The genome in every cell is constantly attacked by a large variety of exogenous and endogenous genotoxins. DNA damage has long been known as causal factor for cancer development and has more recently also been recognized as driving force of the aging process.1 Recent insights have shed light on how organisms respond to DNA damage accumulation by employing bona fide longevity assurance pathways.
A key longevity assurance pathway had been revealed more that 20 years ago by seminal work of the Kenyon, Johnson, and Ruvkun labs. C. elegans that carry mutations in the daf-2 gene encoding for the homolog of the insulin-like growth factor-1 receptor (IGF-1R) or the downstream PI3 kinase age-1 show a remarkable long lifespan that entirely depends on the activity of the FOXO transcription factor DAF-16. The IGF-1 pathway also regulates longevity in mammals, where it is systemically controlled by growth hormone (GH) signaling. The GH/IGF-1 signaling circuit is known as the somatic growth axis and during development controls the growth of the body. When mice are born with defects in pituitary development resulting in low GH levels or they lack the GH receptor (GHR), they remain small and –just like nematodes with mutations in daf-2- live significantly longer than their normal littermates. Aging humans show a decline in their endocrine growth parameters, including reduced GH and IGF-1 levels. Mice that are born with defects in various DNA repair genes that lead to premature aging show a reduction of the somatic growth axis.2 The attenuation of the somatic growth axis in response to persistent DNA lesions that interfere with transcription is mediated by reduction of the key mediators, the receptors for GH and IGF-1R.3 It was, therefore, suggested that the attenuation of the somatic growth axis is driven by the accumulation of DNA damage with aging.4
What is the consequence of the reduced IGF-1 signaling when DNA damage persists? Our recent results in the genetic C. elegans system demonstrate that the activation of the FOXO transcription factor DAF-16 could indeed overcome the detrimental consequences of persistent DNA damage (Fig. 1).5 Nematodes that carry defects in the transcription-coupled nucleotide excision repair (NER) genes csa-1 of csb-1 cease developmental growth already at low levels of DNA lesions inflicted by UV irradiation.5,6 In humans, CSA or CSB mutations lead to the premature aging disorder Cockayne syndrome (CS). CS patients cease developmental growth during the first years of childhood. Shortly thereafter, they suffer from mental retardation, retinal degeneration, cachexia, and die in their teenage.1 The complexity of human CS is thus phenotypically mirrored in the simple metazoan C. elegans. Transcriptome analysis revealed that the daf-2 pathway indeed responds to DNA damage during animal development leading to activation of DAF-16. DAF-16 was known to respond when worms suffer from starvation through arresting developmental growth. As soon as food becomes available the worms resume development to maturity and produce offspring in the nutritious environment. However, when responding to DNA damage, DAF-16 activity promoted developmental growth, instead of arrest.5 Even in completely NER defective animals, strong activation of DAF-16 could overcome the developmental arrest. How could DAF-16 assume such a distinct function in the DNA damage response, as opposed to the growth halting function during starvation? Intriguingly, the DAF-16 target genes that are induced upon DNA damage but repressed upon starvation contained to a large extend DAF-16 associated elements that comprise target sites for GATA transcription factors. A survey of GATA mutant strains revealed that the GATA-factor EGL-27 was required for inducing DAF-16 target genes upon DNA damage. Indeed, EGL-27 directly interacts with DAF-16 and together they promote developmental growth upon DNA damage (Fig. 1).
Figure 1.
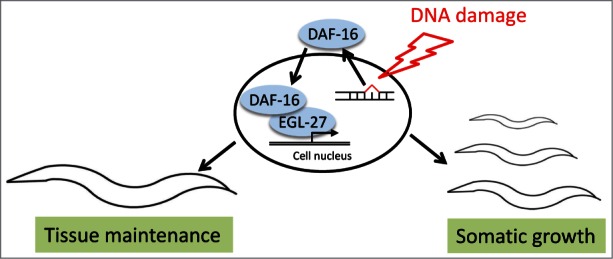
In response to DNA damage the FOXO transcription factor DAF-16 translocates into the nucleus where it interacts with the GATA transcription factor EGL-27 to induce developmental growth genes that allow the growth of somatic tissues even in the presence of persistent DNA damage. In adult animals, DAF-16 enhances the maintenance of tissue functionality to withstand persistent DNA damage. However, as worms age DAF-16 responsiveness to DNA damage declines rendering the somatic tissues increasingly defenseless against the accumulating DNA damage with aging.
DAF-16 also responds to similar DNA lesions in adult worms and ensures the functional maintenance of tissues even when the DNA damage cannot be repaired. However, with advancing age, the responsiveness of DAF-16 to DNA damage declines. In the absence of DAF-16 activation, the worms then succumb to the damage. DAF-16 thus promotes tolerance to persistent DNA damage.
Development can proceed and tissues remain functional for as long as DAF-16 responds to DNA damage. The DNA damage tolerance mechanisms might be particularly useful in postmitotic tissues, which cannot be replenished by stem cells. In C. elegans, differentiated cells cannot be replaced, as somatic tissues in the adult worms are entirely postmitotic. Only germ cells continuously proliferate and go through mitotic and meiotic cell divisions. Most developmental cell divisions occur during embryogenesis before the somatic cells terminally differentiate. It is thus conceivable that the DAF-16 mediated DNA damage tolerance might predominantly benefit postmitotic tissues. Indeed, reduced IGF-1R signaling can benefit neuronal tissues in mammals and protect them from neurodegeneration. In contrast, IGF-1 signaling is an important mitogenic factor that is required to maintain proliferation for the replenishment of tissues.7
References
- 1. Wolters S, Schumacher B. Genome maintenance and transcription integrity in aging and disease. Front Genet 2013; 4:19; PMID:23443494; http://dx.doi.org/ 10.3389/fgene.2013.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 2006; 444:1038-43; PMID:17183314; http://dx.doi.org/ 10.1038/nature05456 [DOI] [PubMed] [Google Scholar]
- 3. Garinis GA, Uittenboogaard LM, Stachelscheid H, Fousteri M, van Ijcken W, Breit TM, van Steeg H, Mullenders LHF, van der Horst GTJ, Brüning JC, et al. Persistent transcription-blocking DNA lesions trigger somatic growth attenuation associated with longevity. Nat Cell Biol 2009; 11:604-15; PMID:19363488; http://dx.doi.org/ 10.1038/ncb1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garinis GA, Schumacher B. Transcription-blocking DNA damage in aging and longevity. Cell Cycle 2009; 8:2134-5; PMID:19556874; http://dx.doi.org/ 10.4161/cc.8.14.9049 [DOI] [PubMed] [Google Scholar]
- 5. Mueller MM, Castells-Roca L, Babu V, Ermolaeva MA, Müller R-U, Frommolt P, Williams AB, Greiss S, Schneider JI, Benzing T, et al. DAF-16/FOXO and EGL-27/GATA promote developmental growth in response to persistent somatic DNA damage. Nat Cell Biol 2014; 16:1168-79; PMID:25419847; http://dx.doi.org/ 10.1038/ncb3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Babu V, Hofmann K, Schumacher B. A C. elegans homolog of the Cockayne syndrome complementation group A gene. DNA Repair (Amst) 2014; 24:57-62; PMID:25453470; http://dx.doi.org/ 10.1016/j.dnarep.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Behrens A, van Deursen JM, Rudolph KL, Schumacher B. Impact of genomic damage and ageing on stem cell function. Nat Cell Biol 2014; 16:201-7; PMID:24576896; http://dx.doi.org/ 10.1038/ncb2928 [DOI] [PMC free article] [PubMed] [Google Scholar]


