Abstract
Patient: Female, 44
Final Diagnosis: Tubulointerstitial nephritis • uveitis syndrome
Symptoms: —
Medication: Loxoprofen sodium hydrate
Clinical Procedure: Renal biopsy
Specialty: Nephrology
Objective:
Rare disease
Background:
Although TINU syndrome is characterized by idiopathic TIN with bilateral anterior uveitis, few reports have provided a comprehensive summary of the features of this disorder. Previous reports have suggested that many Japanese patients had HLA-A2 and -A24 (7), but there is no evidence.
Case Report:
A 44-year-old female was referred to our hospital due to renal dysfunction in March 2012. After admission, her symptoms improved spontaneously without medication within 2 weeks. In the outpatient clinic, she was diagnosed with idiopathic bilateral anterior uveitis in May, and her renal dysfunction relapsed in November. A renal biopsy showed diffuse TIN. We made a diagnosis of TINU syndrome because we could not explain the origin, and treated her with a systemic corticosteroid. Her renal function and ocular symptoms have been improving. The patient had HLA-A24, -B7, -DR1, -C*07: 02 and -DQB1*05: 01: 01. We collected 102 Japanese cases in PubMed, Ovid MEDLINE, and the Japanese Medical Abstracts Society and compared our case with the previous cases.
Conclusions:
This disorder affects primarily young females (median age, 14 years), and the most common symptom is fever (44/102 cases). We conducted a statistical analysis using contingency table and Pearson’s chi-square test, for HLA-A2 and A24, and calculated the odds ratio (OR). There are no significant differences (A2 was present in 7/22 cases and in 19/50 controls, p value (P) 0.61, OR 0.76 (95% confidence interval (CI)) 0.27–2.2; A24 was present in 10/22 cases and in 33/50 controls, P 0.10, OR 0.43, CI 0.16–1.2).
MeSH Keywords: HLA Antigens, Nephritis, Interstitial, Uveitis, Anterior
Background
Since tubulointerstitial nephritis and uveitis (TINU) syndrome was first reported by Dobrin et al. in 1975 [1], many clinicians have submitted case reports to journals around the world. Many clinicians have inferred that TNIU syndrome is an immunological abnormality. More recently, the pathogenesis of TINU syndrome has been gradually becoming clearer. For example, the existence of common antigens present in tubular cells and eyes [2], modified C reactive protein levels [3], and IgG4-related systemic disease [4,5] were all demonstrated to be related to TINU. However, a great deal of uncertainty still remains, such as the optimal treatment and the relevance of the HLA type.
We recently treated an adult patient with this disorder. We collected the previous reports of Japanese cases and compared our case with the average Japanese case. A previous report suggested that particular HLA types are related to this disorder, so patients who have these HLA types tend to be affected with TINU syndrome [6]. Many Japanese patients have HLA-A2 and -A24, which have both been reported to be associated with TINU [7]. To determine the significance of these types in Japanese patients, we conducted a statistical analysis of the reported cases.
Case Reports
A 44-year-old female, who had been healthy until approximately 2 months before admission, was referred to our hospital because of a 2-month history of a low grade fever, weight loss (her weight had decreased by 2.0 kg), and mild renal dysfunction (serum creatinine 1.08 mg/dl) in March 2012. The symptoms had started with common cold-like symptoms, and she had seen a family doctor in late January. She had been taking loxoprofen sodium hydrate and sometimes Chinese herbs (Hochuekkito and Bakumondouto) for approximately 2 months.
At the first admission, she complained of general fatigue, anorexia, and arthralgias. The physical findings indicated cervical lymphadenopathy but no history of skin rash or edema. She had a low-grade fever (37.1°C), and her blood pressure, pulse, and respiration rate were within the normal range.
The laboratory tests showed blood urea nitrogen level of 17.6 mg/dl, creatinine 1.27 mg/dl, and estimated glomerular filtration rate (eGFR) of 37.5 ml/min/1.73 m2. The serum levels of total protein, albumin, globulin, electrolytes, lipase, and amylase were normal, as were tests of her liver function. The urinalysis showed that β2-Microglobulin levels were 184 ng/ml, N-acetyl-β-D-glucosaminidase 23.7 U/liter (normal range 0–10 U/liter). The urinary sediment contained 5–9 red blood cells/high power field (hpf) without any casts. A chest X-ray, ultrasonic abdominal images, and thoracic computed tomography scans were normal. These results and the patient’s clinical history suggested drug-induced tubulointerstitial nephritis, probably due to loxoprofen sodium hydrate. After the administration of NSAIDs was stopped and the patient received rehydration, her renal function and disease presentation gradually returned to normal after 3 days. Therefore, she was discharged from our hospital after 2 weeks. Since then, we have taken a wait-and-see approach. In May 2012, she reported severe pain in both eyes. A general ophthalmologist diagnosed idiopathic bilateral anterior uveitis, but there were no apparent abnormalities of the fundus oculi. The ophthalmologist prescribed topical corticosteroid for the eyes. Three months later, her eye symptoms were found to have improved.
We noted that her serum creatinine and C-reactive protein levels were barely elevated (1.17 mg/dl and 0.38 mg/dl, respectively), and a screening urine dipstick showed occult blood 1+ in the outpatient clinic in November. Although she had no symptoms at that time, we assumed that she had a relapse of tubulointerstitial nephritis and admitted her to our hospital to perform a renal biopsy (Figure 1).
Figure 1.
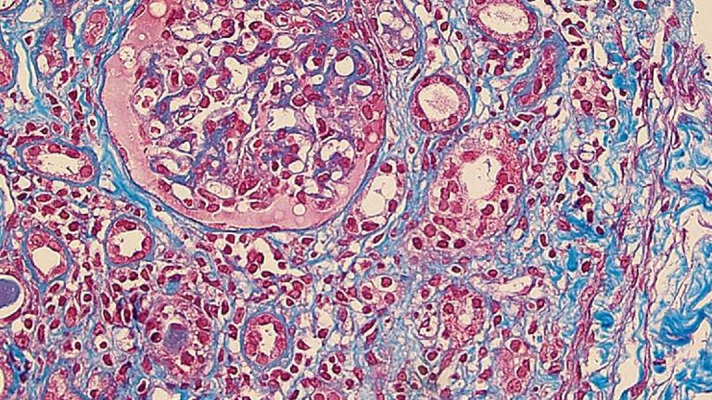
The renal biopsy specimen. This panel shows a high-power image (×400) of a slide prepared from the renal biopsy specimen (Azan and Masson trichrome stain). The optical microscopic study showed almost normal glomeruli and diffuse infiltration of mononuclear cells in the interstitium. The mononuclear cells were mostly lymphocytes, with a few plasma cells. Furthermore, this panel shows that mononuclear cells were infiltrating the tubules. A low-power image (×20) revealed that fibrosis was present in 30% of the whole tissue.
The renal biopsy showed tubulointerstitial nephritis and normal glomeruli; there were 13 glomeruli, 1 was located just beneath the cortices with global sclerosis, but the other 12 glomeruli were almost normal. The most characteristic optical microscopic finding was the infiltration of mononuclear cells, largely composed of lymphocytes, with a few plasma cells. There was fibrosis involving approximately 80% of the interstitium. Immunofluorescence microscopy revealed that there was very weak IgG staining in the glomeruli, but not any other immunoglobulins or complement components. There were no electron-dense deposits detected by electron microscopy.
During hospitalization, an ophthalmologist diagnosed her to have bilateral anterior uveitis, because there was a keratic precipitate, but the retina and vitreous body had nonspecific findings.
As a differential diagnosis, we considered Sjögren’s syndrome, sarcoidosis, IgG4-related systemic disease, Behçet’s syndrome, and infectious mononucleosis. Although further tests were administered, they were all negative. For example, there were no anti-nuclear antibodies, anti-double stranded DNA antibodies, anti-SS-A/B antibodies, anti-neutrophil cytoplasmic antibodies, or anti-Smith antibodies. Chest X-rays showed no characteristic findings of sarcoidosis, such as bilateral hilar lymphadenopathy. Furthermore, at that time, she did not have any overt findings suggesting infection or Behçet’s syndrome. Therefore, we diagnosed TINU syndrome.
We had started a systemic corticosteroid, prednisolone, at 60 mg (1 mg/kg) daily, and tapered the dose for 8 months. Her renal function had stopped deteriorating and started to slowly improve. While the observation is still ongoing, the most recent data showed a serum creatinine level of 0.96 mg/dl and an eGFR of 50.3 ml/min/1.73 m2. In addition, the ophthalmological keratic precipitate disappeared in December 2012, and she was able to stop the topical corticosteroids in February 2013. During a follow-up visit, we checked her human leukocyte antigens and alleles; the A locus was A24 and the B locus was B7. The DR locus had DR1, and the allele of C, and the DQB1 were C*07: 02 and -DQB1*05: 01: 01, respectively. The TIN and uveitis have not relapsed for approximately 12 months.
Discussion
Tubulointerstitial nephritis and uveitis (TINU) syndrome was first reported by Dobrin et al. in 1975 [1]. Over 200 cases have since been reported by physicians all over the world. Mannideville et al. assembled 133 cases and reported that this disorder affects primarily young females (the median age of onset is 14 years), with about 3 times as many females as males developing the syndrome. They also mentioned that the tubulointerstitial nephritis (TIN) generally precedes the uveitis by a few months, and proposed criteria for diagnosing this disorder in 2001. Our patient had typical symptoms (chronic low-grade-fever, weight loss, and anorexia) according to the diagnostic criteria [7].
We were able to identify 102 cases (including our case) of TINU syndrome reported in Japan, 36 cases were reported in English, and the others were reported in Japanese, from 1977 to 2013. We searched the literature in PubMed, Ovid MEDLINE, and the Japanese Medical Abstracts Society. Similar to the study by Mannideville, there was a female predominance of approximately 2.5: 1, and a higher rate of younger patients (the median age was 14 years, range: 8–69 years) in the Japanese patients. Fever (44/102 cases, about 43%) was the most common symptom of this disorder in Japan, as in the previous report [7].
Seventy-eight of the 102 cases (76.5%) were administered systemic corticosteroids, and 9 cases were administered the steroid for ophthalmic symptoms, not renal indications. Forty-six of the 78 patients (59%) who received systemic steroids recovered their baseline renal function by the end of the observation period. However, 16 of the 22 patients (72%) who did not receive systemic corticosteroids also recovered their baseline values. Topical corticosteroids were used to treat the uveitis in 76 cases: 46 (60.5%) had a good response, but 24 (31.6%) were non-responders. We compared the 2 groups (treated/untreated) to determine whether systemic corticosteroids were effective for TINU syndrome, and did not find any significant advantages for steroid treatment. Each of the previous reports had reported different outcomes (e.g., the length of therapy, renal function, and dosage of corticosteroid used) because there is no standard for reporting. TINU syndrome is very rare; therefore, large, higher-powered studies will be required to determine the basis for the therapeutic indications.
Our patient’s serological HLA tests showed -A24, -B7, -DR1 and genomic -C and -DQB1 typing revealed C*07: 02 and -DQB1*05: 01: 01, respectively. HLA-DQB1*05 was reported to be strongly associated with TINU syndrome by Ralph et al. [6]. Mandeville et al. suggested that HLA-A2 and -A24 were important antigens associated with this disorder in Japanese subjects, because these 2 antigens had been identified in many (75%) Japanese patients in 2001. Obviously, they also knew that these 2 antigens were often observed in healthy Asian people [7]. In the 102 Japanese cases identified for the present study, we analyzed the findings for 25 cases where the results of HLA typing were reported (Table 1). We conducted a statistical analysis using a 2×2 contingency table and Pearson’s chi-square test for these 2 antigens and calculated the odds ratio (OR). Thanks to the HLA Laboratory, there is extensive data available about the frequency of Japanese serological and genomic HLA tests (Kyoto, Japan), so we were able to obtained data on the HLA results from 50 healthy Japanese subjects. Seven of the 22 TINU patients (32%) had HLA-A2 and 19 of the 50 controls (48%) had it; P value (P) 0.61, OR 0.76, 95% confidence interval (CI) 0.27–2.2. Twelve of the 22 patients (54.5%) had HLA-A24, and 33 of the 50 controls (64%) had it; P 0.10, OR 0.43, 95% CI 0.16–1.2. There were therefore no significant differences in the specific HLA types between those with and without the disease.
Table 1.
The serological and genomic HLA typing.
| No. | HLA-A | HLA-B | HLA-C | HLA-DQ | HLA-DR |
|---|---|---|---|---|---|
| [14] | A24/A31 | Bw54/Bw55 | Cw1/Cw3 | – | DR4/DRw6 |
| [14] | A24/A31 | Bw48/Bw7 | Cw7 | – | DR4/DRw12 |
| [14] | A24/A2 | Bw54/Bw61 | Cw3 | – | DRw8 |
| [14] | A24/A33 | Bw52/Bw60 | Cw3 | – | DRw8 |
| [15] | A11 | B54/B35 | Cw1/Cw3 | DQB1 0401 | DR4(DRB1 04051) |
| [16] | – | – | – | – | DR6/DR9 |
| [16] | – | – | – | – | DR4/DR6 |
| [17] | A2/A11 | Bw62/Bw46 | Cw4 | – | – |
| [17] | A2/A11 | B54/B35 | Cw1/Cw3 | – | DR4/DR11 |
| [17] | A24/A26 | B70/B51 | Cw7 | – | DR4/DR2 |
| [17] | A26/A11 | Bw61/Bw46 | Cw3 | – | – |
| [17] | A2/A24 | B55/B62 | Cw1/Cw7 | – | DR12 |
| [18] | A9 | Bw54 | C1 | – | DR4 |
| [19] | A24/A33 | B44 | Cw3 | – | – |
| [20] | A24(9)/A26(10) | B35/Bw62(15) | Cw3 | – | DRw12(5) |
| [21] | A24/A26 | B7/56 | C04011/C0702 | – | B101/B104 |
| [22] | A11/A24 | B54/B61 | Cw1/Cw3 | DQ1 | DR14 |
| [23] | A2/A33 | B44/B61 | Cw3 | DQ13/DQ1/DQ4 | DR4 |
| [24] | – | – | – | – | DR4/DR12 |
| [25] | A2/A11 | Bw48/Bw54 | Cw1 | – | – |
| [26] | A33 | B44/B61 | C3 | DQ1/DQ3 | DR9/DR13 |
| [26] | A26 | B61/B62 | C3/C7 | DQ1/DQ3 | DR8/DR9 |
| [27] | A29 | Bw52/Bw59 | Cw1 | – | – |
| [28] | A2/A24 | B51/B61 | Cw10/Cw14 | – | DR4/DR14 |
| Our patient | A24 | B7 | C*07: 02/– | DQB1 05: 01: 01 | DR1 |
Number is same as references. Each report described serological or genomic HLA typing. The bar (–) was unsurveyed or undescribed articles.
We first diagnosed the present patient with drug-induced tubulointerstitial nephritis (TIN) because of her clinical course and the results of the lymphocyte transformation test (LTT), which is called the drug-induced lymphocyte stimulation test (DLST) in Japan. Domenico et al. suggested that drug-induced TIN could cause TINU syndrome [9]. We hypothesized the patient could have developed TINU syndrome because she had an allergic susceptibility to loxoprofen sodium hydrate, Hochuekkito, or Bakumondoto. However, the LTT has been disputed, and has been concluded to be an insufficient method. Moreover, some reports have pointed out that Chinese herbs have mitogenic effects [10]. Although the challenge test is the most reliable method of diagnosing allergic patients, it is difficult to perform the test in most cases because of the risk (e.g., anaphylactic shock) and ethical concerns. Therefore, many clinicians have noted the presence of basophils and their functions in allergic inflammation, and have recently focused on a new method, the basophil activation test (BAT) [11,12]. We performed the BAT in the present case and obtained contradictory results to the LTT. The BAT is still being optimized, but it is a favorable method to elucidate the cause of allergic disorders [11–13]. There have been no previous reports about performing the BAT for tubulointerstitial nephritis. Further study is needed to evaluate the results and their significance for individual patients.
Conclusion
We assembled characteristics of Japanese patients and found no significant differences between both HLA antigens and TINU syndrome in Japanese patients.
Acknowledgments
We thank Hiroto Kojima, manager of the HLA Laboratory, and Hiroo Saji, director of the HLA laboratory, for offering the HLA data from the healthy Japanese subjects.
References:
- 1.Dobrin RS, Vernier RL, Fish AL. Acute eosinophilic interstitial nephritis and renal failure with bone marrow lymph node granulomas and anterior uveitis. A new syndrome. Am J Med. 1975;59:325–33. doi: 10.1016/0002-9343(75)90390-3. [DOI] [PubMed] [Google Scholar]
- 2.Kaori S, Guy VJ, Eddy V, et al. Tubulointerstitial Nephritis and Uveitis Syndrome: A Case with an Autoimmune Reactivity Against Retinal and Renal Antigens. Ocular Immunol Inflamm. 2008;16:51–53. doi: 10.1080/09273940801899772. [DOI] [PubMed] [Google Scholar]
- 3.Ying T, Feng Y, Zhen Q, et al. Modified C-Reactive Protein Might be a Target Autoantigen of TINU Syndrome. Clin J Am Soc Nephrol. 2011;6:93–100. doi: 10.2215/CJN.09051209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toshiro S, Yuki T, Yoshikata M, et al. Is Tubulointerstitial Nephritis and Uveitis Syndrome Associated with IgG4-Related Systemic Disease? Nephrology. 2008;13(1):89. doi: 10.1111/j.1440-1797.2007.00870.x. [DOI] [PubMed] [Google Scholar]
- 5.Houghton D, Troxell M, Fox E, et al. TINU (tubulointerstitial nephritis and uveitis) syndrome is not usually associated with IgG4 sclerosing disease. Am J Kidney Dis. 2012;59:583–84. doi: 10.1053/j.ajkd.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Ralph DL, Min SP, Sarah MR, et al. Strong Associations between Specific HLA-DQ and HLA-DR Alleles and the Tubulointerstitial Nephritis and Uveitis Syndrome. Invest Ophthalmol Vis Sci. 2003;44:653–57. doi: 10.1167/iovs.02-0376. [DOI] [PubMed] [Google Scholar]
- 7.Mandeville JTH, Levinson RD, Holland GN. The tubulointerstitial nephritis and uveitis syndrome. Surv Ophthalmol. 2001;46:195–208. doi: 10.1016/s0039-6257(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 8. HLA Laboratory: http://www.hla.or.jp.
- 9.Domenico S, Giuseppe V, Stefania R, et al. Drug-induced TINU syndrome and genetic characterization. Clin Neph. 2011;3:230–36. doi: 10.5414/cn107119. [DOI] [PubMed] [Google Scholar]
- 10.Naoki M, Toshiaki K, Junichi T, et al. Lymphocyte Transformation Test for Medicinal Herbs Yields False-Positive Results for First-Visit Patients. Clin Diagn Lab Immunol. 2003;10(3):479–80. doi: 10.1128/CDLI.10.3.479-480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mark CS, Brian SK, Jonathan MS, David A. Basophils and allergic inflammation. J Allergy Clin Immunol. 2013;132:789–98. doi: 10.1016/j.jaci.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donald WM., Jr Baltimore. Basophil activation testing. J Allergy Clin Immunol. 2013;132:777–87. doi: 10.1016/j.jaci.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 13.de Weck AL, Sanz ML, Gamboa PM, et al. Diagnostic Tests Based on Human Basophils: More Potentials and Perspectives than Pitfalls. Int Arch Allergy Immunol. 2008;146:177–89. doi: 10.1159/000115885. [DOI] [PubMed] [Google Scholar]
- 14.Tadashi I, Naoto Y, Masaki K, et al. [HLA tissue types in patients with acute tubulointerstitial nephritis accompanying uveitis] Nippon Jinzo Gakkai Shi. 1993;45(6):723–31. [in Japanese] [PubMed] [Google Scholar]
- 15.Hiroki M, Kiyofumi M, Yukiko H, et al. [Ocular hypertension in case of tubulointerstitial nephritis and uveitis syndrome] Rinsho Ganka. 2005;59(3):353–57. [in Japanese] [Google Scholar]
- 16.Hiroshi T, Shinobu W, Tohru N, et al. Tubulointerstitial Nephritis and Uveitis Syndrome in Two Siblings. Tohoku J Exp Med. 2001;193:71–74. doi: 10.1620/tjem.194.71. [DOI] [PubMed] [Google Scholar]
- 17.Tsukasa T, Mitsuru O, Satoshi H, et al. Course and Outcome of Tubulointerstitial Nephritis and Uveitis Syndrome. Am J Kidney Dis. 1999;34(6):1016–21. doi: 10.1016/S0272-6386(99)70006-5. [DOI] [PubMed] [Google Scholar]
- 18.Masaaki M, Kiyoshi I, Toshiki K, et al. Acute tubulointerstitial nephritis in two siblings and concomitant uveitis in one. Acta Paediatr Jpn. 1991;33(1):93–98. doi: 10.1111/j.1442-200x.1991.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 19.Takashi I, Hidehiko K, Shigehiko K, et al. Acute tubulointerstitial nephritis with uveitis syndrome presenting as multiple tubular dysfunction including Fanconi’s syndrome. Pediatr Nephrol. 1992;6:547–49. doi: 10.1007/BF00866499. [DOI] [PubMed] [Google Scholar]
- 20.Kiyoshi H, Yasuhiko T, Hideaki M, et al. A case of acute tubulointerstitial nephritis and uveitis syndrome with a dramatic response to corticosteroid therapy. Am J Nephrol. 1989;9:499–503. doi: 10.1159/000168020. [DOI] [PubMed] [Google Scholar]
- 21.Hiroki M, Kiyofumi M, Yuka Y, Shinji Y. [A case of tubulointerstitial nephritis and uveitis syndrome] Chuno Byoin Nenpo. 2004;4:24–27. [in Japanese] [Google Scholar]
- 22.Masato K, Yoshihiro I, Kouhei N. [A case of uveitis associated with interstitial nephritis] Ganka Rinsho I Ho. 1997;91(4):595–98. [in Japanese] [Google Scholar]
- 23.Shigeko Y, Norio T, Atsuko U, et al. [Juvenile Uveitis in Two Siblings] Nippon Ganka Kiyou. 2004;55(7):547–52. [in Japanese] [Google Scholar]
- 24.Yoko S, Hiromi K, Kenichi S, Ako A. [Two cases of tubulointerstitial nephritis and uveitis syndrome] Shiritsu Shu Byo I Shi. 2004;14:39–43. [in Japanese] [Google Scholar]
- 25.Koko A, Masato W, Yutaka K, Naoki U. [Renal-Ocular syndrome] Rinsho Ganka. 1986;40(1):35–39. [in Japanese] [Google Scholar]
- 26.Hitoshi K, Kazumi N, Jiro A. [Two cases of tubulointerstitial nephritis and uveitis syndrome] Rinsho Ganka. 1994;48(6):1085–89. [in Japanese] [Google Scholar]
- 27.Toshiko T, Shigeru T, Takao T, et al. [A case of juvenile uveitis associated with acute interstitial nephritis] Ganka Rinsho I Ho. 1990;84(5):1065–69. [in Japanese] [Google Scholar]
- 28.Shinsuke F, Keisuke S, Akane I, Tsukasa T. A boy with IgA nephropathy complicated by tubulointerstitial nephritis and uveitis (TINU) syndrome. Clin Nephrol. 2013 doi: 10.5414/CN108139. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


