PCTAIRE1 (also known as PCTK1 and cyclin-dependent kinase 16 (Cdk16)) is a member of the PCTAIRE family, a branch of kinases related to the Cdk family, which includes PCTAIRE1, 2, and 3.1 The human PCTAIRE1 gene is located on chromosome Xp11.23. A physiological function for PCTAIRE1 was suggested by neurons from mutant PCT-1 nematodes, which show altered vesicle transport,2 while gene knockout studies in mice found that mammalian PCTAIRE1 is indispensable for spermatogenesis,3 regulation of intracellular vesicles, and neurite outgrowth. PCTAIRE1 has unique N-terminal and C-terminal domains that flank a central kinase domain that has amino acid sequence similarity to Cdks. While PCTAIRE1 contains a motif reminiscent of cyclin-binding sites found in other Cdk family members, the mechanisms involved in its activation remain unknown. The catalytic domain of PCTAIRE1 contains a putative activation loop (T-loop) that is associated with activation-driving phosphorylation of other kinases, but whether PCTAIRE1 acts through this mechanism is uncertain.
To investigate the effect of PCTAIRE1 knockdown in cancer cells, we performed siRNA experiments that target PCTAIRE1.4,5 In prostate, breast, cervical cancer, and melanoma cell lines, gene knockdown of PCTAIRE1 using siRNA and shRNA diminished cancer cell proliferation. Moreover, annexinV-PI staining showed increases in apoptosis over time in cultures of PCTAIRE1 RNAi-treated cells. However, a role for PCTAIRE1 was not detected in proliferation of non-transformed cells, including the normal fibroblast cell line IMR-90. To examine the mechanisms of cell growth suppression and apoptosis caused by PCTAIRE1 knockdown, cell cycle analyses by FACS were performed. In cultures of PCTAIRE1 knockdown cells, the proportion of M-phase cells first increased, then declined as hypoploid (apoptotic) cells appeared in prostate cancer cultures.4
To identify potential targets of PCTAIRE1, we used yeast 2-hybrid screens of cDNA libraries with human PCTAIRE1 protein as bait. Through these screens we identified tumor suppressor p27 (also known as Kip1, cyclin-dependent kinase inhibitor 1B) as a PCTAIRE1 interactor, and in vitro kinase assays demonstrated that PCTAIRE1 phosphorylates p27 at Ser10. Consistent with a tumor suppressor function for p27, the loss of nuclear p27 is observed in human malignancies and is associated with high-grade tumors and poor prognosis.6 Knockdown of PCTAIRE1 by siRNA/shRNA modulated p27 (Ser10) phosphorylation and led to p27 accumulation in cancerous cells but not non-transformed cells. In tumor xenografts of prostate cancer cells, doxycycline-inducible conditional knockdown of PCTAIRE1 restored expression of p27 protein and suppressed tumor growth. These results prompted us to analyze the phosphorylation of p27 by PCTAIRE1 during the cell cycle. Mechanistic studies using synchronized cervical cancer HeLa cells demonstrated that PCTAIRE1 phosphorylates p27 during the S and M phases of the cell cycle. Furthermore, p27 silencing was sufficient to rescue cells from mitotic arrest caused by PCTAIRE1 silencing.
We also studied primary tumor samples to evaluate the clinical importance of PCTAIRE1. In primary tumors from patients with breast or prostate cancers, PCTAIRE1 was highly expressed in tumor lesions compared to adjacent normal tissues. In prostate cancers, comparison of PCTAIRE1 immunostaining with Gleason grade revealed low expression levels in very well-differentiated tumors relative to less- differentiated tumors. To further assess the role of PCTAIRE1 in cancers, we used the Oncomine database to evaluate levels of PCTAIRE1 mRNA expression. When multiple studies containing informative PCTAIRE1 expression levels were combined, PCTAIRE1 was identified as one of the most upregulated genes in cancers compared with corresponding normal tissues. We also assessed the prognostic value of PCTAIRE1 using Kaplan-Meier Plotter, an online tool that correlates survival with gene expression based upon microarray data. Our analyses showed that high PCTAIRE1 mRNA levels were significantly correlated with lower overall survival in lung and breast cancers.
Very recently, we observed that gene knockdown of PCTAIRE1 sensitized various types of cancer cells to TNF-family cytokines, such as Fas-ligand and TNF-related apoptosis-inducing ligand (TRAIL) (unpublished data). However, knockdown of p27 does not restore Fas/TRAIL-resistance in PCTAIRE1-knockdown cells, suggesting that accumulation of p27 does not affect the sensitivity of PCTAIRE1-knockdown cells to Fas/TRAIL-induced apoptosis. Therefore, other PCTAIRE1 substrates might mediate the resistance of cancer cells to Fas/TRAIL, and as such further studies will be essential for discovering the details of this important regulatory pathway as well as to unlock new avenues for PCTAIRE1-related cancer therapeutics.
In summary, our findings reveal an unknown role for PCTAIRE1 in regulating p27 stability, mitosis, apoptosis, and tumor growth (Fig. 1). Notably, we observed these essential functions for PCTAIRE1 in multiple cell lines (prostate, breast, cervical cancers, and melanomas), which implies that the p27 regulation by PCTAIRE1 may be a common mechanism in human cancers. Taken together, inhibition of PCTAIRE1 in tumors containing pathological elevations of this kinase may provide a strategy for improved treatments of some human cancers.
Figure 1.
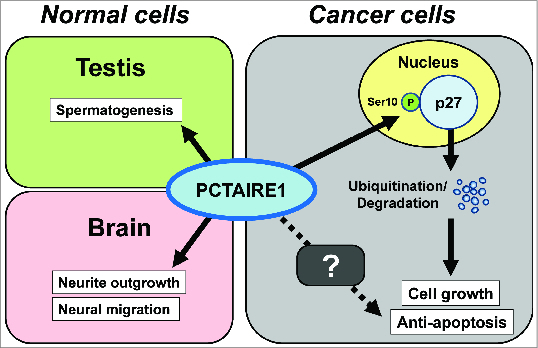
A tumorigenic role for PCTAIRE1. In normal tissues (left), PCTAIRE1 is required for spermatogenesis (in testis) and neuron differentiation (in brain). In cancer cells (right), PCTAIRE1 phosphorylates p27 at Ser10, thereby promoting p27 ubiquitination/degradation, which leads to cell cycle progression and decreased levels of apoptosis. Unknown mechanism(s) may also exist in the PCTAIRE1-apoptosis pathway.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Cole AR. Neurosignals 2009; 17: 288-97; PMID:19816065; http://dx.doi.org/ 10.1159/000231895 [DOI] [PubMed] [Google Scholar]
- 2. Ou CY, et al. . Cell 2010; 141: 846-58; PMID:20510931; http://dx.doi.org/ 10.1016/j.cell.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mikolcevic P, et al. . Cell Cycle 2012; 11: 3758-68.; PMID:22895054; http://dx.doi.org/ 10.4161/cc.21592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yanagi T, et al. . Cancer Res 2014; 74: 5795-807; PMID:25205104; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-0872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yanagi T, et al. . Oncoscience 2014; 1: 624-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chu IM, et al. . Nat Rev Cancer 2008; 8: 253-67; PMID:18354415; http://dx.doi.org/ 10.1038/nrc2347 [DOI] [PubMed] [Google Scholar]


