Abstract
Background
Post-stroke memory impairment is more common among older adults, women, and blacks. It is unclear whether post-stroke differences reflect differential effects of stroke per se, or differences in pre-stroke functioning. We compare memory trajectories before and after stroke by age, sex and race.
Methods
Health and Retirement Study participants age 50+ (n=17,341) with no stroke history at baseline, were interviewed biennially up to 10 years for first self- or proxy-reported stroke (n=1,574). Segmented linear regression models were used to compare annual rate of memory change before and after stroke among 1,169 stroke survivors, 405 stroke decedents, and 15,767 stroke-free participants. Effect modification was evaluated with analyses stratified by baseline age (≤70 vs. >70), sex and race (white vs. nonwhite), and using interaction terms between age/sex/race indicators and annual memory change.
Results
Older (>70 years) adults experienced faster memory decline before stroke (−0.19 vs. −0.10 points/year for survivors, −0.24 vs. −0.13 points/year for decedents, p<0.001 for both interactions), and among stroke survivors, larger memory decrements (−0.64 vs. −0.26 points, p<0.001) at stroke and faster memory decline (−0.15 vs. −0.07 points/year, p=0.003) after stroke onset, compared to younger adults. Female stroke survivors experienced faster pre-stroke memory decline than male stroke survivors (−0.14 vs. −0.10 points/year, p<0.001). However, no sex differences were seen for other contrasts. Although whites had higher post-stroke memory scores than non-whites, race was not associated with rate of memory decline during any period of time; i.e., race did not significantly modify the rate of decline pre- or post-stroke or the immediate effect of stroke on memory.
Conclusions
Older age and predicted worse memory change before, at and after stroke onset. Sex and race differences in post-stroke memory outcomes might be attributable to pre-stroke disparities, which may be unrelated to cerebrovascular disease.
Keywords: Memory change, Stroke, Effect Modifier
Introduction
Stroke survivors often have substantial cognitive impairments1–9, but the prevalence of impairments differs by major demographic characteristics. For example, many studies have shown that memory impairments or dementia are more common among older10–22, female10,12,21,22, and black stroke survivors10,20–24. Therefore, we hypothesize adults who are older, female or black have faster memory decline after stroke compared to those who are younger, male or white. The differential outcomes might be attributable to unequal quality of care for acute stroke or differences in stroke severity by age, sex, or race. However, because most studies on cognitive functions and stroke begin at the time of stroke hospitalization, it is unclear if post-stroke differences in outcomes reflect differences in the effects of stroke per se, or differences in functioning that were apparent prior to stroke. Our previous research suggests that long before stroke onset, individuals who subsequently have stroke are already experiencing cognitive impairment and significantly accelerated rates of memory decline, compared to age-matched individuals who remained stroke free25,26. Comparisons of memory outcomes post-stroke may therefore conflate pre-stroke differences with the consequences of stroke. Any risk factor correlated with pre-stroke memory functioning will also correlate with post-stroke memory functioning, even if that factor does not modify effects of stroke per se. Longitudinal analyses can establish whether post-stroke memory differences are due to differential effects of stroke, or memory differences that existed before stroke.
Building on our prior research25, we used a US nationally representative prospective cohort to test whether age, sex and race modify rates of memory change before stroke, the time of stroke, or in the years following stroke, and compare these changes to annual memory declines among otherwise similar stroke-free participants.
Methods
Study population
The Health and Retirement Study (HRS) was initiated in 1992 with additional enrollments in 1993 and 1998. Information on participants was collected in biennial follow-up interviews. Our analyses used the 1998 survey as baseline and included follow-up data through 2008.
From the 20,567 HRS participants aged 50+ in 1998, we restricted to 17,544 (85.3%) non-Hispanics who were stroke-free at baseline; we additionally excluded respondents with no memory scores at any wave (n=100) or missing risk factor information at baseline (n=103), for a final analytic sample of 17,341 (91.3% of HRS participants aged 50+ in 1998). Hispanics were excluded because the composite memory score we developed could not be calculated for this group. We found evidence that the component score weights differed for Hispanics than non-Hispanics27, but there were not enough Hispanic participants to calculate reliable weights in HRS. Compared with subjects included in the analysis, those who were eligible but excluded due to missing memory scores or risk factors were younger, more likely to be nonwhites, having lower socioeconomic status (Appendix 1).
Dependent variable
Memory was assessed by immediate and (approximately 5-minute) delayed recall tests of a 10-word list of common nouns. Additionally, for individuals too impaired to directly participate in memory assessments, proxy informants, typically spouses, were asked to assess the participants’ memory on a 5-item Likert scale and completed a 16-item version of the Informant Questionnaire for Cognitive Decline (IQCODE)28,29,30. We previously developed a composite memory score combining proxy and direct memory assessments for longitudinal analyses27. The composite memory score was standardized so that every unit change in composite memory score corresponds to one standard deviation at baseline 1998. The composite memory score ranged from −5.5 to 5.9 and each decade of age was associated with approximately 1 point decrease at baseline.
Independent variables
We examined interactions of stroke timing and demographic indicators as predictors of memory. Incidence of stroke was defined as self-reported doctor’s diagnosis of stroke based on the question “Has a doctor ever told you that you had a stroke?” When patients were deceased or unavailable for direct interview, HRS asked proxy informants (usually patients’ spouses) to report on stroke status. Participants or proxies reporting stroke also reported the month and year of event (n=1,574). A small number of participants did not report the month (n=131, 8.3%) or year (n=166, 10.5%) of event between biennial interview waves, and we used the midpoint of last known stroke-free date and date when the stroke was first reported to retain these people. Because our study focused on the effect of first stroke onset, only the first stroke during follow-up was identified; many stroke survivors may have had subsequent strokes but we consider all memory reports after the first stroke as “post-stroke”.
We categorized participants into three groups: 1) stroke survivors, who reported a first stroke during the follow-up period and survived to provide at least one subsequent memory assessment score (n=1,169); 2) stroke decedents, who experienced (per proxy-report) a first stroke during the follow-up period and died prior to their next interview (n=405); 3) stroke-free participants (n=15,767), for whom no stroke was reported during follow-up. For all respondents, follow-up continued until 2008, the respondent’s death, or respondent’s attrition from the sample. We included individuals in the category of “stroke decedents” regardless of whether the primary cause of death was stroke.
The primary exposure was the timing of memory assessment with respect to stroke. We defined the year of stroke onset as time zero and, for each memory assessment, calculated the years until stroke (to describe the pre-stroke memory trajectory) or the years since stroke (to describe the post-stroke memory trajectory). We tested for effect modification by baseline age, sex and race. Age was dichotomized as ≤70 (66.4%) vs. > 70 years old (32.6%); sex was defined as female (57.4%) vs. male (42.6%); race was defined as white (83.3%) vs. nonwhite (16.7%). We combined all nonwhite races (assessed as either “black” or “other”) due to the limited number of subjects who are of other races (2%).
Other Covariates
Baseline covariates included demographics: race (white vs. other), sex, marital status (married/partnered, separated/divorced/widowed, never married), birth place (born in the Northeast, Midwest, South, West, or outside the US); and socioeconomic status indicators, including height (short stature is an indicator of childhood deprivation)31, years of education (0–17), mother’s years of education (<8 years, ≥8 years, or unknown mother’s education), and natural log of household wealth (household wealth less than 1 was set to be 1). Continuous variables were mean centered and reference categories for categorical variables were chosen as the most common category (married/partnered white women with mother’s education ≥8 years and born in the Midwest).
In main effect models, age was defined in two ways. First, time-updated age at interview was included for individuals who remained stroke free (set to 0 for participants who experienced stroke) and second, time-fixed age at stroke was included for those who had a stroke (set to 0 for participants who remained stroke-free). Both age terms were centered at 70 in analyses testing for effect modification by sex and race; for the test of effect modification by age, the younger stratum was centered at 65 and the older stratum was centered at 75 years old.
Statistical analysis
We used segmented linear regression models to describe longitudinal trajectories of memory score. In addition to regression coefficients, we illustrate predicted trajectories graphically for reference categories (described above). Because all models are linear, the choice of reference category is arbitrary and does not influence graphical patterns.
The main model contained all stroke survivors, stroke decedents and stroke-free participants. To describe memory function change, we specified variables corresponding to time with respect to date of stroke onset for each stroke status group. For example, for stroke survivors, we specified a slope for annual rate of memory change prior to non-fatal stroke, a discontinuity term for change in memory function at the time of stroke, and a slope for rate of memory change in the years following non-fatal stroke; for stroke decedents, we specified a rate of annual memory change prior to fatal stroke; and for stroke-free participants, we specified a slope of memory change with each additional year of age (described previously as updated age). Additionally, we adjusted the model for all baseline covariates and age at stroke onset.
To separately estimate and compare the trajectory of memory change pre and post stroke in different sub-groups of age, sex and race, we stratified the cohort into participants who were ≤70 vs. >70 years at baseline; male vs. female; and white vs. nonwhite and estimated the main model within each stratum. Next, to test for effect modification by age, sex or race, we included interaction terms for each time period (years prior to stroke, stroke onset, and years after stroke) by age/sex/race indicators. An example of full model parameterization for participants in the younger stratum is shown in Appendix 2.
For all models, we used generalized linear regression with robust variance estimates to account for repeated measures on the same individual. Models were estimated in SUDAAN to account for the complex sample design used to draw the HRS sample32. All analyses were weighted according to the 1998 sample weights to be representative of the 1998 community-dwelling US population born 1947 or earlier.
Role of funding source
HRS is sponsored by the National Institute on Aging (U01AG009740) and conducted by the University of Michigan, whose institutional review board approved the original study procedures. The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Results
Of 17,341 eligible participants (139,663 person-years), 6508 (37%) were dropped before death or the end of follow-up. Attrition was associated with being older, unmarried, with lower socioeconomic status or last composite memory score (Appendix 3). The crude incidence rate of stroke was 11.3 cases/1,000 person-years (Table 1) and the unadjusted stroke incidence rates were higher for older individuals, males, and nonwhites. Among participants who experienced stroke, mortality was higher among older (p<0.001) and female (p=0.03) participants. We have previously shown that stroke decedents experienced significantly faster memory declines than stroke survivors prior to stroke onset25. This association was also true across all subgroups of age, sex and race in this study (p<0.04 for all comparisons).
Table 1.
Stroke Incidence and Follow-up Time of Participants in Strata of Age, Sex and Race
| All Participants | Stratified by Age | Stratified by Sex | Stratified by Race | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| N=17,341 | <=70 years N=11,512 |
>70 years N=5,829 |
Male N=7,388 |
Female N=9,953 |
Non-white N=2,902 |
White N=14,439 |
|
| Incidence | |||||||
| All Stroke | |||||||
| Individuals with incident first strokes | 1,574 | 737 | 837 | 659 | 915 | 327 | 1247 |
| Crude incidence rate, any first stroke (/1000 person-years) | 11.27 | 7.37 | 21.11 | 11.31 | 11.24 | 14.09 | 10.71 |
| Stroke Survivors | |||||||
| Number of stroke survivors | 1,169 | 619 | 550 | 508 | 661 | 234 | 935 |
| Crude incidence rate, survived first strokes (/1000 person-years) | 8.37 | 6.19 | 13.87 | 8.72 | 8.12 | 10.08 | 8.03 |
| Stroke Decedents | |||||||
| Number of stroke decedents | 405 | 118 | 287 | 151 | 254 | 93 | 312 |
| Crude incidence rate, first strokes not survived (/1000 person-years) | 2.90 | 1.18 | 7.24 | 2.59 | 3.12 | 4.01 | 2.68 |
| Follow-up Time | |||||||
| Total number of person-years | 139,663 | 100,005 | 39,658 | 58,228 | 81,435 | 23,211 | 116,452 |
| Stroke Survivors | |||||||
| Number of person-years before stroke onset | 5,672 | 3,142 | 2,530 | 2,483 | 3,189 | 1,142 | 4,530 |
| Number of person-years after stroke onset | 4,590 | 2,563 | 2,027 | 1,998 | 2,592 | 927 | 3,663 |
| Stroke Decedents | |||||||
| Number of person-years before stroke onset | 1,905 | 609 | 1,296 | 648 | 1,257 | 407 | 1,498 |
| Stroke Free Participants | |||||||
| Number of person-years | 127,496 | 93,691 | 33,805 | 53,099 | 74,397 | 20,735 | 106,761 |
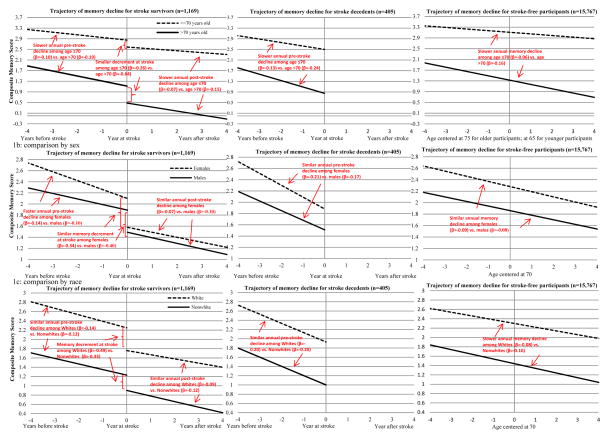
For stroke survivors, the annual rate of memory decline before stroke was −0.19 points/year in the older age stratum and −0.10 points/year in the younger age stratum (p<0.001 for test of interaction; Table 2 and Figure 1a). In addition, stroke survivors in the older stratum experienced a larger decrement of memory function at stroke onset (−0.64 vs. −0.26 points, p<0.001) and a faster annual decline after stroke (−0.15 vs. −0.07 points/year, p=0.003) compared to those in the younger age stratum. For stroke decedents, the pre-stroke memory decline rate was faster (p=0.006) in the older stratum (−0.24 points/year) than that in the younger stratum (−0.13 points/year). Among stroke-free participants, annual rate of memory decline was also faster among people in the older vs. younger stratum (−0.16 vs. −0.06 points/year, p<0.001).
Table 2.
Description of crude incidence and follow-up time by stroke status and demographic factors (n=17,341)
| Annual rate of memory decline prior to stroke | Memory decrement at stroke onset | Annual rate of memory decline post to stroke | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Beta coefficient |
95% confidence interval |
P-value* | Beta coefficient |
95% confidence interval |
P-value* | Beta coefficient |
95% confidence interval |
P-value* | ||
| Stroke Survivors (n=1169) | −0.13 | (−0.15, −0.11) | −0.46 | (−0.56, −0.36) | −0.09 | (−0.11, −0.07) | ||||
| Age | >70 years old | −0.19 | (−0.21, −0.17) | <0.001 | −0.64 | (−0.78, −0.50) | <0.001 | −0.15 | (−0.19, −0.11) | 0.003 |
| ≤70 years old | −0.10 | (−0.12, −0.08) | −0.26 | (−0.36, −0.16) | −0.07 | (−0.09, −0.05) | ||||
| Sex | Male | −0.10 | (−0.12, −0.08) | <0.001 | −0.40 | (−0.52, −0.28) | 0.10 | −0.10 | (−0.12, −0.08) | 0.32 |
| Female | −0.14 | (−0.16, −0.12) | −0.34 | (−0.48, −0.20) | −0.07 | (−0.09, −0.05) | ||||
| Race | White | −0.14 | (−0.16, −0.12) | 0.32 | −0.49 | (−0.59, −0.39) | 0.11 | −0.09 | (−0.11, −0.07) | 0.13 |
| Nonwhite | −0.12 | (−0.14, −0.10) | −0.33 | (−0.53, −0.13) | −0.12 | (−0.16, −0.08) | ||||
| Stroke Decedents (n=405) | −0.20 | (−0.22, −0.18) | ||||||||
| Age | >70 years old | −0.24 | (−0.28, −0.20) | 0.006 | ||||||
| ≤70 years old | −0.13 | (−0.17, −0.09) | ||||||||
| Sex | Male | −0.17 | (−0.21, −0.13) | 0.18 | ||||||
| Female | −0.21 | (−0.25, −0.17) | ||||||||
| Race | White | −0.20 | (−0.22, −0.18) | 0.99 | ||||||
| Nonwhite | −0.20 | (−0.26, −0.14) | ||||||||
| Stroke-free Participants (n=15767) | −0.09 | (−0.10, −0.08) | ||||||||
| Age | >70 years old | −0.16 | (−0.17, −0.15) | <0.001 | ||||||
| ≤70 years old | −0.06 | (−0.07, −0.05) | ||||||||
| Sex | Male | −0.08 | (−0.09, −0.07) | <0.001 | ||||||
| Female | −0.09 | (−0.10, −0.08) | ||||||||
| Race | White | −0.08 | (−0.09, −0.07) | <0.001 | ||||||
| Nonwhite | −0.10 | (−0.11, −0.09) | ||||||||
Tests the null hypothesis that rate of memory decline is equal for the two groups
Figure 1.
Trajectory of memory decline for stroke survivors, stroke decedents and stroke-free participants by age, sex and race
Predicted trajectories of memory scores for stroke survivors (n=1,169), stroke decedents (n=405) and stroke-free cohort members (n=15,767). Figure 1a (top panel) compares memory trajectories for participants ≤70 years old (dashed line) and >70 years old at baseline (solid line); Figure 1b (middle panel) compares memory trajectories for females (dashed line) and males (solid line); Figure 1c (bottom panel) compares memory trajectories for white participants (dashed line) and nonwhite participants (solid line). For Figure 1a, Time 0 indicates the time of stroke onset for participant who experienced stroke (centered at age 65 if participants were ≤70 at baseline or at age 75 if they were >70 at baseline), whereas for stroke-free participants, it represents age 65 years for those ≤70 at baseline or age 75 for those >70 at baseline. For Figure 1b and 1c, Time 0 indicates the time of stroke onset for participants who experienced stroke (centered at 70 years old), while it represents age 70 for stroke-free cohort members. Decline at stroke onset indicates the immediate decrement of memory scores at stroke onset among stroke survivors. The curves to the left of stroke onset indicate change in memory scores before stroke onset and the curve to the right indicates the change after stroke onset.
Women who survived stroke experienced faster memory declines before stroke compared to men who survived stroke (−0.14 vs. −0.10 points/year, p<0.001, Table 2, Figure 1b). However, memory decrements at the time of stroke and post-stroke memory declines were not significantly different between male and female stroke survivors. For stroke decedents, females also experienced slightly faster pre-stroke memory decline (−0.21 points/year) compared to males stroke decedents (−0.17 points/year) though the difference was not statistically significant (p=0.18). The annual rate of memory decline with aging was similar among female and male (−0.09 vs. −0.08 points/year) stroke-free participants.
There was no evidence for significant effect modification by race among participants who experienced first stroke incidence during follow-up (Figure 1c): no significant differences between white and nonwhite stroke survivors could be found with respect to the annual rate of memory decline prior to stroke (−0.14 vs. −0.12 points/year), the change in functioning at stroke onset (−0.49 vs. −0.33 points), or the annual rate of memory decline after stroke (−0.09 vs. −0.12 points/year) (Table 2). For stroke decedents, the annual decline rate was also similar (−0.20 points/year for each) among whites and nonwhites. Annual rate of memory decline among stroke-free participants was slightly faster among non-white (−0.10 points/year), compared to white (−0.08 points/year) participants (p<0.001).
Discussion
In this large, nationally representative cohort, older adults experienced accelerated memory declines before stroke onset and, for those who survived stroke, a larger decrement in functioning at the time of stroke as well as faster memory decline after stroke onset. Additionally, women experienced significant faster pre-stroke memory declines than men, but there was no significant sex difference in post-stroke changes. We found no evidence that rate of memory changes prior to stroke, at the time of stroke, or after stroke differed by race.
Comparison to prior literature
Building on previous evidence that individuals who died shortly after stroke were experiencing more rapid memory declines prior to stroke than comparable individuals who later had a stroke but survived25, we found in this study that such pattern was consistent across all subgroups stratified by age, sex and race.
With respect to our findings in analyses stratified by age, sex, and race, several previous studies have reported mixed patterns of association between demographic factors and memory related outcomes. Most studies found older age to be a strong predictor for both pre-21,22,33–36 and post-stroke21,22,37,38 dementia, but a few reported inconsistent results8,39. Building on our previous study25, our analysis showed that participants in the older stratum at baseline experienced accelerated memory decline throughout the period before, at, and after stroke onset, compared to those in the younger stratum. Both pre-stroke memory differences and the differential effects of acute stroke contribute to post-stroke cognitive differences associated with age.
Being female was identified as an effect modifier for pre-stroke memory decline among stroke survivors in our analysis. Given the close link between memory loss and dementia, this is consistent with findings of several studies that female sex is an important predictor for pre-stroke dementia21,22,40,41. The faster pre-stroke memory declines in females could be attributable to differences in stroke risk factors that also affect cognitive function. For example, after menopause, females may have higher prevalence of hypertension than males of similar ethnic background42,43. Therefore, the inadequate management of hypertension in older females may partially account for pre-stroke memory differences compared to older males. Findings on the effect of sex on post-stroke dementia have not been consistent8,18,39, and an indication was made that female sex was a much stronger predictor of pre- than post-stroke dementia21,22. Our study suggests that the worse post-stroke cognitive function among women reported in many previous studies may largely reflect faster pre-stroke cognitive decline. We do not know whether pre-stroke memory scores between men and women are due to biological differences in the aging process or social patterns in neuropsychological test familiarity. In our data, rate of change after stroke differed little by gender; it is not possible from our data to explain this apparent equalization. One possibility is that there are gender disparities in major risk factors such as hypertension or depression, which may attenuate in the wake of an acute event such as stroke. Another possibility is that women experience silent strokes more frequently or identification of cerebrovascular disease is delayed in women, who may more frequently present with “non-traditional” symptoms44. The small sex differences among stroke free participants may have little clinical relevance, despite statistical significance.
Dementia (without regard to stroke status) is more common among African Americans45–48 and evidence suggests blacks experience higher post-stroke dementia than whites10,20–24, but it has been difficult to rule out differences in educational background as the explanation for racial disparities in dementia49,50. We found no evidence of effect modification by race in any time period with respect to stroke. However, nonwhites, predominantly African American in this sample, consistently averaged lower neuropsychological test scores than whites. Race differences in neuropsychology test scores are very likely attributable to differences in school quality49 or test-taking patterns51, rather than difference in underlying function or neurological pathology. Therefore, the elevated prevalence of post-stroke dementia among blacks reported in previous studies may be attributable to disparities in other domains or neuropsychological test performance that emerged long before stroke onset, rather than to the differential effects of cerebrovascular disease or acute stroke on memory.
Limitations and strengths
Some limitations to this study should be noted. Self- or proxy-reported strokes were not verified using medical records so recall bias could occur, especially in older and cognitively impaired subjects. However, previous studies have shown that the incidence of self-reported stroke was similar to other cohorts with medical-record verification of stroke outcomes52. Missing data and imprecision in the reported date of stroke could lead to information bias if the accuracy of recall differed by age, sex, or race. The composite memory score was developed based on all Informant Questionnaire for Cognitive Decline items, which included assessments of cognitive domains other than memory. This score may therefore be influenced by other cognitive domains, such as executive function or reasoning skills. Although we used composite memory score to retain impaired respondents to reduce attrition, some individuals had neither direct nor proxy memory assessments. Additionally, attrition before the final follow-up was associated with being older, low socioeconomic status and having low last memory scores, compared to those who remained in the study. Prior research suggests that study dropout is related to underlying cognitive decline53,54, therefore potential bias due to selective attrition remains. We restricted the study population to HRS participants with at least 1 memory score and no missing risk factors. Because individuals with no memory score at any wave or missing risk factors were more likely to be blacks or have lower socioeconomic status compared to those included in our study sample, this may introduce bias, potentially attenuating the association between race and memory. However, due to the very small percent missing, any bias would likely be small. Most importantly, because many of these demographic factors are related both to the risk of stroke and the memory function, analyses such as ours which stratify on stroke status can induce a special type of selection bias if there are other, unmeasured, common causes of stroke and memory55. Sensitivity analyses suggest that within plausible ranges of unmeasured confounding, this type of bias is unlikely to qualitatively affect our findings (Appendix 4).
Despite these limitations, this study has a number of important strengths that advance the empirical understanding of how changes in memory associated with stroke differ by age, sex, and race. Most notably, HRS is a large, nationally representative sample, with long follow-up, comprehensive socio-demographic assessments, prospectively assessed repeated pre-stroke measurements of memory, and high follow-up rates, even for memory impaired individuals. In addition, by using a segmented linear regression model, our analysis simultaneously tested the effects of demographic factors on rate of change in the years preceding stroke, at the time of stroke, and in the years following stroke. To our knowledge, no prior study has simultaneously assessed these patterns in order to comprehensively describe the change in memory function associated with cerebrovascular disease.
Conclusion
Our study found that older age predicted faster memory declines before and after stroke as well as worse memory decrements at the time of stroke. Female sex was associated with faster pre-stroke memory declines, but post-stroke memory declines did not differ by sex. Race was not associated with rate of memory decline among participants with first stroke. Although cognitive outcomes after stroke may be worse among nonwhites than whites, this is likely attributable to pre-existing disparities, rather than differential effects of cerebrovascular disease or acute stroke on memory among nonwhites.
Supplementary Material
References
- 1.Elias MF, Sullivan LM, D’Agostino RB, et al. Framingham stroke risk profile and lowered cognitive performance. Stroke. 2004 Feb;35(2):404–409. doi: 10.1161/01.STR.0000103141.82869.77. [DOI] [PubMed] [Google Scholar]
- 2.Llewellyn DJ, Lang IA, Xie J, Huppert FA, Melzer D, Langa KM. Framingham Stroke Risk Profile and poor cognitive function: a population-based study. BMC Neurol. 2008;8:12. doi: 10.1186/1471-2377-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linden T, Skoog I, Fagerberg B, Steen B, Blomstrand C. Cognitive impairment and dementia 20 months after stroke. Neuroepidemiology. 2004 Jan-Apr;23(1–2):45–52. doi: 10.1159/000073974. [DOI] [PubMed] [Google Scholar]
- 4.Bejot Y, Aboa-Eboule C, Durier J, et al. Prevalence of early dementia after first-ever stroke: a 24-year population-based study. Stroke. Mar;42(3):607–612. doi: 10.1161/STROKEAHA.110.595553. [DOI] [PubMed] [Google Scholar]
- 5.Srikanth VK, Anderson JF, Donnan GA, et al. Progressive dementia after first-ever stroke: a community-based follow-up study. Neurology. 2004 Sep 14;63(5):785–792. doi: 10.1212/01.wnl.0000137042.01774.33. [DOI] [PubMed] [Google Scholar]
- 6.Desmond DW, Moroney JT, Sano M, Stern Y. Incidence of dementia after ischemic stroke: results of a longitudinal study. Stroke. 2002 Sep;33(9):2254–2260. doi: 10.1161/01.str.0000028235.91778.95. [DOI] [PubMed] [Google Scholar]
- 7.Censori B, Manara O, Agostinis C, et al. Dementia after first stroke. Stroke. 1996 Jul;27(7):1205–1210. doi: 10.1161/01.str.27.7.1205. [DOI] [PubMed] [Google Scholar]
- 8.Ivan CS, Seshadri S, Beiser A, et al. Dementia after stroke: the Framingham Study. Stroke. 2004 Jun;35(6):1264–1268. doi: 10.1161/01.STR.0000127810.92616.78. [DOI] [PubMed] [Google Scholar]
- 9.Lowery K, Ballard C, Rodgers H, et al. Cognitive decline in a prospectively studied group of stroke survivors, with a particular emphasis on the >75’s. Age Ageing. 2002 Nov;31 (Suppl 3):24–27. doi: 10.1093/ageing/31.suppl_3.24. [DOI] [PubMed] [Google Scholar]
- 10.Desmond DW, Moroney JT, Paik MC, et al. Frequency and clinical determinants of dementia after ischemic stroke. Neurology. 2000;54(5):1124–1131. doi: 10.1212/wnl.54.5.1124. [DOI] [PubMed] [Google Scholar]
- 11.Pohjasvaara T, Erkinjuntti T, Ylikoski R, Hietanen M, Vataja R, Kaste M. Clinical determinants of poststroke dementia. Stroke. 1998 Jan;29(1):75–81. doi: 10.1161/01.str.29.1.75. [DOI] [PubMed] [Google Scholar]
- 12.Inzitari D, Di Carlo A, Pracucci G, et al. Incidence and determinants of poststroke dementia as defined by an informant interview method in a hospital-based stroke registry. Stroke. 1998 Oct;29(10):2087–2093. doi: 10.1161/01.str.29.10.2087. [DOI] [PubMed] [Google Scholar]
- 13.Henon H, Durieu I, Guerouaou D, Lebert F, Pasquier F, Leys D. Poststroke dementia: incidence and relationship to prestroke cognitive decline. Neurology. 2001 Oct 9;57(7):1216–1222. doi: 10.1212/wnl.57.7.1216. [DOI] [PubMed] [Google Scholar]
- 14.Barba R, Martinez-Espinosa S, Rodriguez-Garcia E, Pondal M, Vivancos J, Del Ser T. Poststroke dementia : clinical features and risk factors. Stroke. 2000 Jul;31(7):1494–1501. doi: 10.1161/01.str.31.7.1494. [DOI] [PubMed] [Google Scholar]
- 15.Altieri M, Di Piero V, Pasquini M, et al. Delayed poststroke dementia: a 4-year follow-up study. Neurology. 2004 Jun 22;62(12):2193–2197. doi: 10.1212/01.wnl.0000130501.79012.1a. [DOI] [PubMed] [Google Scholar]
- 16.Lin JH, Lin RT, Tai CT, Hsieh CL, Hsiao SF, Liu CK. Prediction of poststroke dementia. Neurology. 2003 Aug 12;61(3):343–348. doi: 10.1212/01.wnl.0000078891.27052.10. [DOI] [PubMed] [Google Scholar]
- 17.Zhou DH, Wang JY, Li J, Deng J, Gao C, Chen M. Study on frequency and predictors of dementia after ischemic stroke: the Chongqing stroke study. J Neurol. 2004 Apr;251(4):421–427. doi: 10.1007/s00415-004-0337-z. [DOI] [PubMed] [Google Scholar]
- 18.Klimkowicz-Mrowiec A, Dziedzic T, Slowik A, Szczudlik A. Predictors of poststroke dementia: results of a hospital-based study in poland. Dement Geriatr Cogn Disord. 2006;21(5–6):328–334. doi: 10.1159/000091788. [DOI] [PubMed] [Google Scholar]
- 19.Sachdev PS, Brodaty H, Valenzuela MJ, et al. Clinical determinants of dementia and mild cognitive impairment following ischaemic stroke: the Sydney Stroke Study. Dement Geriatr Cogn Disord. 2006;21(5–6):275–283. doi: 10.1159/000091434. [DOI] [PubMed] [Google Scholar]
- 20.Patel MD, Coshall C, Rudd AG, Wolfe CD. Cognitive impairment after stroke: clinical determinants and its associations with long-term stroke outcomes. J Am Geriatr Soc. 2002 Apr;50(4):700–706. doi: 10.1046/j.1532-5415.2002.50165.x. [DOI] [PubMed] [Google Scholar]
- 21.Pendlebury ST. Stroke-related dementia: rates, risk factors and implications for future research. Maturitas. 2009 Nov 20;64(3):165–171. doi: 10.1016/j.maturitas.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Pendlebury ST. Dementia in patients hospitalized with stroke: rates, time course, and clinico-pathologic factors. International journal of stroke : official journal of the International Stroke Society. 2012 Oct;7(7):570–581. doi: 10.1111/j.1747-4949.2012.00837.x. [DOI] [PubMed] [Google Scholar]
- 23.Tatemichi TK, Foulkes MA, Mohr JP, et al. Dementia in stroke survivors in the Stroke Data Bank cohort. Prevalence, incidence, risk factors, and computed tomographic findings. Stroke. 1990 Jun;21(6):858–866. doi: 10.1161/01.str.21.6.858. [DOI] [PubMed] [Google Scholar]
- 24.Tatemichi TK, Desmond DW, Paik M, et al. Clinical determinants of dementia related to stroke. Ann Neurol. 1993 Jun;33(6):568–575. doi: 10.1002/ana.410330603. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Capistrant BD, Ehntholt A, Glymour MM. Long-term rate of change in memory functioning before and after stroke onset. Stroke. 2012 Oct;43(10):2561–2566. doi: 10.1161/STROKEAHA.112.661587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFries T, Avendano M, Glymour MM. Level and change in cognitive test scores predict risk of first stroke. J Am Geriatr Soc. 2009 Mar;57(3):499–505. doi: 10.1111/j.1532-5415.2008.02132.x. [DOI] [PubMed] [Google Scholar]
- 27.Wu Q, Tchetgen Tchetgen EJ, Osypuk TL, White K, Mujahid M, Maria Glymour M. Combining Direct and Proxy Assessments to Reduce Attrition Bias in a Longitudinal Study. Alzheimer Dis Assoc Disord. 2012 Sep 18; doi: 10.1097/WAD.0b013e31826cfe90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994 Feb;24(1):145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 29.Jorm AF, Christensen H, Korten AE, Jacomb PA, Henderson AS. Informant ratings of cognitive decline in old age: validation against change on cognitive tests over 7 to 8 years. Psychol Med. 2000 Jul;30(4):981–985. doi: 10.1017/s0033291799002299. [DOI] [PubMed] [Google Scholar]
- 30.Ofstedal MB, Fisher GF, Herzog AR. Documentation of cognitive functioning measures in the Health and Retirement Study. [Accessed March, 2005];HRS Documentation Report. 2005 :DR-006. http://hrsonline.isr.umich.edu/sitedocs/userg/dr-006.pdf.
- 31.Glymour MM, Avendano M, Haas S, Berkman LF. Lifecourse social conditions and racial disparities in incidence of first stroke. Ann Epidemiol. 2008 Dec;18(12):904–912. doi: 10.1016/j.annepidem.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heeringa SG, Connor JH. Technical Description of the Health and Retirement Study Sample Design. 1995. [Google Scholar]
- 33.Lefebvre C, Deplanque D, Touze E, et al. Prestroke dementia in patients with atrial fibrillation. Frequency and associated factors. J Neurol. 2005 Dec;252(12):1504–1509. doi: 10.1007/s00415-005-0900-2. [DOI] [PubMed] [Google Scholar]
- 34.Pohjasvaara T, Mantyla R, Aronen HJ, et al. Clinical and radiological determinants of prestroke cognitive decline in a stroke cohort. J Neurol Neurosurg Psychiatry. 1999 Dec;67(6):742–748. doi: 10.1136/jnnp.67.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barba R, Castro MD, del Mar Morin M, et al. Prestroke dementia. Cerebrovasc Dis. 2001;11(3):216–224. doi: 10.1159/000047642. [DOI] [PubMed] [Google Scholar]
- 36.Tang WK, Chan SS, Chiu HF, et al. Frequency and determinants of prestroke dementia in a Chinese cohort. J Neurol. 2004 May;251(5):604–608. doi: 10.1007/s00415-004-0385-4. [DOI] [PubMed] [Google Scholar]
- 37.Gottesman RF, Hillis AE. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol. Sep;9(9):895–905. doi: 10.1016/S1474-4422(10)70164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005 Nov;4(11):752–759. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- 39.Kokmen E, Whisnant JP, O’Fallon WM, Chu CP, Beard CM. Dementia after ischemic stroke: a population-based study in Rochester, Minnesota (1960–1984) Neurology. 1996 Jan;46(1):154–159. doi: 10.1212/wnl.46.1.154. [DOI] [PubMed] [Google Scholar]
- 40.Klimkowicz A, Dziedzic T, Polczyk R, Pera J, Slowik A, Szczudlik A. Factors associated with pre-stroke dementia: the cracow stroke database. J Neurol. 2004 May;251(5):599–603. doi: 10.1007/s00415-004-0384-5. [DOI] [PubMed] [Google Scholar]
- 41.Henon H, Pasquier F, Durieu I, et al. Preexisting dementia in stroke patients. Baseline frequency, associated factors, and outcome. Stroke. 1997 Dec;28(12):2429–2436. doi: 10.1161/01.str.28.12.2429. [DOI] [PubMed] [Google Scholar]
- 42.Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995 Mar;25(3):305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 43.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001 May;37(5):1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 44.Appelros P, Stegmayr B, Terent A. A review on sex differences in stroke treatment and outcome. Acta neurologica Scandinavica. 2010 Jun;121(6):359–369. doi: 10.1111/j.1600-0404.2009.01258.x. [DOI] [PubMed] [Google Scholar]
- 45.Demirovic J, Prineas R, Loewenstein D, et al. Prevalence of dementia in three ethnic groups: the South Florida program on aging and health. Ann Epidemiol. 2003 Jul;13(6):472–478. doi: 10.1016/s1047-2797(02)00437-4. [DOI] [PubMed] [Google Scholar]
- 46.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001 Jan 9;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 47.Potter GG, Plassman BL, Burke JR, et al. Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2009 Nov;5(6):445–453. doi: 10.1016/j.jalz.2009.04.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gurland BJ, Wilder DE, Lantigua R, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999 Jun;14(6):481–493. [PubMed] [Google Scholar]
- 49.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008 Sep;18(3):223–254. doi: 10.1007/s11065-008-9064-z. [DOI] [PubMed] [Google Scholar]
- 50.Manly JJ, Schupf N, Tang MX, Stern Y. Cognitive decline and literacy among ethnically diverse elders. Journal of geriatric psychiatry and neurology. 2005 Dec;18(4):213–217. doi: 10.1177/0891988705281868. [DOI] [PubMed] [Google Scholar]
- 51.Goff PA, Steele CM, Davies PG. The space between us: stereotype threat and distance in interracial contexts. J Pers Soc Psychol. 2008 Jan;94(1):91–107. doi: 10.1037/0022-3514.94.1.91. [DOI] [PubMed] [Google Scholar]
- 52.Glymour MM, Avendano M. Can self-reported strokes be used to study stroke incidence and risk factors?: evidence from the health and retirement study. Stroke. 2009 Mar;40(3):873–879. doi: 10.1161/STROKEAHA.108.529479. [DOI] [PubMed] [Google Scholar]
- 53.Glymour MM, Chene G, Tzourio C, Dufouil C. Brain MRI markers and dropout in a longitudinal study of cognitive aging: the Three-City Dijon Study. Neurology. 2012 Sep 25;79(13):1340–1348. doi: 10.1212/WNL.0b013e31826cd62a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chatfield MD, Brayne CE, Matthews FE. A systematic literature review of attrition between waves in longitudinal studies in the elderly shows a consistent pattern of dropout between differing studies. J Clin Epidemiol. 2005 Jan;58(1):13–19. doi: 10.1016/j.jclinepi.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 55.VanderWeele TJ. Bias formulas for sensitivity analysis for direct and indirect effects. Epidemiology. Jul;21(4):540–551. doi: 10.1097/EDE.0b013e3181df191c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.