Abstract:
Background:
Hypoglycemia is associated with a higher risk of death. This study analyzed various body mass index (BMI) categories and mortalities of severe hypoglycemic patients with type 2 diabetes mellitus (DM) in a hospital emergency department.
Methods:
The study included 566 adults with type 2 diabetes who were admitted to 1 medical center in Taiwan between 2008 and 2009 with a diagnosis of severe hypoglycemia. Mortality data, demographics, clinical characteristics and the Charlson's Comorbidity Index were obtained from the electronic medical records. Patients were stratified into 4 study groups as determined by the National Institute of Health (NIH) and World Health Organization classification for BMI, and the demographics were compared using the analysis of variance and χ2 test. Kaplan-Meier's analysis and the Cox proportional-hazards regression model were used for mortality, and adjusted hazard ratios were adjusted for each BMI category among participants.
Results:
After controlling for other possible confounding variables, BMI <18.5 kg/m2 was independently associated with low survival rates in the Cox regression analysis of the entire cohort of type 2 DM patients who encountered a hypoglycemic event. Compared to patients with normal BMI, the mortality risk was higher (adjusted hazard ratios = 4.9; 95% confidence interval [CI] = 2.4–9.9) in underweight patients. Infection-related causes of death were observed in 101 cases (69.2%) and were the leading cause of death.
Conclusions:
An independent association was observed between BMI less than 18.5 kg/m2 and mortality among type 2 DM patient with severe hypoglycemic episode. Deaths were predominantly infection related.
Key Indexing Terms: Hypoglycemia, Body mass index, Mortality, Emergency department, Diabetes mellitus
Severe hypoglycemia is an acute complication of diabetes therapy. Although less common in patients with type 2 diabetes, severe hypoglycemia is a threat for the diabetic patient treated with glucose-lowering drugs. Population-based data indicate that the overall event rate for severe hypoglycemia in type 2 diabetes would be equivalent to 0.35 events per patient per year.1 The precipitating cause of severe hypoglycemia is not always easy to find. However, overtreatment of diabetes was passed for an important cause of severe hypoglycemic reactions.2 Studies have demonstrated that intensive glycemic control lowers micro- and macrovascular event risks,3,4 yet a number of large randomized controlled trials have failed to demonstrate a clear reduction in mortality with intensification of treatment.5–7 In addition, 3 large randomized trials (ACCORD, ADVANCE and VADT) showed that a favorable glycemic control (HbA1c 6.4%–6.9%) is associated with an increased incidence of hypoglycemia with type 2 diabetes.7–9 Furthermore, recent studies have linked hypoglycemia with angina, myocardial infarction and acute cerebrovascular events, which results in an increased risk of cardiovascular disease and all-cause mortality.8,10,11 The association between hypoglycemia and increased risk of mortality in an intensive care unit and general ward settings has also been demonstrated.12,13 McCoy et al14 also indicated that self-reported severe hypoglycemia is associated with a 3.4-fold increase in the risk of death.
We were interested in the body weight and outcome of severe hypoglycemic patients with type 2 diabetes. Each 5 kg/m2 increase in body mass index (BMI) is associated with a significant increase in mortality from diabetes in overweight and obese patients.15 We evaluated the association between severe hypoglycemia and mortality after discharge and focused on various BMI and other independent risk factors to quantify the risk of mortality in the cohort. In addition, we examined the major causes of mortality among these patients.
METHODS
Setting
This study was conducted at Kaohsiung Chang Gung Memorial Hospital (KCGMH) in Taiwan. The hospital is the largest medical center in Southern Taiwan. Its central location serves as a primary national referral center for specialized care for over 3 million people.
Design
This was a cohort study for patients with severe hypoglycemic type 2 diabetes in an emergency department (ED). We identified all patients who visited the ED of the hospital between January 1, 2008, and December 31, 2009 with a primary discharge diagnosis of hypoglycemia (ICD-9 codes 251.0–251.2) and then evaluated them as then prospective follow-up. Participants were recruited based on the following inclusion criteria: (1) 18 years of age or older, (2) type 2 diabetes mellitus (DM), (3) severe hypoglycemic episode (ie, loss of consciousness or major alteration of mental status that required the assistance of another person on ED arrival) and (4) the symptoms of severe hypoglycemia and impairment of mental function completely relieved after resolution of hypoglycemia. The exclusion criteria were the following: (1) traumatic event, (2) pregnant women, (3) transferred to another acute care hospital and (4) no in-hospital follow-up for at least 3 months. DM was diagnosed according to the criteria of The American Diabetic Association and Expert Committee (1997). If a patient visited more than once during the study period, only the first hospitalization was abstracted. Data from each patient who visited the ED at KCGMH were entered into the electronic medical record (EMR) computerized registry. These data included information on demographics, weight, height, laboratory test values and current diabetes therapy. Each ED visit included the measurement and calculation of blood sugar levels, vital signs, Glasgow Coma Scale (GCS), serum creatinine levels and the Charlson's Comorbidity Index (CCI). Because the heights and weights of all patients were not routinely measured and recorded during their ED visits, they were obtained from the hospitalized medical records or during the preceding 1 month of the index ED visiting in the EMRs for calculation of the BMI. If the data were unavailable, each patient and/or primary caregiver was questioned and answers were recorded as self-reported data. The CCI, which is the most widely used comorbidity index, is a scoring system that includes weight factors on the basis of disease severity and is used for measurement of the comorbid conditions of patients. The CCI was previously validated for use in patients with diabetes16 and ED-revisiting patients.17 We used capillary blood glucose levels for analysis. The BMI values were used to indicate body fat status and were calculated from height and weight data using the kg/m2 equation. Glycosylated hemoglobin (HbA1c) levels recorded over 3 months were obtained from charts. The primary outcome was all-cause mortality, and mortality data were obtained from the EMRs. Participants were considered alive if there was a clinical encounter and censored if they did not have an encounter without a documented death in the EMR during the follow-up period. The information on the causes of death was also recorded. All patients were subsequently evaluated, and we followed this cohort of 566 patients for an average of 29.1 months.
Definition of Hypoglycemia
A plasma glucose concentration below 70 mg/dL is the most common threshold used to define hypoglycemia.18 Hypoglycemia can be considered symptomatic and documented. Patients with hypoglycemia who are brought to the ED usually have at least 1 typical symptom of hypoglycemia (eg, palpitations, hunger, sweating, tremulousness and/or dizziness). The cutoff blood glucose level of 70 mg/dL was selected to define biochemical hypoglycemia. Severe hypoglycemia was defined as an event of loss of consciousness or other major alteration of mental status caused by hypoglycemia that required the assistance of another person to treat the condition.
Patients
This study was conducted at a tertiary hospital that serves the port city of Southern Taiwan. Among the 742 patients identified using a primary discharge diagnosis of hypoglycemia in the computerized registry between January 2008 and December 2009, 29 (3.9%) were younger than 18 years, 52 (7.0%) were not type 2 DM patients (including type 1 diabetes and nondiabetes), 18 (2.4%) were not severe hypoglycemia patients, 17 (2.3%) without complete remission of the symptoms of severe hypoglycemia and impairment of mental function after resolution of hypoglycemia, 6 (0.8%) were patients with trauma and 6 (0.8%) were transferred to other health care systems after their ED visits. Among the 614 recruited patients, 48 (7.8%) were unable to attend our hospital for further evaluation over the 3-month period. A retrospective analysis of all patient data was approved by the Institutional Review Board of KCGMH.
Statistical Analyses
All statistical analyses were performed using Stata software version 11.0 (STATA Corp., TX). Continuous variables were compared using analysis of variance and were expressed as mean ± SD. The χ2 test was used for comparison of categorical variables. Kaplan-Meier's estimates were used to evaluate survival over time, with differences evaluated using the log-rank test for equality of survivor functions. Cox proportional hazard models were used to assess confounders. Age, sex, BMI and CCI were included in the multivariate Cox proportional hazard model, and the independent effects were produced to estimate predictor variables on survival. The adjusted hazard ratio (aHR) and 95% confidence intervals (CIs) were also obtained. Other potential confounders were added, such as blood pressure, heart rate, body temperature, treatment modalities, serum creatinine, white-cell count, initial blood glucose and GCS. Age, sex and CCI were retained in the final model based on clinical interest or an appreciable change (±10%) in the HR associated with BMI. All P values were 2 tailed, and a P value of < 0.05 was considered statistically significant.
RESULTS
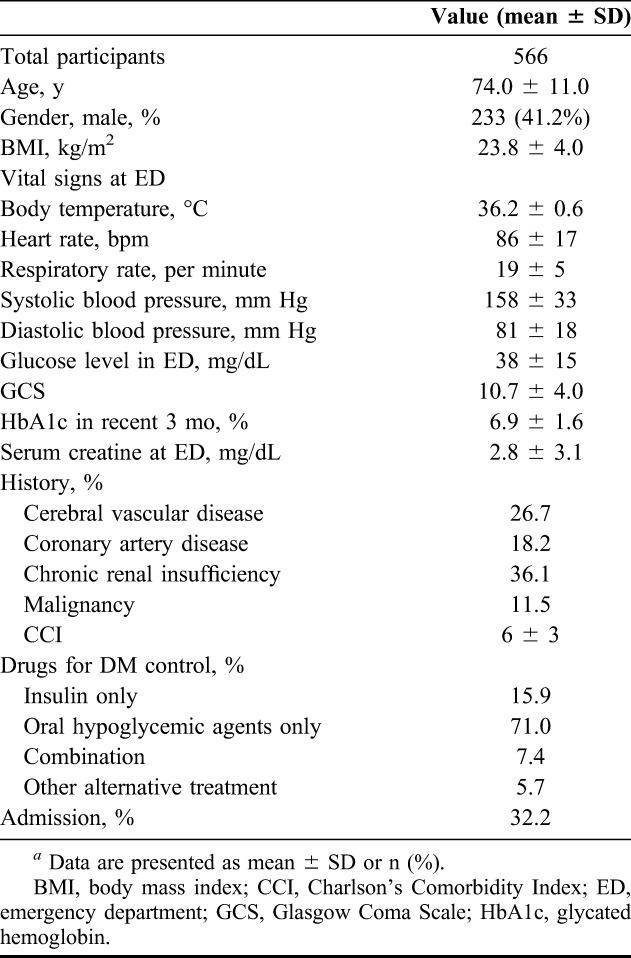
Data were obtained for 566 patients at the hospital. The characteristics and demographics of these patients with severe hypoglycemia from the indexed ED visits are shown in Table 1. Patient age ranged from 21 to 99 years (mean: 74.0 ± 11.0 years). Approximately, half (41.2%) of all patients were men. The mean BMI was 23.8 ± 4.0 kg/m2, the initial blood glucose value was 37.7 ± 14.6 mg/dL, GCS was 10.7 ± 4.0, HbA1c was 5.4 ± 3.1%, serum creatinine was 3.9 ± 3.1 mg/dL and CCI was 6.2 ± 2.7. The treatment modalities included insulin only (15.9%), oral hypoglycemic agents only (71.0%), combination of insulin and oral hypoglycemic agents (7.4%) and other alternative treatment. Approximately one-third (32.2%) of all participants were admitted after emergency treatment.
TABLE 1.
Demographic characteristics of the participantsa
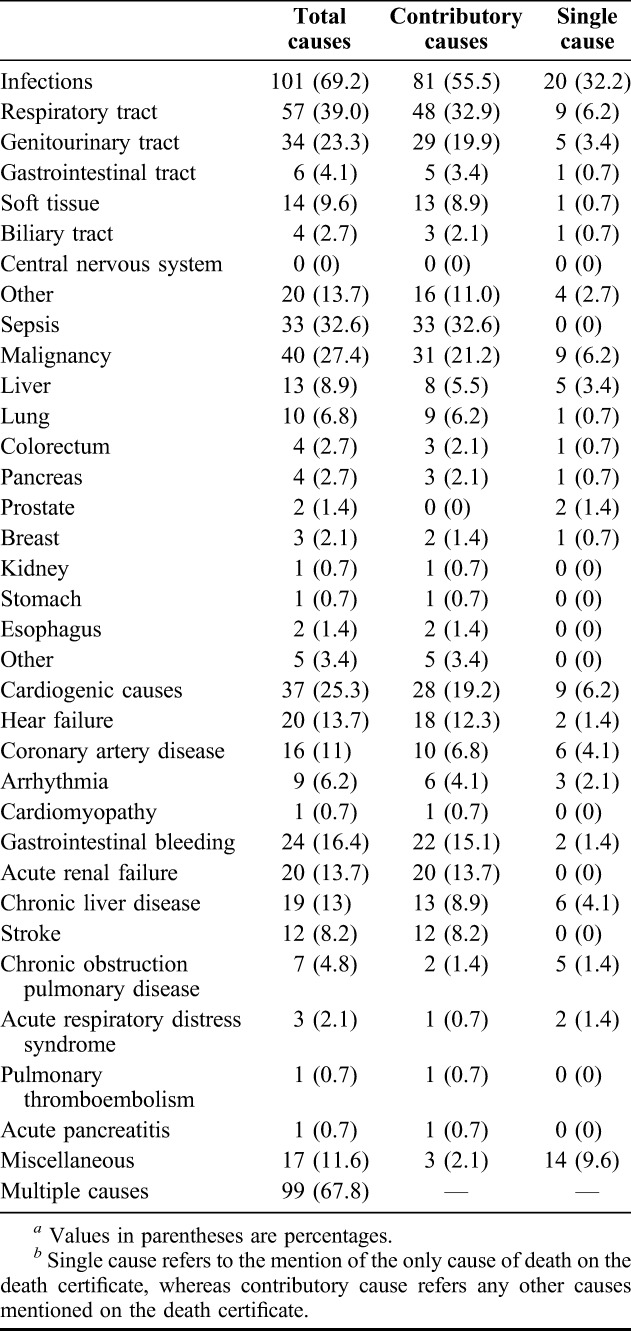
BMI classifications were obtained from the NIH19 and WHO.20 The patients were classified into four groups: underweight (BMI <18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (≥25.0–29.9 kg/m2) and obese (≥30 kg/m2). Table 2 shows the demographic data, serum creatinine, HbA1c, GCS and CCI among 4 BMI groups at the beginning of the study. The differences did not reach statistical significance among these factors, except for age (P = 0.0175). Post hoc test reveals that the significant difference was between BMI <18.5 and BMI = 25–29.9. Table 3 and Figure 1 show assessments for mortality and aHR for various BMI categories among patients with type 2 DM with severe hypoglycemia. The mean time from the index date to the occurrence of the first hypoglycemic episode was 29.1 months, and the median time was 33.4 months. A total of 146 (25.8%) deaths occurred after reporting an episode of severe hypoglycemia. Patients with a BMI of less than 18.5 kg/m2 had a higher mortality density than patients with a BMI between 18.5 and 25 kg/m2 (24.8 versus 9.8 per 1000 people-months and aHR = 4.9). Patients with a BMI between 25 and 29.9 and more than 30 had a lower mortality density than those in the reference group (5.2, 2.4 versus 9.8 per 1000 people-months); however, the difference did not reach statistical significance (aHR = 1.2 and 0.16; 95% CI = 0.6–2.2 and 0.0–1.2). The difference in cumulative mortality incidence in the cohort was 16.1% at the 12-month follow-up, 21.1% at the 24-month follow-up, 26.6% at the 36-month follow-up and 28.9% at the 48-month follow-up. The highest mortality incidence was noted at the first 12-month follow-up, and the incidence was 5%, 5.1% and 2.7% at the subsequent 12-month follow-up. The cumulative mortality incidence difference between the underweight group and reference group was 14.7% (32.7% versus 18.0%) at the 12-month follow-up, 22.4% (43.9% versus 21.5%) at the 24-month follow-up, 28.7% (56.4% versus 27.1%) at the 36-month follow-up and 31.3% (62.7% versus 31.4%) at the 48-month follow-up. By contrast, mortality rates increased during the follow-up period in the underweight group. Cox regression analysis was used to model aHR for mortality for each BMI category. After controlling for other possible confounding variables, BMI <18.5 kg/m2 was independently associated with low survival in Cox regression analysis of the entire cohort of type 2 DM patients with a hypoglycemic event. Compared with patients with a reference BMI, the mortality risk was higher (aHR = 4.9; 95% CI = 2.4–9.9) in underweight patients. No statistical significance was achieved for mortality risk in overweight (aHR = 1.2; 95% CI = 0.6–2.2) and obese patients (aHR = 0.16; 95% CI = 0.0–1.2). The adverse interaction indicates that a BMI of less than 18.5 kg/m2 considerably modifies the effect of a hypoglycemic event on survival. The Kaplan-Meier's survival estimates are shown in Figure 2. A statistically significant difference among 4 BMI categories (log-rank test, χ2 = 41.23; P < 0.0001) was observed during the follow-up period. Cause of death was reviewed for 146 nonsurvivors, and infection was found to be the leading cause of death in the patients with severe hypoglycemia (101 cases, 69.2%). More than half (57 cases, 56.4%) of all infected deceased patients had respiratory tract infections and approximately one-third of deceased patients (34 cases, 33.7%) had urinary tract infections. There were 33 patients (32.6%) presented as sepsis (Table 4).
TABLE 2.
BMI categories in the severe hypoglycemic type 2 DM follow-up cohorta
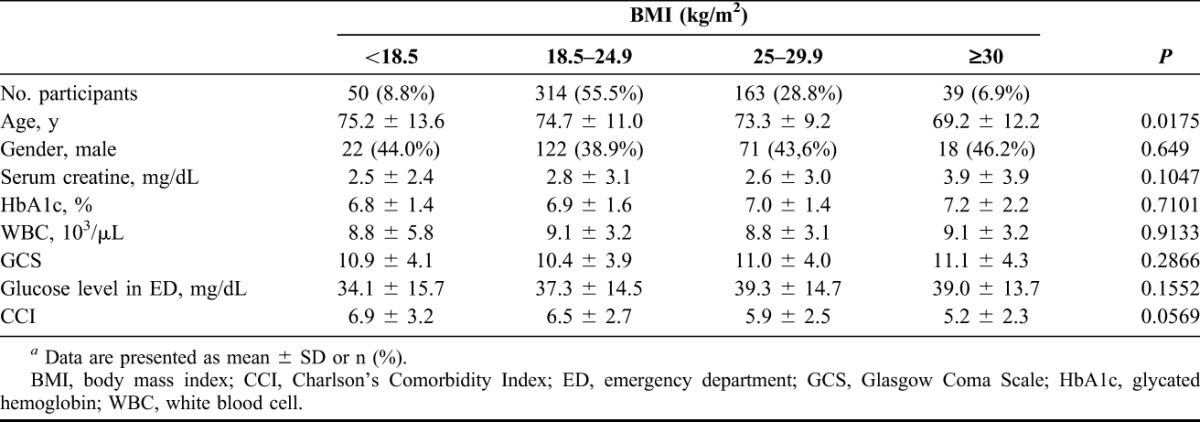
TABLE 3.
Mortality density, CM and aHR associated with BMI (kg/m2) among type 2 DM patients with severe hypoglycemia
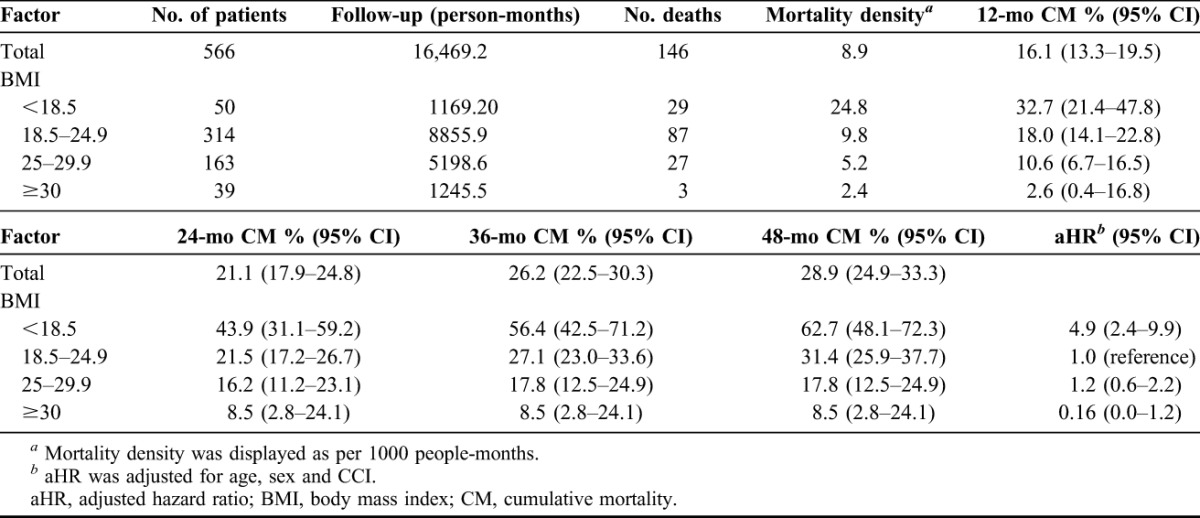
FIGURE 1.
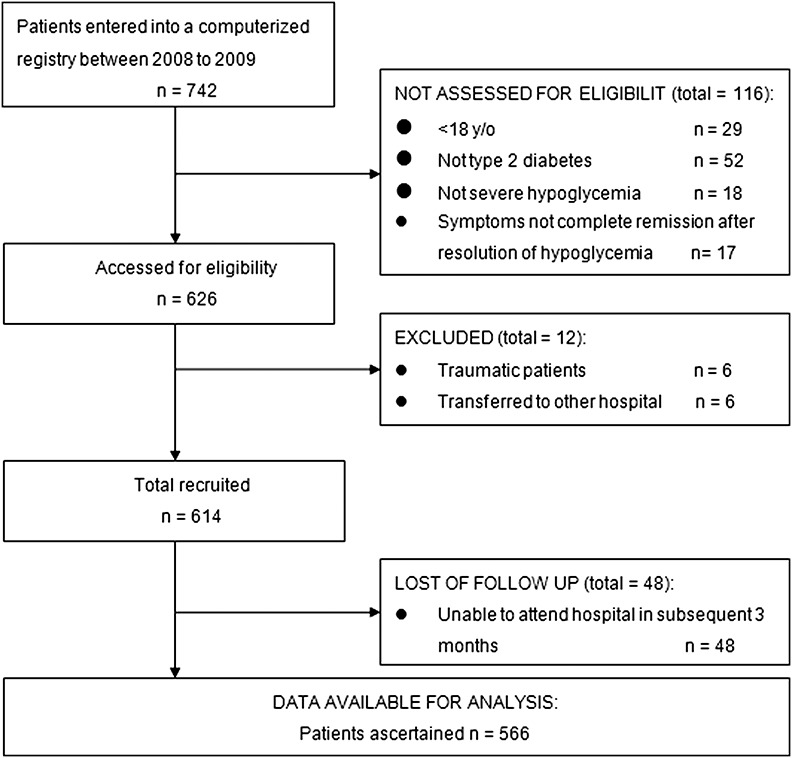
Flow diagram summarizing sample recruitment of the study.
FIGURE 2.
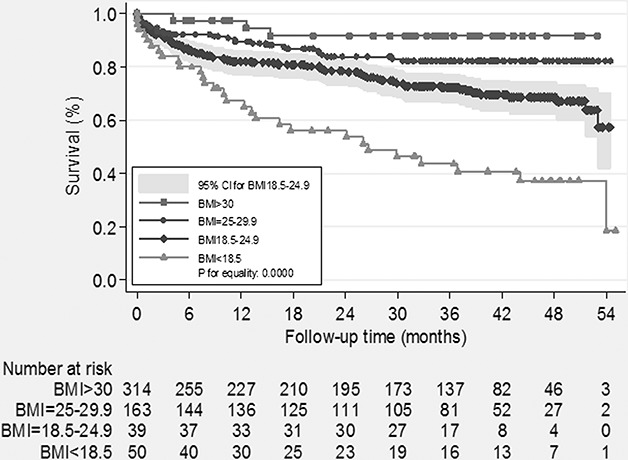
Kaplan-Meier's survival estimates by each BMI categories of severe hypoglycemia with type 2 DM patient. BMI, body mass index; DM, diabetes mellitus.
TABLE 4.
Causes of death in 146 diabetic patients with severe hypoglycemiaa,b
DISCUSSION
Meticulous glycemic control is a major concern for patients with diabetes in recent decades. Several epidemiological studies have reported an increased risk of death and cardiovascular disease with increased levels of HbA1c,21–23 and the importance of efficient glycemic control for protection against microvascular and cardivascular disease was established for patients with type 1 diabetes. Although the role of glycemic control on microvascular disease in patients with type 2 diabetes was documented in the United Kingdom Prospective Diabetes Study24 and other recent studies,4,7 the role of glycemic control for reducing mortality has not yet been established for patients with type 2 diabetes.5–7 Although most clinicians agree that effective glycemic control is a desirable intervention for patients with diabetes, the association with increased incidences of hypoglycemia demonstrated in ACCORD, ADVANCE and VADT trails has limited the treatment. The frequency of severe hypoglycemia was low initially; it was increasing in the latter part of the study in the United Kingdom Prospective Diabetes Study.24 Patients who were clinically diagnosed with severe hypoglycemia had 2.3- and 3.3-fold excess mortality rates in the standard treatment arms of ACCORD25 and ADVANCE,10 respectively. A recent observational and prospective study also indicated that patients who self-reported severe hypoglycemia had a 3.4-fold higher risk of death after 5 years compared with those who reported mild or no hypoglycemic symptoms.14 Therefore, patients with diabetes who developed severe hypoglycemia had inferior outcomes and higher mortality rates than patients with diabetes.
This study describes the clinical characteristics of a cohort of 566 consecutive patients admitted to the ED, and each patient was diagnosed with type 2 diabetes and sustained at least 1 episode of severe hypoglycemia, defined as plasma glucose level less than 70 mg/dL and associated with an event of loss of consciousness or other major alteration of mental status that required the assistance of another person. Our findings reveal that underweight was associated with a high risk of mortality in our cohort. Patients with a BMI of less than 18.5 kg/m2 exhibited a higher risk (aHR = 4.9) of mortality (independent of age, sex and CCI) than patients with a standard BMI in the Cox analysis model. This was a remarkable finding, despite the established association between obesity and increased mortality.26
For individuals with type 2 diabetes, studies have demonstrated that moderate weight loss (5% of body weight) is associated with decreased insulin resistance and improved measures of glycemia.27 However, the higher mortality among patients in the underweight group was unexpected, and the higher risk cannot be accounted for other covariables. A lower BMI may partially reflect the effects of coexisting conditions, such as malnutrition, especially in the presence of severe hypoglycemia. Malnutrition is demonstrated to be associated with increased morbidity and mortality in patients with acute and chronic diseases,28 and it impairs recovery and convalescence and prolongs hospital stays.29 There was no objective evidence that malnutrition was found in these 50 patients; even so, the observations made are very suggestive. We noted that the patients with the highest CCI were the lower BMI group and those with the lowest CCI were the obese patients, but we may not be powered to demonstrate the difference. This suggests that the lower BMI patients with diabetes are fundamentally different and have an underlying pathophysiology different from the other BMI groups, and as the CCI suggests, have a higher prediction for mortality, which may explain their poor outcome. In addition, we observed that the obese group had a lower mortality risk compared with the standard BMI group (aHR = 0.16; 95% CI = 0.0–1.2); however, this difference did not reach statistical significance. It is possible that the study population was underpowered, and the observational period was insufficient to detect differences. However, the prognoses for overweight and obese participants were better than those for underweight participants. This phenomenon is referred to as the “obesity paradox.” Although its mechanism is unknown, the obesity paradox has been reported in several patient categories, such as heart failure, stroke, coronary heart disease, chronic hemodialysis, renal failure, COPD, cancer and rheumatoid arthritis. Overweight and obese patients may have a superior prognosis because obesity correlates with a superior nutritional status.30 The results highlight the negative effect of malnutrition instead of the positive influence of obesity in patients with severe hypoglycemia; this indicates that a standard BMI or being overweight are crucial determinants of risk reduction in type 2 DM patients with severe hypoglycemia.
Infections alone or in combination with other causes accounted for more than 60% of deaths in this study. Limited information is available on the cause of death in patients with severe hypoglycemia. Previous studies have indicated that infection is one of the main causes of mortality in patients with hypoglycemia. The NICE-SUGAR study indicated that patients with severe hypoglycemia had a significantly higher HR for death from distributive shock compared with patients without hypoglycemia (aHR = 4.35; 95% CI = 2.49–7.61).31 Egi et al32 indicated that hypoglycemia was independently associated with the death caused by infectious disease (51.6%, 128 of 248 cases). The prevalence of infection may have been higher in this study. The unfavorable causal relationship is plausible because hypoglycemia may increase mortality by impairment of autonomic function, alteration of blood flow and composition, vasoconstriction, white-cell activation and the release of inflammatory mediators and cytokines.33,34
The all-cause mortality rate of adults with severe hypoglycemia was 25.8% at 4 years in this study, which was higher than the 23.7% in the self-reported severe hypoglycemia study at 5 years14 and the 19.5% in the ADVANCE study at 5 years.10 A significant correlation between severe hypoglycemia and increased mortality was consistently demonstrated. The discrepancies and high mortality rate of patients with severe hypoglycemia may be partially attributed to the small sample size and the problems that are inherent to ED population-based studies, such as an older population, higher patient comorbidity, more severe and critical diseases, higher patient flow, delayed or missed diagnoses and overcrowding. These factors may present a therapeutic challenge for physicians when treating a specific group of patients. Regardless of the fact that high mortality was observed, it was striking that only one-third of these patients were admitted after emergency treatment.
Limitations
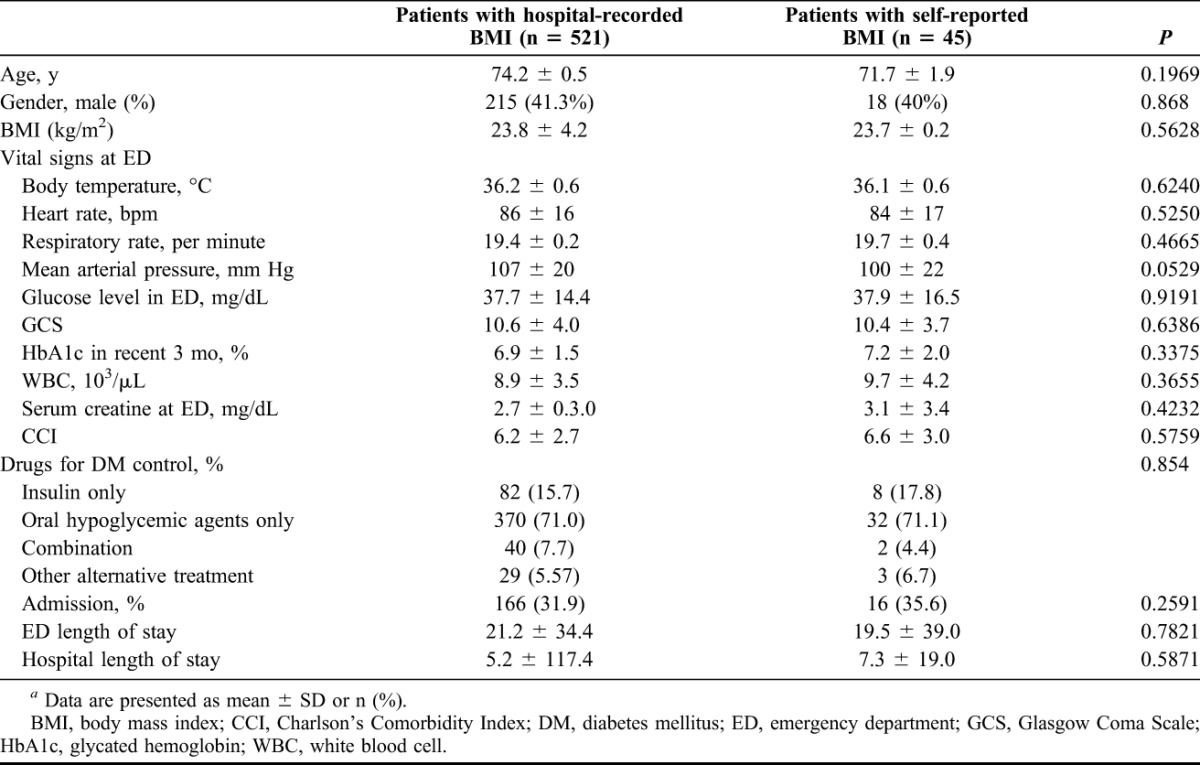
The main strengths of this study include its complete recruitment, comprehensive data collection and follow-up. However, this study had certain limitations. First, the main limitation was the small sample size, and the results must be confirmed in larger registries in the future. Second, this study was a single-center examination of patients in the ED, although the assessment of an accurate weight and height in critical patients may be difficult. Because the heights and weights of all patients were not routinely measured and recorded during their ED visits, we had to rely on self-report if these data were not available. We relied on only 1 weight and height measurement from the ED, which is a major limitation of this study. Forty-five participants (7.9%) were recorded by self-report among all precipitants. To assess bias, we compared the participants whose BMI were hospital recorded and self-reported. However, self-reported BMI did not differ importantly from the rest of the cohort with respect to baseline characteristics (Table 5) (all P values < 0.05) and would not potentially bias outcome data in a more favorable direction.
TABLE 5.
Baseline characteristics of the 2 groups of hospital-recorded BMI and self-reported BMIa
Finally, we were unable to obtain information on the frequency of severe hypoglycemic episodes and whether the index visit was the first hypoglycemia event. The fortuitous severe hypoglycemia could not be differentiated as to whether it was the first-ever or recurrent hypoglycemic episode for each patient. The differences of clinical characteristics, pathophysiology, mechanisms and medical care between the first hypoglycemia event and recurrent hypoglycemia events were not effectively examined. Merging these patients may cause nondifferential misclassification and bias the results.
CONCLUSIONS
To the best of our knowledge, this is the first study on severe hypoglycemia in an ED. The results of this study showed that the crucial prognostic factor with statistical significance for increased risk of death was BMI less than 18.5 kg/m2 among adult patients with type 2 diabetes with severe hypoglycemia. Excessive low weight may identify patients with particularly high mortality risks and may be a marker or trigger to exacerbate underlying diseases. The results reveal an association between type 2 diabetes with a low BMI and increased short- and long-term mortality and highlight the requirement for a new therapeutic approach for severe hypoglycemia in patients with type 2 diabetes, especially for underweight group. According to the result of the study, admission after emergency treatment for advanced surveying possible concomitant diseases such as infection is recommended. Physician, dieticians and nurses must collaborate to develop an interdisciplinary assessment of these patients to maintain an optimal nutritional status and beware of underweight if severe hypoglycemia was identified. In conclusion, an independent association was observed between a BMI of less than 18.5 kg/m2 and mortality among type 2 DM patients with severe hypoglycemic episode when compared to those with normal or high BMIs. Deaths for all patients were predominantly infection related.
Footnotes
The authors have no financial or other conflicts of interest to disclose.
REFERENCES
- 1.Donnelly LA, Morris AD, Frier BM, et al. Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: a population-based study. Diabet Med 2005;22:749–55. [DOI] [PubMed] [Google Scholar]
- 2.Casparie AF, Elving LD. Severe hypoglycemia in diabetic patients: frequency, causes, prevention. Diabetes Care 1985;8:141–5. [DOI] [PubMed] [Google Scholar]
- 3.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977– 86. [DOI] [PubMed] [Google Scholar]
- 4.Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89. [DOI] [PubMed] [Google Scholar]
- 5.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–39. [DOI] [PubMed] [Google Scholar]
- 6.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72. [DOI] [PubMed] [Google Scholar]
- 8.Gerstein HC, Riddle MC, Kendall DM, et al. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007;99:34i–43. [DOI] [PubMed] [Google Scholar]
- 9.Duckworth WC, Abraira C, Moritz TE, et al. The duration of diabetes affects the response to intensive glucose control in type 2 subjects: the VA Diabetes Trial. J Diabetes Complications 2011;25:355–61. [DOI] [PubMed] [Google Scholar]
- 10.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–8. [DOI] [PubMed] [Google Scholar]
- 11.Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care 2010;33:1389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turchin A, Matheny ME, Shubina M, et al. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care 2009;32:1153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krinsley JS, Schultz MJ, Spronk PE, et al. Mild hypoglycemia is independently associated with increased mortality in the critically ill. Crit Care 2011;15:R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCoy RG, Van Houten HK, Ziegenfuss JY, et al. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012;35:1897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEwen LN, Kim C, Karter AJ, et al. Risk factors for mortality among patients with diabetes: the Translating Research into Action for Diabetes (TRIAD) Study. Diabetes Care 2007;30:1736–41. [DOI] [PubMed] [Google Scholar]
- 17.Wang HY, Chew G, Kung CT, et al. The use of Charlson comorbidity index for patients revisiting the emergency department within 72 hours. Chang Gung Med J 2007;30:437–44. [PubMed] [Google Scholar]
- 18.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003;26:1902–12. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a who convention, Geneva, 1999. WHO technical report series 894. Geneva, Switzerland: WHO; 2000. [PubMed] [Google Scholar]
- 20.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–the evidence report. National Institutes of health. Obes Res 1998;6 (suppl 2):51S– 209S. [PubMed] [Google Scholar]
- 21.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141:421–31. [DOI] [PubMed] [Google Scholar]
- 23.Gerstein HC, Pogue J, Mann JF, et al. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia 2005;48:1749–55. [DOI] [PubMed] [Google Scholar]
- 24.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837– 53. [PubMed] [Google Scholar]
- 25.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. JAMA 2003;289:187–93. [DOI] [PubMed] [Google Scholar]
- 27.Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med 2010;170:1566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norman K, Pichard C, Lochs H, et al. Prognostic impact of disease-related malnutrition. Clin Nutr 2008;27:5–15. [DOI] [PubMed] [Google Scholar]
- 29.Caccialanza R, Klersy C, Cereda E, et al. Nutritional parameters associated with prolonged hospital stay among ambulatory adult patients. CMAJ 2010;182:1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kastorini CM, Panagiotakos DB. The obesity paradox: methodological considerations based on epidemiological and clinical evidence-new insights. Maturitas 2012;72:220–4. [DOI] [PubMed] [Google Scholar]
- 31.Finfer S, Liu B, Chittock DR, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012;367:1108–18. [DOI] [PubMed] [Google Scholar]
- 32.Egi M, Bellomo R, Stachowski E, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc 2010;85:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adler GK, Bonyhay I, Failing H, et al. Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes 2009;58:360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev 2008;24:353–63. [DOI] [PubMed] [Google Scholar]


