Abstract
Recent studies have shown that long-term antihypertensive action of soluble epoxide hydrolase inhibition (sEHi) in angiotensin II (ANG II)-dependent hypertension might be mediated by the suppression of intrarenal ANG II levels. To test this hypothesis, we examined the effects of acute (2 days) and chronic (14 days) sEH inhibition on blood pressure (BP) in transgenic rats with inducible ANG II-dependent hypertension. ANG II-dependent malignant hypertension was induced by 10 days’ dietary administration of indole-3-carbinol (I3C), a natural xenobiotic that activates the mouse renin gene in Cyp1a1-Ren-2 transgenic rats. BP was monitored by radiotelemetry. Acute and chronic sEH inhibition was achieved using cis-4-[4-(3-adamantan-1-yl-ureido)cyclohexyloxy]benzoic acid (c-AUCB), given at doses of 0.3, 3, 13, 26, 60 and 130 mg/L in drinking water. At the end of experiments renal concentrations of epoxyeicosatrienoic acids (EETs), their inactive metabolites dihydroxyeicosatrienoic acids (DHETEs), and ANG II were measured. Acute BP-lowering effects of sEH inhibition in I3C-induced rats was associated with a marked increase in renal EETs/DHETEs ratio and acute natriuresis. Chronic treatment with c-AUCB in I3C-induced rats elicited dose-dependent persistent BP-lowering associated with a significant reduction of plasma and kidney ANG II levels. Our findings show that acute BP-lowering effect of sEH inhibition in I3C-induced Cyp1a1-Ren-2 transgenic rats is mediated by a substantial increase in intrarenal EETs and their natriuretic action without altering intrarenal RAS activity. Long-term antihypertensive action of c-AUCB in I3C-induced Cyp1a1-Ren-2 transgenic rats is mediated mostly by suppression of intrarenal ANG II concentration.
Introduction
An increasing body of evidence indicates that cytochrome P-450 (CYP) dependent metabolites of arachidonic acid, including epoxyeicosatrienoic acids (EETs), play an important role in regulation of cardiovascular and renal functions 1–4. Numerous independent studies have shown that increasing bioavailability of EETs in tissues by preventing their degradation to the biologically inactive dihydroxyeicosatrienoic acids (DHETEs) employing soluble epoxide hydrolase (sEH) inhibition has antihypertensive effects 5–15. Based on these studies it has been proposed that the EETs-mediated antihypertensive actions are related to their immediate vasodilatory effects and direct influence on renal tubular transport of sodium 3,4,16.
We found recently that cis-4-[4-(3-adamantan-1-yl-ureido) cyclohexyloxy]benzoic acid (c-AUCB), an sEH inhibitor, induced a significant reduction in plasma ANG II levels and normalization of kidney ANG II concentration in Ren-2 transgenic rat model of angiotensin II (ANG II)-dependent hypertension (TGR) 17. Though, c-AUCB significantly increased the intrarenal availability of biologically active EETs in TGR, detailed analyses of the results of this study made us propose that the antihypertensive action of sEH inhibition in TGR is predominantly mediated by suppression of systemic and intrarenal renin-angiotensin system (RAS) 17. Since the dose of c-AUCB employed in this study was in the upper range of the dosage recommended (26 mg/l in drinking water) 18, a double of the dose we usually utilize 9–12,14,15, we thought it important to examine whether the effect of sEH inhibition on the RAS might be dose-dependent.
Of note, the most common model of ANG II-dependent hypertension is the one obtained by chronic infusion of subpressor doses of ANG II, which induces suppression of endogenous RAS 19–21. Another possibility is the two-kidney, one-clip (2K1C) Goldblatt hypertension, where BP elevation is the consequence of surgically induced unilateral renal artery stenosis 22. Thus, induction of ANG II-dependent hypertension in these models results either from application of exogenous ANG II leading to unphysiological suppression of RAS or from a surgical intervention that yields a variable degree of RAS activation 19–24. Moreover, the exact onset and the severity of hypertension in these models often vary and cannot be precisely controlled. To avoid these limitations, we used an inbred transgenic rat line [strain name: TGR(Cyp1a1-Ren-2)] that offers a unique possibility to precisely control the development of ANG II-dependent hypertension 25. This transgenic rat model allows assessment of the pathophysiological mechanisms of ANG II-dependent hypertension involving increased activity of endogenous RAS. Moreover, we and others 11,12,15,26–30 have demonstrated that the gene expression, the level of endogenously produced ANG II and, consequently the degree of hypertension can be precisely controlled.
Our aim was to find out if sEH inhibition would result in dose-dependent inhibition of the circulating and intrarenal RAS in hypertensive Cyp1a1-Ren-2 transgenic rats. Therefore we evaluated the effects of acute (2 days) and chronic (14 days) c-AUCB treatment on BP and ANG II, EETs and DHETEs concentrations as affected by doses ranging from 0.3 to the highest suggested dose of 130 mg/l of drinking water 18. Furthermore, to elucidate the mechanism(s) responsible for the effects of sEH inhibition on RAS activity, Ren-2 renin gene expression and activities/concentrations of individual components of the RAS were assessed in untreated and c-AUCB-treated Cyp1a1-Ren-2 transgenic rats; this was done under control conditions and after induction of the renin gene. Finally, our recent data 17 suggested that after high-dose c-AUCB treatment the pattern of changes was similar as that observed in ANG II-dependent hypertension treated with angiotensin-converting enzyme inhibitor (ACEi) 19,20,31,32. Therefore we also determined the effects of ACEi treatment in noninduced animals and in Cyp1a1-Ren-2 transgenic rats after induction of the renin gene on the profiles of hypertension and EETs and DHETEs concentrations and expression and activities/concentrations of individual components of the RAS. This was done to gain a deeper insight into possible role of interaction between CYP-dependent metabolites and the RAS in the antihypertensive potency of sEH inhibition.
Results
Series 1. Effects of c-AUCB and ACEi in noninduced or I3C-induced Cyp1a1-Ren-2 transgenic rats on arterial blood pressure, body weight, urinary sodium excretion, plasma c-AUCB concentrations and Ren-2 renin gene expression
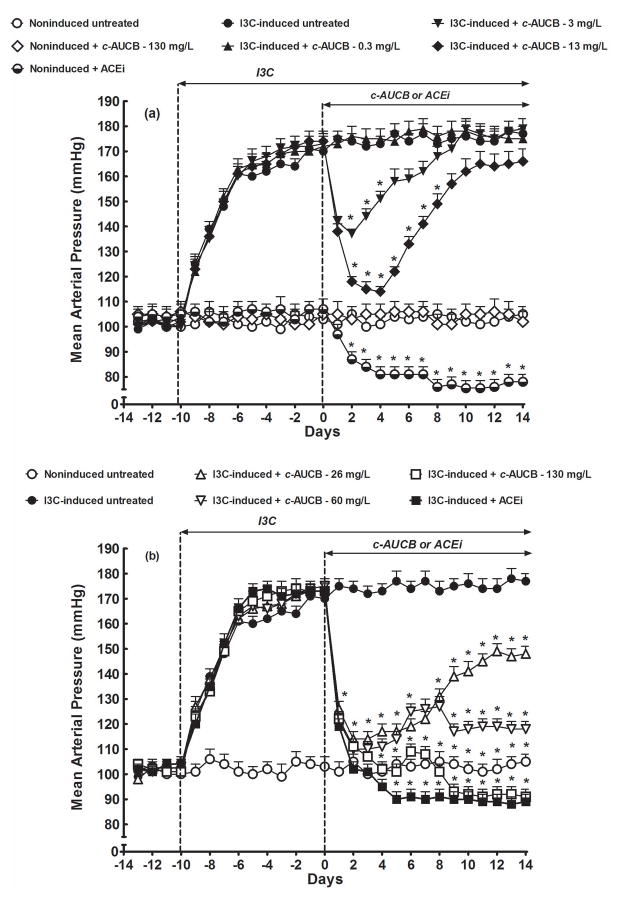
As shown in figure 1, basal values of mean arterial pressure (MAP) were not significantly different among experimental groups. In untreated animals, I3C-induction resulted in the rise of MAP from 102 ± 3 mmHg (day −10) to 171 ± 3 mmHg (day 0) (p<0.05). Noninduced rats remained normotensive throughout the experiment, and the treatment with the highest dose of c-AUCB (130 mg/L) did not have any significant effect on their MAP. However, the treatment with ACEi (trandolapril, 6 mg/L) resulted in a significant decrease in MAP as compared with untreated noninduced rats (78 ± 3 vs. 105 ± 3 mmHg, on day 14) (figure 1A). As also shown in figure 1A, treatment with 0.3 mg/L of c-AUCB did not affect the course of hypertension in I3C-induced rats as compared with untreated I3C-induced rats (175 ± 4 vs. 177 ± 3 mmHg measured on day 14). Treatment with 3 mg/L of c-AUCB significantly decreased MAP in I3C-induced rats, with maximal BP-lowering effect observed 2 days after initiation of c-AUCB administration (from 173 ± 4 to 137 ± 2 mmHg, p<0.05), but then, already on day 7, MAP returned to levels observed in I3C-induced untreated rats. Treatment with 13 mg/L of c-AUCB caused marked decreases in MAP with maximum BP-lowering effect on day 4 of treatment (from 174 ± 4 to 114 ± 2 mmHg, p<0.05), followed by gradual return to levels observed in untreated I3C-induced rats (figure 1A).
Figure 1.
Time-course of mean arterial pressure (measured by radiotelemetry) in indole-3-carbinol (I3C)-induced and noninduced Cyp1a1-Ren-2 transgenic rats, and effects of c-AUCB or ACEi treatment. *P<0.05 versus baseline values.
As shown in figure 1B, treatment with 26 mg/L c-AUCB elicited a profound decrease in MAP to normotensive levels (from 173 ± 4 to 113 ± 3 mmHg, p<0.05), then MAP slowly increased but at the end of experiments still remained significantly lower compared to untreated I3C-induced rats (148 ± 3 vs. 177 ± 3 mmHg, p<0.05). Treatment with 60 mg/L of c-AUCB had similar rapid BP-lowering effects; however, the MAP remained within normotensive range until the end of experiment (118 ± 3 mmHg on day 14). Treatment with 130 mg/L c-AUCB and administration of ACEi caused similar profound decreases in MAP to normotensive levels, and at the end of experiment it even dropped below those observed in untreated noninduced rats (91 ± 3 and 89 ± 3 vs. 105 ± 3 mmHg, p<0.05 in both cases) (figure 1B).
As shown in supplemental figure 1A, untreated and c-AUCB- and ACEi-treated noninduced rats showed a significant body weight (BW) gain throughout the experiment, with final increases by +57 ± 3, +56 ± 3 and +54 ±4 g, respectively (p<0.05 vs. initial body weight). In I3C-induced rats the development of hypertension was associated with profound loss of BW (from 320 ± 8 to 207 ± 7 g, p<0.05). In I3C-induced rats neither the treatment with the highest dose of c-AUCB (130 mg/L) nor that with ACEi fully prevented the BW losses (supplemental figure 1B).
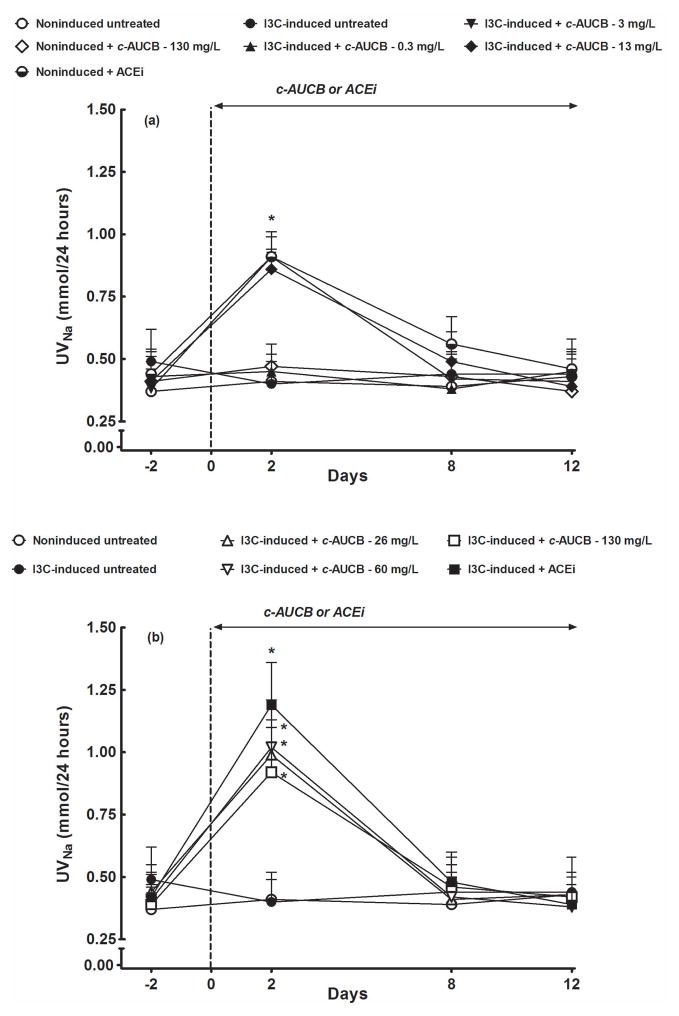
As shown in figure 2, the basal daily sodium excretion did not significantly differ among the groups and remained unaltered in untreated noninduced as well as I3C-induced rats. The treatment with 130 mg/L of c-AUCB in noninduced rats as well as the treatment with the lowest dose of c-AUCB (0.3 mg/L) in I3C-induced rats did not significantly alter daily sodium excretion (figure 2A). In contrast, the treatment with each dose of c-AUCB (from 3 mg/L to 130 mg/L) and ACEi elicited marked increases in daily sodium excretion in I3C-induced rats when measured on the second day of treatment (figures 2A and 2B). In addition, the treatment with ACEi caused a substantial increase in daily sodium excretion in noninduced rats on second day of treatment (figure 2A). However, already on day 8, sodium excretion returned to the levels observed in untreated noninduced rats and remained at those levels until the end of experiment.
Figure 2.
Effects of c-AUCB or ACEi treatment on daily sodium excretion (UNaV) in indole-3-carbinol (I3C)-induced and noninduced Cyp1a1-Ren-2 transgenic rats. *P<0.05 versus baseline values.
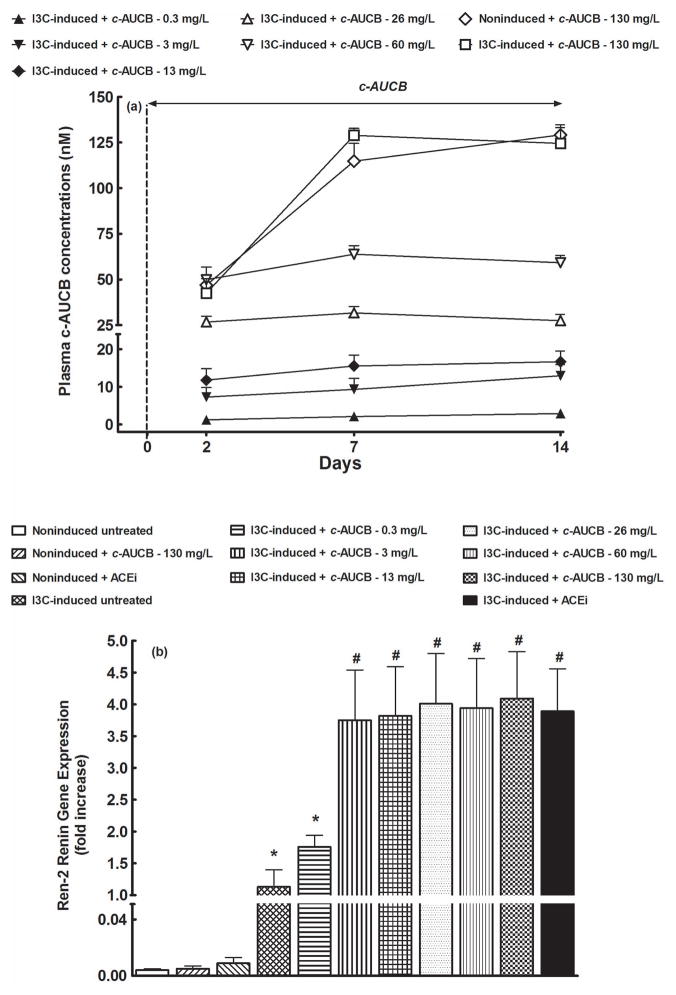
As shown in figure 3A, plasma concentrations of c-AUCB in groups that were not treated with this sEH inhibitor were at zero level whereas in c-AUCB-treated groups they increased in dose-dependent manner. Starting with the dose 3 mg/L, c-AUCB plasma concentration was already on the second day of treatment above the value of IC50 for the specific sEH inhibition. This concentration was observed to effectively inhibit sEH activity in vitro as well as in vivo 18. Of special interest is the finding that treatment with the highest dose of c-AUCB (130 mg/L) resulted in maximum plasma concentrations on day 7 of administration and the levels were about 20 times higher than the IC50 value required for the specific sEH inhibition 18.
Figure 3.
Plasma c-AUCB concentrations (A) and liver Ren-2 renin gene expression (B) in indole-3-carbinol (I3C)-induced and noninduced Cyp1a1-Ren-2 transgenic rats, and effects c-AUCB or ACEi. *P<0.05 versus unmarked values, # P<0.05 versus all the other values.
As shown in figure 3B, the liver expression of Ren-2 renin gene in the noninduced rats was virtually undetectable. Treatment with 130 mg/L of c-AUCB and ACEi did not change liver Ren-2 renin gene expression in noninduced rats. I3C administration resulted in marked liver Ren-2 renin gene expression and it was substantially potentiated by effective sEH and ACE inhibition.
Series 2. Effects of c-AUCB and ACEi in noninduced or I3C-induced Cyp1a1-Ren-2 transgenic rats on EETs, DHETEs, plasma and kidney tissue ANG II concentrations, plasma renin activity and plasma and kidney tissue ACE activity
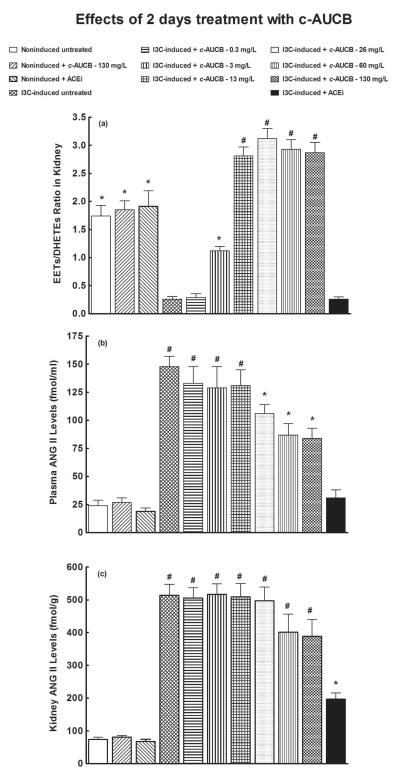
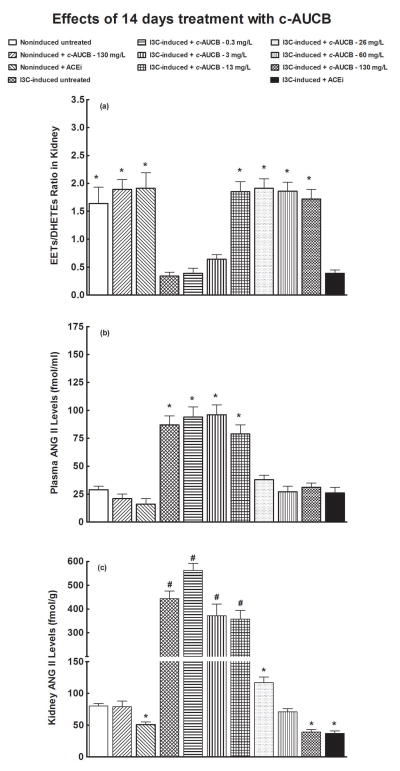
Figures 4A and 5A summarize the availability of biologically active epoxygenase products in kidney tissue expressed as the ratio of EETs to DHETEs. The ratios in untreated I3C-induced rats were significantly lower than in untreated noninduced rats. The treatment with 130 mg/L of c-AUCB did not significantly change the ratios in noninduced rats. As shown in figures 4A and 5A, acute (two days) as well as chronic (14 days) treatment with c-AUCB beginning with the dose of 3 mg/L caused significant increases in the EETs/DHETEs ratio. In addition, the increases in EETs/DHETEs ratios were similar in I3C-induced rats treated with each of the other doses of c-AUCB (from 13 mg/L to 130 mg/L). However, the increases in the EETs/DHETEs ratio were greater in acutely than in chronically treated I3C-induced rats for all doses c-AUCB. The treatment with ACEi did not significantly change the ratios of EETs/DHETEs in I3C-induced rats (figures 4A and 5A).
Figure 4.
Effects of acute (2 days) treatment with c-AUCB or ACEi on kidney tissue EETs/DHETEs ratio (A), plasma ANG II levels (B) and kidney ANG II levels (C) in indole-3-carbinol (I3C)-induced and noninduced Cyp1a1-Ren-2 transgenic rats. *P<0.05 versus unmarked values, # P<0.05 versus all the other values.
Figure 5.
Effects of chronic (14 days) treatment with c-AUCB or ACEi on the kidney tissue EETs/DHETEs ratio (A), plasma ANG II levels (B) and kidney ANG II levels (C) in indole-3-carbinol (I3C)-induced and noninduced Cyp1a1-Ren-2 transgenic rats. *P<0.05 versus unmarked values, #P<0.05 versus all the other values.
As shown in figures 4B and 5B, plasma ANG II levels were significantly higher in untreated I3C-induced rats than in untreated noninduced rats. Neither acute (two days) nor chronic (14 days) treatment with 130 mg/L of c-AUCB significantly changed plasma ANG II levels in noninduced rats. Figure 4B shows that acute treatment with 0.3, 3 or 13 mg/L of c-AUCB did not alter plasma ANG II levels in I3C-induced as compared with untreated I3C-induced rats. In addition, acute administration of 26, 60 and 130 mg/L of c-AUCB in I3C-induced rats resulted in significant decreases in plasma ANG II levels as compared with untreated I3C-induced rats (106 ± 6, 77 ± 9 and 74 ± 8 vs. 148 ± 9 fmol/ml, different at p<0.05 in each case), but they remained significantly higher than observed in untreated noninduced rats. Acute treatment with ACEi normalized plasma ANG II levels to values observed in untreated noninduced rats (31 ± 3 vs. 24 ± 5 fmol/ml). Acute treatment with ACEi did not alter plasma ANG II levels in noninduced rats (figure 4B).
Figure 5B shows that, similarly as with acute treatment, chronic administration of 0.3, 3 and 13 mg/L of c-AUCB did not significantly change plasma ANG II levels in I3C-induced rats. In contrast to acute treatment, chronic administration of 26, 60 and 130 mg/L of c-AUCB decreased plasma ANG II levels to values observed in untreated noninduced rats. As with acute treatment, chronic treatment with ACEi in I3C-induced rats also decreased plasma ANG II levels to values observed in untreated noninduced rats. Plasma ANG II levels in I3C-induced rats decapitated on day 2 were higher compared to the values on day 14; in untreated I3C-induced rats the respective values were 148 ± 9 and 87 ± 8 fmol/ml (different at p<0.05). Chronic treatment with ACEi did not significantly change plasma ANG II levels in noninduced rats (figure 5B).
As shown in figures 4C and 5C, kidney ANG II concentrations were substantially higher in untreated I3C-induced than in untreated noninduced rats. Acute treatment with any dose of c-AUCB did not affect kidney ANG II concentrations in I3C-induced rats. On the other hand, acute treatment with ACEi significantly decreased kidney ANG II concentrations as compared with untreated I3C-induced rats (197 ± 19 vs. 514 ± 34 fmol/g of tissue, p<0.05), but they still remained significantly higher compared to values observed in noninduced rats (197 ± 19 vs. 74 ± 7 fmol/g of tissue, p<0.05). Acute treatment with ACEi did not alter kidney ANG II concentrations in noninduced rats (figure 4C).
In contrast to the results of acute treatment, chronic treatment with 26 mg/L c-AUCB (figure 5C) markedly decreased kidney ANG II concentrations in I3C-induced rats compared to untreated I3C-induced rats (117 ± 9 vs. 443 ± 32 fmol/g of tissue, p<0.05). Chronic application of 60 mg/L of c-AUCB in I3C-induced rats decreased kidney ANG II concentrations to values observed in noninduced rats. Moreover, the treatment with 130 mg/L c-AUCB and ACEi elicited significant decreases in kidney ANG II concentrations, even below the values observed in untreated noninduced rats (39 ± 4 and 37 ± 4 vs. 80 ± 4 fmol/g of tissue, p<0.05 in each case). Finally, chronic treatment with ACEi resulted in a significant decrease in kidney ANG II concentrations in noninduced rats (figure 5C).
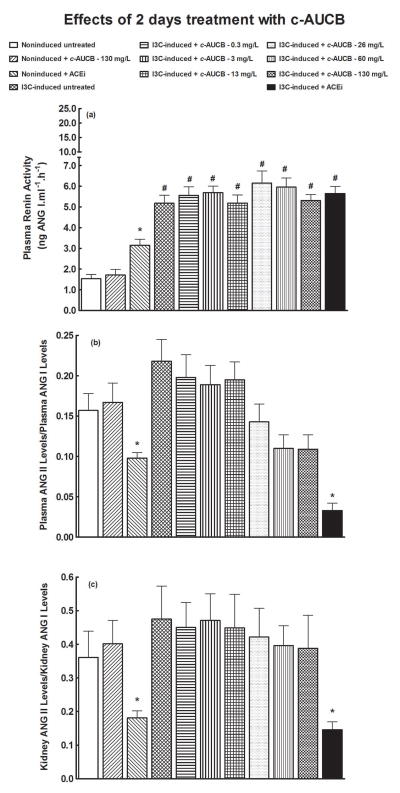
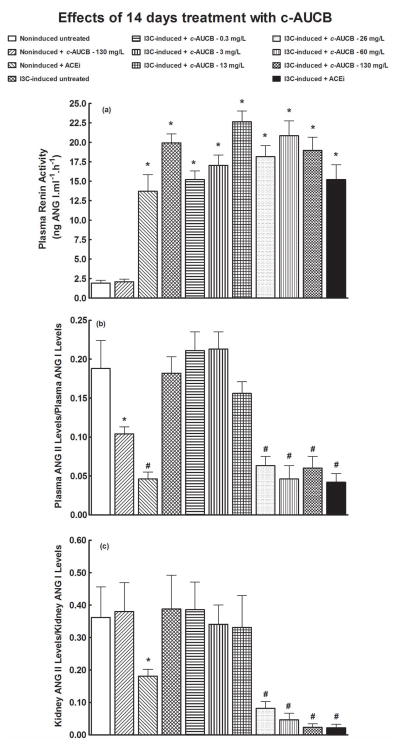
As shown in figures 6A and 7A, plasma renin activities were markedly higher in untreated I3C-induced rats than in untreated noninduced rats. As expected, plasma renin activity in untreated I3C-induced rats from the chronic treatment protocol (i.e. 14 days) was noticeably higher as compared with untreated I3C-induced rats from the acute treatment protocol (2 days) Neither acute nor chronic treatment with c-AUCB significantly changed plasma renin activities in noninduced or I3C-induced rats. In contrast, acute as well as chronic treatment with ACEi elicited significant increases in plasma renin activities in noninduced rats. Remarkably, chronic treatment with ACEi increased plasma renin activity in noninduced rats to values that were observed in untreated I3C-induced rats. (figure 7A).
Figure 6.
Effects of acute (2 days) treatment with c-AUCB or ACEi on plasma renin activities (A), ratios of plasma ANG II to ANG I levels (B), ratios of kidney ANG II to ANG I levels (C) in indole-3-carbinol (I3C)-induced and noninduced Cyp1a1-Ren-2 transgenic rats. *P<0.05 versus unmarked values, # P<0.05 versus all the other values.
Figure 7.
Effects of chronic (14 days) treatment with c-AUCB or ACEi on on plasma renin activities (A), ratios of plasma ANG II to ANG I levels (B), ratios of kidney ANG II to ANG I levels (C) in indole-3-carbinol (I3C)-induced and noninduced Cyp1a1-Ren-2 transgenic rats. *P<0.05 versus unmarked values, # P<0.05 versus all the other values.
As shown in figures 6B, 6C and 7B, there were no significant differences in plasma and kidney ACE activities estimated as the ratio of ANG II-to-ANG I among noninduced and I3C-induced rats, and acute treatment with any dose of c-AUCB did not significantly alter them (figures 6B and 6C). In contrast, acute treatment with ACEi caused significant decreases in plasma and kidney ACE activities in noninduced as well as in I3C-induced rats (figures 6B and 6C).
Figures 7B and 7C show that chronic treatment with 0.3, 3 and 13 mg/L of c-AUCB did not change plasma and kidney ACE activities in I3C-induced rats. In contrast, chronic treatment with 26, 60 and 130 mg/L of c-AUCB profoundly decreased plasma and kidney ACE activities in I3C-induced rats, bringing them close to levels observed in I3C-induced rats exposed to chronic ACEi treatment. Similar results regarding plasma and kidney ACE activities were obtained by direct measurement of ACE activity
Discussion
The first major finding of this study is that acute (two days’ treatment) BP-lowering effect of sEH inhibition in our model of ANGII-dependent hypertension was associated with a substantial increase in renal bioavailability of EETs (increased EETs/DHETEs) and natriuresis, while intrarenal ANG II concentration remained unaltered. This is in accordance with the vast evidence indicating anti-hypertensive properties of EETs are largely related to their natriuretic potency 1–4,9–16. Indeed, EETs have been shown to inhibit sodium reabsorption in the proximal tubule by blocking the sodium-hydrogen exchanger 33 and in the cortical collecting duct by blocking the epithelial sodium channels 34. In agreement with our previous studies 11,12,15 the I3C-induced Cyp1a1-Ren-2 transgenic rats exhibited reduced availability of biologically active epoxygenase metabolites. The data accord also with our recent finding that chronic inhibition of sEH normalized intrarenal EETs bioavailability and improved the pressure-natriuresis relationship in 2K1C Goldblatt hypertensive and I3C-induced Cyp1a1-Ren-2 transgenic rats 9,11,12 which further supports the concept that net intrarenal deficiency of EETs contributes to the impairment of the pressure-natriuresis mechanism 12,35. In accordance with the concept first proposed by Guyton et al. 36 and validated by several other groups 33–40, the impairment of this mechanism is the crucial factor responsible for the development and maintenance of hypertension.
Acute administration of c-AUCB at the dose 3 mg/L significantly increased intrarenal availability of biologically active EETs and other epoxy fatty acids, and beginning from doses of 13 mg/L even the intrarenal EETs in I3C-induced Cyp1a1-Ren-2 transgenic rats increased to values significantly higher than those in noninduced rats. Of note, acute administration of c-AUCB at doses below 13 mg/L did not alter plasma ANG II levels in I3C-induced Cyp1a1-Ren-2 transgenic rats while beginning from the dose of 26 mg/L these levels were reduced but still remained distinctly higher than in noninduced rats. Furthermore, acute administration of c-AUCB at any dose did not significantly change kidney ANG II in our hypertensive rats. Taken together, these findings strongly suggest that acute sEH inhibition does not alter intrarenal RAS activity whereas acute ACEi treatment results in plasma ANG II normalization and in striking suppression of kidney ANG II concentrations. Therefore, based on our earlier 9–12,14 and present findings, we propose that the mechanism underlying BP-lowering in response to acute sEH inhibition is related to markedly enhanced intrarenal availability of EETs and their direct inhibitory influence on renal tubular sodium transport and is not crucially dependent on the alterations in RAS activity.
The second critically important finding of the present study is that sustained antihypertensive action of chronic (14 days’ treatment) sEH inhibition with c-AUCB is dose-dependent; it is associated both with normalization of intrarenal EETs and significant reduction of plasma and kidney ANG II levels. This is in agreement with of our recent findings that high-dosage of c-AUCB (26 mg/L) led to a significant reduction in plasma ANG II and normalization of kidney ANG II concentrations in TGR, another rat model of ANG II-dependent hypertension, whereas low-dose treatment (3 mg/L), which still efficiently blocked sEH, did not alter circulating and tissue RAS 17,41. The c-AUCB induced BP-lowering persisted throughout the two-week period of treatment. Two features of this response were very remarkable: first, the BP-lowering effect of c-AUCB was dose-dependent. Secondly, the degree of concurrent suppression of ANG II concentrations was also dose-dependent and parallel to the BP change. In contrast, in I3C-induced Cyp1a1-Ren-2 transgenic rats chronic c-AUCB treatment did not alter intrarenal EETs in a dose-dependent manner: the maximal effect was already observed at the dose of 13 mg/L. Admittedly, our assays of plasma c-AUCB concentration revealed that each dose was highly above the range of IC50 for the specific sEH inhibition 18. Moreover, although chronic c-AUCB treatment increased intrarenal EETs in our hypertensive rats to values observed in noninduced rats, the effect of chronic treatment on the ratio of EETs/DHETEs was significantly smaller compared to that of acute c-AUCB treatment. Taken together, these data strongly suggest that sustained antihypertensive actions of sEH inhibition with c-AUCB were specifically mediated by suppression of circulating and, especially, intrarenal ANG II levels rather than by normalization of the intrarenal availability of biologically active EETs. This conclusion fits well the recent data demonstrating the crucial importance of an enhanced intrarenal ANG II in the pathophysiology of ANG II-dependent hypertension 20,27,31,32.
What are the mechanisms responsible for the effects of chronic sEH inhibition by c-AUCB on plasma and kidney ANG II levels in I3C-induced Cyp1a1-Ren-2 transgenic rats?
We found that chronic c-AUCB treatment caused dose-dependent suppression of plasma as well as kidney ANG II concentrations. It will be recalled that inhibition of RAS activity by pharmacological blockade of ANG II type 1 receptor (AT1) leads to a marked elevation of circulating ANG II levels. This is the consequence of the interruption of the short-loop negative feedback wherein AT1 activation suppresses renin secretion and decreases plasma ANG II levels 42,43 It is, therefore, expected that the blockade of the RAS activity by chronic c-AUCB treatment must be either at the renin or at the ACE level. In this context, a further important finding of the present study is that chronic sEH inhibition by c-AUCB did not suppress the expression of the Ren-2 renin gene in I3C-induced Cyp1a1-Ren-2 transgenic rats. In contrast, these animals exhibited a marked increase in the expression of Ren-2 renin gene, suggesting that I3C-induced Cyp1a1-Ren-2 transgenic rats responded to suppression of ANG II concentrations by a compensatory rise in renin gene expression. This is consistent with the concept of disruption of the short-loop negative effect of ANG II on the renin gene expression and secretion 42,43. In addition, our present results clearly show that chronic sEH inhibition by c-AUCB did enhance plasma renin activity in I3C-induced Cyp1a1-Ren-2 transgenic rats. These findings strongly suggest that the blockade of the RAS activity by chronic c-AUCB treatment did not occur at the renin level.
In this regard, of special interest is our finding that I3C-induced Cyp1a1-Ren-2 transgenic rats treated chronically with ACEi or with high doses of c-AUCB (either treatment leading to sustained normalization of BP) affected ANG II concentrations and plasma and kidney ACE activity in the same way. This suggest strongly that c-AUCB, a new orally active sEH inhibitor, blocks the activity of the RAS at the ACE level, an unexpected finding in a study that was not designed to explore the ACEi-like action of c-AUCB. In addition, the limitation of our present study is that it was not designed to delineate the strict correlation between decreases in BP and BW in response to dose-dependent treatment and changes in intrarenal ANG II concentrations in I3C-induced Cyp1a1-Ren-2 transgenic rats. It is clear that additional studies are required to address this issue more precisely.
Furthermore, the evidence that chronic treatment with ACEi and with the highest dose of c-AUCB (130 mg/L) decreased in kidney ANG II concentrations in I3C-induced Cyp1a1-Ren-2 transgenic rats even below the levels observed in noninduced rats further emphasizes the critical role of the intrarenal ANG II content in the pathophysiology of hypertension in this model. The present results corroborate those of the previous studies showing that suppression of intrarenal RAS activity is the crucial mechanism underlying effective long-term antihypertensive treatment in ANG II-dependent models of hypertension 20,22,31,32.
With regards to BW lost in I3C-induced Cyp1a1-Ren-2 transgenic rats, previous studies have revealed that malignant hypertension in this model is characterized by augmented activity of the RAS, rapid increase in BP and an intensive pressure diuresis associated with profound loss of BW, identically as in our present study 25–29. In our present study, the decrease in BW in untreated I3C-induced Cyp1a1-Ren-2 transgenic rats was not simply the result of reduced food intake (and consequently loss in muscle weight etc.), because our data from monitoring food intake show that these animals reduced food intake only about 7 %, which was not significantly different from food intake of either noninduced rats or I3C-induced and pharmacologically treated rats. Taken together, our present data rather indicate that the loss in BW is consequence of the accentuated pressure diuresis accompanying the development of malignant hypertension. However, it is obvious that future studies should address the mechanism(s) responsible for the BW loss after induction of renin gene in Cyp1a1-Ren-2 transgenic rats more complexly.
In conclusion, our findings indicate that acute BP-lowering effects of sEH inhibition in I3C-induced Cyp1a1-Ren-2 transgenic rats are mediated by a substantial increase of intrarenal biologically active epoxygenase products and their natriuretic action. In contrast, the long-term antihypertensive actions of sEH inhibition in this rat model are chiefly due to a suppression of intrarenal angiotensin II concentration, which is mediated by the ACEi-like action of c-AUCB.
Methods
The studies were performed in accordance with guidelines and practices established by, Committee for Animal Care and Use at the Institute of Clinical and Experimental Medicine (IKEM, Prague, Czech Republic, protocol #26-2010 issued by Ministry of Health of Czech Republic)
Animals and diets
Experiments were performed in male Cyp1a1-Ren-2 transgenic rats generated by inserting the mouse Ren-2 renin gene, fused to the cytochrome P-450 (Cyp1a1) promoter, into the genome of the Fischer 344 rat. The Cyp1a1 promoter is not constitutively expressed in the liver; however, after exposure to various natural xenobiotics, such as indole-3-carbinol (I3C) which can be easily administrated in the diet, the expression of the Cyp1a1 promoter is rapidly enhanced and results in a marked increase of the expression of the Ren-2 renin gene in the liver. Enhanced expression of the Ren-2 renin gene increases ANG II levels 25–30. All animals used in the present study were bred at the Center for Experimental Medicine of the Institute for Clinical and Experimental Medicine from stock animals supplied from the Center for Cardiovascular Science, University of Edinburgh, UK (we acknowledge the generous gift from Professor Mullins). All diets used in the present study were produced by Albert Weber - Services to Experimental Medicine (SEMED Ltd., Prague 4, Czech Republic). Rats were fed either a rat chow without I3C (noninduced groups) or a rat chow containing 0.3 % I3C (I3C-induced groups). It has been shown that I3C is a dietary supplement that does not exhibit any harmful biological effects in transgene-negative rats but causes a very strong induction of Cyp1a1 through activation of the aryl hydrocarbon receptor, which is a basic helix-loop-helix-transcription factor that binds to the Cyp1a1 promoter 25.
Chemicals
Cis-4-[4-(3-adamantan-1-yl-ureido)cyclohexyloxy]benzoic acid (c-AUCB), an sEH inhibitor, was prepared freshly and given in drinking water as described previously 9–12. The following doses of c-AUCB per 1 L of water were examined: 0.3 mg, 3 mg, 13 mg, 26 mg, 60 mg and 130 mg. Trandolapril (Gopten, Abbot, Prague, Czech Republic), an ACEi, was used at a dose of 6 mg/L drinking water. Our recent studies have demonstrated that this high dose completely blocks the development of ANG II-dependent form of hypertension 41,44,45.
Series 1. Measurements of BP (radiotelemetry), urine collections in conscious animals, and measurement of plasma c-AUCB levels and Ren-2 renin gene expression
In accordance with the recommendation for BP measurement in experimental animals, we employed a radiotelemetry system for direct BP measurements 46. Rats were anaesthetized with a combination of tiletamine, zolazepam (Zoletil, Virbac SA, Carros Cedex, France; 8 mg/kg), and xylazine (Rometar, Spofa, Czech Republic; 4 mg/kg) intramuscularly, and TA11PA-C40 radiotelemetric probes (Data Sciences International, St. Paul, MN, USA) were implanted for direct BP measurements as described previously 9,11,12,15. Rats were allowed 10 days to recover before basal BP was recorded. Basal BP was determined for 4 days and then induction of renin gene was performed until the end of the experiment that lasted 24 days.
Our previous studies have shown that after 10 days of induction a stable, severe hypertension develops 11,12,26,27. Then, the treatment with either c-AUCB or ACEi was conducted for 14 days. Untreated conscious noninduced and I3C-induced rats and noninduced rats treated with ACEi served as controls. The animals were housed individually in metabolic cages, BP was continuously recorded, and urine was collected for 24 hours into sterile tubes. 24-hour urine samples were collected prior to the start of pharmacological treatment (day −2) and then on days 2, 8 and 12 of treatment. Urine volumes were determined for each urine collection and the samples were centrifuged (3,000 rpm/5 min; 4°C) and preserved for analysis. Urinary concentration of sodium was assessed by flame photometry. The whole above procedure was recently described in detail 9,11,41,44,45. At the end of experiments, all rats were decapitated to collect blood for measurement of plasma c-AUCB as described previously 10,11 and the livers were removed for analysis of Ren-2 renin gene expression by technique described in detail previously 47. Briefly, total RNA was extracted from liver tissue using Trizol (Invitrogen) according to the manufacturer’s directions. Total RNA treated with DNase I (Fermentas, Thermoscientific, Waltham, MA, USA) was reverse transcribed and amplified using One Step SYBR® PrimeScript™ RT-PCR Kit II (TAKARA BIO INC, Shiga, Japan) following the manufacturer’s recommendations, with total volume of 20 μl. All samples were analyzed in triplicates. The primer sequences for renin-2 were: forward: 5′-GCCTCAGCAAGACTGATTCC-3′, reverse: 5′-ATATTCATGTAGTCTCTTCTCC-3′ and for β-actin: forward: 5′-TGACTGACTACCTCATGAAGA-3′ and reverse: 5′-CACGTCACACTTCATGATG-3′. PCR amplifications were performed using the ABI Prism 7300 sequence detection system (Applied Biosystems) following the reaction parameters recommended by the manufacturer, using 2 mg RNA per sample. β-actin was used as an endogenous control gene and negative controls contained water instead of cDNA. In all experiments, relative gene expression was calculated by the Δ cycle threshold (Ct) method. Briefly, the resultant mRNA was normalized to a calibrator; in each case, the calibrator chosen was the basal sample. Final results were expressed as the n-fold difference in gene expression relative to β-actin mRNA and calibrator as follows: n-fold = 2 − (ΔCt sample/ΔCt basal), where ΔCt values of the sample and calibrator were determined by subtracting the average Ct value of the transcript under investigation from the average Ct value of the β-actin mRNA gene for each sample.
Only animals with stable BP records during the basal period were used; they were randomly divided into the following experimental groups:
Group 1: Noninduced + untreated (n = 6)
Group 2: Noninduced + c-AUCB (130 mg/L, n = 7)
Group 3: Noninduced + ACEi (n = 7)
Group 3: I3C-induced + untreated (n = 7)
Group 4: I3C-induced + c-AUCB (0.3 mg/L, n = 7)
Group 5: I3C-induced + c-AUCB (3 mg/L, n = 7)
Group 6: I3C-induced + c-AUCB (13 mg/L, n = 8)
Group 7: I3C-induced + c-AUCB (26 mg/L, n = 8)
Group 8: I3C-induced + c-AUCB (60 mg/L, n = 7)
Group 9: I3C-induced + c-AUCB (130 mg/L, n = 8)
Group 10: I3C-induced + ACEi (n = 7)
Series 2. Effects of c-AUCB and ACEi in noninduced or I3C-induced Cyp1a1-Ren-2 transgenic rats on EETs, DHETEs, plasma and kidney tissue ANG II concentrations, plasma renin activity and plasma and kidney tissue ACE activity
It is now generally recognized that plasma and tissue ANG II concentrations in anaesthetized animals are higher than those obtained from rats decapitated while conscious and that normotensive animals exhibit a greater increase in renin secretion in response to anaesthesia and surgery than ANG II-induced hypertensive intrarenal renin-depleted animals 9–12,14,15,19,20,22,31. Therefore, in this study we determined ANG II levels in separate groups of conscious rats (as described in series 1) that were decapitated either on day 2 (to evaluate acute effects) or on day 14 (to evaluate chronic effects) after introduction of pharmacological treatment (n = 8 in each group). Plasma and whole-kidney ANG I and ANG II concentrations were assessed by radioimmunoassay as described in detail in our previous studies 26,27. In this study for measurements of plasma renin activities, the commercially available RIA kit was used (Cisbio Bioassays, Paris, France) and for measurements of plasma and kidney ACE activities the commercially available RIA kit was also employed (Bühlmann Laboratories, Allschwil, Switzerland). In this protocol, 1 unit ACE activity was defined as the amount of enzyme required to release 1 μmol of hippuric acid per minute per liter of sample at 37° C. In addition, in this study ACE activities were also estimated as the ratio of ANG II to ANG I, because we have recently found, in accordance with one original study in ANG II-infused hypertensive rats, that in this ANG II-dependent model of hypertension the ratio is a very reliable index for assessment of circulating and especially tissue ACE activity 19,26,27. The level of the arachidonic acid metabolites, EETs and DHETEs, were measured in the kidney cortex. Samples were extracted, separated by reverse-phase, high performance liquid chromatography, and analysed by negative-mode electrospray ionization and tandem mass spectroscopy as described previously 9–12. The procedure of blood sampling and the assays of individual components of RAS and EETs and DHETEs assays are routinely employed in our laboratory and this standardized approach allows us to compare the results with those of our previous studies evaluating the role of the RAS and CYP-dependent metabolites in the pathophysiology of ANG II-dependent form of hypertension 9–12,14,15,17,26,27.
Statistical Analysis
All values are expressed as means ± SEM. Graph-Pad Prism software (Graph Pad Software, San Diego, CA, USA) was used and, when appropriate, statistical analysis was performed using one-way analysis of variance (ANOVA). ANOVA for repeated measurements, followed by Student-Newman-Keuls test was performed for the analysis within groups (e.g. before and after either I3C or pharmacological treatment). Values exceeding the 95% probability limits (p<0.05) were considered statistically significant.
Supplementary Material
Acknowledgments
This study was principally supported by grant No. NT/12171-5 awarded by the Internal Grant Agency of the Ministry of Health to Z.H. A.S. was supported by a Marie Curie Fellowship from the European Commission Program PEOPLE (IRG 247847). Institute for Clinical and Experimental Medicine (IKEM) is recipient of the project of the Ministry of Health of the Czech Republic for the development of research organization 00023001 (institutional support). S.J. is supported the Grant Agency of Charles University No. 266213. The Center for Experimental Medicine (IKEM) received financial support from the European Commission within the Operational Program Prague–Competitiveness; project “Rozvoj infraskruktury PEM” (#CZ.2.16/3.1.00/28025). J.D.I. was supported by NIH grants HL59699 and DK38226. S.H.H. was supported by a fellowship from the NIEHS Supported Basic Research Program. Partial support was provided by NIEHS Grant R01 ES02710, R01 ES013933 and P42 ES013933 and by West Coast Center U24 DK097154 awarded to B.D.H. B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Foundation.
Footnotes
DISCLOSURE
B.D.H. was founded by EicOsis to develop soluble epoxide hydrolase inhibitors for treating inflammatory and neuropathic pain. The other authors declare no potential conflict of interest.
References
- 1.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent response. Pfugers Arch. 2010;459:881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 3.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92:101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capdevilla J, Wang W. Role of cytochrome P450 epoxygenase in regulating renal membrane transport and hypertension. Curr Opin Nephrol Hypertens. 2013;22:163–169. doi: 10.1097/MNH.0b013e32835d911e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Carroll MA, Chander PN, Falck JR, Sangras B, Stier CT. Soluble epoxide hydrolase inhibitor, AUDA, prevents early salt-sensitive hypertension. Front Biosci. 2008;13:3480–3487. doi: 10.2741/2942. [DOI] [PubMed] [Google Scholar]
- 6.Huang H, Morisseau C, Wang JF, et al. Increasing or stabilizing renal epoxyeicosatrienoic acid production attenuates abnormal renal function and hypertension in obese rats. Am J Physiol. 2007;293:F342–F349. doi: 10.1152/ajprenal.00004.2007. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Quilley J, Doumad AB, et al. Increases in plasma trans-EETs and blood pressure reduction in spontaneously hypertensive rats. Am J Physiol. 2011;300:H1990–H1996. doi: 10.1152/ajpheart.01267.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kompanowska-Jezierska E, Kuczeriszka M. Cytochrome P-450 metabolites in renal circulation and excretion - interaction with the nitric oxide (NO) system. J Physiol Pharmacol. 2008;59(Suppl 9):137–149. [PubMed] [Google Scholar]
- 9.Sporkova A, Kopkan L, Varcabová A, et al. Role of cytochrome P450 metabolites in the regulation of renal function and blood pressure in 2-kidney, 1-clip hypertensive rats. Am J Physiol. 2011;300:R1468–R1475. doi: 10.1152/ajpregu.00215.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neckář J, Kopkan L, Husková Z, et al. Inhibition of soluble epoxide hydrolase by cis-4-[4-(3-adamantan-I-ylureido)cyclohexyl-oxy]benzoic acid exhibits antihypertensive and cardioprotective actions in transgenic rats with angiotensin II-dependent hypertension. Clin Sci. 2012;122:513–525. doi: 10.1042/CS20110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honetschlägerová Z, Husková Z, Vaňourková Z, et al. Renal mechanisms contributing to the antihypertensive action of soluble epoxide hydrolase inhibition in Ren-2 transgenic rats with inducible hypertension. J Physiol. 2011;589:207–219. doi: 10.1113/jphysiol.2010.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honetschlägerová Z, Sporková A, Kopkan L, et al. Inhibition of soluble epoxide hydrolase improves the impaired pressure-natriuresis relationship and attenuates the development of hypertension and hypertension-associated end-organ damage in Cyp1a1-Ren-2 transgenic rats. J Hypertens. 2011;29:1590–1601. doi: 10.1097/HJH.0b013e328349062f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CR, Imig JD, Edin ML, Foley J, et al. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010;24:3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopkan L, Husková Z, Sporková A, et al. Soluble epoxide hydrolase inhibition exhibits antihypertensive actions independently of nitric oxide in mice with renovascular hypertension. Kidney Blood Press Res. 2012;35:595–607. doi: 10.1159/000339883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honetschlägerová Z, Kitada K, Husková Z, et al. Antihypertensive and renoprotective actions of soluble epoxide hydrolase inhibition in ANG II-dependent malignant hypertension are abolished by pretreatment with L-NAME. J Hypertens. 2013;31:321–332. doi: 10.1097/HJH.0b013e32835b50aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmarakby AA. Reno-protective mechanisms of epoxyeicosatrienoic acids in cardiovascular disease. Am J Physiol. 2012;302:R321–R330. doi: 10.1152/ajpregu.00606.2011. [DOI] [PubMed] [Google Scholar]
- 17.Varcabová Š, Husková Z, Kramer HJ, et al. Antihypertensive action of soluble epoxide hydrolase inhibition in Ren-2 transgenic rats is mediated by suppression of the intrarenal renin-angiotensin system. Clin Exp Pharmacol Physiol. 2013;40:273–281. doi: 10.1111/1440-1681.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50:3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Thun, Vari RC, El-Dahr SD, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol. 1994;266:F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 20.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 21.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol. 2003;284:R893–R912. doi: 10.1152/ajpregu.00491.2002. [DOI] [PubMed] [Google Scholar]
- 22.Navar LG, Zou L, Von Thun A, Wang CT, Imig JD, Mitchell KD. Unraveling the mystery of Goldblatt hypertension. News Physiol Sci. 1998;13:170–176. doi: 10.1152/physiologyonline.1998.13.4.170. [DOI] [PubMed] [Google Scholar]
- 23.Pinto YM, Paul M, Ganten D. Lessons from rat models of hypertension: from Goldblatt to genetic engineering. Cardiovasc Res. 1998;39:77–88. doi: 10.1016/s0008-6363(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 24.Mullins LJ, Mullins J. Current successes and limitations of using genetic modification for blood pressure research. Pflugers Arch. 2003;445:491–494. doi: 10.1007/s00424-002-0968-9. [DOI] [PubMed] [Google Scholar]
- 25.Kantachuvesiri S, Fleming S, Peters J, et al. Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem. 2001;276:36727–36733. doi: 10.1074/jbc.M103296200. [DOI] [PubMed] [Google Scholar]
- 26.Vaňourková Z, Kramer HJ, Husková Z, et al. AT1 receptor blockade is superior to conventional triple therapy in protecting against end-organ damage Cyp1a1-Ren-2 transgenic rats with inducible hypertension. J Hypertens. 2006;24:2465–2472. doi: 10.1097/01.hjh.0000251909.00923.22. [DOI] [PubMed] [Google Scholar]
- 27.Husková Z, Vaňourková Z, Erbanová M, et al. Inappropriately high circulating and intrarenal angiotensin II levels during dietary salt loading exacerbate hypertension in Cyp1a1-Ren-2 transgenic rats. J Hypertens. 2010;28:495–509. doi: 10.1097/HJH.0b013e3283345d69. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell KD, Bagatell SJ, Miller CS, Mouton CR, Seth DM, Mullins JJ. Genetic clamping of renin gene expression induces hypertension and elevation of intrarenal II levels of graded severity in Cyp1a1-Ren2 transgenic rats. JRAAS. 2006;7:74–86. doi: 10.3317/jraas.2006.013. [DOI] [PubMed] [Google Scholar]
- 29.Peters B, Grisk O, Becher B, et al. Dose-dependent titration of prorenin and blood pressure in Cyp1a1-Ren-2 transgenic rats: absence of prorenin-induced glomerulosclerosis. J Hypertens. 2008;26:102–109. doi: 10.1097/HJH.0b013e3282f0ab66. [DOI] [PubMed] [Google Scholar]
- 30.Peters BS, Dornaika R, Hosten N, et al. Regression of cardiac hypertrophy in Cyp1a1-Ren-2 transgenic rat. J Magn Reson Imaging. 2012;36:373–378. doi: 10.1002/jmri.23661. [DOI] [PubMed] [Google Scholar]
- 31.Navar LG, Prieto MC, Satou R, Kobori H. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol. 2011;11:180–186. doi: 10.1016/j.coph.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Vilalobos RA, Janjoulia T, Fletecher NK, et al. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;125:2011–2023. doi: 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madhun ZT, Goldthwait DA, McKay D, Hopfer U, Douglas JG. An epoxygenase metabolite of arachidonic acid mediates angiotensin II-induced rises in cytosolic calcium in rabbit proximal tubule epithelial cells. J Clin Invest. 1991;88:456–461. doi: 10.1172/JCI115325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakairi Y, Jacobson HR, Noland DT, Capdevila JH, Falck JR, Breyer MD. 5,6-EET inhibits ion transport in collecting duct by stimulating endogenous prostaglandin synthesis. Am J Physiol. 1995;268:F931–F939. doi: 10.1152/ajprenal.1995.268.5.F931. [DOI] [PubMed] [Google Scholar]
- 35.Erbanová M, Thumová M, Husková Z, et al. Impairment of the autoregulation of renal hemodynamics and of the pressure-natriuresis relationship precedes the development of hypertension in Cyp1a1-Ren-2 transgenic rats. J Hypertens. 2009;27:575–586. doi: 10.1097/hjh.0b013e32831cbd5a. [DOI] [PubMed] [Google Scholar]
- 36.Guyton AC, Hall JE, Coleman TG, Manning RD., Jr . The dominant role of the kidneys in the long term regulation of arterial pressure in normal and hypertensive states. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis and Management. New York, NY: Raven Press, Publishers; 1990. pp. 1029–1052. [Google Scholar]
- 37.Roman RJ, Cowley AW., Jr Abnormal pressure-diuresis-natriuresis response in spontaneously hypertensive rats. Am J Physiol. 1985;248:F199–F205. doi: 10.1152/ajprenal.1985.248.2.F199. [DOI] [PubMed] [Google Scholar]
- 38.Miao CY, Liu KL, Benzoni D, Sassard J. Acute pressure-natriuresis function shows early impairment in Lyon hypertensive rats. J Hypertens. 2005;23:1225–1231. doi: 10.1097/01.hjh.0000170386.84450.e3. [DOI] [PubMed] [Google Scholar]
- 39.Van der Mark J, Kline RL. Altered pressure natriuresis in chronic angiotensin II hypertension in rats. Am J Physiol. 1994;266:F739–F748. doi: 10.1152/ajpregu.1994.266.3.R739. [DOI] [PubMed] [Google Scholar]
- 40.Hall JE, Mizelle HL, Brands MV, Hildebrandt DA. Pressure natriuresis and angiotensin II in reduced kidney mass, salt-induced hypertension. Am J Physiol. 1992;262:R61–R71. doi: 10.1152/ajpregu.1992.262.1.R61. [DOI] [PubMed] [Google Scholar]
- 41.Kujal P, Čertíková Chábová V, Škaroupková P, et al. Inhibition of soluble epoxide hydrolase is renoprotective in 5/6 nephrectomized Ren-2 transgenic hypertensive rats. Clin Exp Pharmacol Physiol. 2014;41:227–237. doi: 10.1111/1440-1681.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castrop H, Höcherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of kidney renin. Physiol Rev. 2010;90:607–673. doi: 10.1152/physrev.00011.2009. [DOI] [PubMed] [Google Scholar]
- 43.Hall JE, Brands MW. The renin-angiotensin-aldosterone system: renal mechanisms and circulatory homeostasis. In: Seldin DW, Giebisch G, editors. The Kidney: Physiology and Pathophysiology. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 1009–1046. [Google Scholar]
- 44.Kujal P, Čertíková Chábová V, Vernerova Z, et al. Similar renoprotection after renin-angiotensin-dependent and -independent antihypertensive therapy in 5/6-nephrectomized Ren-2 transgenic rats: are there blood pressure-independent effects? Clin Exp Pharmacol Physiol. 2010;37:1159–1169. doi: 10.1111/j.1440-1681.2010.05453.x. [DOI] [PubMed] [Google Scholar]
- 45.Vaněčková I, Kujal P, Husková Z, et al. Effects of combined endothelin A receptor blockade and renin-angiotensin system blockade on the course end-organ damage in 5/6 nephrectomized Ren-2 hypertensive rats. Kidney Blood Press Res. 2012;35:382–392. doi: 10.1159/000336823. [DOI] [PubMed] [Google Scholar]
- 46.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Recommendations for blood pressure measurements in humans and experimental animals. Part 2: Blood pressure measurements in experimental animals. Hypertension. 2005;45:299–310. doi: 10.1161/01.HYP.0000150857.39919.cb. [DOI] [PubMed] [Google Scholar]
- 47.Nogueira EF, Vargas CA, Otis M, Gallo-Payet N, Bollag WB, Rainey WE. Angiotensin-II acute regulation of rapid response genes in human, bovine, and rat adrenocortical cells. J Mol Endocrinol. 2007;39:365–374. doi: 10.1677/JME-07-0094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.