Abstract
Rationale: Pulmonary arterial hypertension (PAH) is a medically incurable disease resulting in death from right ventricular (RV) failure. Both pulmonary vascular and RV remodeling are linked to dynamic changes in the microvasculature. Therefore, we hypothesized that circulating angiostatic factors could be linked to outcomes and represent novel biomarkers of disease severity in PAH.
Objectives: We sought to determine the relationship of a potent angiostatic factor, endostatin (ES), with disease severity and mortality in PAH. Furthermore, we assessed genetic predictors of ES expression and/or function and their association with outcomes in PAH.
Methods: We measured levels of serum ES in two independent cohorts of patients with PAH. Contemporaneous clinical data included New York Heart Association functional class, 6-minute-walk distance, invasive hemodynamics, and laboratory chemistries.
Measurements and Main Results: Serum ES correlated with poor functional status, decreased exercise tolerance, and invasive hemodynamics variables. Furthermore, serum ES was a strong predictor of mortality. A loss-of-function, missense variant in the gene encoding ES, Col18a1, was linked to lower circulating protein and was independently associated with reduced mortality.
Conclusions: Our data link increased expression of ES to disease severity in PAH and demonstrate a significant relationship with adverse outcomes. Circulating ES levels can be genetically influenced, implicating ES as a genetically determined modifier of disease severity impacting on survival. These observations support serum ES as a potential biomarker in PAH with the capacity to predict poor outcomes. More importantly, this study implicates Col18a1/ES as a potential new therapeutic target in PAH.
Keywords: pulmonary arterial hypertension, endostatin, collagen 18a1, genetics, survival
At a Glance Commentary
Scientific Knowledge on the Subject
Pulmonary arterial hypertension (PAH) is characterized by dynamic alteration in the cardiopulmonary microvasculature. Endostatin (ES) is a potent angiostatic factor linked to decreased myocardial vascular density. The clinical significance of ES in PAH is uncertain.
What This Study Adds to the Field
In PAH, serum ES levels correlate with clinical, biochemical, and hemodynamic markers of disease severity. Serum ES levels predict mortality. Heritable loss-of-function variants in the gene encoding ES (Col18a1) are linked to altered serum levels and independently predict mortality. We propose that ES is a genetically influenced modifier of disease severity and outcomes in PAH.
Pulmonary arterial hypertension (PAH) is a progressive, debilitating, and medically incurable disease of the pulmonary vasculature. In PAH, remodeling of the pulmonary vessels results in increased pulmonary vascular resistance (PVR), increased right ventricular (RV) pressure overload, and death through right ventricular failure (RVF). Despite increasing treatment options, mortality from PAH is unacceptably high, and median survival remains dismal. For Group I diseases of the World classification of pulmonary hypertension, which include idiopathic PAH (IPAH) and connective tissue disease–associated (CTD) PAH (CTD-PAH, predominantly scleroderma), the reported 3-year survival rates are 72 and 52%, respectively (1–3).
Cardiac function strongly predicts outcomes in these patients (4, 5). Global ventricular performance is highly dependent on oxygen supply and thus myocardial perfusion. An essential component of myocardial perfusion during ventricular hypertrophy is the myocyte–microvascular balance (6, 7). In preclinical models of disease, the transition from RV adaptation to RVF is linked to diminished angiogenesis within the hypertrophic RV (8) and decreased myocardial capillary density in the failed RV (9). In humans, studies using adenosine coupled with cardiac magnetic resonance imaging (cMRI) indicate that RV myocardial perfusion reserve is reduced in patients with PAH and is linked to poor RV function (10). Thus, we predict that disruption, or direct antagonism, of the microcirculatory balance within the RV would predispose to RV dysfunction, negatively impacting on outcomes in PAH. Angiogenesis within the myocardium is dependent on the interplay between multiple angiogenic and angiostatic factors, yet neither the clinical significance of angiostatic factors on cardiac function and hemodynamics nor their impact on PAH morbidity and mortality have been addressed.
Endostatin (ES) is a potent angiostatic peptide derived from the carboxy terminus of the extracellular matrix protein, collagen XVIII, alpha 1 (Col18a1), initially identified by its capacity to inhibit tumor angiogenesis in vitro and in vivo (11–13). In ischemic heart disease, elevated ES levels in the circulation, pericardial space, and myocardial tissue have been associated with diminished collateral circulation within the heart (14–16). These small studies in humans with left heart disease indicate that the myocardium is a source of serum ES, which can be detected in the peripheral circulation (14). Moreover, they provide evidence that ES levels in humans negatively correlate with coronary collateral density. Most recently, elevated ES levels have been linked to increased mortality in the setting of left ventricular systolic dysfunction (17).
We hypothesized that circulating ES is altered in patients with PAH and that elevated levels are associated with worse morbidity and mortality. We also assessed the potential influence of genetic variants of ES on serum levels and clinical outcomes.
Methods
Full methodological details are available in the online supplement.
Results
Study Population, Derivation Serum Cohort
Table 1 shows the demographics, hemodynamic parameters, 6-minute-walk distance (6 MWD), New York Heart Association functional class (NYHA-FC), serum creatinine, sodium, and N-terminal pro-brain natriuretic peptide (NT-proBNP). Greater than 45% of the cohort had NYHA-FC III or IV symptoms at enrollment. Consistent with this, the mean 6 MWD was 384 m and the mean NT-proBNP was greater than two times the upper limit of normal. The median follow-up was 2.3 years, with a maximal follow-up of 7.2 years. The overall mortality was 27%, with 22 deaths observed. Details of the demographics of the validation cohort (exclusively patients with IPAH) are summarized in Table E2 in the online supplement. The median follow-up was 3.4 years (maximum of 7.5 yr), with an overall mortality of 22%.
Table 1.
Demographics and Characteristics of Derivation Serum Cohort
| N | 82 |
| Age, yr | 59.8 ± 13.4 |
| Female, n (%) | 65 (80) |
| Race, EA/AA/other, n (%) | 68/12/2 (82.9/14.6/2.4) |
| IPAH/CTD-PAH, n (%) | 37/45 (45/54) |
| 6 MWD, mean (range), m | 384 (211–671) |
| NYHA-FC, n (%) | |
| I | 11 (13.7) |
| II | 37 (45.1) |
| III | 30 (36.6) |
| IV | 4 (9.0) |
| Laboratory chemistries, mean (range) | |
| Serum sodium | 139 (123–147) |
| Serum creatinine | 1.0 (0.4–2.4) |
| NT-proBNP, pg/ml | 2,021 (20–11,698) |
| Hemodynamics | |
| RAP, mm Hg | 8 ± 5 |
| mPAP, mm Hg | 43 ± 14 |
| PCWP, mm Hg | 11 ± 4 |
| PVR, Wood units | 9.4 ± 6.3 |
| CO, L/min | 4.8 ± 1.6 |
| Cardiac index, L/min/m2 | 2.6 ± 0.7 |
Definition of abbreviations: 6 MWD = 6-minute-walk distance; AA = African American; CO = cardiac output; CTD-PAH = connective tissue disease–associated pulmonary arterial hypertension; EA = European American; IPAH = idiopathic pulmonary arterial hypertension; mPAP = mean pulmonary artery pressure; NT-proBNP = N-terminal pro-brain natriuretic peptide; NYHA-FC = New York Heart Association functional class; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RAP = right atrial pressure.
Data expressed as mean ± SD unless otherwise indicated.
Serum Endostatin Correlates with Clinical Markers of Disease Severity
We evaluated the relationship between serum ES and clinical markers of exercise performance and function, specifically 6 MWD and NYHA-FC, and serum chemistries previously linked to outcomes in PAH, notably NT-proBNP (18–20), creatinine (21), and serum sodium (22) (Table 2).
Table 2.
Correlation Table
| Correlation Coefficient |
|||
|---|---|---|---|
| Derivation Cohort |
Multicenter Cohort (Derivation and Validation) |
||
| Group I (n = 82) | Group I (n = 132) | IPAH Only (n = 87) | |
| Hemodynamics | |||
| RAP, mm Hg | 0.1* | 0.2* | 0.3† |
| mPAP, mm Hg | 0.2* | 0.3‡ | 0.5§ |
| PVR, Wood units | 0.2* | 0.3‡ | 0.5§ |
| CO, L/min | −0.2* | −0.2† | −0.3‡ |
| Cardiac index, L/min/m2 | −0.2* | −0.2† | −0.3† |
| Demographics | |||
| Age, yr | 0.3† | 0.2* | 0.03* |
| 6 MWD, m | −0.4§ | |
|
| NYHA-FC | 0.5§ | |
|
| Laboratory chemistries | |||
| Serum sodium | −0.2† | |
|
| Serum creatinine | 0.3† | |
|
| NT-proBNP, pg/ml | 0.4† | ||
Definitions of abbreviations: 6 MWD = 6-minute-walk distance; CO = cardiac output; IPAH = idiopathic pulmonary arterial hypertension; mPAP = mean pulmonary artery pressure; NT-proBNP = N-terminal pro-brain natriuretic peptide; NYHA-FC = New York Heart Association functional class; PVR = pulmonary vascular resistance; RAP = right atrial pressure.
P ≥ 0.05.
P < 0.05.
P < 0.005.
P < 0.001.
We evaluated the relationship between serum ES and invasive resting hemodynamics. Serum ES correlated positively with mean pulmonary arterial pressure (mPAP, r = 0.3, P < 0.005) and PVR (r = 0.3, P < 0.005) and negatively with cardiac output (CO, r = −0.2, P ≤ 0.05) and cardiac index (r = −0.2, P < 0.05). In the subset of patients with IPAH, serum ES also correlated positively with RAP (r = 0.3, P < 0.05), and the correlations were stronger with mPAP (r = −0.5, P < 0.001), PVR (r = 0.5, P < 0.001), CO (r = −0.3, P < 0.005), and cardiac index (r = −0.3, P < 0.05) (Table 2).
Circulating ES levels correlated positively with NYHA-FC (r = 0.4, P < 0.001), age (r = 0.3, P = 0.01), serum creatinine (r = 0.3, P = 0.03), and NT-proBNP (r = 0.4, P = 0.003) and negatively with 6 MWD (r = −0.4, P < 0.001) and serum sodium (r = −0.2, P = 0.04).
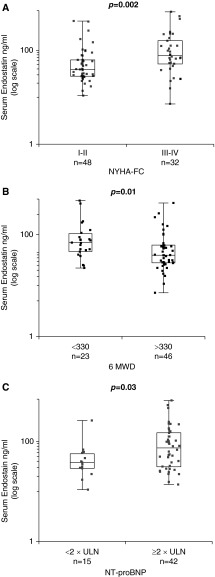
Functional class, functional capacity, and NT-proBNP are established metrics of disease severity in PAH (23–25). We evaluated serum ES as a function of NYHA-FC and observed that ES was higher in patients with NYHA-FC III to IV PAH relative to those in class I to II (80.0 vs. 40.2 ng/ml, P = 0.002) (Figure 1A). Similarly, patients with a 6 MWD of less than 330 m, a parameter previously linked to poor prognosis in PAH, had significantly higher ES than those with a 6 MWD greater than 330 m (69.6 vs. 39.5 ng/ml, P = 0.01) (Figure 1B). Using a cut-off of two times the upper limit of normal, patients with elevated NT-proBNP had significantly higher serum ES (73.9 vs. 37.7 ng/ml, P = 0.03) (Figure 1C).
Figure 1.
Box and whisker plots of serum endostatin (ES) levels in patients with pulmonary arterial hypertension (PAH) with (A) New York Heart Association functional class (NYHA-FC) I to II versus III to IV symptoms, (B) 6-minute-walk distance (6 MWD) < 330 versus ≥330 m, and (C) N-terminal pro-brain natriuretic peptide (NT-proBNP) ≥2 × upper limit of normal (ULN) versus <2 × ULN. Boxes represent the interquartile range (IQR) and the horizontal lines are the medians. Whiskers represent the closest values within 1.5 times the IQR.
Serum Endostatin and Disease Severity
There is no established normal value for serum ES in humans. To define a threshold value for ES, serum ES was measured in healthy and disease control subjects (see Table E1 in the online supplement). We observed significantly elevated ES in patients with PAH relative to these healthy control subjects (62.9 vs. 28.1 ng/ml, P < 0.0001) (Figure E2A). In addition, we also observed elevated serum ES levels in patients with Group I PAH relative to age-matched patients with interstitial lung disease (62.9 vs. 44.3 ng/ml, P < 0.04; data not shown). Using the observed values, a receiver operating characteristics (ROC) curve was generated (Figure E2B). Serum ES had the ability to identify PAH with an area under the curve of 0.879, P < 0.0001. From this ROC analysis, we established a threshold value of serum ES set at 66 ng/ml to distinguish healthy subjects from patients with PAH. This threshold value had a sensitivity and specificity for PAH of 76 and 91%, respectively.
Based on this threshold ES level, the derivation cohort of patients with PAH was divided into two subgroups, designated ESLow (i.e., serum ES < 66 ng/ml) and ESHigh (ES ≥ 66 ng/ml). Table 3 shows demographics, laboratory chemistries, hemodynamic parameters, and PAH-specific therapy for each subgroup. The ESHigh arm had significantly lower 6 MWD (331 vs. 411 m, P < 0.0001) than ESLow. Additionally, more than half the patients within ESHigh were NYHA-FC III to IV, whereas the majority of ESLow patients were NYHA class I to II. NT-proBNP was significantly lower in ESLow versus ESHigh patients (1,045 vs. 3,362 ng/ml, P = 0.003). However, there were no statistically significant differences observed in resting hemodynamics between the arms. There were also no discernable differences in PAH-specific therapy. Thus, patients with elevated serum ES (ESHigh) had diminished functional capacity, worse functional status, and increased NT-proBNP, implicating ES as a prognostic marker of disease severity in PAH.
Table 3.
Clinical Characteristic as a Function of Serum Endostatin
| ESLow (n = 50) | ESHigh (n = 32) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age, mean ± SD, yr | 58.5 ± 11.9 | 61.1 ± 15.4 | 0.3 |
| Female, n (%) | 41 (82) | 27 (84) | 1.0 |
| Race, EA/AA/other (EA%) | 40/7/3 (80) | 26/6/0 (81) | 0.1 |
| 6 MWD, mean (range), m | 411 (228–671) | 331 (211–550) | <0.0001 |
| NYHA-FC, n (%) | |||
| I | 10 (20) | 0 (0) | 0.0003 |
| II | 27 (54) | 10 (31.3) | |
| III | 13 (26) | 17 (53.1) | |
| IV | 0 (0) | 4 (12.5) | |
| Laboratory chemistries, mean (range) | |||
| Serum sodium | 140 (135–147) | 138 (23–144) | 0.06 |
| Serum creatinine | 0.9 (0.4–2.1) | 1.2 (0.6–2.5) | 0.0007 |
| NT-proBNP, pg/ml | 1,045 (20–6,922) | 3,362 (94–11,698) | 0.003 |
| PAH-specific therapy, n (%) | |||
| PDE5 inhibitor | 30 (60.0) | 22(68.8) | 0.4 |
| ETRA | 23 (46.0) | 16 (50.0) | 0.9 |
| Prostacyclin | 11 (12.5) | 4 (18.3) | 0.6 |
| Ca-channel blockers | 5 (10.0) | 0 (0.0) | 0.2 |
| Treatment naive | 6 (12.0) | 7 (21.9) | 0.4 |
| Hemodynamic, mean ± SD | |||
| RAP, mm Hg | 8 ± 5 | 8 ± 5 | 0.9 |
| mPAP, mm Hg | 41 ± 13 | 46 ± 16 | 0.1 |
| PCWP, mm Hg | 10 ± 4 | 12 ± 4 | 0.3 |
| PVR, Wood units | 8.7 ± 5.9 | 10.5 ± 6.9 | 0.2 |
| CO, L/min | 5.0 ± 1.7 | 4.5 ± 1.4 | 0.2 |
| Cardiac index, L/min/m2 | 2.7 ± 0.8 | 2.4 ± 0.6 | 0.1 |
Definition of abbreviations: 6 MWD = 6-minute-walk distance; AA = African American; Ca = calcium; CO = cardiac output; EA = European American; ESHigh = endostatin ≥ 66 ng/ml; ESlow = endostatin < 66 ng/ml; ETRA = endothelin receptor antagonist; mPAP = mean pulmonary artery pressure; NT-proBNP = N-terminal pro-brain natriuretic peptide; NYHA-FC = New York Heart Association functional class; PAH = pulmonary arterial hypertension; PCWP = pulmonary capillary wedge pressure; PDE5 = phosphodiesterase type five; PVR = pulmonary vascular resistance; RAP = right atrial pressure.
Endostatin and Outcomes in PAH
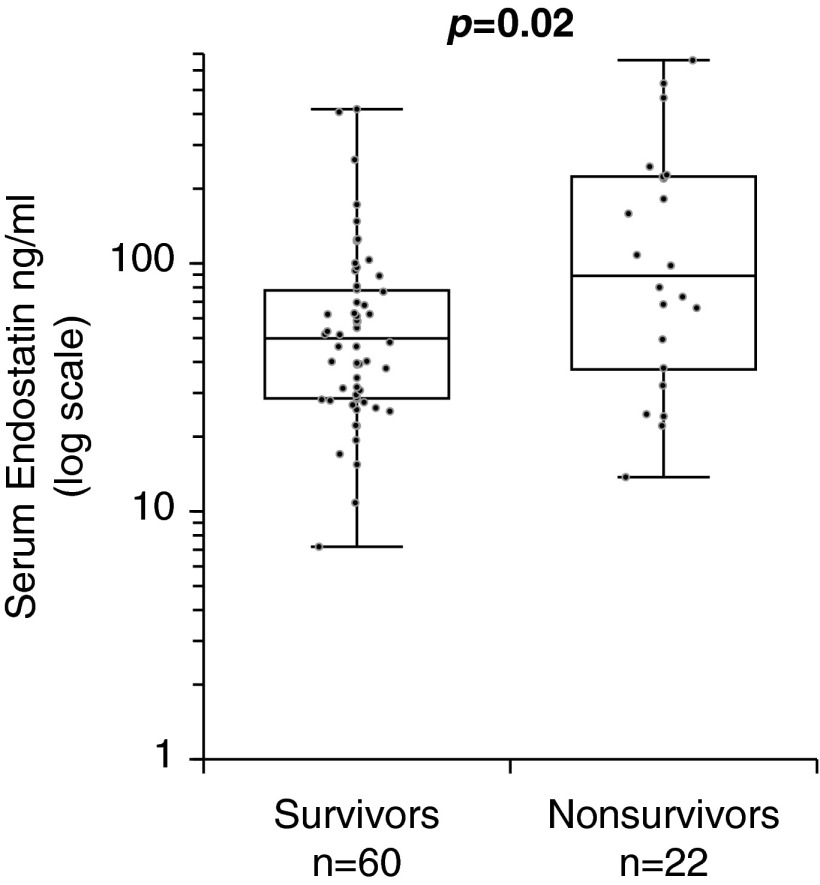
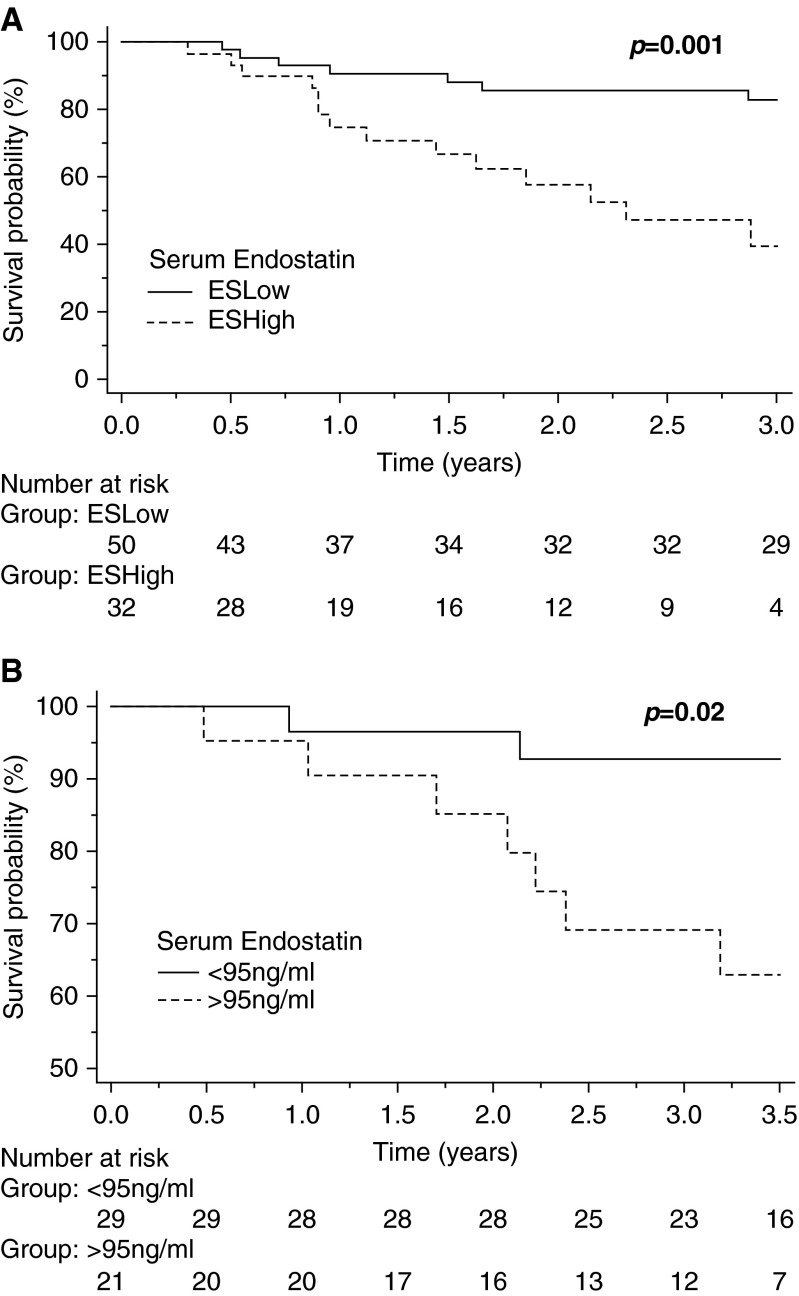
Comparison of serum ES in survivors and nonsurvivors demonstrated a significantly higher concentration of ES in nonsurvivors of PAH (89.0 vs. 49.8 ng/ml, P = 0.015) (Figure 2). Kaplan-Meier analysis demonstrated that high ES expression (ESHigh subgroup) was associated with a significantly increased risk of death, with an unadjusted hazard ratio [HR] of 3.7 (95% confidence interval [CI], 1.5–9.2; log-rank P = 0.001) (Figure 3A). Cox proportional hazard models examining the relationship between serum ES (dichotomized at the identified threshold) and outcomes were constructed. High ES expression was a significant predictor of adverse outcomes, with an unadjusted HR of 4.0 (95% CI, 1.6–9.6; P = 0.002) (Table 4). Analysis of the IPAH and CTD-PAH subsets independently linked serum ES with mortality (HR, 10.4; 95% CI, 1.1–99.1; and HR, 2.7; 95% CI, 1.0–7.3, respectively). The relationship between ES levels and outcomes persisted in multivariable Cox proportional hazard models. ES predicted death after adjusting for age, etiology of PAH (i.e., IPAH vs. CTD-PAH), invasive hemodynamic variables (RAP, mean PA, PVR, PCWP, CO, and cardiac index), serum sodium, and creatinine (Table 4). The strength of association was attenuated when adjusting for some clinical parameters, specifically NYHA-FC, 6 MWD, and NT-proBNP, suggesting these variables either confound the relationship or share a common causal pathway between ES and outcomes.
Figure 2.
Box and whisker plots of serum endostatin levels in surviving and nonsurviving patients with pulmonary arterial hypertension. Boxes represent the interquartile range (IQR), and the horizontal lines are the medians. Whiskers represent the closest values within 1.5 times the IQR.
Figure 3.
Survival curve for serum endostatin (ES) in pulmonary arterial hypertension (PAH). (A) Kaplan-Meier survival analysis of derivation cohort dichotomized by serum ES levels; n = 82, P = 0.001. (B) Kaplan-Meier survival analysis of a validation idiopathic PAH cohort; n = 50, P = 0.02.
Table 4.
Bivariable Cox Proportional Hazard Models
| HR (95% CI) | |
|---|---|
| Endostatin (serum) | 4.0 (1.6–9.6)* |
| Adjusted for | |
| Demographics | |
| Age, per yr | 3.3 (1.3–8.3)* |
| Etiology, CTD-PAH | 3.3 (1.3–8.2)† |
| NYHA-FC, III–IV vs. I–II | 2.2 (0.9–5.5)‡ |
| 6 MWD, per 100 m | 1.9 (0.5–6.6)‡ |
| Laboratory chemistries | |
| Serum creatinine | 3.5 (1.4–9.0)* |
| Serum sodium | 3.2 (1.2–8.3)† |
| NT-proBNP, log | 2.0 (0.7–5.6)‡ |
| Hemodynamics | |
| RAP, mm Hg | 4.3 (1.7–10.5)§ |
| mPAP, mm Hg | 4.2 (1.7–10.2)* |
| PCWP, mm Hg | 4.3 (1.7–10.6)* |
| PVR, Wood units | 3.8 (1.5–9.2)* |
| CO, L/min | 3.6 (1.5–8.5)* |
| Cardiac index, L/min/m2 | 3.9 (1.6–9.7)* |
Definition of abbreviations: 6 MWD = 6-minute-walk distance; CI = confidence interval; CO = cardiac output; CTD-PAH = connective tissue disease–associated pulmonary arterial hypertension; HR = hazard ratio; mPAP = mean pulmonary artery pressure; NT-proBNP = N-terminal pro-brain natriuretic peptide; NYHA-FC = New York Heart Association functional class; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RAP = right atrial pressure.
P < 0.01.
P < 0.05.
P ≥ 0.05.
P < 0.001.
To validate the observed relationship between ES and poor outcomes, we generated a second ROC curve from the derivation cohort to define a threshold value to predict mortality in PAH (Figure E3). Serum ES had the ability to identify nonsurvivors with an area under the curve of 0.675, P = 0.02. From this analysis, we established a cutoff for serum ES for predicting increased mortality, which was set at 95 ng/ml and provided a relatively high specificity of 83% and a sensitivity of 50%. Similar to the initial analysis, this threshold value predicted poor outcomes in the derivation cohort with an unadjusted HR of 3.4 (95% CI, 1.2–9.1; log-rank P = 0.002). We then applied this cutoff to the validation cohort. In the validation cohort, serum ES levels were significantly elevated relative to age-matched healthy or disease control subjects (88.3 vs. 28.1 vs. 44.1 ng/ml, respectively; P < 0.0001). Kaplan-Meier analysis demonstrated that high ES expression (>95 ng/ml) was associated with a significantly increased risk of death, with an unadjusted HR of 4.2 (95% CI, 1.3–14.6; log-rank P = 0.02) (Figure 3B) in this second cohort of patients with IPAH. Using this validation PAH cohort, Cox proportional hazard models were constructed to examine the relationship between serum ES and outcomes. High ES expression was validated as a significant predictor of adverse outcomes with an unadjusted HR of 4.3 (95% CI, 1.1–8.3; P < 0.05), and the relationship persisted in multivariable Cox proportional hazard models after adjusting for age, sex, cardiac index, or PVR (Table E3).
Col18a1 Genetic Variant and PAH
In a parallel study, genotyping was performed on genomic DNA samples from subjects with PAH (IPAH and CTD-PAH), scleroderma without pulmonary hypertension, and control subjects. Baseline demographics and index hemodynamic indices for patients are listed in Table E4. Because of differences in minor allele frequency (MAF) between ancestries, association studies were restricted to cases and control subjects of European American ancestry. Twelve potential variants in the gene encoding ES, Col18a1, were included within the analysis. Of these, a missense single nucleotide polymorphism (SNP) in Col18a1 (rs12483377) was observed at an increased frequency in patients with PAH (MAF 21.6) relative to published control subjects (MAF 7.5) and/or patients with scleroderma without PH (MAF 12.5) (Table E5). In the validation cohort of patients with IPAH, the frequency was 15.8. The genotype distribution of the studied SNP was in Hardy-Weinberg equilibrium in cases and control subjects. Using an in silico analysis tool, polymorphism phenotyping (26), one would predict that this missense variant altered the function of the encoded protein (polymorphism phenotyping score, 0.984). This variant encodes an uncharged amino acid asparagine (N) at residue 104 of ES in place of the ancestral, negatively charged aspartic acid (D) (D104 ES). More importantly, molecular biological studies of this variant ES peptide in vitro indicate that it represents a loss-of-function mutation, with altered binding to one of its receptors, laminin (27).
Col18a1 Genetic Variant and Serum Endostatin
To determine if serum ES levels were genetically influenced in PAH, we evaluated the relationship between carrier status of the identified Col18a1 variant (rs12483377) and circulating ES levels. A subgroup of patients donated both genomic DNA and serum (n = 48). Carriers of a single minor allele A (genotype AG), N104ES, had significantly lower serum ES levels relative to those who carried two copies of the ancestral, negatively charged aspartic acid (D) at residue 104 (protein D104 ES, genotype GG) (33.6 vs. 70.6 ng/ml, P = 0.02) (Figure E4).
Col18a1 Variant and PAH Outcomes
We next analyzed the relationship between carrier status of the N104ES natural variant versus the ancestral D104ES and mortality within the larger derivation PAH cohort providing genomic DNA (n = 100 European American). Demographics and index hemodynamics are included in the online supplement (Table E4). The median follow-up post diagnosis of PAH was 5.3 years, with a range of 0.7 to 16.0 years. During follow-up, 47 deaths were observed, with an overall mortality of 47%. Comparison of demographics and baseline hemodynamics revealed no identifiable differences in age of diagnosis, etiology of PAH, or index hemodynamics in the two genotypes (D/D104ES vs. N/D104ES) (Table E6).
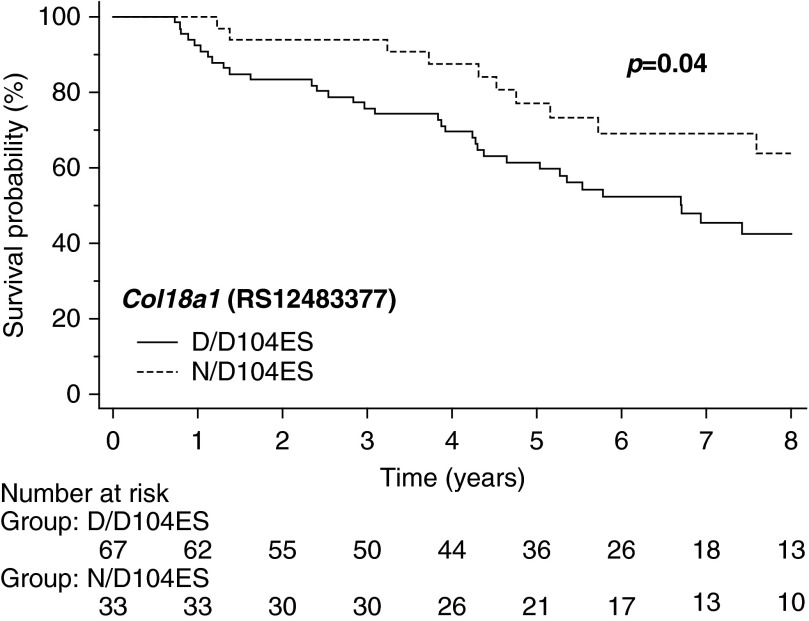
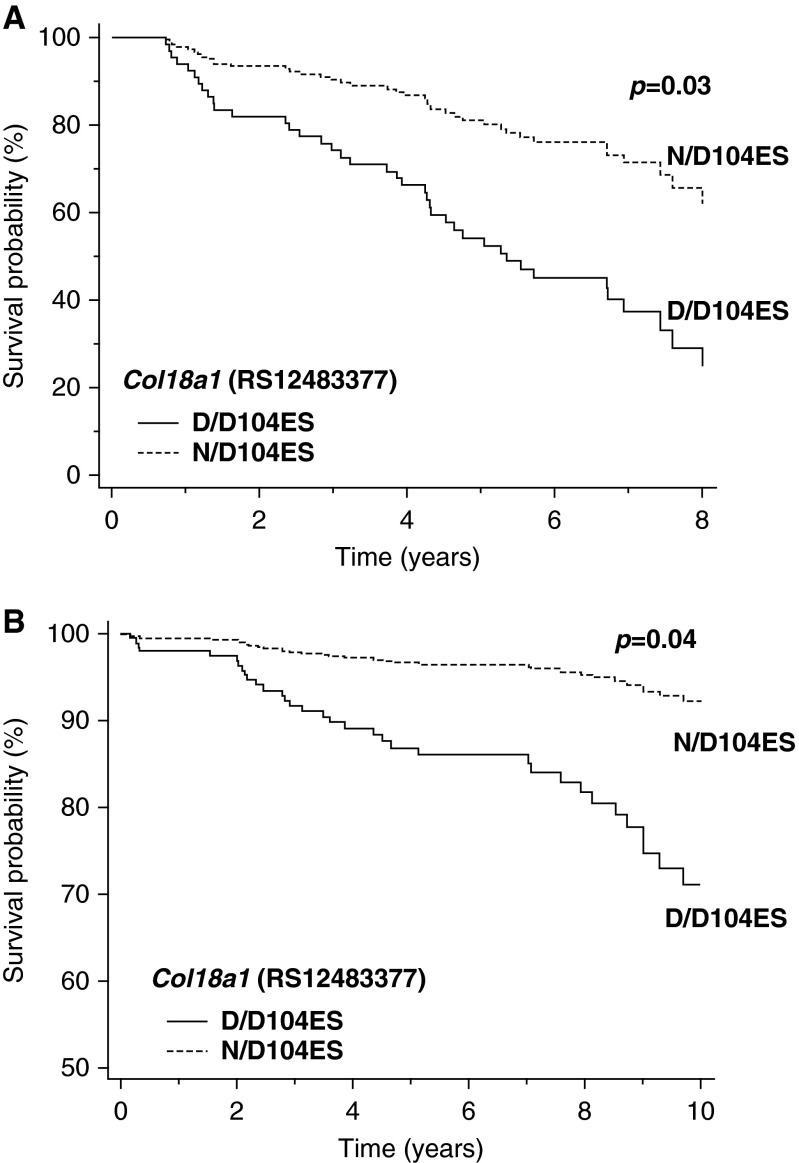
Kaplan-Meier analysis demonstrated that the Col18a1 genotype encoding D/D104ES was associated with increased mortality with an unadjusted HR of 2.0 (95% CI, 1.1–3.6; P = 0.04) (Figure 4). We then constructed Cox proportional hazard models to examine predictors of the relationship between Col18a1 genotype and outcomes. The unadjusted risk of death in patients with the GG genotype encoding only the D104ES variant was 2.0 (95% CI, 1.1–3.9; P = 0.04) (Table 5).
Figure 4.
Survival curve for Col18a1/endostatin (ES) variant status in pulmonary arterial hypertension. Kaplan-Meier survival analysis of derivation cohort dichotomized by carrier status of ES variants. n = 100, P = 0.04.
Table 5.
Multivariable Cox Proportional Hazard Ratio Models
| HR (95% CI) | |||
|---|---|---|---|
| Genotype (GG) protein D/D104ES | 2.0 (1.1–3.9)* |
||
| Model adjusted for: | Age, Cardiac Index, and | Age, CTD-PAH, and | Sex, CTD-PAH, and |
| Age | — | — | 2.4 (1.2–4.8)* |
| Sex | 3.2 (1.5–7.0)† | 2.4 (1.2–4.8)* | — |
| CTD-PAH | 3.2 (1.5–6.9)† | — | — |
| RAP | 3.0 (1.3–6.8)† | 2.4 (1.2–5.0)* | 2.2 (1.1–4.6)* |
| PVR | 2.8 (1.3–6.2)† | 2.8 (1.4–5.9)† | 2.2 (1.1–4.6)* |
| Cardiac index | — | 3.2 (1.5–6.7)† | 2.9 (1.4–6.3)† |
Definition of abbreviations: CI = confidence interval; CTD-PAH = connective tissue disease–associated pulmonary arterial hypertension; HR = hazard ratio; PVR = pulmonary vascular resistance; RAP = right atrial pressure.
P < 0.05.
P < 0.01.
Multivariable models were built using carrier status adjusting for age of diagnosis, sex, or etiology of PAH (i.e., CTD-PAH vs. IPAH), and individual hemodynamic variables (Table 5). These variables were included based both on our univariable Cox proportional hazard models and/or demonstration of their capacity to predict outcome in previous studies (18, 25, 28). The GG genotype encoding only D104ES remained a significant predictor of increased mortality in these models. Survival analysis based on Col181/ES carrier status after adjusting for demographics variables or hemodynamic variables linked a genetic variation in this gene to risk of death in PAH (Figure 5A). Thus, carriers of ES variant N104ES, which impairs receptor binding (27) and is associated with decreased serum ES in our analysis, had improved survival even after adjusting for invasive cardiopulmonary markers of disease severity, implicating the Col18a1 gene product as a heritable determinant of mortality in PAH.
Figure 5.
Cox proportional hazard models of Col18a1/endostatin (ES) carrier status adjusted for demographics (age, sex, and cardiac index). (A) Derivation cohort of Group I pulmonary arterial hypertension (PAH); n = 100, P = 0.03. (B) Validation cohort of patients with idiopathic PAH; n = 169, P = 0.04.
To strengthen this observation, the relationship between Col18a1 genotype and outcomes was analyzed in the validation cohort of adults with Group I, specifically IPAH. Baseline demographics and index hemodynamic indices for this cohort of patients are listed in Table E7. Multivariable models were built using carrier status (D/D104ES) adjusting for age of diagnosis, sex, or individual hemodynamic variables (Table E8). In Cox proportional hazard models, the GG genotype encoding only D104ES variant again remained a significant predictor of increased mortality in patients with IPAH. Thus, Col181/ES carrier status was associated with an increased risk of death in IPAH after adjusting for demographics and hemodynamic variables in two independent cohorts (Figure 5B and Table E8).
Discussion
This study demonstrates that serum ES is associated with disease severity and predicts outcomes in two independent cohorts of patients with PAH. Circulating ES inversely correlates with diminished functional capacity (6 MWD) and directly correlates with impaired functional status (NYHA-FC) and NT-proBNP. Furthermore, we observe significant differences in ES between survivors and nonsurvivors, implicating ES as a predictor of poor outcomes. This is supported by multivariable Cox proportional hazard models that demonstrate an increased risk of death in patients with elevated ES levels. Survival analysis demonstrates that ES levels predict outcomes in patients with PAH with an unadjusted risk of death of 4.0 (95% CI, 1.6–9.6; P = 0.002). Additionally, we provided evidence that a loss-of-function variant in the gene encoding ES (Col18a-SNP rs12483377) was linked to altered serum ES levels in PAH. More importantly, we demonstrate that Col18a1/ES genotype is independently associated with mortality in PAH. Thus, we provide the first evidence linking an angiostatic factor to disease severity and poor outcomes in PAH.
In PAH, global performance in the remodeled RV is highly dependent on oxygen supply. Disruption of the myocardial–microvascular balance would predictably impact on RV function, clinical severity of disease, and outcomes. Clinical support of such a principle in PAH comes from adenosine magnetic resonance imaging data, which demonstrate decreased RV myocardial perfusion reserve index in PAH (10). The RV myocardial perfusion reserve index is inversely associated with RV systolic function and RV remodeling (i.e., RV mass). These data indicate that reduced myocardial perfusion is associated with poor RV performance and remodeling in human PAH. Such functional studies cannot yet address the etiology of diminished RV perfusion, and imaging of the ventricular microcirculation is not yet feasible in humans. However, preclinical studies in rodent models of disease provide evidence that decreased RV capillary density is linked to RVF (8, 29).
ES is a potent inhibitor of angiogenesis with the capacity to induce endothelial cell apoptosis and inhibit endothelial cell proliferation and migration. Although a detailed understanding of the molecular mechanisms behind the angiostatic properties of ES has remained elusive, the capacity of ES to inhibit the angiogenic effects of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor has been long recognized (11). Clinical interest in ES has focused primarily on attempts to capitalize on its angiostatic properties in the treatment of solid tumors. Exogenous ES has been shown to antagonize tumor angiogenesis in preclinical models (30) and has been exploited in clinical trials as an adjuvant to chemotherapy. Far less is known about the effects of endogenous ES in human disease states. Multiple studies have demonstrated an inverse correlation between ES and the density of coronary vessels in the heart (14–16), and, during the preparation of this manuscript, ES has been linked to increased mortality in left heart disease (17). Collectively, this implicates ES as a potential negative regulator of myocardial angiogenesis, but this remains to be directly tested. The contribution of ES to vascular remodeling in the lung is less clear, and no published data have linked ES to altered vascular remodeling in the human lung. Preclinical models demonstrate that pharmacologic inhibition of VEGF under hypoxic conditions can recapitulate pulmonary vascular lesions similar to those observed in human PAH (31). Furthermore, loss and/or rarefaction of capillaries with the RV has been observed in this model and linked to RVF (32). These observations indirectly link an angiostatic effect (i.e., inhibition of VEGF signaling) to both pulmonary vascular remodeling and RVF in preclinical studies. Studies to address the capacity of ES to alter both the pulmonary and myocardial remodeling are ongoing. Ultimately, understanding the mechanism(s) by which ES negatively impacts disease outcomes in PAH is necessary.
Serum ES levels correlated with unfavorable hemodynamics in Group I patients and most strongly within the patients with IPAH. Specifically, ES levels positively correlated with RAP, mPAP, and PVR and inversely correlated with CO and cardiac index. These relationships were strongest in patients with IPAH and were not observed in the smaller CTD-PAH subset alone (data not shown), suggesting this study may be underpowered to observe these associations. Irrespective of this potential difference based on etiology of disease, serum ES did correlate strongly with 6 MWD, NYHA-FC, and NT-proBNP and, more importantly, predicted poor outcomes in both disease subsets, implicating ES as a novel marker of functional capacity and disease severity in PAH.
Preclinical models of PAH provide strong experimental evidence that the inhibition of angiogenesis, specifically with the VEGF receptor 2 antagonist (i.e., Sugen), can contribute to both pathologic pulmonary vascular remodeling (33) and RV dysfunction (9). Despite the preclinical data, few studies have addressed the relationship between circulating VEGF levels, hemodynamics, and/or outcomes in human PAH (34, 35). Clinical and preclinical data have also implicated increased expression of angiogenic factors in PAH. Specifically, angiopoietin, a vascular growth factor, and its receptor Tie2, are elevated in pathologic pulmonary vascular remodeling in humans (36). Furthermore, elevated circulating angiopoietin 2 has been linked to hemodynamics and poor outcomes in IPAH (37). Angiostatin, another endogenous angiostatic mediator, has been tested in the chronic hypoxic animal model of disease and, when delivered to the lung directly, exacerbates pathologic pulmonary vascular remodeling (38). As for clinical data, a very small (n = 6) study has suggested that angiostatin is elevated in patients with PAH (39).
Although not included in this study population, Down syndrome (trisomy 21) accounts for more than 10% of pediatric PAH (40, 41) and appears to have an accelerated and more severe disease course than patients matched for similar congenital heart defects (42). Intriguingly, patients with Down syndrome have elevated circulating ES levels secondary to the duplication of Col18a1 encoded on chromosome 21 (43). Furthermore, data link altered ES expression to altered pulmonary vascular remodeling in the lungs of newborns with Down syndrome (44). The relationship between Col18a1/ES gene dose and PAH risk/outcomes have not been addressed in this at-risk cohort.
We observed a link between a natural variant in Col18a1/ES and serum levels of ES in PAH. The identified Col18a1 SNP results in the substitution of a negatively charged aspartic acid (D) to a hydrophobic asparagine (N) at residue 104 (D104ES and N104ES, respectively). Published work indicates that this substitution disrupts the binding of ES with one of its receptors, laminin (27), making this a loss-of-function mutation. Importantly, carriage of two copies of D104ES (GG genotype) was associated with a median survival of 6.7 years after diagnosis of PAH. In contrast, carrier status of a single loss-of-function, N014ES variant (AG genotype) was linked with improved survival (unadjusted HR, 0.5; 95% CI, 0.3–0.9; P = 0.04), reflected in a median survival of greater than 14.5 years. This effect persisted after adjustments for age of diagnosis, sex, etiology of PAH, and invasive hemodynamic parameters. Although carriers of the N104ES predictably have lower functional ES protein, the mechanism(s) by which this genetic variation impacts on circulating ES levels remains to be determined. Variants in protein sequence and structure can impact on intracellular processing, secretion, cleavage, and/or turnover (i.e., stability), leading to altered steady state levels. Alternatively, rs12483377 may directly, or indirectly, impact on gene expression to account for the differences in circulating ES observed. Further studies will be needed to address the mechanisms regulating ES levels in PAH and their genetic determinates. Collectively, we predict that carriers of the N014ES variant (AG genotype) have improved outcomes as a consequence of decreased effective ES, both with lower serum levels and decreased functional peptide.
Natural variants of Col18a1 have been observed in other human disease states. Most notably, nonsense mutations in Col18a1 are linked to Knobloch syndrome (45, 46), a recessive form of macular degeneration. One Knobloch kindred has been identified that carries the N104ES variant (27). This substitution alters ES binding to its receptor, laminin, a basement membrane protein. The capacity of ES to bind lamina has been implicated in the molecule’s angiostatic properties (47). Additional studies will be necessary to define the molecular effects of this nonconservative change on ES function in the context of PAH. However, based on the documented properties of N104ES, it is provocative to speculate that this functional variant impacts on PAH outcomes through the diminished angiostatic activity of the altered peptide. Although multiple studies have examined, with variable results, the association of Col18a1 SNPs with development and severity of solid and liquid tumors (48–52), our data are the first to formally associate natural variants in Col18a1 to cardiovascular disease, altered serum levels, and PAH mortality.
Data regarding the genetic basis for the development and clinical heterogeneity of PAH are limited. Mutations in BMPR2 (53, 54) and other members of the transforming growth factor-β superfamily (ligands, receptors, and activators) have been found in patients with PAH and hereditary hemorrhagic telangiectasia including ALK1, ENG, SMAD4, and SMAD8 (55–62). More recently, variants in caveolin1 (CAV1) and KCNK3 have also been described in familial PAH and IPAH (63, 64). We observed an increased frequency of the A allele (rs12483377) in Col18a1 in our derivation cohort (MAF 21.6) relative to our control subjects (MAF 13.0) and the frequency observed to date in the National Heart, Lung, and Blood Institute exome sequencing project (MAF 7.5). Furthermore, within the validation cohorts we also observed an increase in the MAF relative to control subjects (15.8 vs. 7.5) (Table E5).These associations could implicate this variant as a risk allele for PAH; however, in light of the demonstrated relationship with survival, we suspect this increase in MAF reflects survival bias. Here we provide unique evidence supporting a genetic basis for clinical heterogeneity in PAH, linking it to altered serum levels and, more importantly, mortality.
There are several potential limitations to this study. First, subjects were referred to pulmonary hypertension programs; thus, ascertainment bias could favor inclusion of patients with severe disease. Second, survival estimates may be influenced by lead-time bias because the majority of patients received PAH-specific therapy at the time of analysis. Third, our cohorts include both prevalent and incident PAH and may introduce survival bias (65). Although additional studies would be necessary to prove this, we suspect such a bias accounts for the increased frequency of the loss-of-function ES variant (N104ES) observed in our PAH cohorts. Similar observations have been made in the MUC5B promoter polymorphisms and pulmonary fibrosis (66). In this case there is a clear increase in the frequency of a genetic variant in patients with disease. However, carriers of this common “risk” polymorphism have improved survival (67). We did not observe appreciable differences in age, sex, disease subgroup, or index hemodynamics based on N104ES carrier status (Table E6) to suggest that lead-time bias contributed to the differences in outcomes; however, this remains a potential limitation. Furthermore, the impact of therapy on ES levels has not yet been defined, and we cannot assess the effects of specific therapies. We have attempted to minimize sampling bias through the use of standardized collection, processing, and storage of samples, including avoidance of freeze-thaws of banked serum, and batched analysis. Finally, we cannot yet define the tissue source or target of circulating ES. Although it is clear that circulating ES can be produced by the human heart in cardiovascular disease (14–16), it remains to be determined if this is the case in PAH. Our unpublished data indicate that Col18a1 mRNA and ES protein are selectively up-regulated in the RV, not the left ventricle, in preclinical models of pulmonary hypertension. Additionally, skeletal muscle is both a potential source and possible target of ES (68), and thus peripheral effects of ES may contribute to the observed association between serum ES and poor 6 MWD or NYHA-FC. However, additional clinical and preclinical studies will be needed to address these remaining important questions.
Conclusions
In summary, this work provides strong evidence that serum ES and a heritable variation in its gene, Col18a1, are potential determinants of disease severity, hemodynamics, and outcomes in PAH. We hypothesize this is based on the ability of ES to negatively impact pathologic vascular remodeling in PAH. Furthermore, our data support a novel role for ES as a serum biomarker linked to poor functional class, decreased functional capacity, adverse hemodynamics, and increased mortality in PAH. Angiostatic factors, typified by ES, may represent novel therapeutic targets in PAH.
Footnotes
Supported by the National Institutes of Health/National Heart, Lung, and Blood Institute awards P50 HL084946/R01 and HL114910 (P.M.H.), K08HL088320 (R.D.), and K23HL90038491 (S.C.M.).
Author Contributions: R.D. and P.M.H. planned the project, recruited subjects, analyzed the data, and wrote the manuscript; R.D., S.C.M., M.B.D., and N.R. performed statistical analyses; L.V., L.W., L.G., and N.R. performed experiments and interpreted the results; T.M.K, T.H., R.J.T., D.A.K., R.L.B., J.L.R., R.H., J.E.L., I.M.R., A.R.H., W.K.C., E.D.A., and S.C.M. enrolled subjects and performed the research; J.L.R., L.G., N.R., and K.C.B. performed the genotyping; all authors reviewed, revised, and approved the manuscript for submission.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201409-1742OC on December 9, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lefèvre G, Dauchet L, Hachulla E, Montani D, Sobanski V, Lambert M, Hatron PY, Humbert M, Launay D. Survival and prognostic factors in systemic sclerosis-associated pulmonary hypertension: a systematic review and meta-analysis. Arthritis Rheum. 2013;65:2412–2423. doi: 10.1002/art.38029. [DOI] [PubMed] [Google Scholar]
- 2.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142:448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 3.McGoon MD, Benza RL, Escribano-Subias P, Jiang X, Miller DP, Peacock AJ, Pepke-Zaba J, Pulido T, Rich S, Rosenkranz S, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62:D51–D59. doi: 10.1016/j.jacc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62:D22–D33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 5.Vonk-Noordegraaf A, Westerhof N. Describing right ventricular function. Eur Respir J. 2013;41:1419–1423. doi: 10.1183/09031936.00160712. [DOI] [PubMed] [Google Scholar]
- 6.Rakusan K, Cicutti N, Kolar F. Effect of anemia on cardiac function, microvascular structure, and capillary hematocrit in rat hearts. Am J Physiol Heart Circ Physiol. 2001;280:H1407–H1414. doi: 10.1152/ajpheart.2001.280.3.H1407. [DOI] [PubMed] [Google Scholar]
- 7.Rakusan K, Flanagan MF, Geva T, Southern J, Van Praagh R. Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation. 1992;86:38–46. doi: 10.1161/01.cir.86.1.38. [DOI] [PubMed] [Google Scholar]
- 8.Sutendra G, Dromparis P, Paulin R, Zervopoulos S, Haromy A, Nagendran J, Michelakis ED. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med (Berl) 2013;91:1315–1327. doi: 10.1007/s00109-013-1059-4. [DOI] [PubMed] [Google Scholar]
- 9.Drake JI, Bogaard HJ, Mizuno S, Clifton B, Xie B, Gao Y, Dumur CI, Fawcett P, Voelkel NF, Natarajan R. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Respir Cell Mol Biol. 2011;45:1239–1247. doi: 10.1165/rcmb.2010-0412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogel-Claussen J, Skrok J, Shehata ML, Singh S, Sibley CT, Boyce DM, Lechtzin N, Girgis RE, Mathai SC, Goldstein TA, et al. Right and left ventricular myocardial perfusion reserves correlate with right ventricular function and pulmonary hemodynamics in patients with pulmonary arterial hypertension. Radiology. 2011;258:119–127. doi: 10.1148/radiol.10100725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taddei L, Chiarugi P, Brogelli L, Cirri P, Magnelli L, Raugei G, Ziche M, Granger HJ, Chiarugi V, Ramponi G. Inhibitory effect of full-length human endostatin on in vitro angiogenesis. Biochem Biophys Res Commun. 1999;263:340–345. doi: 10.1006/bbrc.1999.1342. [DOI] [PubMed] [Google Scholar]
- 12.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 13.Chen QR, Kumar D, Stass SA, Mixson AJ. Liposomes complexed to plasmids encoding angiostatin and endostatin inhibit breast cancer in nude mice. Cancer Res. 1999;59:3308–3312. [PubMed] [Google Scholar]
- 14.Mitsuma W, Kodama M, Hanawa H, Ito M, Ramadan MM, Hirono S, Obata H, Okada S, Sanada F, Yanagawa T, et al. Serum endostatin in the coronary circulation of patients with coronary heart disease and its relation to coronary collateral formation. Am J Cardiol. 2007;99:494–498. doi: 10.1016/j.amjcard.2006.09.095. [DOI] [PubMed] [Google Scholar]
- 15.Panchal VR, Rehman J, Nguyen AT, Brown JW, Turrentine MW, Mahomed Y, March KL. Reduced pericardial levels of endostatin correlate with collateral development in patients with ischemic heart disease. J Am Coll Cardiol. 2004;43:1383–1387. doi: 10.1016/j.jacc.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 16.Sodha NR, Clements RT, Boodhwani M, Xu SH, Laham RJ, Bianchi C, Sellke FW. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am J Physiol Heart Circ Physiol. 2009;296:H428–H434. doi: 10.1152/ajpheart.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gouya G, Siller-Matula JM, Fritzer-Szekeres M, Neuhold S, Storka A, Neuhofer LM, Clodi M, Hulsmann M, Pacher R, Wolzt M. Association of endostatin with mortality in patients with chronic heart failure. Eur J Clin Invest. 2014;44:125–135. doi: 10.1111/eci.12197. [DOI] [PubMed] [Google Scholar]
- 18.Mathai SC, Bueso M, Hummers LK, Boyce D, Lechtzin N, Le Pavec J, Campo A, Champion HC, Housten T, Forfia PR, et al. Disproportionate elevation of N-terminal pro-brain natriuretic peptide in scleroderma-related pulmonary hypertension. Eur Respir J. 2010;35:95–104. doi: 10.1183/09031936.00074309. [DOI] [PubMed] [Google Scholar]
- 19.Fijalkowska A, Kurzyna M, Torbicki A, Szewczyk G, Florczyk M, Pruszczyk P, Szturmowicz M. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest. 2006;129:1313–1321. doi: 10.1378/chest.129.5.1313. [DOI] [PubMed] [Google Scholar]
- 20.Souza R, Jardim C, Julio Cesar Fernandes C, Silveira Lapa M, Rabelo R, Humbert M. NT-proBNP as a tool to stratify disease severity in pulmonary arterial hypertension. Respir Med. 2007;101:69–75. doi: 10.1016/j.rmed.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Shah SJ, Thenappan T, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Association of serum creatinine with abnormal hemodynamics and mortality in pulmonary arterial hypertension. Circulation. 2008;117:2475–2483. doi: 10.1161/CIRCULATIONAHA.107.719500. [DOI] [PubMed] [Google Scholar]
- 22.Forfia PR, Mathai SC, Fisher MR, Housten-Harris T, Hemnes AR, Champion HC, Girgis RE, Hassoun PM. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:1364–1369. doi: 10.1164/rccm.200712-1876OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106:1477–1482. doi: 10.1161/01.cir.0000029100.82385.58. [DOI] [PubMed] [Google Scholar]
- 24.Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, Badesch DB, McGoon MD. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141:354–362. doi: 10.1378/chest.11-0676. [DOI] [PubMed] [Google Scholar]
- 25.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 26.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menzel O, Bekkeheien RC, Reymond A, Fukai N, Boye E, Kosztolanyi G, Aftimos S, Deutsch S, Scott HS, Olsen BR, et al. Knobloch syndrome: novel mutations in COL18A1, evidence for genetic heterogeneity, and a functionally impaired polymorphism in endostatin. Hum Mutat. 2004;23:77–84. doi: 10.1002/humu.10284. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser R, Seiler S, Held M, Bals R, Wilkens H. Prognostic impact of renal function in precapillary pulmonary hypertension. J Intern Med. 2014;275:116–126. doi: 10.1111/joim.12131. [DOI] [PubMed] [Google Scholar]
- 29.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009;135:794–804. doi: 10.1378/chest.08-0492. [DOI] [PubMed] [Google Scholar]
- 30.Folkman J. Endogenous inhibitors of angiogenesis. Harvey Lect. 1996-1997;92:65–82. [PubMed] [Google Scholar]
- 31.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–2754. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 32.Voelkel NF, Bogaard HJ, Al Husseini A, Farkas L, Gomez-Arroyo J, Natarajan R. Antioxidants for the treatment of patients with severe angioproliferative pulmonary hypertension? Antioxid Redox Signal. 2013;18:1810–1817. doi: 10.1089/ars.2012.4828. [DOI] [PubMed] [Google Scholar]
- 33.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 34.Duncan M, Wagner BD, Murray K, Allen J, Colvin K, Accurso FJ, Ivy DD. Circulating cytokines and growth factors in pediatric pulmonary hypertension. Mediators Inflamm. 2012;2012:143428. doi: 10.1155/2012/143428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pako J, Bikov A, Karlocai K, Csosza G, Kunos L, Losonczy G, Horvath I.Plasma VEGF levels and their relation to right ventricular function in pulmonary hypertension Clin Exp Hypertensonline ahead of print 27 Oct 2014; DOI: 10.3109/10641963.2014.972561 [DOI] [PubMed] [Google Scholar]
- 36.Du L, Sullivan CC, Chu D, Cho AJ, Kido M, Wolf PL, Yuan JX, Deutsch R, Jamieson SW, Thistlethwaite PA. Signaling molecules in nonfamilial pulmonary hypertension. N Engl J Med. 2003;348:500–509. doi: 10.1056/NEJMoa021650. [DOI] [PubMed] [Google Scholar]
- 37.Kümpers P, Nickel N, Lukasz A, Golpon H, Westerkamp V, Olsson KM, Jonigk D, Maegel L, Bockmeyer CL, David S, et al. Circulating angiopoietins in idiopathic pulmonary arterial hypertension. Eur Heart J. 2010;31:2291–2300. doi: 10.1093/eurheartj/ehq226. [DOI] [PubMed] [Google Scholar]
- 38.Pascaud MA, Griscelli F, Raoul W, Marcos E, Opolon P, Raffestin B, Perricaudet M, Adnot S, Eddahibi S. Lung overexpression of angiostatin aggravates pulmonary hypertension in chronically hypoxic mice. Am J Respir Cell Mol Biol. 2003;29:449–457. doi: 10.1165/rcmb.2002-0120OC. [DOI] [PubMed] [Google Scholar]
- 39.Jurasz P, Ng D, Granton JT, Courtman DW, Stewart DJ. Elevated platelet angiostatin and circulating endothelial microfragments in idiopathic pulmonary arterial hypertension: a preliminary study. Thromb Res. 2010;125:53–60. doi: 10.1016/j.thromres.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Rose ML, Strange G, King I, Arnup S, Vidmar S, O’Donnell C, Kermeen F, Grigg L, Weintraub RG, Celermajer DS. Congenital heart disease-associated pulmonary arterial hypertension: preliminary results from a novel registry. Intern Med J. 2012;42:874–879. doi: 10.1111/j.1445-5994.2011.02708.x. [DOI] [PubMed] [Google Scholar]
- 41.Cua CL, Blankenship A, North AL, Hayes J, Nelin LD. Increased incidence of idiopathic persistent pulmonary hypertension in Down syndrome neonates. Pediatr Cardiol. 2007;28:250–254. doi: 10.1007/s00246-006-0011-6. [DOI] [PubMed] [Google Scholar]
- 42.Van de Bruaene A, Delcroix M, Pasquet A, De Backer J, De Pauw M, Naeije R, Vachiéry JL, Paelinck B, Morissens M, Budts W. The Belgian Eisenmenger syndrome registry: implications for treatment strategies? Acta Cardiol. 2009;64:447–453. doi: 10.2143/AC.64.4.2041608. [DOI] [PubMed] [Google Scholar]
- 43.Zorick TS, Mustacchi Z, Bando SY, Zatz M, Moreira-Filho CA, Olsen B, Passos-Bueno MR. High serum endostatin levels in Down syndrome: implications for improved treatment and prevention of solid tumours. Eur J Hum Genet. 2001;9:811–814. doi: 10.1038/sj.ejhg.5200721. [DOI] [PubMed] [Google Scholar]
- 44.Csaba Galambos AM, Nguyen D, Seedorf G, Abman SH.Increased lung endostatin expression impairs perinatal lung development in Down syndromePresented at the Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting. May 3–6, 2014, Vancouver, Canada. Abstract 1455.8 [Google Scholar]
- 45.Cook GR, Knobloch WH. Autosomal recessive vitreoretinopathy and encephaloceles. Am J Ophthalmol. 1982;94:18–25. doi: 10.1016/0002-9394(82)90185-4. [DOI] [PubMed] [Google Scholar]
- 46.Sertié AL, Sossi V, Camargo AA, Zatz M, Brahe C, Passos-Bueno MR. Collagen XVIII, containing an endogenous inhibitor of angiogenesis and tumor growth, plays a critical role in the maintenance of retinal structure and in neural tube closure (Knobloch syndrome) Hum Mol Genet. 2000;9:2051–2058. doi: 10.1093/hmg/9.13.2051. [DOI] [PubMed] [Google Scholar]
- 47.Delaney CE, Weagant BT, Addison CL. The inhibitory effects of endostatin on endothelial cells are modulated by extracellular matrix. Exp Cell Res. 2006;312:2476–2489. doi: 10.1016/j.yexcr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Permuth-Wey J, Chen Z, Tsai YY, Lin HY, Chen YA, Barnholtz-Sloan J, Birrer MJ, Chanock SJ, Cramer DW, Cunningham JM, et al. Ovarian Cancer Association Consortium. MicroRNA processing and binding site polymorphisms are not replicated in the Ovarian Cancer Association Consortium. Cancer Epidemiol Biomarkers Prev. 2011;20:1793–1797. doi: 10.1158/1055-9965.EPI-11-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu X, Wu J, Xin Z, Wang H, Zhu X, Pan L, Li Z, Li H, Liu Y. A 3′ UTR SNP in COL18A1 is associated with susceptibility to HBV related hepatocellular carcinoma in Chinese: three independent case-control studies. PLoS ONE. 2012;7:e33855. doi: 10.1371/journal.pone.0033855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armelin-Correa LM, Lin CJ, Barbosa A, Bagatini K, Winnischofer SM, Sogayar MC, Passos-Bueno MR. Characterization of human collagen XVIII promoter 2: interaction of Sp1, Sp3 and YY1 with the regulatory region and a SNP that increases transcription in hepatocytes. Matrix Biol. 2005;24:550–559. doi: 10.1016/j.matbio.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Park JY, Amankwah EK, Anic GM, Lin HY, Walls B, Park H, Krebs K, Madden M, Maddox K, Marzban S, et al. Gene variants in angiogenesis and lymphangiogenesis and cutaneous melanoma progression. Cancer Epidemiol Biomarkers Prev. 2013;22:827–834. doi: 10.1158/1055-9965.EPI-12-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iughetti P, Suzuki O, Godoi PH, Alves VA, Sertié AL, Zorick T, Soares F, Camargo A, Moreira ES, di Loreto C, et al. A polymorphism in endostatin, an angiogenesis inhibitor, predisposes for the development of prostatic adenocarcinoma. Cancer Res. 2001;61:7375–7378. [PubMed] [Google Scholar]
- 53.Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, Ward K, Yacoub M, Mikhail G, Rogers P, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet. 2000;37:741–745. doi: 10.1136/jmg.37.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soubrier F, Chung WK, Machado R, Grünig E, Aldred M, Geraci M, Loyd JE, Elliott CG, Trembath RC, Newman JH, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:D13–D21. doi: 10.1016/j.jacc.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 55.Thomson J, Machado R, Pauciulo M, Morgan N, Yacoub M, Corris P, McNeil K, Loyd J, Nichols W, Trembath R. Familial and sporadic primary pulmonary hypertension is caused by BMPR2 gene mutations resulting in haploinsufficiency of the bone morphogenetic protein type II receptor. J Heart Lung Transplant. 2001;20:149. doi: 10.1016/s1053-2498(01)00259-5. [DOI] [PubMed] [Google Scholar]
- 56.Trembath RC, Thomson JR, Machado RD, Morgan NV, Atkinson C, Winship I, Simonneau G, Galie N, Loyd JE, Humbert M, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med. 2001;345:325–334. doi: 10.1056/NEJM200108023450503. [DOI] [PubMed] [Google Scholar]
- 57.Trembath RC. Mutations in the TGF-beta type 1 receptor, ALK1, in combined primary pulmonary hypertension and hereditary haemorrhagic telangiectasia, implies pathway specificity. J Heart Lung Transplant. 2001;20:175. doi: 10.1016/s1053-2498(00)00352-1. [DOI] [PubMed] [Google Scholar]
- 58.Chaouat A, Coulet F, Favre C, Simonneau G, Weitzenblum E, Soubrier F, Humbert M. Endoglin germline mutation in a patient with hereditary haemorrhagic telangiectasia and dexfenfluramine associated pulmonary arterial hypertension. Thorax. 2004;59:446–448. doi: 10.1136/thx.2003.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nasim MT, Ogo T, Ahmed M, Randall R, Chowdhury HM, Snape KM, Bradshaw TY, Southgate L, Lee GJ, Jackson I, et al. Molecular genetic characterization of SMAD signaling molecules in pulmonary arterial hypertension. Hum Mutat. 2011;32:1385–1389. doi: 10.1002/humu.21605. [DOI] [PubMed] [Google Scholar]
- 60.Shintani M, Yagi H, Nakayama T, Saji T, Matsuoka R. A new nonsense mutation of SMAD8 associated with pulmonary arterial hypertension. J Med Genet. 2009;46:331–337. doi: 10.1136/jmg.2008.062703. [DOI] [PubMed] [Google Scholar]
- 61.Best DH, Austin ED, Chung WK, Elliott CG. Genetics of pulmonary hypertension. Curr Opin Cardiol. 2014;29:520–527. doi: 10.1097/HCO.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 62.Ma L, Chung WK. The genetic basis of pulmonary arterial hypertension. Hum Genet. 2014;133:471–479. doi: 10.1007/s00439-014-1419-3. [DOI] [PubMed] [Google Scholar]
- 63.Austin ED, Ma L, LeDuc C, Berman Rosenzweig E, Borczuk A, Phillips JA, III, Palomero T, Sumazin P, Kim HR, Talati MH, et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet. 2012;5:336–343. doi: 10.1161/CIRCGENETICS.111.961888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, Germain M, Trégouët DA, Borczuk A, Rosenzweig EB, et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med. 2013;369:351–361. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Humbert M, Sitbon O, Yaïci A, Montani D, O’Callaghan DS, Jaïs X, Parent F, Savale L, Natali D, Günther S, et al. French Pulmonary Arterial Hypertension Network. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36:549–555. doi: 10.1183/09031936.00057010. [DOI] [PubMed] [Google Scholar]
- 66.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peljto AL, Zhang Y, Fingerlin TE, Ma SF, Garcia JG, Richards TJ, Silveira LJ, Lindell KO, Steele MP, Loyd JE, et al. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA. 2013;309:2232–2239. doi: 10.1001/jama.2013.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gu JW, Shparago M, Tan W, Bailey AP. Tissue endostatin correlates inversely with capillary network in rat heart and skeletal muscles. Angiogenesis. 2006;9:93–99. doi: 10.1007/s10456-006-9035-z. [DOI] [PubMed] [Google Scholar]