Abstract
Background:
Cardiac autonomic dysfunction, clinically expressed by reduced heart rate variability (HRV), is present in patients with congestive heart failure (CHF) and is related to the degree of left ventricular dysfunction. In athletes, HRV is an indicator of ability to improve performance. No similar data are available for CHF.
Objectives:
The aim of this study was to assess whether HRV could predict the capability of CHF patients to improve physical fitness after a short period of exercise-based cardiac rehabilitation (CR).
Patients and Methods:
This was an observational, non-randomized study, conducted on 57 patients with advanced CHF, admitted to a residential cardiac rehabilitation unit 32 ± 22 days after an episode of acute heart failure. Inclusion criteria were sinus rhythm, stable clinical conditions, no diabetes and ejection fraction ≤ 35%. HRV (time-domain) and mean and minimum heart rate (HR) were evaluated using 24-h Holter at admission. Patients’ physical fitness was evaluated at admission by 6-minute walking test (6MWT) and reassessed after two weeks of intensive exercise-based CR. Exercise capacity was evaluated by a symptom-limited cardiopulmonary exercise test (CPET).
Results:
Patients with very depressed HRV (SDNN 55.8 ± 10.0 ms) had no improvement in their walking capacity after short CR, walked shorter absolute distances at final 6MWT (348 ± 118 vs. 470 ± 109 m; P = 0.027) and developed a peak-VO2 at CPET significantly lower than patients with greater HRV parameters (11.4 ± 3.7 vs. an average > 16 ± 4 mL/kg/min). Minimum HR, but not mean HR, showed a negative correlation (ρ = -0.319) with CPET performance.
Conclusions:
In patients with advanced CHF, depressed HRV and higher minimum HR were predictors of poor working capacity after a short period of exercise-based CR. An individualized and intensive rehabilitative intervention should be considered for these patients.
Keywords: Heart Failure, Heart, Rehabilitation
1. Background
A profound cardiac autonomic derangement occurs in patients with chronic heart failure (CHF) demonstrated using different methodological approaches (1, 2). Cardiac dysautonomia can be clinically assessed by the analysis of heart rate variability (HRV), which provides a noninvasive and semiquantitative method to evaluate the sympathovagal balance of the heart in a reliable and reproducible way (3, 4). HRV decline seems to be significantly associated with the degree of left ventricular dysfunction and progression and prognosis of disease (5-7). An increasing number of studies used HRV as an indicator of training status in athletes, even though there are conflicting reports on the association between physical fitness and HRV parameters (8-12). Some studies suggested a possible association between basal evaluation of HRV and ability to improve performance measures in athletes (13, 14). It is not known whether a basal evaluation of HRV could also give useful information about physical fitness achievable by CHF patients after exercise training, and whether it could help selecting those patients requiring a more intensive and personalized rehabilitative intervention.
2. Objectives
The aim of this study was to assess whether an impaired autonomic function, as assessed by reduced HRV, could indicate a limited capacity of patients with CHF to improve their physical performance after a short period of intensive, exercise-based cardiac rehabilitation.
3. Patients and Methods
We investigated 57 patients with advanced CHF (46 males; a mean age of 60.5 ± 11.4 years, ranged 32-79 years), consecutively admitted to our residential cardiac rehabilitation unit (CR), 31.6 ± 22.4 days after an episode of acute systolic heart failure. Inclusion criteria were presence of sinus rhythm, stabilized clinical conditions, no diabetes nor other known causes of HRV alteration and echocardiographic ejection fraction ≤ 35%. At the time of study, all patients were receiving beta-blockers and ACE-Inhibitors or Angiotensin Receptor Blockers at maximum tolerated dosage. On the day of admission to CR, patients were submitted to 24-h Holter recording, while beginning light training activities according to the hospital protocol. Their initial physical fitness was evaluated using a 6-minute walking test (6MWT), performed in a 30-meter long, unobstructed indoor corridor according to the American Thoracic Society recommendations (15). The same test was repeated on the day of discharge, and the difference between initial and final tests (Δ-6MWT) was recorded. In the two weeks of residential rehabilitation, patients participated in three daily sessions of exercise-based training, six days per week, including respiratory exercises, aerobic training and calisthenics. The left ventricle (LV) ejection fraction was assessed using a standard two-dimensional transthoracic echocardiogram (the Simpson’s method when good quality echo windows were available, area-length method in other cases). On the day before discharge, patients’ physical performance was evaluated using a symptom-limited cardiopulmonary exercise test (CPET).
3.1. HRV Evaluation
After cleansing of arrhythmias and artifacts, the following HRV time-domain parameters were evaluated from the 24-h Holter (Del Mar-Reynolds Impresario Holter Analysis System, version 2.8.0024; time-domain HRV Analyzer, version 1.0.8.4, CENTUM & Del Mar Reynolds Medical Inc., Irvine, CA, USA; sampling rate of 128 Hz): mean and minimum heart rates (mean-HR, min-HR), standard deviation of all normal RR intervals (SDNN), square root of the mean square differences of successive NN intervals (RMSSD), standard deviation of the 5-minute average of NN intervals (SDANN), proportion of successive beats with differences in NN intervals > 50 ms (pNN50), average of the standard deviations of all NN intervals for each 5-minute segments (SDNN index) and HRV Triangular Index. HRV parameters were evaluated both on the whole 24-hour recording and subdivided by day and night. We defined “day” as the period between 06:00 and 22:59 and “night” as 23:00 to 05:59.
3.2. CPET Methodology and Parameters
The tests were performed in late morning, at least three hours after a light meal, on a computer driven bicycle ergometer (Cardiovit CS-200 Ergo-Spiro, Schiller AG, Baar, CH; Ergoselect 100 ergometer, Ergoline GmbH, Bitz, D). A progressive ramp protocol was used with a load of 5 to 10 W/min (adapted to age, gender and weight of patients to produce a stress test of about 10 minutes duration) until subjective exhaustion or appearance of criteria of interruption. Expired gas was collected using a tightly fitting facemask and continuously analyzed during the exercise test (Schiller Ganshor CS-200 Power Cube). The oxygen consumption at the peak of exercise (peak-VO2) was calculated as the average over a 20-second period and expressed relative to body weight (mL/kg/min). Peak exercise capacity was expressed in Watt as the maximum sustained workload (Watt-max) (16).
3.3. Statistical Analysis
SPSS 15 Statistics Package for Social Sciences (SPSS Inc., Chicago, Illinois, USA) was used for data analysis. Descriptive statistics were expressed as mean ± standard deviation for continuous variables. Categorical variables were presented as absolute values with percentages. Differences between day and night values of HRV in the single cases were evaluated using the paired-samples student t-test. Differences between groups were evaluated using the student t-test for unpaired samples. Categorical variables were compared using the Spearman chi-square test. P value < 0.05 was considered statistically significant.
3.4. Statement
During CR hospitalization, all participants were informed about the procedures they were undergoing. A written consent was obtained from all patients before CPET. The usual diagnostic and follow-up routine for CR was applied; no special test or treatment was performed. The research was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. An approval was obtained from the Provincial Ethics Committee (Provincial Health Directorate, Belluno, Italy).
4. Results
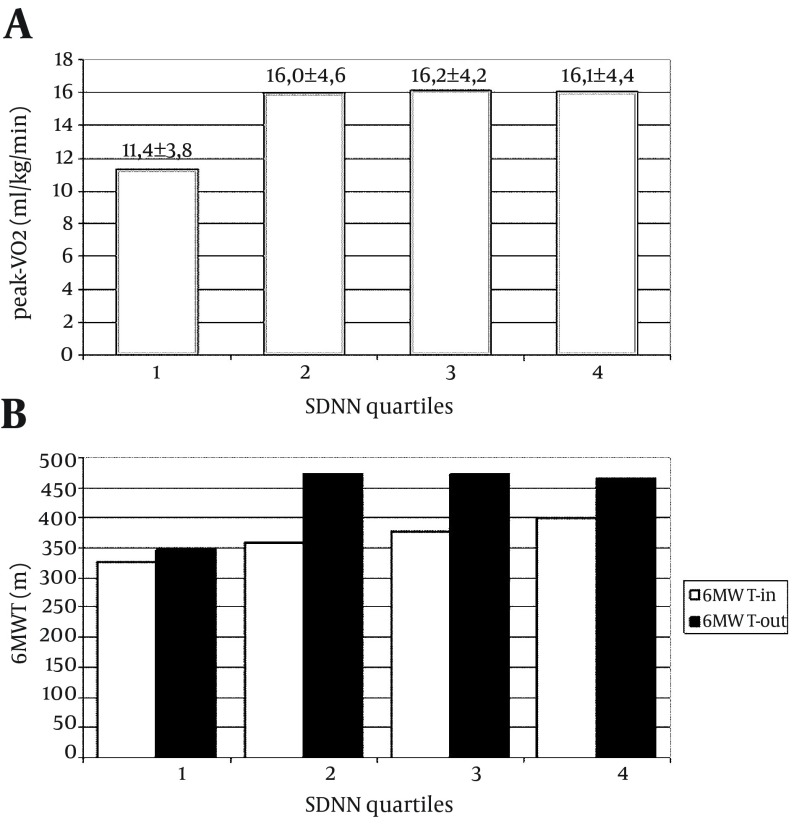
Main results of HRV and performance parameters for all patients are summarized in Tables 1 and 2, respectively. HRV parameters showed wide variations among patients, while in some subjects, SDNN was in the range of normality, in other cases the same parameters were markedly low. When distributed into four quartiles according to their SDNN value, 15 cases were in the lowest group, with a mean value of 55.8 ± 10.0 ms; 14 patients were in each of the other quartiles, with mean SDNN values of 85.0 ± 5.7, 106.4 ± 6.6 ms and 149.3 ± 30.3, respectively. In 11 patients, the SDNN values were < 60 ms (range 38.5-59.3 ms). The min-HR recorded during 24-h Holter recording ranged from 39 to 81 bpm; it was negatively correlated with all HRV parameters (pNN50: ρ = -0.442; SDNN: ρ = -0.701; RMSSD: ρ = -0.475; Triangular Index: ρ = -0.590; SDNN Index: ρ = -0.528; SDANN ρ = -0.679; all P < 0.001). In particular, patients in the lowest SDNN quartile presented a more elevated min-HR (in SDNN-quartile-1: min-HR 65.0 ± 10.4 bpm), while the other quartiles of SDNN were associated with progressively lower min-HR (respectively 53.2 ± 7.1, 52.5 ± 6.0 and 46.6 ± 7.5 bpm; P < 0.005 between SDNN-quartile-1 and SDNN-quartiles 2, 3 and 4; P = ns between SDNN-quartile-2 and 3; P < 0.05 between SDNN-quartile-4 and both 2 and 3). In the analysis of circadian oscillations of HRV, our group of CHF patients did not show significant day/night variations of any HRV parameter (Table 1). Because of selection criteria, patients in our study group presented a markedly depressed ejection fraction (EF). Besides, they presented poor performance parameters. At pre-discharge CPET, nine patients had a peak-VO2 < 10 mL/kg/min. No significant correlation was found between peak-VO2 and EF (Spearman ρ = 0.229, P = 0.126). In contrast, peak-VO2 demonstrated a weak but statistically significant correlation with some HRV parameters: SDNN (ρ = 0.367, P = 0.012), Triangular Index (ρ = 0.395, P = 0.007), SDANN (ρ = 0.387, P = 0.009); see Figure 2. In particular, patients in the lowest SDNN quartile reached a significantly lower peak-VO2 at pre-discharge CPET (Figure 1 A) compared to patients in other three quartiles (student t-test for unpaired samples t = 2.6398, P = 0.0153). Peak-VO2 presented a weak (statistically significant) negative correlation with the min-HR recorded in 24-h Holter monitoring (ρ = -0.319, P = 0.045), while no correlation was found with the mean-HR. No correlation was found between any of the HRV parameters and the values of EF. At the initial 6MWT evaluation, the absolute amount of distance walked differed largely among patients, ranging from 60 to 560 meters. Although, patients in the lowest SDNN quartile showed the poorest walking capacity and a tendency was evident towards a better walking capacity in higher SDNN quartiles (Figure 1 B); the differences were not statistically significant. After a short period of intensive cardiac rehabilitation, the distance walked by patients showed a wide range of improvement, from 0 to 355 meters. The Δ-6MWT of patients did not show significant correlations with any HRV parameter (SDNN: ρ = 0.077, P = 0.600; RMSSD: ρ = 0.066, P = 0.652; triangular index: ρ = 0.104, P = 0.481; SDANN: ρ = 0.062, P = 0.679) (Figure 2). Nevertheless, comparing the performance of group with the lowest quartile of SDNN with patients in other SDNN quartiles indicated that cases in the lowest quartile did not have significant improvement in their walking capacity (P = 0.5207), while different amounts of improvement were obtained by other groups of patients (Figure 1 B).
Table 1. HRV Parameters for all Patients a, b.
| HRV Parameter | Total | Day | Night | P Value (day/night) |
|---|---|---|---|---|
| Mean-HR, bpm | 68.1 ± 11.1 | - | - | - |
| Minimum HR, bpm | 55.4 ± 10.4 | - | - | - |
| pNN50, % | 8.16 ± 9.03 | - | - | - |
| SDNN, ms | 98.74 ± 37.99 | 87.80 ± 36.55 | 93.57 ± 44.11 | ns (0.295) |
| RMSSD, ms | 44.31 ± 33.69 | 44.32 ± 39.94 | 41.91 ± 24.98 | ns (0.604) |
| Triangular Index | 12.86 ± 4.21 | |||
| SDNN Index | 36.29 ± 18.96 | 35.70 ± 21.81 | 37.43 ± 17.92 | ns (0.481) |
| SDANN, ms | 88.05 ± 35.77 | 76.75 ± 29.85 | 82.32 ± 42.60 | ns (0.202) |
a Abbreviations: Mean-HR and Minimum HR, mean and minimum heart rates recorded at the 24-h Holter monitoring; pNN50, proportion of successive beats with differences in NN intervals > 50 ms; SDNN, standard deviation of all normal RR intervals; RMSSD, square root of the mean square differences of successive NN intervals; SDNN Index, average of the standard deviations of all NN intervals for each 5-minute segments; SDANN, standard deviation of the 5-min average of NN intervals; P (day/night): level of significance in student t-test for paired samples, between day and night values of HRV parameters.
b Data are presented as Mean ± SD.
Table 2. Performance Parameters for all Patients and the Four Subgroups According to the SDNN Quartiles a, b.
| All Patients (n = 57) | SDNN-1 (n = 15) | SDNN-2 (n = 14) | SDNN-3 (n = 14) | SDNN-4 (n = 14) | SDNN quart. 2-4 (n = 42) | P Value | |
|---|---|---|---|---|---|---|---|
| Ejection Fraction, % | 28.8 ± 6.5 | 27.7 ± 7.7 | 26.0 ± 6.2 | 29.8 ± 6.3 | 30.2 ± 7.3 | 28.7 ± 6.8 | ns (0.6150) |
| Peak-VO2, mL/kg/min | 14.9 ± 4.6 | 11.4 ± 3.8 | 16.0 ± 4.6 | 16.2 ± 4.2 | 16.1 ± 4.4 | 16.1 ± 4.3 | < 0.0005 (0.0004) |
| Watt-max, watt | 69.1 ± 23.8 | 55.0 ± 23.8 | 71.4 ± 22.7 | 72.6 ± 22.9 | 59.8 ± 28.9 | 67.2 ± 25.2 | ns (0.1075) |
| Initial 6MWT, m | 366.3 ± 126.2 | 325.7 ± 63.6 | 357.4 ± 142.3 | 377.2 ± 157.7 | 400.0 ± 141.2 | 381.6 ± 142.6 | ns (0.1496) |
| Final 6MWT, m | 437.5 ± 123.1 | 348.2 ± 118.3 | 473.8 ± 74.2 | 472.8 ± 144.5 | 466.3 ± 112.7 | 469.9 ± 109.3 | < 0.0005 (0.0006) |
| Δ-6MWT, m | 70.96 ± 74.29 | 51.2 ± 47.8 | 116.8 ± 95.8 | 95.6 ± 99.0 | 65.3 ± 55.1 | 88.3 ± 81.6 | ns (0.1035) |
a Abbreviations: SDNN, standard deviation of all normal RR intervals; SDNN-1, SDNN-2, SDNN-3, SDNN-4: groups of patients stratified according to the SDNN quartiles; peak-VO2, oxygen consumption at the peak of exercise; Watt-max: peak exercise capacity in Watt; 6MWT, six-minute walking test; Δ-6MWT, difference between initial and final 6MWT; p, level of significance in student t-test for unpaired samples between patients in SDNN quartile 1 vs. patients in other quartiles of SDNN.
b data are presented as Mean ± SD.
Figure 2. Correlation Between SDNN and Functional Parameters: peak-VO2 at Cardiopulmonary Exercise Test and Absolute Distance Walked at Initial and Final 6MWT.
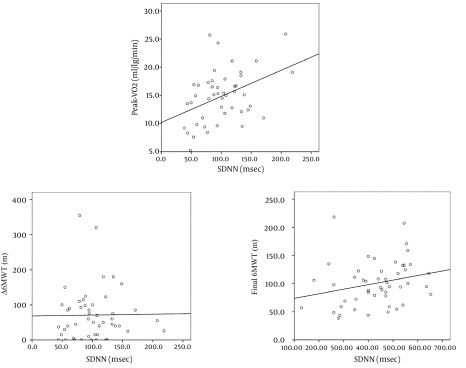
Peak-VO2, peak oxygen uptake measured at cardiopulmonary exercise test, in mL/kg/min; SDNN, standard deviation of all normal RR intervals recorded in 24-hour Holter; ms, milliseconds; 6MWT, six-minute walking test; m, meters.
Figure 1. Physical Performance of Patients With CHF According to Their SDNN Quartiles.
Peak-VO2, peak oxygen uptake measured at cardiopulmonary exercise test, in mL/kg/min; 6MWT-in, initial evaluation of the six-minute walk test; 6MWT-out, final evaluation of six-minute walk test; SDNN, standard deviation of all normal RR intervals recorded in 24-hour Holter; ms, milliseconds.
Differently, the absolute distance walked by patients in the final 6MWT was significantly (although weakly) correlated to some HRV parameters (SDNN: ρ = 0.315, P = 0.027; SDNN Index: ρ = 0.338, P = 0.017; Triangular Index: ρ = 0.287, P = 0.048); Figure 2. There was no direct correlation between peak-VO2 and Δ-6MWT (respectively: ρ = 0.223, P = ns), while a significant correlation was found between both peak-VO2 and Watt-max with absolute walked distance at the final 6MWT (respectively: ρ = 0.537, P < 0.001 and ρ = 0.512, P < 0.001).
5. Discussion
Different levels of autonomic dysregulation are evident in patients with advanced CHF; in these cases, SDNN (a parameter indicating the total amount of variability, induced by both sympathetic and parasympathetic activities) is highly depressed in a quarter of cases, reaching values not dissimilar from those reported in literature for patients with advanced left ventricular systolic dysfunction (17). Eleven of our patients had SDNN values < 60 ms, that, according to the UK-Heart study (5), are considered as a group with a high-risk ratio and a high annual mortality rate. The degree of cardiac dysautonomia showed an inverse correlation with exercise capacity evaluated by the 6MWT performed at the beginning of CR period. Even though the association between the two parameters was not statistically significant, patients with more depressed HRV parameters showed the lowest walking capacity, somehow confirming the known correlation among cardiac dysautonomia, stage of disease and physical performance in patients with CHF (18-20). Despite advanced stage of CHF, our patients succeeded to improve their physical performance in two weeks of intensive exercise-based cardiac rehabilitation. They were able to increase their walking capacity by an average of 71 meters, a distance generally considered as a prognostic indicator of favorable outcome (21). The amount of improvement was extremely different among patients, ranging from 0 to 355 meters. A significant but weak correlation was found between physical performance attained after short-term CR (evaluated both final 6MWT and pre-discharge CPET) and autonomic parameters recorded at the beginning of CR. Somehow, similar findings were described for normal subjects undergoing 8-week aerobic exercise training intervention, in which the baseline evaluated HRV parameters were the most powerful determinants associated with future training response, independent from age, baseline fitness and body mass index (22, 23). Several reports supported the evidence of an association between HRV parameters and training level in athletes (8-14). At our best knowledge, similar data have not been published for patients with CHF yet, and our study is the first report assessing depressed HRV parameters and impaired training capacity in patients with CHF. Patients with worse parameters of cardiac dysautonomia (SDNN, Triangular Index and SDANN) at admission had a lower gain after short period of exercise-based rehabilitation, with lower increase and shorter absolute walking distance at 6MWT, as well as poorer levels of performance at pre-discharge CPET (24). The knowledge of a correlation between highly depressed basal HRV and low physical improvement after CR could help identifying those patients with CHF who need special attention during CR, and perhaps require a more individualized, intensive and prolonged rehabilitative intervention. Further studies are needed to evaluate whether a more targeted cardiac rehabilitation for patients with CHF with highly reduced HRV could be effective in producing a long-term modification of their physical recovery. Differently from HRV, echocardiographic EF, a widely used parameter of LV function, showed no significant correlation with performance measures (6MWT, peak-VO2, Watt-max), confirming previous studies on patients with CHF (25). Indeed, LV systolic dysfunction, clinically evaluated as a reduction of LVEF, is the “primum movens” of heart failure syndrome, but it is only one aspect of the complex interrelationship involving right heart, cardiac and peripheral responses mediated by the autonomic nervous system, neurohumonal responses, endothelial factors, pulmonary function, chemoreceptor responses, muscle fibers composition and physical activity. Evaluating HRV, an indicator of overall degree of intervention of the autonomic nervous system, seems to give more comprehensive information about the degree of deterioration of LV function and physical fitness of patients with CHF. In our cases, finding a high minimum heart rate was predictive of poor working capacity at the end of short rehabilitative period (negative correlation between minimum heart rate evaluated at basal Holter recording and peak-VO2 at final CPET). Elevated minimum HR (26) observed in some of patients with CHF, despite ongoing beta-blocker therapy, may suggest an elevated sympathetic activity (1, 27) and blunted parasympathetic activity (28), as a result of a more severe stage of heart failure (3). It is known that sympathetic over activity contributes to skeletal myopathy and brings to inadequate regulation of muscle blood flow, leading to progressive worsening of exercise tolerance in patients with CHF (1). This simple parameter is known to carry prognostic significance, indicating an increased relative risk for sudden death over a 2-year period (29).
5.1. Limitations of the Study
This study was performed in a cardiac rehabilitation center, where patients were admitted (on average of one month) after the index event. Consecutively admitted patients were included in the study, according to our inclusion/exclusion criteria. Therefore, it is an observational, nonrandomized study and patients may not represent the average situation of other patients with similar pathology. Patients with atrial fibrillation or pacemaker induced heart rhythm were excluded from the study. Although they may have significant autonomic dysfunction, HRV could not be measured in such patients and consequently it is not known how cardiovascular dysautonomia could affect their physical performance. We were unable to perform frequency domain HRV analyses that could have allowed a more specific determination of variations of vagal tone and sympathovagal balance. Anyway, previous studies determined that time-domain HRV indices measured over a 24-hour period are well correlated with frequency domain indices, at least in general population (29). No follow-up has yet been performed to analyze probable occurrence of cardiovascular events in these patients. In patients with advanced stage CHF, a profound cardiac autonomic derangement is evident. HRV parameters are related to measures of cardiopulmonary fitness, such as peak-VO2 and 6MWT. A depressed HRV predicts impaired capability of improving physical fitness after a short period of exercise-based cardiac rehabilitation. In CHF patients with very low HRV parameters, an individualized cycle of cardiac rehabilitation should be considered.
References
- 1.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54(19):1747–62. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118(8):863–71. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- 3.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–81. [PubMed] [Google Scholar]
- 4.Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51(18):1725–33. doi: 10.1016/j.jacc.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart). Circulation. 1998;98(15):1510–6. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
- 6.Bilchick KC, Fetics B, Djoukeng R, Fisher SG, Fletcher RD, Singh SN, et al. Prognostic value of heart rate variability in chronic congestive heart failure (Veterans Affairs' Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure). Am J Cardiol. 2002;90(1):24–8. doi: 10.1016/s0002-9149(02)02380-9. [DOI] [PubMed] [Google Scholar]
- 7.Rashba EJ, Estes NA, Wang P, Schaechter A, Howard A, Zareba W, et al. Preserved heart rate variability identifies low-risk patients with nonischemic dilated cardiomyopathy: results from the DEFINITE trial. Heart Rhythm. 2006;3(3):281–6. doi: 10.1016/j.hrthm.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 8.Achten J, Jeukendrup AE. Heart rate monitoring: applications and limitations. Sports Med. 2003;33(7):517–38. doi: 10.2165/00007256-200333070-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kouidi E, Haritonidis K, Koutlianos N, Deligiannis A. Effects of athletic training on heart rate variability triangular index. Clin Physiol Funct Imaging. 2002;22(4):279–84. doi: 10.1046/j.1475-097x.2002.00431.x. [DOI] [PubMed] [Google Scholar]
- 10.Hottenrott K, Hoos O, Esperer HD. [Heart rate variability and physical exercise. Current status]. Herz. 2006;31(6):544–52. doi: 10.1007/s00059-006-2855-1. [DOI] [PubMed] [Google Scholar]
- 11.Grant CC, Clark JR, Janse van Rensburg DC, Viljoen M. Relationship between exercise capacity and heart rate variability: supine and in response to an orthostatic stressor. Auton Neurosci. 2009;151(2):186–8. doi: 10.1016/j.autneu.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Lee CM, Mendoza A. Dissociation of heart rate variability and heart rate recovery in well-trained athletes. Eur J Appl Physiol. 2012;112(7):2757–66. doi: 10.1007/s00421-011-2258-8. [DOI] [PubMed] [Google Scholar]
- 13.Hedelin R, Bjerle P, Henriksson-Larsen K. Heart rate variability in athletes: relationship with central and peripheral performance. Med Sci Sports Exerc. 2001;33(8):1394–8. doi: 10.1097/00005768-200108000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Stanley J, D'Auria S, Buchheit M. Cardiac Parasympathetic Activity and Race Performance: An Elite Triathlete Case Study. Int J Sports Physiol Perform. 2014 doi: 10.1123/ijspp.2014-0196. [DOI] [PubMed] [Google Scholar]
- 15.A. T. S. Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories.. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 16.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 17.de Sousa MR, Barbosa MP, Lombardi F, Ribeiro AL. Standard Deviation of normal interbeat intervals as a risk marker in patients with left ventricular systolic dysfunction: a meta-analysis. Int J Cardiol. 2010;141(3):313–6. doi: 10.1016/j.ijcard.2008.11.128. [DOI] [PubMed] [Google Scholar]
- 18.Yi G, Goldman JH, Keeling PJ, Reardon M, McKenna WJ, Malik M. Heart rate variability in idiopathic dilated cardiomyopathy: relation to disease severity and prognosis. Heart. 1997;77(2):108–14. doi: 10.1136/hrt.77.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fauchier L, Babuty D, Cosnay P, Autret ML, Fauchier JP. Heart rate variability in idiopathic dilated cardiomyopathy: characteristics and prognostic value. J Am Coll Cardiol. 1997;30(4):1009–14. doi: 10.1016/s0735-1097(97)00265-9. [DOI] [PubMed] [Google Scholar]
- 20.Hombach V. Electrocardiography of the failing heart. Cardiol Clin. 2006;24(3):413–26. doi: 10.1016/j.ccl.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Passantino A, Lagioia R, Mastropasqua F, Scrutinio D. Short-term change in distance walked in 6 min is an indicator of outcome in patients with chronic heart failure in clinical practice. J Am Coll Cardiol. 2006;48(1):99–105. doi: 10.1016/j.jacc.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 22.Hautala AJ, Makikallio TH, Kiviniemi A, Laukkanen RT, Nissila S, Huikuri HV, et al. Cardiovascular autonomic function correlates with the response to aerobic training in healthy sedentary subjects. Am J Physiol Heart Circ Physiol. 2003;285(4):H1747–52. doi: 10.1152/ajpheart.00202.2003. [DOI] [PubMed] [Google Scholar]
- 23.Hautala AJ, Kiviniemi AM, Tulppo MP. Individual responses to aerobic exercise: the role of the autonomic nervous system. Neurosci Biobehav Rev. 2009;33(2):107–15. doi: 10.1016/j.neubiorev.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110(2):325–32. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 25.Donal E, Coquerel N, Bodi S, Kervio G, Schnell F, Daubert JC, et al. Importance of ventricular longitudinal function in chronic heart failure. Eur J Echocardiogr. 2011;12(8):619–27. doi: 10.1093/ejechocard/jer089. [DOI] [PubMed] [Google Scholar]
- 26.Swedberg K, Komajda M. The beat goes on: on the importance of heart rate in chronic heart failure. Eur Heart J. 2012;33(9):1044–5. doi: 10.1093/eurheartj/ehr483. [DOI] [PubMed] [Google Scholar]
- 27.Pepper GS, Lee RW. Sympathetic activation in heart failure and its treatment with beta-blockade. Arch Intern Med. 1999;159(3):225–34. doi: 10.1001/archinte.159.3.225. [DOI] [PubMed] [Google Scholar]
- 28.Binkley PF, Nunziata E, Haas GJ, Nelson SD, Cody RJ. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: demonstration in human subjects and verification in a paced canine model of ventricular failure. J Am Coll Cardiol. 1991;18(2):464–72. doi: 10.1016/0735-1097(91)90602-6. [DOI] [PubMed] [Google Scholar]
- 29.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. Heart rate variability from 24-hour electrocardiography and the 2-year risk for sudden death. Circulation. 1993;88(1):180–5. doi: 10.1161/01.cir.88.1.180. [DOI] [PubMed] [Google Scholar]