Full text
PDF
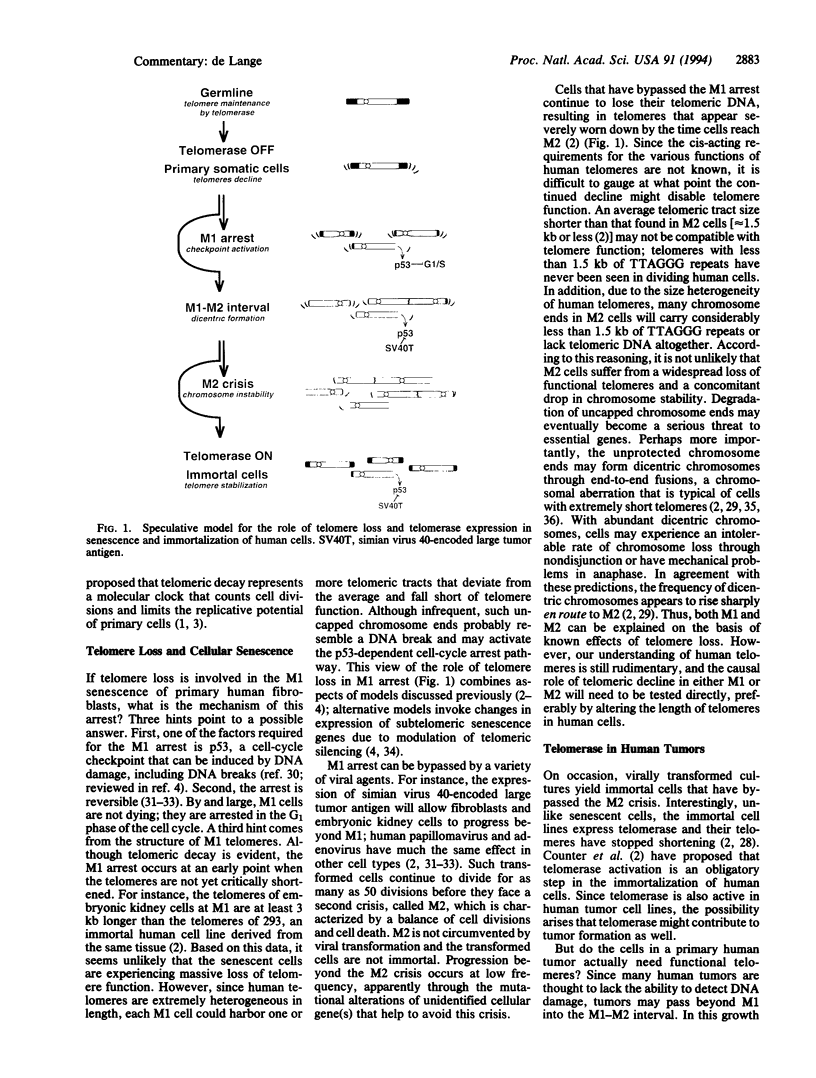


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allshire R. C., Gosden J. R., Cross S. H., Cranston G., Rout D., Sugawara N., Szostak J. W., Fantes P. A., Hastie N. D. Telomeric repeat from T. thermophila cross hybridizes with human telomeres. Nature. 1988 Apr 14;332(6165):656–659. doi: 10.1038/332656a0. [DOI] [PubMed] [Google Scholar]
- Allsopp R. C., Vaziri H., Patterson C., Goldstein S., Younglai E. V., Futcher A. B., Greider C. W., Harley C. B. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Cardenas M. E., Bianchi A., de Lange T. A Xenopus egg factor with DNA-binding properties characteristic of terminus-specific telomeric proteins. Genes Dev. 1993 May;7(5):883–894. doi: 10.1101/gad.7.5.883. [DOI] [PubMed] [Google Scholar]
- Cooke H. J., Smith B. A. Variability at the telomeres of the human X/Y pseudoautosomal region. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):213–219. doi: 10.1101/sqb.1986.051.01.026. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter C. M., Hirte H. W., Bacchetti S., Harley C. B. Telomerase activity in human ovarian carcinoma. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2900–2904. doi: 10.1073/pnas.91.8.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis H., Lacroix J. C. The dichotomy between germ line and somatic line, and the origin of cell mortality. Trends Genet. 1993 Jan;9(1):7–11. doi: 10.1016/0168-9525(93)90065-P. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Vaziri H., Counter C. M., Allsopp R. C. The telomere hypothesis of cellular aging. Exp Gerontol. 1992 Jul-Aug;27(4):375–382. doi: 10.1016/0531-5565(92)90068-b. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Dempster M., Dunlop M. G., Thompson A. M., Green D. K., Allshire R. C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990 Aug 30;346(6287):866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Holzmann K., Blin N., Welter C., Zang K. D., Seitz G., Henn W. Telomeric associations and loss of telomeric DNA repeats in renal tumors. Genes Chromosomes Cancer. 1993 Mar;6(3):178–181. doi: 10.1002/gcc.2870060308. [DOI] [PubMed] [Google Scholar]
- Ide T., Tsuji Y., Nakashima T., Ishibashi S. Progress of aging in human diploid cells transformed with a tsA mutant of simian virus 40. Exp Cell Res. 1984 Feb;150(2):321–328. doi: 10.1016/0014-4827(84)90575-5. [DOI] [PubMed] [Google Scholar]
- Kipling D., Cooke H. J. Hypervariable ultra-long telomeres in mice. Nature. 1990 Sep 27;347(6291):400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- Klingelhutz A. J., Barber S. A., Smith P. P., Dyer K., McDougall J. K. Restoration of telomeres in human papillomavirus-immortalized human anogenital epithelial cells. Mol Cell Biol. 1994 Feb;14(2):961–969. doi: 10.1128/mcb.14.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R. W., Ganesan R., Houtchens K., Tolar L. A., Sheen F. M. Transposons in place of telomeric repeats at a Drosophila telomere. Cell. 1993 Dec 17;75(6):1083–1093. doi: 10.1016/0092-8674(93)90318-k. [DOI] [PubMed] [Google Scholar]
- Lindsey J., McGill N. I., Lindsey L. A., Green D. K., Cooke H. J. In vivo loss of telomeric repeats with age in humans. Mutat Res. 1991 Jan;256(1):45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- Lundblad V., Blackburn E. H. An alternative pathway for yeast telomere maintenance rescues est1- senescence. Cell. 1993 Apr 23;73(2):347–360. doi: 10.1016/0092-8674(93)90234-h. [DOI] [PubMed] [Google Scholar]
- Lundblad V., Szostak J. W. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989 May 19;57(4):633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. G., Kastan M. B. DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol. 1994 Mar;14(3):1815–1823. doi: 10.1128/mcb.14.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak S., Wang Z., Dhaliwal M. K., Sacks P. C. Telomeric association: another characteristic of cancer chromosomes? Cytogenet Cell Genet. 1988;47(4):227–229. doi: 10.1159/000132555. [DOI] [PubMed] [Google Scholar]
- Price C. M. Centromeres and telomeres. Curr Opin Cell Biol. 1992 Jun;4(3):379–384. doi: 10.1016/0955-0674(92)90002-t. [DOI] [PubMed] [Google Scholar]
- Radna R. L., Caton Y., Jha K. K., Kaplan P., Li G., Traganos F., Ozer H. L. Growth of immortal simian virus 40 tsA-transformed human fibroblasts is temperature dependent. Mol Cell Biol. 1989 Jul;9(7):3093–3096. doi: 10.1128/mcb.9.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltman D., Morgan R., Cleary M. L., de Lange T. Telomeric structure in cells with chromosome end associations. Chromosoma. 1993 Jan;102(2):121–128. doi: 10.1007/BF00356029. [DOI] [PubMed] [Google Scholar]
- Sandell L. L., Zakian V. A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993 Nov 19;75(4):729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- Shay J. W., Wright W. E., Brasiskyte D., Van der Haegen B. A. E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene. 1993 Jun;8(6):1407–1413. [PubMed] [Google Scholar]
- Starling J. A., Maule J., Hastie N. D., Allshire R. C. Extensive telomere repeat arrays in mouse are hypervariable. Nucleic Acids Res. 1990 Dec 11;18(23):6881–6888. doi: 10.1093/nar/18.23.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri H., Schächter F., Uchida I., Wei L., Zhu X., Effros R., Cohen D., Harley C. B. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet. 1993 Apr;52(4):661–667. [PMC free article] [PubMed] [Google Scholar]
- Weinert T. A., Hartwell L. H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988 Jul 15;241(4863):317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Pereira-Smith O. M., Shay J. W. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol Cell Biol. 1989 Jul;9(7):3088–3092. doi: 10.1128/mcb.9.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Shay J. W. Telomere positional effects and the regulation of cellular senescence. Trends Genet. 1992 Jun;8(6):193–197. doi: 10.1016/0168-9525(92)90232-s. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Bradley J. D., Attardi L. D., Blackburn E. H. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990 Mar 8;344(6262):126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Shiue L., Kaplan S., de Lange T. A mammalian factor that binds telomeric TTAGGG repeats in vitro. Mol Cell Biol. 1992 Nov;12(11):4834–4843. doi: 10.1128/mcb.12.11.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T., Shiue L., Myers R. M., Cox D. R., Naylor S. L., Killery A. M., Varmus H. E. Structure and variability of human chromosome ends. Mol Cell Biol. 1990 Feb;10(2):518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]