Defects in cell death pathways promote tumor development and progression, contributing to drug resistance and poor outcomes in cancer treatment. Direct activation of cell death signaling routes, independently of the cellular proliferation state and of the frequently mutated tumor suppressor genes, constitutes an appealing approach for overcoming the natural reluctance of cancer cells to trigger their own demise in tumor-targeted therapy.
The ether phospholipid edelfosine, considered the prototype molecule of a family of structurally related antitumor compounds collectively known as alkylphospholipid analogs or antitumor lipids, shows the ability to kill a wide number of distinct human cancer cells by affecting the molecular machinery closely involved in cell death. This cellular demise displays major morphological and biochemical hallmarks of apoptosis, involving death receptor-mediated extrinsic and mitochondria-mediated intrinsic apoptotic signaling pathways in hematological cancer cells1,2 as well as endoplasmic reticulum stress response in solid tumor cells.3 All of these apoptotic signaling routes eventually require the involvement of mitochondria, and are blocked by protecting mitochondria through overexpression of Bcl-2 or Bcl-xL.2,3 Interestingly, we have recently found that edelfosine also induces a rapid necroptotic cell death involving receptor-interacting protein kinase 1 (RIPK1) and RIPK3 in U118 glioblastoma cells, which have low levels of proteins involved in the extrinsic apoptotic pathway (Fas/CD95, FADD and 57-kDa procaspase-8).4 Inhibition of RIPK1 or silencing of RIPK3 inhibited edelfosine-induced necroptosis, while increased apoptosis.4 Thus, these data indicate that this antitumor drug is able to promote distinct types of cell death in tumor cells depending on the cell's ability to activate specific signaling pathways that modulate cell demise, ranging from apoptosis to necrosis/necroptosis, which has led us to propose the model depicted in Figure 1 for the triggering, initiating and execution phases in edelfosine-induced cell death. Edelfosine induces different cell death signals (cell death triggers) forcing the cancer cell to commit suicide through a highly regulated process (Fig. 1). The killing activity of edelfosine, which is a rather selective drug for cancer cells through its preferential uptake by malignant cells, involves its interaction with cell membranes, particularly lipid rafts, leading to the reorganization of the lipid raft protein composition,1,2,5 followed by raft internalization and interactions with other subcellular structures that are not well understood.6 These processes could lead to apoptosis in apoptosis-prone cells or to necroptosis in apoptosis-reluctant, but necroptosis-prone cells. Lipid rafts/death receptors, endoplasmic reticulum, mitochondria and the necrosome have been shown to participate in edelfosine-induced cell death in different cell systems,1-6 and it is important to explore in more detail their role in the initiating phase of both apoptosis and necrosis/necroptosis (Fig. 1), in order to get new insights into the mechanism of action of the drug as well as into the distinct interconnected underlying signaling pathways that result in different cell death types (Fig. 1). For e.g. mitochondria have an important role amplifying the apoptotic signal transduced from death receptors (especially in type II cells that require mitochondrial amplification of apoptosis), but mitochondria may also be a direct target of edelfosine,6 acting as a trigger, or promote necroptosis by generating ROS.7
Figure 1.
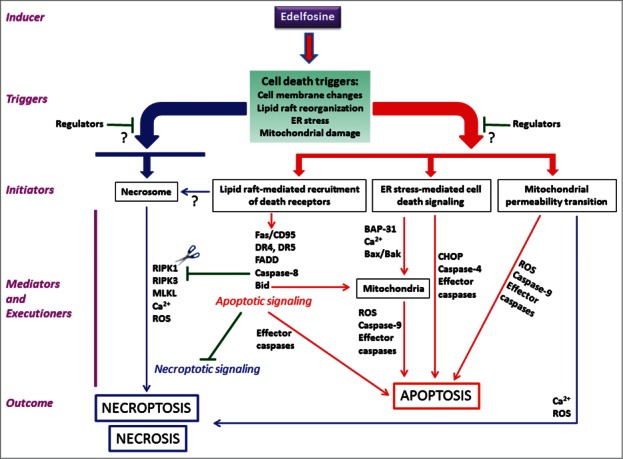
Schematic view of the putative phases involved in edelfosine-induced cell death. Edelfosine induces a series of cell death triggers that together with the initiators set off distinct signaling and cross-talk processes leading to different types of cell death, ranging from apoptosis (red) to necrosis/necroptosis (blue). The whole process is highly modulated by not yet fully characterized regulators (?), acting at different levels of the cell death process. ROS, reactive oxygen species.
Rafts serve as foci for recruitment and concentration of signaling molecules and have been implicated in signal transduction from cell surface receptors, linking exogenous stimuli to the intracellular signaling machinery that mediates and executes cell death. Necroptosis is a programmed and regulated necrosis process that is dependent on RIPK1 and RIPK3-MLKL (mixed lineage kinase domain-like) axis and has been linked to death receptor activation.7 Edelfosine has been found to induce apoptosis in a number of cancer cells through the participation of Fas/CD95 death receptor,1,2 but the triggering of apoptosis or necrosis seems to be dependent on the molecular features and gene expression profiles of the cell target, such as the relative ratio of RIPK1/procaspase-8 level, which is much higher in U118 cells (undergoing necroptosis) than in HeLa and Jurkat cells (undergoing apoptosis).4 On these grounds, edelfosine acting on the cell membrane seems to turn on cell death triggers, committing the cell to a cell death process that is executed through either apoptosis or necroptosis depending on the cellular phenotype. Despite the onset of different types of cell death can be detected simultaneously at some degree, the initiation of a determined mechanism of cell death seems to influence negatively on the other types of cell death as it happens in U118 cells.4 Unlike apoptotic cell death that preserves physically the cell membrane with no release of intracellular contents, necrotic cell death results in permeabilization of the cell membrane and release of cellular contents to the extracellular milieu that could lead to secondary damage in surrounding cells. On these grounds the induction of distinct types of cell death can be of major importance depending of the tissue or development stage where the cell demise process occurs in order to avoid collateral damage. Unveiling the molecular and regulatory features of the early/primary cell death triggers and initiators as well as of the switches leading to the different types of cell death will be of major importance in delineating new approaches to trigger and modulate cell death processes of critical importance in the treatment of tumors and neurodegenerative diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Gajate C, et al. J Exp Med 2004; 200:353-65; PMID:15289504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gajate C, et al. Blood 2007; 109:711-9; PMID:17003375 [DOI] [PubMed] [Google Scholar]
- 3. Gajate C, et al. Oncogene 2012; 31:2627-39; PMID:22056873; http://dx.doi.org/ 10.1038/onc.2011.446 [DOI] [PubMed] [Google Scholar]
- 4. Melo-Lima S, et al. Oncoscience 2014; 1:649-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cuesta-Marban A, et al. J Biol Chem 2013; 288:8405-18; PMID:23335509; http://dx.doi.org/ 10.1074/jbc.M112.425769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gajate C, et al. Anticancer Agents Med Chem 2014; 14:509-27; PMID:24628241 [DOI] [PubMed] [Google Scholar]
- 7. Vanden Berghe T, et al. Nat Rev Mol Cell Biol 2014; 15:135-47; PMID:24452471; http://dx.doi.org/ 10.1038/nrm3737 [DOI] [PubMed] [Google Scholar]


