25,001–$50,000, $50,001–$100,00, >$100,000), parental history of asthma (yes, no), and alcohol consumption (never, current, past). A total of 842 women (including 18 cases) were missing information on pack-years, and 266 women (including eight cases) were missing information on age started smoking; 438 women (including eight cases) were missing information on BMI.
In a sensitivity analysis using the more stringent asthma case definition (i.e., cases who reported use of a preventive asthma medication; n = 988 cases), the multivariable HRs for passive, former, and current smoking were 1.36 (95% CI, 1.08–1.72), 1.52 (95% CI, 1.18–1.97), and 1.49 (95% CI, 1.13–2.97), respectively. In the second sensitivity analysis using the less stringent case definition (all physician-diagnosed asthma regardless of medication use) (2,934 cases), the comparable HRs were 1.20 (95% CI, 1.05–1.36), 1.36 (95% CI, 1.17–1.56), and 1.32 (95% CI, 1.14–1.55). When we confined the cases to those who confirmed their asthma diagnosis on a supplementary questionnaire (842 cases), the comparable HRs were 1.35 (95% CI, 1.06–1.73), 1.50 (95% CI, 1.14–1.96), and 1.34 (95% CI, 1.00–1.82).
Discussion
In this large population of African American women, the incidence of asthma was significantly increased among current and past smokers compared with women with no active or passive smoking, and incidence increased as pack-years increased. Asthma incidence was 2.3 times higher among women who accumulated 20 or more pack-years and had started smoking before 18 years of age compared with women with no active or passive smoke exposure. There was also a 21% increase in asthma incidence among women exposed only to passive smoke. The association of active and passive smoking with increased asthma incidence was most apparent among nonobese women and women 50 years of age or older.
The airway epithelium, which appears to play a key role in the pathogenesis of asthma, is the tissue that comes into the most direct contact with inhaled tobacco smoke. Smoking has profound effects on the airway epithelium, as indicated by smoking-induced alterations of mRNA and miRNA expression (24, 27). By effects on epithelial permeability (23, 28) and other aspects of epithelial regulation of inflammation (21), tobacco smoke exposure increases allergic sensitization in animal models of inhaled allergen exposure (29, 30). Human studies have shown that acute exposure to environmental tobacco smoke worsens allergic airway responses to simultaneously inhaled aeroallergens (31). In an animal model, chronic tobacco smoke exposure has been reported to increase airway responsiveness, an effect that may be mediated in part by IL-13, a cytokine important in the pathophysiology of asthma (30). In addition to immune system–mediated effects of airway responsiveness, in vitro data suggest that cigarette smoke induces neurotrophin and neurotrophin receptor in human airway smooth muscle, thereby increasing neurotrophin signaling, which may contribute to cigarette smoke–induced airway hyperresponsiveness (32). Although the precise mechanisms that link smoking to the development of adult-onset asthma are uncertain, it appears that adverse effects on the airway epithelium, immune cells in the airways, and airway smooth muscle may play roles.
Several longitudinal studies of active smoking and asthma have reported increased asthma incidence among past and/or current smokers. In data from northern Europe (n = 14,731) in the European Community Respiratory Health Survey, subjects were interviewed about asthma prevalence and smoking at baseline and 10 years later were interviewed again about asthma incidence and smoking status in the intervening years (8). The incidence rate ratios (IRRs) for current and ex-smoking compared with never smoking were 1.3 (95% CI, 1.0–1.6) and 1.4 (95% CI, 1.0–2.0), respectively, similar to those in the present study. Lack of control for passive smoking and retrospective report of asthma onset and smoking status for the period between surveys may have introduced some degree of bias in either direction. In a follow-up study of 4,741 Swedish citizens interviewed in 1985 and again 10 years later (10), the odds ratios for current and former smokers were 1.4 (95% CI, 0.95–2.0) and 2.0 (95% CI, 1.5–2.8), respectively; odds ratios (ORs) were adjusted for occupational status and potential exposure to dust and fumes, but not for passive smoking. In a study of 325 allergic rhinitis patients interviewed at their initial visit to an allergy clinic and again 10 years later, the ORs for asthma among subjects who were current and former smokers at baseline were 2.93 (95% CI, 1.81–4.92) and 1.93 (95% CI, 0.91–4.10), respectively, and the ORs increased as pack-years of smoking increased (11). Although a detailed smoking history was taken at the initial clinic visit, smoking history in the 10 years between the baseline and follow-up visit was not updated, and passive smoke exposure was not accounted for in the analysis. In a large cohort study of ∼15,000 Japanese men and women, smoking status was ascertained at baseline, and a follow-up interview ascertained asthma incidence in the intervening 10 years; smoking status was not updated (12). Asthma incidence was increased among male current smokers (HR, 2.79; 95% CI, 1.18–6.55) or past smokers (HR, 1.86; 95% CI, 0.75–4.63), but there were too few female smokers for informative analyses. Smoking was not associated with asthma incidence in 2 years of follow-up in the Canadian National Population Health Survey (n = 12,636) except among women who had pets at home (relative risk for current smoking, 2.50, 95% CI, 1.24–5.05) (7). The analysis was limited by short follow-up and lack of accounting for passive smoke exposure.
Finally, several studies observed inverse associations. In the Nurse’s Health Study (NHS), in which 671 incident cases occurred over 10 years of follow-up, the age-adjusted IRR for asthma among current smokers compared with never smokers was 0.57 (95% CI, 0.47–0.71); risk did not vary with number of cigarettes per day (15). An increased IRR among former smokers immediately after quitting declined to ∼1.0 within 5 years of quitting. The authors interpreted findings for current and past smokers as evidence of a healthy smoker effect (i.e., people with sensitive airways elect not to initiate smoking, or those with sensitive airways who do smoke are more likely to give up smoking than those with a greater tolerance for smoking). Like the BWHS, the NHS updated smoking data biennially and had the same case definition (self-reported physician-diagnosed asthma with use of asthma medication). Major differences were that NHS participants were almost all white, whereas BWHS participants were African American; in addition, the IRRs from the NHS were adjusted for age only and did not account for passive smoke exposure. In Spanish data from the European Community Respiratory Health Survey, the cumulative incidence of asthma between two surveys conducted on average 7 years apart was reduced by a nonsignificant 17% among those who were current smokers at baseline (6). This study lacked time-varying data on smoking and relied on retrospective recall of year of asthma onset. In a pooled analysis of two small studies from Tuscon, Arizona and Cracow, Poland, asthma risk was reduced 40% (CI not given) among women 19 to 40 years of age who reported smoking on two surveys conducted ∼10 years apart compared with never smokers (16). However, among older smokers the risk was doubled. Differences in populations studied, timing of exposure ascertainment, adjustment for different covariates, and handling of passive smoking likely contributed to differences in effect estimates across studies. It is not clear why a healthy smoker effect would manifest itself in some but not in other cohorts.
Two well-designed prospective studies of adolescents found positive associations between smoking and asthma incidence, taking into account passive smoke exposure. In both studies, active smoking increased asthma incidence up to 20 years of age by 2- to 3-fold, with significant increases as duration and intensity of smoking increased (3, 4). However, the lung is still developing during adolescence (33), and the airways may be more vulnerable to the effects of tobacco smoke.
Three studies have specifically assessed passive smoking and adult-onset asthma. In a Swedish case-control study of nonsmokers, subjects were queried as to secondhand smoke exposure in their six most recent homes (20). The OR associated with exposure to environmental tobacco smoke was 2.4 (95% CI, 1.4–4.0). In a Finnish case-control study of nonsmokers, the interaction of parental history of asthma and secondhand smoke exposure was assessed (18). Compared with subjects with no parental history of asthma and no secondhand smoke exposure, the multivariable OR for secondhand smoke exposure in the 12 months before diagnosis or index date was 1.85 (95% CI, 0.97–3.50) among subjects with no parental history of asthma and 14.8 (95% CI, 3.11–70.59) among subjects with such a history. Finally, in a Norwegian study, the effect of secondhand smoke exposure was assessed among smokers and nonsmokers, controlling for smoking status: the OR associated with subject’s mother smoking during childhood was 2.9 (95% CI, 1.1–5.5), and the OR for other household member smoking was 1.2 (95% CI, 0.7–2.0) (19).
Secondhand smoke exposure is difficult to ascertain and quantify, and case-control studies are vulnerable to recall bias. We found only a weak association of passive smoke exposure with adult-onset asthma, but our data on passive smoking were limited. The best estimate of the association between lifetime passive smoke exposure and adult-onset asthma requires a cohort with good measures of passive smoke exposure, good control for active smoking, and follow-up through adulthood for incident asthma.
No previous study of smoking and asthma was stratified by BMI. Our finding of a stronger association of smoking and asthma incidence among nonobese women may reflect the lower background rate of disease in that group compared with obese women. On the other hand, the finding may reflect different pathogenetic mechanisms underlying adult-onset asthma in nonobese and obese women. Two cluster analyses of large samples of adults with asthma have observed a distinct subphenotype of adults with asthma; individuals with this phenotype are more likely to be female and obese and to have asthma that is of later onset and less atopic than in other subgroups (34, 35). The occurrence of adult-onset asthma (or symptoms diagnosed as asthma) in this subgroup could be due to inflammation related to obesity and adipokine dysregulation rather than allergy or other mechanisms (36), to impaired pulmonary function (36) or dyspnea (37) related to mechanical effects of obesity rather than inflammatory airways disease, and/or to gastroesophageal reflux (38). These factors may overshadow the effects of tobacco smoking in the subset of women who are obese and have adult-onset asthma or asthma-like symptoms due to mechanisms other than epithelial injury related to environmental factors. Lung function declines with age (33), and the higher HRs observed in women 50 years of age and older may reflect the greater vulnerability of the aging lung to past smoke exposure. We found no evidence of effect modification by parental history of asthma.
The present study has numerous strengths. It has more cases and longer follow-up than previous longitudinal studies of smoking and adult asthma incidence. Active smoking was ascertained every 2 years, and report of smoking status preceded asthma diagnosis, obviating recall bias. The reference group comprised women with no exposure to active or passive smoking. This reduced potential confounding of the effect of active smoking by passive smoking and vice versa. Limitations include the use of self-reported passive smoke exposure and lack of information on intensity and duration of passive smoke exposure. In addition, “current” passive smoke exposure reported for the year 1997 may not have been good indicator of exposure over subsequent follow-up, especially in the office, because smoking bans began to be implemented in the late 1990s. However, passive smoke exposure before 20 years of age would not have been affected by these changes because all women were older than 20 years of age when they reported their passive smoke exposure in 1997. Misclassification would have tended to bias effect estimates for passive smoking to the null. We had no information on occupational or other environmental exposures that might be relevant, including indoor and outdoor air contaminants, animal dander, and mold.
We relied on self-report of doctor-diagnosed asthma. The standard method of identifying asthma cases in large national cohort studies is self-report (6–8, 15, 39–41) because it is not feasible in such studies to conduct detailed medical evaluation or physiologic testing to confirm case and noncase status. Results were consistent when we used several case definitions, including one where all cases had confirmed their asthma diagnosis on a supplemental questionnaire. In addition, in a small validation study among 43 BWHS participants who met our case definition and gave us permission to contact their physicians, 39 (91%) were confirmed by their physicians as having asthma. Nevertheless, in the absence of physiologic test data, one cannot exclude some degree of misclassification, on the part of subjects and their physicians, between asthma and chronic obstructive pulmonary disease (COPD), the latter being a condition for which smoking is an established risk factor. We did not query BWHS participants about COPD. Inclusion of COPD cases in the asthma case series would have inflated the observed HR for the association of smoking with asthma.
In conclusion, our results support the hypothesis that active smoking increases the risk of adult-onset asthma and that passive smoke exposure may increase the risk among nonsmokers. In recent decades, rates of smoking have declined in African American and white women. In 1985, 31% of African American women and 28% of white women smoked; in 2007, the corresponding figures were 16 and 19% (42). Continued efforts to reduce rates of smoking and exposure to secondhand smoke may have a beneficial effect on the incidence of adult onset asthma.
Footnotes
Supported by National Institutes of Health/National Heart, Lung, and Blood Institute grant HL107314 and by National Cancer Institute grant CA058420.
Author Contributions: Conception, hypothesis delineation, and design of the study, acquisition of the data, analysis and interpretation, and writing the article: P.F.C., J.R.P., and L.R. Data analysis and interpretation and writing the article: N.C.-W., J.Y., and G.T.O’C.
Originally Published in Press as DOI: 10.1164/rccm.201406-1108OC on November 11, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Polosa R, Thomson NC. Smoking and asthma: dangerous liaisons. Eur Respir J. 2013;41:716–726. doi: 10.1183/09031936.00073312. [DOI] [PubMed] [Google Scholar]
- 2.Baena-Cagnani CE, Gómez RM, Baena-Cagnani R, Canonica GW. Impact of environmental tobacco smoke and active tobacco smoking on the development and outcomes of asthma and rhinitis. Curr Opin Allergy Clin Immunol. 2009;9:136–140. doi: 10.1097/ACI.0b013e3283294038. [DOI] [PubMed] [Google Scholar]
- 3.Genuneit J, Weinmayr G, Radon K, Dressel H, Windstetter D, Rzehak P, Vogelberg C, Leupold W, Nowak D, von Mutius E, et al. Smoking and the incidence of asthma during adolescence: results of a large cohort study in Germany. Thorax. 2006;61:572–578. doi: 10.1136/thx.2005.051227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilliland FD, Islam T, Berhane K, Gauderman WJ, McConnell R, Avol E, Peters JM. Regular smoking and asthma incidence in adolescents. Am J Respir Crit Care Med. 2006;174:1094–1100. doi: 10.1164/rccm.200605-722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLeish AC, Zvolensky MJ. Asthma and cigarette smoking: a review of the empirical literature. J Asthma. 2010;47:345–361. doi: 10.3109/02770900903556413. [DOI] [PubMed] [Google Scholar]
- 6.Basagaña X, Sunyer J, Zock JP, Kogevinas M, Urrutia I, Maldonado JA, Almar E, Payo F, Antó JM Spanish Working Group of the European Community Respiratory Health Survey. Incidence of asthma and its determinants among adults in Spain. Am J Respir Crit Care Med. 2001;164:1133–1137. doi: 10.1164/ajrccm.164.7.2012143. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Dales R, Tang M, Krewski D. Sex-related interactive effect of smoking and household pets on asthma incidence. Eur Respir J. 2002;20:1162–1166. doi: 10.1183/09031936.02.00273102. [DOI] [PubMed] [Google Scholar]
- 8.Torén K, Gislason T, Omenaas E, Jögi R, Forsberg B, Nyström L, Olin AC, Svanes C, Janson C RHINE Group. A prospective study of asthma incidence and its predictors: the RHINE study. Eur Respir J. 2004;24:942–946. doi: 10.1183/09031936.04.00044804. [DOI] [PubMed] [Google Scholar]
- 9.Plaschke PP, Janson C, Norrman E, Björnsson E, Ellbjär S, Järvholm B. Onset and remission of allergic rhinitis and asthma and the relationship with atopic sensitization and smoking. Am J Respir Crit Care Med. 2000;162:920–924. doi: 10.1164/ajrccm.162.3.9912030. [DOI] [PubMed] [Google Scholar]
- 10.Hedlund U, Eriksson K, Rönmark E. Socio-economic status is related to incidence of asthma and respiratory symptoms in adults. Eur Respir J. 2006;28:303–310. doi: 10.1183/09031936.06.00108105. [DOI] [PubMed] [Google Scholar]
- 11.Polosa R, Knoke JD, Russo C, Piccillo G, Caponnetto P, Sarvà M, Proietti L, Al-Delaimy WK. Cigarette smoking is associated with a greater risk of incident asthma in allergic rhinitis. J Allergy Clin Immunol. 2008;121:1428–1434. doi: 10.1016/j.jaci.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Nagata C, Fujii K, Kawachi T, Takatsuka N, Oba S, Shimizu H. Cigarette smoking and the adult onset of bronchial asthma in Japanese men and women. Ann Allergy Asthma Immunol. 2009;102:288–293. doi: 10.1016/S1081-1206(10)60333-X. [DOI] [PubMed] [Google Scholar]
- 13.Vesterinen E, Kaprio J, Koskenvuo M. Prospective study of asthma in relation to smoking habits among 14,729 adults. Thorax. 1988;43:534–539. doi: 10.1136/thx.43.7.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siroux V, Pin I, Oryszczyn MP, Le Moual N, Kauffmann F. Relationships of active smoking to asthma and asthma severity in the EGEA study: epidemiological study on the genetics and environment of asthma. Eur Respir J. 2000;15:470–477. doi: 10.1034/j.1399-3003.2000.15.08.x. [DOI] [PubMed] [Google Scholar]
- 15.Troisi RJ, Speizer FE, Rosner B, Trichopoulos D, Willett WC. Cigarette smoking and incidence of chronic bronchitis and asthma in women. Chest. 1995;108:1557–1561. doi: 10.1378/chest.108.6.1557. [DOI] [PubMed] [Google Scholar]
- 16.Krzyzanowski M, Lebowitz MD. Changes in chronic respiratory symptoms in two populations of adults studied longitudinally over 13 years. Eur Respir J. 1992;5:12–20. [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services The health consequences of smoking: 50 years of progress. A report of the Surgeon General Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014 (printed with corrections January 2014 [Google Scholar]
- 18.Lajunen TK, Jaakkola JJ, Jaakkola MS. The synergistic effect of heredity and exposure to second-hand smoke on adult-onset asthma. Am J Respir Crit Care Med. 2013;188:776–782. doi: 10.1164/rccm.201304-0773OC. [DOI] [PubMed] [Google Scholar]
- 19.Skorge TD, Eagan TM, Eide GE, Gulsvik A, Bakke PS. The adult incidence of asthma and respiratory symptoms by passive smoking in uterus or in childhood. Am J Respir Crit Care Med. 2005;172:61–66. doi: 10.1164/rccm.200409-1158OC. [DOI] [PubMed] [Google Scholar]
- 20.Thorn J, Brisman J, Torén K. Adult-onset asthma is associated with self-reported mold or environmental tobacco smoke exposures in the home. Allergy. 2001;56:287–292. doi: 10.1034/j.1398-9995.2001.00805.x. [DOI] [PubMed] [Google Scholar]
- 21.Hallstrand TS, Hackett TL, Altemeier WA, Matute-Bello G, Hansbro PM, Knight DA. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin Immunol. 2014;151:1–15. doi: 10.1016/j.clim.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Wright JL, Lawson LM, Pare PD, Wiggs BJ, Kennedy S, Hogg JC. Morphology of peripheral airways in current smokers and ex-smokers. Am Rev Respir Dis. 1983;127:474–477. doi: 10.1164/arrd.1983.127.4.474. [DOI] [PubMed] [Google Scholar]
- 23.Rusznak C, Mills PR, Devalia JL, Sapsford RJ, Davies RJ, Lozewicz S. Effect of cigarette smoke on the permeability and IL-1beta and sICAM-1 release from cultured human bronchial epithelial cells of never-smokers, smokers, and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2000;23:530–536. doi: 10.1165/ajrcmb.23.4.3959. [DOI] [PubMed] [Google Scholar]
- 24.Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, Palma J, Brody JS. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci USA. 2004;101:10143–10148. doi: 10.1073/pnas.0401422101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erle DJ, Sheppard D. The cell biology of asthma. J Cell Biol. 2014;205:621–631. doi: 10.1083/jcb.201401050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg L, Adams-Campbell L, Palmer JR. The Black Women’s Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc. 1995;50:56–58. [PubMed] [Google Scholar]
- 27.Schembri F, Sridhar S, Perdomo C, Gustafson AM, Zhang X, Ergun A, Lu J, Liu G, Zhang X, Bowers J, et al. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc Natl Acad Sci USA. 2009;106:2319–2324. doi: 10.1073/pnas.0806383106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rusznak C, Sapsford RJ, Devalia JL, Justin John R, Hewitt EL, Lamont AG, Wood AJ, Shah SS, Davies RJ, Lozewicz S. Cigarette smoke potentiates house dust mite allergen-induced increase in the permeability of human bronchial epithelial cells in vitro. Am J Respir Cell Mol Biol. 1999;20:1238–1250. doi: 10.1165/ajrcmb.20.6.3226. [DOI] [PubMed] [Google Scholar]
- 29.Lanckacker EA, Tournoy KG, Hammad H, Holtappels G, Lambrecht BN, Joos GF, Maes T. Short cigarette smoke exposure facilitates sensitisation and asthma development in mice. Eur Respir J. 2013;41:1189–1199. doi: 10.1183/09031936.00096612. [DOI] [PubMed] [Google Scholar]
- 30.Trimble NJ, Botelho FM, Bauer CM, Fattouh R, Stämpfli MR. Adjuvant and anti-inflammatory properties of cigarette smoke in murine allergic airway inflammation. Am J Respir Cell Mol Biol. 2009;40:38–46. doi: 10.1165/rcmb.2008-0107OC. [DOI] [PubMed] [Google Scholar]
- 31.Diaz-Sanchez D, Rumold R, Gong H., Jr Challenge with environmental tobacco smoke exacerbates allergic airway disease in human beings. J Allergy Clin Immunol. 2006;118:441–446. doi: 10.1016/j.jaci.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 32.Sathish V, Vanoosten SK, Miller BS, Aravamudan B, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Brain-derived neurotrophic factor in cigarette smoke-induced airway hyperreactivity. Am J Respir Cell Mol Biol. 2013;48:431–438. doi: 10.1165/rcmb.2012-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1:253–260. doi: 10.2147/ciia.2006.1.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SH, Sutherland ER, Gelfand EW. Is there a link between obesity and asthma? Allergy Asthma Immunol Res. 2014;6:189–195. doi: 10.4168/aair.2014.6.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. 2002;162:1477–1481. doi: 10.1001/archinte.162.13.1477. [DOI] [PubMed] [Google Scholar]
- 38.Moore JM, Vaezi MF. Extraesophageal manifestations of gastroesophageal reflux disease: real or imagined? Curr Opin Gastroenterol. 2010;26:389–394. doi: 10.1097/MOG.0b013e32833adc8d. [DOI] [PubMed] [Google Scholar]
- 39.Beckett WS, Jacobs DR, Jr, Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med. 2001;164:2045–2050. doi: 10.1164/ajrccm.164.11.2004235. [DOI] [PubMed] [Google Scholar]
- 40.Burr ML. Diagnosing asthma by questionnaire in epidemiological surveys. Clin Exp Allergy. 1992;22:509–510. doi: 10.1111/j.1365-2222.1992.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 41.Romieu I, Avenel V, Leynaert B, Kauffmann F, Clavel-Chapelon F. Body mass index, change in body silhouette, and risk of asthma in the E3N cohort study. Am J Epidemiol. 2003;158:165–174. doi: 10.1093/aje/kwg131. [DOI] [PubMed] [Google Scholar]
- 42.Bayer-Oglesby L, Schindler C, Hazenkamp-von Arx ME, Braun-Fahrländer C, Keidel D, Rapp R, Künzli N, Braendli O, Burdet L, Sally Liu LJ, et al. SAPALDIA Team. Living near main streets and respiratory symptoms in adults: the Swiss Cohort Study on Air Pollution and Lung Diseases in Adults. Am J Epidemiol. 2006;164:1190–1198. doi: 10.1093/aje/kwj338. [DOI] [PubMed] [Google Scholar]