To the Editor:
Clinical tests that predict clinical outcomes in cystic fibrosis (CF) such as exacerbation risk and lung function decline are lacking. Calprotectin (MRP8/14, S100A8/A9) is an abundant neutrophil protein found in bronchoalveolar lavage fluid (1), sputum (2), and serum of patients with CF (3). We have demonstrated that serum calprotectin changes with treatment of exacerbation (3, 4), and predicts time to re-exacerbation (3). We hypothesized that stable patients with higher serum calprotectin at baseline would exacerbate sooner and have more rapid lung function decline.
Methods
Patients were recruited while clinically stable (no exacerbation treatment with oral, inhaled, or intravenous antibiotics in the preceding 2 wk). Exclusion criteria were previous lung transplant, nonpulmonary chronic inflammatory condition, immunosuppressant therapy, or malignancy. The Lothian Research Ethics Committee approved all study procedures and protocols and patients gave written informed consent (LREC no. 09/S1101/43).
Patients were monitored as part of their routine clinical care. Pulmonary function was measured as per guidelines (5). Exacerbation was defined as an acute requirement for intravenous antibiotics, determined by the CF clinical team, among patients who had an increase in symptoms and a decrease from baseline FEV1. Exacerbation episodes that did not fulfill modified Fuchs criteria (6) were excluded from the analysis. Serum calprotectin and C-reactive protein (CRP) were measured by ELISA at baseline; complete blood counts were not obtained. Statistical analyses were performed in R (v2.15.2) (7).
Time to exacerbation by calprotectin was modeled in Cox proportional hazard models, using a penalized spline function to examine for nonlinearity. Decline in FEV1 and FVC was estimated using linear mixed models. Age, sex, previous exacerbation rate, symptom score, and high-sensitivity CRP were selected for inclusion in the regression models (in CRP analyses baseline calprotectin was substituted). There were no missing data for any of the baseline covariates, except for CRP, where three individuals with missing data were excluded in a complete case analysis. Because we used linear mixed models, missingness in pulmonary function measures was accommodated. Missing pulmonary function due to death was treated as missing at random for the main analyses. Calprotectin was treated as a continuous variable for the main analysis, but results are also presented by quantile. Tertiles are presented for time to exacerbation to ensure there were a reasonable number of events in each category. Rates of decline in lung function are presented by quintile rather than tertile as FEV1 and FVC are continuous variables.
Results
The 57 patients enrolled had a mean (SD) age of 26.4 (7.7) years. Twenty-seven (47%) were male. Patients with higher baseline calprotectin were older and had more severe disease (see Table E1 in the online supplement). The sample was representative of our regional CF clinic population as also seen in Table E1. Calprotectin and CRP at baseline were moderately associated (r = 0.35, P = 0.005). Patients were monitored for exacerbations for 1 year with none lost to follow-up. Three patients died during lung function follow-up (calprotectin levels of 7.9, 15.3, and 17.7) with the remainder being censored at 3 years.
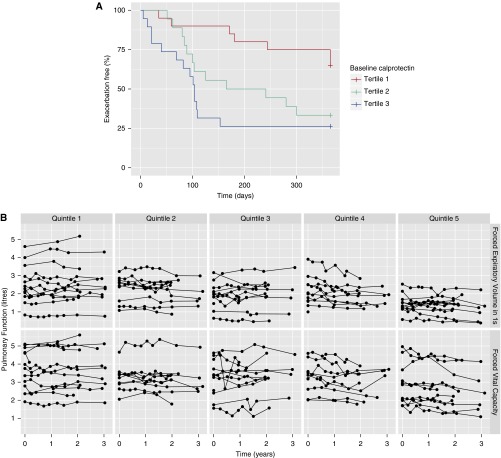
Thirty-three patients (58%) exacerbated within 1 year of baseline calprotectin. The median time to exacerbation was less in the upper (104 d) and middle (203 d) tertiles for calprotectin compared with the lower tertile (no median) (Figure 1A and Table 1). There was a 2.15-fold increase in hazard ratio (HR) for first exacerbation per twofold increment in calprotectin (95% confidence interval [CI], 1.45–3.18; P < 0.001). This association persisted after adjustment (HR, 2.05; 95% CI, 1.29–3.24; P = 0.002). CRP was similarly, but more weakly, associated with time to first exacerbation (HR, 1.40 per twofold increment in baseline CRP; 95% CI, 1.13–1.874; P = 0.002). This association was attenuated after adjustment (HR, 1.14 per twofold increment in baseline CRP; 95% CI, 0.90–1.46; P = 0.28).
Figure 1.
(A) Kaplan–Meier plot of time to first exacerbation. Subjects were divided into three groups based on median calprotectin level at baseline from low (tertile 1) to high (tertile 3). Median time to exacerbation from enrollment was lower in tertiles 2 and 3 compared with tertile 1 (P < 0.001). (B) Spaghetti plots of pulmonary function over time, by FEV1 and FVC and according to baseline calprotectin. Subjects were divided into five groups based on median calprotectin level at baseline from low (quintile 1) to high (quintile 5). Baseline lung function was higher in the lower quintiles. Rate of pulmonary function decline was fastest in the higher quintiles. The association of calprotectin with decline in FEV1 and FVC was significant at the conventional level (FEV1, P < 0.05; FVC, P < 0.01) when corrected for other variables (see Table 2).
Table 1.
Time to Exacerbation Prediction by Serum Calprotectin Level
| Tertile 1 (n = 20) | Tertile 2 (n = 18) | Tertile 3 (n = 19) | Per Twofold Increment in Baseline Calprotectin or CRP | P Value (Compared with Tertile 1) | |
|---|---|---|---|---|---|
| Median calprotectin (range), μg/ml | 5.4 (2.2–6.8) | 10.9 (6.9–15.9) | 21.2 (16.4–93.9) | ||
| Exacerbations, n | 7 | 12 | 14 | ||
| Median time to exacerbation, d | No median | 203 | 104 | ||
| Hazard ratio, unadjusted | — | 2.58 (1.01–6.59) | 3.90 (1.56–9.75) | 2.15 (1.45–3.18) | <0.001 |
| Hazard ratio, adjusted* | — | 2.79 (0.90–8.64) | 4.21 (1.39–12.71) | 2.05 (1.29–3.24) | 0.002 |
Hazard ratio for time to first exacerbation in unadjusted analysis and adjusting for age, sex, previous exacerbation rate, and FEV1 at baseline and for CRP at baseline.
Note that three patients did not have a baseline high-sensitivity CRP model and were excluded from this model.
There were a total of 341 spirometry measures with a median (interquartile range) of 6 (5–8), obtained over a median of 2.26 (1.96–3.17) years of follow-up. Decline in pulmonary function was associated with baseline calprotectin (Figure 1B and Table 2). Patients with higher baseline calprotectin had lower baseline FEV1 and FVC and more rapid decline in FEV1 and FVC. Overall, the mean (SD) decline in FEV1 and FVC was 55 (126) and 65 (91) ml/yr, respectively. The rate of decline was faster per twofold increment in baseline calprotectin, with the relationship being strongest for FVC (51 ml/yr; 95% CI, –21 to 81 ml/yr; P < 0.001, P = 0.009 adjusted), with FEV1 following a similar trend (33 ml/yr; 95% CI, –2 to 68 ml/yr; P = 0.06, P = 0.03 adjusted). CRP was not associated with decline in FEV1 or FVC in analyses adjusting for age, sex, previous exacerbation rate, and symptom score (per twofold increment in CRP: –5 ml/yr; 95% CI, –31 to 20 ml/yr; P = 0.68 decline in FEV1 and 15 ml/yr; 95% CI, –10 to 39 ml/yr; P = 0.24 decline in FVC).
Table 2.
Decline in Lung Function Based on Baseline Calprotectin Level
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Per Twofold Increment in Baseline Calprotectin | P Value | |
|---|---|---|---|---|---|---|---|
| Median calprotectin, μg/ml | 4.9 | 6.6 | 10.9 | 16.7 | 24.2 | — | — |
| FEV1, ml | |||||||
| Unadjusted | −35 | 84 | 0 | 186 | 68 | 33 (–2 to 68) | 0.06 |
| Adjusted* | −59 | 66 | −51 | 195 | 65 | 44 (3 to 84) | 0.03 |
| FVC, ml | |||||||
| Unadjusted | −14 | 54 | −22 | 134 | 175 | 51 (21 to 81) | <0.001 |
| Adjusted* | −78 | −17 | −122 | 88 | 89 | 50 (12 to 87) | 0.009 |
The rate of lung function decline per year increases with increasing baseline calprotectin. Data are presented for both unadjusted and adjusted (for age, sex, previous exacerbation rate, symptom score, and high-sensitivity CRP).
Note that three patients did not have a baseline high-sensitivity CRP model and were excluded from the analysis for this model.
Discussion
This study demonstrates that serum calprotectin levels are significantly associated with key future clinical events in CF including pulmonary exacerbations and lung function decline. Exacerbations are coupled with declines in lung function, which may not return to baseline after treatment (8, 9), and are associated with mortality (10). One study has suggested that severity and quality of life scoring predict risk of future exacerbation but that inflammatory biomarkers only predict re-exacerbation (11). Sputum high mobility group box 1 (HMGB1) levels may predict CF exacerbation (12), but this requires specialized sample processing that is not available in most clinics. The lung clearance index, a physiological measurement, also predicts exacerbation frequency and onset in children (13), but again requires specialized equipment. In contrast, calprotectin may be measured in serum with relative ease, underlined by its use in a pediatric study of azithromycin (14).
Rate of decline in lung function is also a marker of disease severity and disease progression. No serum biomarker has been identified that can predict lung function decline, although Sagel and colleagues have demonstrated a strong association between sputum neutrophil elastase levels (measured at multiple time points) and more rapid declines in lung function (15). We demonstrate that the rate of decline in FVC is approximately 50 ml/yr faster per twofold increment in baseline serum calprotectin (Table 2), and this association is independent of all other variables tested.
In conclusion, single measurement of serum calprotectin in stability predicts changes in disease activity manifest by time to next exacerbation and decline in lung function. Measurement of calprotectin in the clinic may allow the identification of patients at high risk of exacerbation and/or lung function decline and allow appropriate tailoring of therapy. Larger multicenter studies are now required to confirm these findings.
Acknowledgments
Acknowledgment
The authors thank Sachinkumar Singh for critical reading of the manuscript.
Footnotes
Supported by the Wellcome Trust (WT 093767; R.D.G. is a Wellcome Trust fellow). P.A.R. was funded by the CF Trust.
Author Contributions: R.D.G., A.P.G., and P.A.R. generated the hypothesis, analyzed data, and wrote the manuscript. D.A.M. performed the statistical analysis and contributed to manuscript writing. A.C.B., D.P., and J.A.I. were involved in the conception of the study, manuscript preparation, and interpretation of data.
This letter has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.MacGregor G, Gray RD, Hilliard TN, Imrie M, Boyd AC, Alton EW, Bush A, Davies JC, Innes JA, Porteous DJ, et al. Biomarkers for cystic fibrosis lung disease: application of SELDI-TOF mass spectrometry to BAL fluid. J Cyst Fibros. 2008;7:352–358. doi: 10.1016/j.jcf.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Gray RD, MacGregor G, Noble D, Imrie M, Dewar M, Boyd AC, Innes JA, Porteous DJ, Greening AP. Sputum proteomics in inflammatory and suppurative respiratory diseases. Am J Respir Crit Care Med. 2008;178:444–452. doi: 10.1164/rccm.200703-409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray RD, Imrie M, Boyd AC, Porteous D, Innes JA, Greening AP. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. J Cyst Fibros. 2010;9:193–198. doi: 10.1016/j.jcf.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Horsley AR, Davies JC, Gray RD, Macleod KA, Donovan J, Aziz ZA, Bell NJ, Rainer M, Mt-Isa S, Voase N, et al. Changes in physiological, functional and structural markers of cystic fibrosis lung disease with treatment of a pulmonary exacerbation. Thorax. 2013;68:532–539. doi: 10.1136/thoraxjnl-2012-202538. [DOI] [PubMed] [Google Scholar]
- 5.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 6.Bilton D, Canny G, Conway S, Dumcius S, Hjelte L, Proesmans M, Tümmler B, Vavrova V, De Boeck K. Pulmonary exacerbation: towards a definition for use in clinical trials: report from the EuroCareCF Working Group on outcome parameters in clinical trials. J Cyst Fibros. 2011;10:S79–S81. doi: 10.1016/S1569-1993(11)60012-X. [DOI] [PubMed] [Google Scholar]
- 7.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. Available from: http://www.R-project.org/
- 8.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1. Epidemiology and pathogenesis. Thorax. 2007;62:360–367. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders DB, Bittner RCL, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182:627–632. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153:345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wojewodka G, De Sanctis JB, Bernier J, Bérubé J, Ahlgren HG, Gruber J, Landry J, Lands LC, Nguyen D, Rousseau S, et al. Candidate markers associated with the probability of future pulmonary exacerbations in cystic fibrosis patients. PLoS One. 2014;9:e88567. doi: 10.1371/journal.pone.0088567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liou TG, Adler FR, Keogh RH, Li Y, Jensen JL, Walsh W, Packer K, Clark T, Carveth H, Chen J, et al. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. PLoS One. 2012;7:e42748. doi: 10.1371/journal.pone.0042748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vermeulen F, Proesmans M, Boon M, Havermans T, De Boeck K. Lung clearance index predicts pulmonary exacerbations in young patients with cystic fibrosis. Thorax. 2014;69:39–45. doi: 10.1136/thoraxjnl-2013-203807. [DOI] [PubMed] [Google Scholar]
- 14.Ratjen F, Saiman L, Mayer-Hamblett N, Lands LC, Kloster M, Thompson V, Emmett P, Marshall B, Accurso F, Sagel S, et al. Effect of azithromycin on systemic markers of inflammation in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa. Chest. 2012;142:1259–1266. doi: 10.1378/chest.12-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2012;186:857–865. doi: 10.1164/rccm.201203-0507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]