Abstract
Substance P (SP) is commonly coexpressed with ACh in enteric motor neurons, and, according to the classical paradigm, both these neurotransmitters excite smooth muscle via parallel pathways. We hypothesized that, in addition, SP was responsible for maintaining the muscular responsiveness to ACh. We tested this hypothesis by using botulinum toxin (BoNT/A), a known blocker of vesicular release of neurotransmitters including ACh and neuropeptides. BoNT/A was injected into rat pyloric sphincter in different doses; as control we used boiled BoNT/A. At the desired time point, pylorus was dissected out and pyloric contractility was measured ex vivo in an organ bath and by measuring phosphorylation of myosin light chain 20 (MLC20). BoNT/A (10 IU) significantly reduced the response of pyloric muscle to exogenous ACh, an effect that was accompanied by reduced MLC20 phosphorylation in the muscle. Both effects were reversed by exogenous SP. CP-96345, a NK1 receptor antagonist, blocked the ability of exogenous SP to reverse the cholinergic hyporesponsiveness as well as the reduction in MLC20 phosphorylation induced by BoNT/A. In conclusion, we have identified a novel role for SP as a coneurotransmitter that appears to be important for the maintenance of muscular responsiveness to the principal excitatory neurotransmitter, ACh. These results also provide new insight into the effects of botulinum toxin on the enteric nervous system and gastrointestinal smooth muscle.
Keywords: botulinum toxin, gut smooth muscle contractility, substance P, cholinergic responsiveness, acetylcholine, coneurotransmission, MLC phosphorylation
botulinum neurotoxin type A (BoNT/A) is one of the most potent blockers of vesicular neurotransmitter release from neurons and is widely used as a therapeutic agent for a variety of spastic muscular disorders including those involving visceral muscle (4, 10, 14, 24, 25, 30, 33). The mechanisms of action of this toxin on skeletal muscle have been extensively studied; by contrast, there is little literature on how it affects gastrointestinal smooth muscle. A previous report has shown BoNT/A to have a dual effect on gut smooth muscle tone, with low concentrations inhibiting neurally mediated contractile responses and higher concentrations directly inhibiting smooth muscle contractility in response to exogenous ACh (12). This latter effect is distinct from that seen in skeletal muscle, where the application of botulinum toxin results in denervation hypersensitivity. However, the mechanism for this phenomenon remains unknown.
Unlike most motor neurons innervating skeletal muscle, which are purely excitatory, enteric motor neurons can be considered as either excitatory or inhibitory in terms of their effects on smooth muscle tone. Furthermore, enteric neurons are distinguished by the phenomenon of coneutrotransmission; as an example, excitatory motor neurons contain both ACh and the tachykinin substance P (SP) (5, 7, 18, 21). According to the classical paradigm, there are both a cholinergic and a tachykininergic component to neurally mediated muscle contraction, with the latter more evident during stronger stimulation (1, 6, 8, 31). Since both ACh and SP are released from vesicles by a process that is sensitive to botulinum toxin, we hypothesized that by blocking both ACh and SP release from enteric neurons, BoNT/A induces paralysis of gastrointestinal smooth muscle not only because of a lack of ACh but also from the lack of endogenous SP that is required to maintain sensitivity to exogenous contractile agonists. Our results provide the basis for a new paradigm for the mechanism of action of BoNT/A as well as a novel role for coneurotransmission in the enteric nervous system with important physiological and clinical implications.
MATERIALS AND METHODS
Chemicals and reagents.
BoNT/A was purchased from Allergan (Irvine, CA). Acetylcholine chloride (ACh), SP, atropine, guanethidine, NG-nitro-l-arginine methyl ester (l-NAME), sodium nitroprusside, and potassium chloride were all from Sigma (St. Louis, MO). Tetrodotoxin was purchased from Calbiochem (San Diego, CA). Anti-phospho-myosin light chain (p-MLC20) antibody was purchase from Cell Signaling (Danvers, MA), and myosin light chain (MLC20) antibody was from Sigma (St. Louis, MO). CP-96345, [(S)-N-methy-N-[4-(4-acetylamino-4-phenylpiperidino)-2-(3,4-di-chlorophenyl)-butyl] benzamide], was a gift from Pfizer Central Research (Groton, CT).
BoNT/A injection.
All experiments were approved by the Institutional Animal Care and Use Committee of Stanford University and Johns Hopkins University. Under anesthesia with a cocktail of intraperitoneal ketamine (80 mg/kg)-xylazine (5 mg/kg), adult male Sprague-Dawley rats (200–250 g) underwent laparotomy to expose the pyloric sphincter. The pylorus was identified as the region from the gastroduodenal junction extending proximally by ∼6 mm. BoNT/A (1 or 10 IU) dissolved in 20 μl saline was injected into each quadrant of the pylorus from the serosal side; sterile saline or BoNT/A inactivated by boiling for 5 min at 100°C was injected in the pylorus of control rats.
Pyloric circular muscle strips preparation.
At the desired time point, the rats were euthanized and the pylorus was dissected out. The tissue on the antral side was trimmed away and the remaining pylorus was pinned down, mucosa side up, on a Sylgard-coated dissecting dish containing ice-cold Krebs bicarbonated buffer (composition in mM: 4.7 KCl, 2.5 CaCl2, 0.5 MgCl2, 118 NaCl, 1.17 NaH2PO4, 11.1 d-glucose, 25 NaHCO3) oxygenated with 95% O2-5% CO2. The mucosa was gently scraped away with a sterile cotton swab, and then each pylorus was further cut in half to yield a circular muscle strip (15). Those strips treated with SP were incubated in 4°C Krebs buffer oxygenated with 95% O2-5% CO2 for 4 h; the strips treated with CP-96345 and SP were incubated with 10−3 M CP-96345 for 30 min (32) and then with 10−3 M SP for 4 h in 4°C Krebs buffer oxygenated with 95% O2-5% CO2 (9, 16).
Organ bath studies.
Pyloric circular muscle strips were placed in organ bath chambers containing oxygenated Krebs bicarbonated buffer at 37°C, and one end was tied to its chamber while the other was tied to a transducer above the organ bath. Then the strips were given 2 g preload tension and allowed to equilibrate for 60 min. After equilibration, the strips were given electrical field stimulation (EFS) or a series of ACh challenges (10−5 to 10−2 M final concentration in bath), respectively (2).
EFS.
EFS (150 V, 0.1 ms, 1–20 Hz, 1 min stimulation every 4 min) was performed to test the effect of BoNT/A on neuronal regulation of smooth muscle activity. At the end of each experiment, the strips were weighed and stored in −80°C for further Western blot analysis.
Measure of p-MLC20 by Western blot analysis.
The strips were homogenized on ice for 30 min in lysis buffer supplemented with protease inhibitors. The homogenate suspension was centrifuged at 15,000 g for 10 min and the supernatant was boiled in loading buffer. For Western blot analysis, protein samples were separated on a 4–12% Bis-Tris-PAGE gel, and then the resolved proteins were transferred onto polyvinylidene difluoride membranes followed by blocking with 5% nonfat dry milk in Tris-buffered saline with Tween-20 (TBST) for 1 h. Membranes were then incubated with the appropriate primary antibodies diluted in 5% nonfat dry milk in TBST overnight at 4°C. After three washes with TBST, membranes were incubated with appropriate horseradish peroxidase-linked secondary antibodies for 1 h in 5% nonfat dry milk in TBST. Protein bands were detected by enhanced chemiluminescence reagent (Pierce). Optical density value of band was analyzed by means of an Image J analysis system (NIH, version 1.44).
Statistical analysis.
Muscle tension was monitored with an isometric force transducer and recorded and analyzed by a digital recording system (Biopac Systems, Santa Barbara, CA). Responses were collected as initial increase or decrease in tension or, in cases where the muscle was involved in rhythmic contraction at the time the chemical agent or EFS was applied, deflection from baseline. Responses were adjusted for mass by dividing the tension generated by the mass of the tissue. The results were expressed as means ± SE. Unless otherwise indicated, results were analyzed by univariate and multivariate analysis of variance and one-way ANOVA by using SPSS statistical analysis software (IBM SPSS, Chicago, IL), with values of P < 0.05 considered statistically significant.
RESULTS
BoNT/A reduces pyloric smooth muscle responsiveness to neural stimulation as well as exogenous ACh.
To investigate the effect of different dose of BoNT/A on pyloric circular muscle, we firstly measured pyloric circular muscle contractility in response to EFS and exogenous ACh at 1 h after BoNT/A or boiled BoNT/A injection. We tested BoNT/A at doses as low as 1 IU and were unable to demonstrate a dissociation between the neural and muscular effects, suggesting that the two are intimately tied to each other. Thus, as can be seen from the Fig. 1, the off-response to EFS (Fig. 1A) is markedly diminished even at 1 IU and is associated with a corresponding decrease in the response to exogenous ACh (Fig. 1B).
Fig. 1.
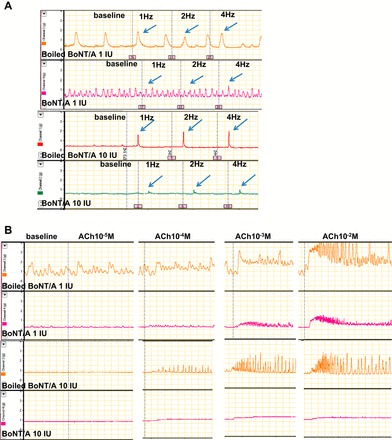
Effect of different dose (1 or 10 IU) of botulinum toxin type A (BoNT/A) or boiled BoNT/A on pyloric circular muscle contractility. A: pyloric circular muscle contractility to electrical field stimulation (EFS). B: pyloric circular muscle contractility to acetylcholine chloride (ACh). Both doses caused similar changes in the pyloric sphincter responsiveness.
We then characterized the response of pyloric muscle to electrical stimulation 24 h after treatment with 10 IU of BoNT/A, saline or boiled BoNT/A. Pyloric circular muscle strips were isolated and subjected to a series of EFSs of 1-min duration at various frequencies (1, 2, 4 Hz). Off-contractions (known to be mediated mostly by the release of ACh and SP) were recorded at the end of each stimulation period. As shown in Fig. 2A, off-contractions were significantly reduced in pyloric circular muscle strips from BoNT/A-injected rats compared with saline-injected controls. In subsequent experiments we used boiled BoNT/A as control instead of saline to eliminate any confounding nonspecific effects from protein injection.
Fig. 2.
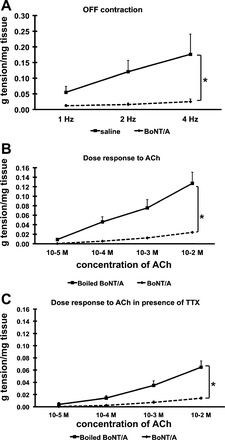
Effect of BoNT/A on pyloric circular muscle contractility. A: pyloric circular muscle contractility to EFS, means of 11–12 experiments (total N = 23). B: pyloric circular muscle contractility to ACh; means of 4 experiments (total N = 8). C: pyloric circular muscle contractility to ACh in presence of TTX, means of 4 experiments (total N = 8). Results were expressed as means ± SE, *P < 0.05.
We next measured the contractility response of the muscle in response to bath applications of ACh. Our results show that prior treatment with BoNT/A significantly reduced ACh-induced contractions of pyloric circular muscle strips 24 h later compared with boiled BoNT/A injected controls (Fig. 2B). This effect was also observed in the presence of TTX (1 μM) (Fig. 2C), suggesting that it was not due to a neural mechanism such as that involving the release of an inhibitor of smooth muscle tone. Furthermore, circular muscle strips were challenged with KCl (125 mM) in the presence of TTX; pretreatment with BoNT/A reduced the contractile response to KCl by ∼60% (expressed as an average of the boiled BoNT/A group) at 1 h.
To further characterize the effect of BoNT/A on pyloric circular muscle, we measured muscle contractility in response to EFS (4 Hz), at 1 h, 24 h, 2 wk, and 1 mo post-BoNT/A injection. The contractility of the BoNT/A-treated muscle was expressed as percentage of the contractility measured in boiled BoNT/A-treated controls. Off-contractions were significantly reduced in the BoNT/A-treated muscle as early as 1 h, stayed reduced for at least 2 wk, and had largely recovered by 4 wk (Fig. 3). To avoid two surgeries on the same animal, all subsequent experiments described in this paper were done at the 1-h time point.
Fig. 3.
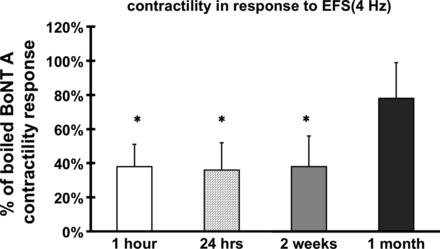
Time course of BoNT/A action. Off-contractions were significantly reduced in the BoNT/A-treated muscle as early as 1 h, stayed reduced for at least 2 wk, and had largely recovered by 4 wk. Results were expressed as a percentage of the average response of the boiled BoNT/A group and represent means ± SE, means of 5–8 experiments (total N = 24), *P < 0.05.
To confirm the role of ACh and SP in the off-contractions, we added atropine and the NK1 receptor antagonist, CP-96345 (32). In the presence of atropine (an ACh receptor antagonist, 1 × 10−6 M), off-contractions in the control groups (treated with boiled BoNT/A) dramatically decreased; furthermore, in the presence of both atropine and CP-96345 (a NK1 receptor antagonist, 1 × 10−3 M), off-contractions were essentially eliminated. No significant differences existed in the effect of various frequencies of EFS (Fig. 4, A and B).
Fig. 4.
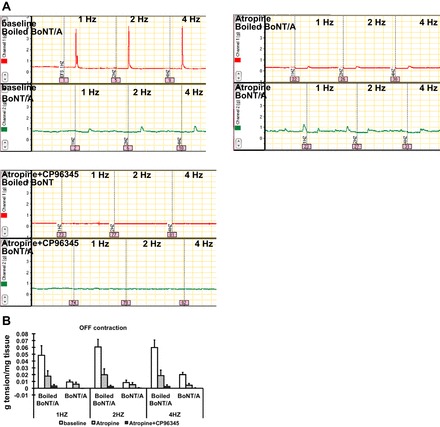
Effect of BoNT/A on neurotransmitter release of substance P (SP). Off-contractions were recorded at the baseline, in the presence of atropine (1 × 10−6 M), and in the presence of both atropine and CP-96345 (1 × 10−3 M). Compared with boiled BoNT/A-treated muscle, off-contractions were significantly reduced in the BoNT/A-treated muscle. In the presence of atropine, off-contractions dramatically decreased; furthermore, in the presence of both atropine and CP-96345, off-contractions nearly totally diminished. No significant differences existed in the effect of various frequencies of EFS (multivariate ANOVA: effect of BoNT/A treatment, P < 0.05; effect of presence of chemicals, P < 0.05; effect of EFS frequency, P = 0.924). Results were expressed as means ± SE, means of 3 experiments (total N = 12).
NK1 receptor blockade can mimic the effects of BoNT/A on pyloric smooth muscle contractility.
On the basis of our hypothesis that the effects of BoNT/A on cholinergic responsiveness are due to an inhibition of SP release, we compared the effects of BoNT/A and the NK1 receptor antagonist, CP-96345, on pyloric circular muscle contractility and MLC20 phosphorylation. BoNT/A, boiled BoNT/A, and saline-treated pyloric muscles were isolated 1 h after in vivo injection as above and the strips were incubated in vitro in Krebs's solution for 30 min. In addition, CP-96345 (10−3 M) was added to the medium of strips treated with boiled BoNT/A. Incubation of muscle strips with CP-96345 mimicked the effect of botulinum toxin on smooth muscle responsiveness to exogenous ACh (Fig. 5, A–C) and caused a similar reduction in MLC20 phosphorylation (Fig. 5, E and F).
Fig. 5.
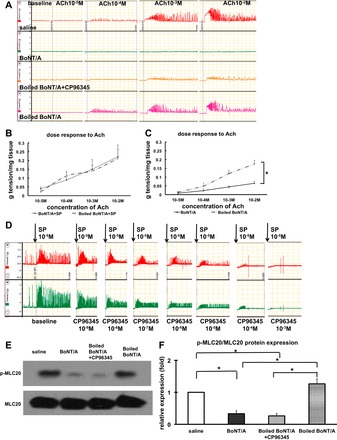
Effect of CP-96345 on pyloric circular muscle contractility and myosin light chain 20 (MLC20) phosphorylation (p-MLC20) expression following injection of BoNT/A. A–C: pyloric circular muscle contractility to ACh. D: effect of CP-96345 on the response to SP in normal pyloric circular muscle contractility. E and F: p-MLC20 expression following injection of BoNT/A. Results were expressed as means ± SE, means of 3 experiments (total N = 12); *P < 0.05.
Effect of exogenous SP on pyloric circular muscle contractility and p-MLC20 expression following injection of BoNT/A.
The effects of BoNT/A on both the contractile response (Fig. 6, A–C) and MLC20 phosphorylation (Fig. 6, D and E) in response to exogenous ACh were reversed by incubation of the tissue with exogenous SP (10−3 M).
Fig. 6.
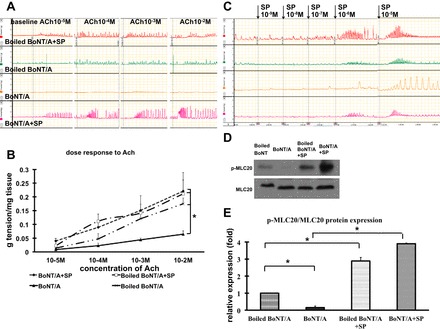
Effect of exogenous substance P (SP; 10−3 M) on pyloric circular muscle contractility and p-MLC20 expression following injection of BoNT/A. A and B: pyloric circular muscle contractility to ACh. C: dose response to SP in normal pyloric circular muscle contractility. D and E: p-MLC20 expression following injection of BoNT/A. Results were expressed as means ± SE, means of 5 experiments (total N = 15); *P < 0.05.
We then incubated the tissue with different concentrations of exogenous SP (10−5, 10−4, and 10−3 M) on BoNT/A-treated pyloric strips and tested their response to exogenous ACh. A dose-dependent increase in contractility was seen with exogenous SP (Fig. 7, A and B), an effect that was accompanied by increased MLC20 phosphorylation in the muscle (Fig. 7, C and D).
Fig. 7.
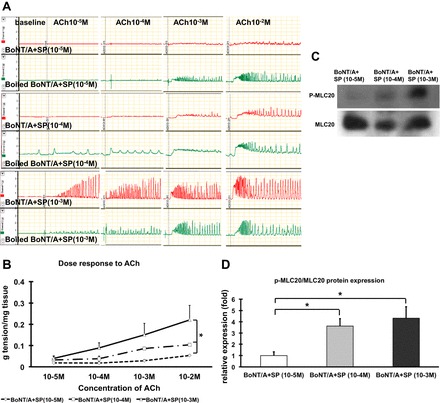
Effect of different concentrations of SP on pyloric circular muscle contractility and p-MLC20 expression following injection of BoNT/A. A and B: pyloric circular muscle contractility to ACh. C and D: p-MLC20 expression following injection of BoNT/A. Results were expressed as means ± SE, means of 3 experiments (total N = 12); *P < 0.05.
Finally, we tested whether the effects of SP (10−3 M) on BoNT/A-induced muscle hypocontractility were mediated by the NK1 receptor antagonist. In the presence of CP 96345, SP had no effect on smooth muscle responsiveness (Fig. 8, A and B) and MLC20 phosphorylation (Fig. 8, C and D) in preparations from BoNT/A-treated pyloric sphincter.
Fig. 8.
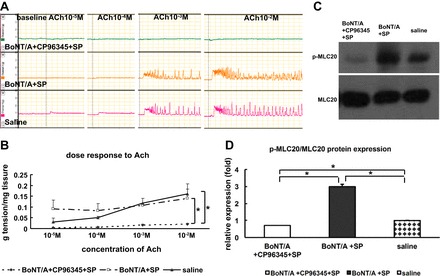
Effect of CP-96345 on pyloric circular muscle contractility and p-MLC20 expression following injection of BoNT/A. A and B: pyloric circular muscle contractility to ACh. C and D: p-MLC20 expression following injection of BoNT/A. Results were expressed as means ± SE, means of 3 experiments (total N = 9); *P < 0.05.
DISCUSSION
The phenomenon of coneurotransmitter expression in enteric motor neurons has been well known for several decades and it has been assumed that this reflects a redundancy in the system necessary for preservation of gastrointestinal motility, which is critical for survival of the organism. Thus, according to the current paradigm, excitatory motor neurons coexpress ACh and SP, both of which can independently induce contraction in gut smooth muscle (26). Indeed, the lack of a significant functional defect in the gastrointestinal tract in mice that lack both the M2 and M3 muscarinic receptors attests to the importance of alternative neurotransmitters (such as SP) that can compensate for the lack of a cholinergic drive (19). However, little is known about whether there is any significant interaction between these coexpressed neurotransmitters in the regulation of smooth muscle contractility in the gut. In this study, we used BoNT/A as a tool to examine the possibility of such cooperation, using its ability to block vesicular release of neurotransmitters and then selectively adding back SP (11).
In this study, we show that BoNT/A strongly impaired exogenous pyloric circular muscle contractility to exogenous ACh, confirming previous observations in guinea pig antral muscle (12). The effect persists in the presence of tetrodotoxin and is thus not mediated by neural mechanisms such as the release of an inhibitory neurotransmitter. Furthermore, the reduction in the response to exogenous KCl suggests that the effect of BoNT/A on circular muscle contractility probably affects mechanisms downstream from specific receptors. However, this effect could be reversed by the addition of SP in a dose-dependent manner and appears to be mediated by the NK1 receptor since it can be blocked by CP-96345 (32). Furthermore, blockade of the NK1 receptor by itself can mimic the effects of BoNT/A on the cholinergic responsiveness of the muscle. We acknowledge that some of these results have to be interpreted with caution because of the high concentrations of this antagonist that were used, giving rise to the possibility of “off-target” or nonspecific effects. However, in our system, such concentrations of CP-96345 (NK1 receptor antagonist) were necessary to completely block the effect of SP.
Both ACh, via the muscarinic M3 receptor, and SP, via the NK1 receptor, can activate Gq/11, which initiates phospholipase C-mediated increases in cytosolic calcium. Smooth muscle contraction depends on both the concentration of free cytosolic calcium as well as the sensitivity of the contractile and regulatory proteins to calcium (28). These mechanisms, which include cAMP/cGMP signaling, CPI-17, MLC kinase, and phosphatase among others, converge on the regulatory MLC20, whose state of phosphorylation is the principal determinant of smooth muscle cell contraction. This is reflected in our results, with the levels of p-MLC20 running parallel to the observed effects on contractility in response to various interventions.
Previous studies have hinted at an interaction between ACh and SP in gastrointestinal smooth muscle. Thus the net effect of partial muscarinic blockade combined with NK1 and NK2 antagonists on colonic peristalsis in the guinea pig is significantly higher than the sum of the individual effects (29). Furthermore, this effect may be unique to the gut since it has not been shown in respiratory muscle (13) or salivary glands (27). The results of our study indicate that tachykininergic and cholinergic pathways in gut smooth muscle interact at a point or points downstream of the respective receptors and upstream of MLC20 phosphorylation, which is a very broad domain with multiple putative targets that interact in a complex manner. It is also possible that the effect of SP is mediated via interstitial cells of Cajal, which prominently express the NK1 receptor, perhaps even more so than smooth muscle itself (26). Eliciting the exact site of cooperation will therefore require considerable more studies. It would also be important in the future to determine whether other neurotransmitters expressed by enteric neurons have similar effects on cholinergic responsiveness.
Our results also provide further insight into the mechanism of action of botulinum toxin on gastrointestinal smooth muscle. Little is known about the mechanism of action of BoNT/A on gastrointestinal muscle contractility, despite the proliferation of therapeutic applications (4, 10, 14, 24, 30, 33). Instead, the prevailing assumption, almost entirely extrapolated from the skeletal muscle literature, is that its effects are confined to blockade of neurotransmitter release from prejunctional enteric neurons with consequent reduction in muscle tone and neurally mediated contractility (3, 17, 20, 22, 23, 25). Indeed, a “neural blockade” has been demonstrated in vivo and in vitro, with effects on those neurotransmitters whose release requires vesicular fusion such as ACh and perhaps others but with sparing of nitric oxide release, which is not a vesicular process (3, 17, 20, 22, 23, 25). This notion has been challenged by experiments by James et al. (12) on guinea pig stomach, in which at a low concentration (2 IU/ml) of BoNT/A was shown to decrease EFS-induced contractile responses without affecting ACh-induced contractions; at higher concentrations, botulinum toxin directly inhibits smooth muscle contractility as evidenced by the decreased contractile response to ACh. We were unable to distinguish this so-called “neural” from the “muscular” effect using BoNT/A in vivo as James et al. did in their in vitro application of BoNT/A. This may be due to the species differences and/or because of methodological differences (they added BoNT/A to the bath in vitro whereas we injected it in vivo). Although this may be seen as a limitation of our study, it does not detract from our main finding that SP restores cholinergic responsiveness to the muscle.
In conclusion, the results of our study have identified an additional novel role for SP as a coneurotransmitter that appears to be important for the maintenance of muscular responsiveness to the principal excitatory neurotransmitter, ACh. These findings are summarized in Fig. 9. This suggests that it is no longer accurate to regard ACh and SP as acting in parallel to stimulate gut muscle. Instead, the relationship appears to be much more complex and intertwined, offering a new paradigm for the function of the enteric nervous system as well as for the mechanism of action of BoNT/A with important physiological and clinical implications.
Fig. 9.
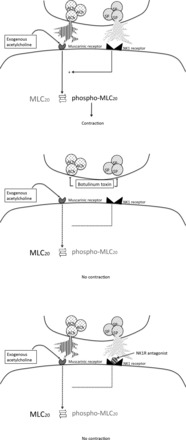
Schematic illustrating the physiological implications of the results of this experiment. Under normal conditions (top), ACh and SP are coexpressed in the terminal of motor neurons of the myenteric plexus and, upon stimulation of the neuron, are released by vesicular-mediated mechanisms. SP, via the NK1 receptor and as yet unknown downstream pathways, is necessary for the ability of acetylcholine (endogenous or exogenous) to phosphorylate MLC20 and initiate contraction. In the presence of botulinum toxin, the release of both neurotransmitters from neurons is prevented (middle). In the absence of SP, the muscle loses its responsiveness to exogenous acetylcholine as well and fails to active MLC20 and initiate contraction. These effects can be mimicked by blocking the NK1 receptor as well, suggesting that some tonic release of SP is necessary to maintain cholinergic responsiveness of smooth muscle under physiological conditions (bottom).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.L. and M.-A.M. performed experiments; C.L., M.-A.M., and P.J.P. analyzed data; C.L., M.-A.M., and P.J.P. interpreted results of experiments; C.L. and M.-A.M. prepared figures; C.L., M.-A.M., and P.J.P. drafted manuscript; C.L., M.-A.M., K.S.M., and P.J.P. edited and revised manuscript; C.L., M.-A.M., K.S.M., and P.J.P. approved final version of manuscript; K.S.M. and P.J.P. conception and design of research.
REFERENCES
- 1.Barthó L, Holzer P, Donnerer J, Lembeck F. Evidence for the involvement of substance P in the atropine-resistant peristalsis of the guinea-pig ileum. Neurosci Lett 32: 69–74, 1982. [DOI] [PubMed] [Google Scholar]
- 2.Berk T, Crochelt RF, Peikin SR. Role of pylorus in mediating cholecystokinin-stimulated satiety in the Zucker rat. Dig Dis Sci 31: 502–505, 1986. [DOI] [PubMed] [Google Scholar]
- 3.Bigalke H, Habermann E. Blockade by tetanus and botulinum toxin A toxin of postganglionic cholinergic nerve endings in the myenteric plexus. Naunyn Schmiedebergs Arch Pharmacol 312: 255–263, 1980. [DOI] [PubMed] [Google Scholar]
- 4.Brisinda G, Civello IM, Albanese A, Maria G. Gastrointestinal smooth muscles and sphincters spasms: treatment with botulinum neurotoxin. Curr Med Chem 10: 603–623, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Costa M, Furness JB, Pullin CO, Bornstein J. Substance P enteric neurons mediate non-cholinergic transmission to the circular muscle of the guinea-pig intestine. Naunyn Schmiedebergs Arch Pharmacol 328: 446–453, 1985. [DOI] [PubMed] [Google Scholar]
- 6.Donnerer J, Barthó L, Holzer P, Lembeck F. Intestinal peristalsis associated with release of immunoreactive substance P. Neuroscience 11: 913–918, 1984. [DOI] [PubMed] [Google Scholar]
- 7.Grider JR. Tachykinins as transmitters of ascending contractile component of the peristaltic reflex. Am J Physiol Gastrointest Liver Physiol 257: G709–G714, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Harrington AM, Hutson JM, Southwell BR. Immunohistochemical localization of substance P NK1 receptor in guinea pig distal colon. Neurogastroenterol Motil 17: 727–737, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Holzer-Petsche U, Lembeck F, Seitz H. Contractile effects of substance P and neurokinin A on the rat stomach in vivo and in vitro. Br J Pharmacol 90: 273–279, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoogerwerf WA, Pasricha PJ. Botulinum toxin for spastic gastrointestinal disorders. Baillieres Best Pract Res Clin Gastroenterol 13: 131–143, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Hou YP, Zhang YP, Song YF, Zhu CM, Wang YC, Xie GL. Botulinum toxin type A inhibits rat pyloric myoelectrical activity and substance P release in vivo. Can J Physiol Pharmacol 85: 209–214, 2007. [DOI] [PubMed] [Google Scholar]
- 12.James AN, Ryan JP, Parkman HP. Inhibitory effects of botulinum toxin on pyloric and antral smooth muscle. Am J Physiol Gastrointest Liver Physiol 285: G291–G297, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Kamikawa Y, Shimo Y. Inhibitory effect of CP-96,345, a non-peptide neurokinin-1-receptor antagonist, on neurogenic responses of guinea-pig isolated airway smooth muscle. J Pharm Pharmacol 45: 913–916, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Klein AW. The therapeutic potential of botulinum toxin. Dermatol Surg 30: 452–455, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Qian W, Hou X. Effect of four medications associated with gastrointestinal motility on Oddi sphincter in the rabbit. Pancreatology 9: 615–620, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Lidberg P, Dahlström A, Ahlman H. On the nature of the contractile motor responses of the rat stomach elicited by serotonin or substance P in vitro. J Neural Transm 63: 73–89, 1985. [DOI] [PubMed] [Google Scholar]
- 17.MacKenzie I, Burnstock G, Dolly JO. The effects of purified botulinum neurotoxin type A on cholinergic, adrenergic and non-adrenergic, atropine-resistant autonomic neuromuscular transmission. Neuroscience 7: 997–1006, 1982. [DOI] [PubMed] [Google Scholar]
- 18.Maggi CA, Catalioto RM, Criscuoli M, Cucchi P, Giuliani S, Lecci A, Lippi A, Meini S, Patacchini R, Renzetti AR, Santicioli P, Tramontana M, Zagorodnyuk V, Giachetti A. Tachykinin receptors and intestinal motility. Can J Physiol Pharmacol 75: 696–703, 1997. [PubMed] [Google Scholar]
- 19.Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi S, Manabe T, Taketo MM. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci 22: 10627–10632, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris JL, Jobling P, Gibbins IL. Differential inhibition by botulinum neurotoxin A of cotransmitters released from autonomic vasodilator neurons. Am J Physiol Heart Circ Physiol 281: H2124–H2132, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Nohr D, Eiden LE, Weihe E. Coexpression of vasoactive intestinal peptide, calcitonin gene-related peptide and substance P immunoreactivity in parasympathetic neurons of the rhesus monkey lung. Neurosci Lett 199: 25–28, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Olgart C, Gustafsson LE, Wiklund NP. Evidence for nonvesicular nitric oxide release evoked by nerve activation. Eur J Neurosci 12: 1303–1309, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Paul ML, Cook MA. Lack of effect of botulinum toxin on nonadrenergic, noncholinergic inhibitory responses of the guinea pig fundus in vitro. Can J Physiol Pharmacol 58: 88–92, 1980. [DOI] [PubMed] [Google Scholar]
- 24.Scott AB. Development of botulinum toxin therapy. Dermatol Clin 22: 131–133, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Sand J, Nordback I, Arvola P, Pörsti I, Kalloo A, Pasricha P. Effects of botulinum toxin A on the sphincter of Odd: an in vivo and in vitro study. Gut 42: 507–510, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu Y, Matsuyama H, Shiina T, Takewaki T, Furness JB. Tachykinins and their functions in the gastrointestinal tract. Cell Mol Life Sci 65: 295–311, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snider RM, Longo KP, Drozda SE, Lowe JA, 3rd, Leeman SE. Effect of CP-96,345, a nonpeptide substance P receptor antagonist, on salivation in rats. Proc Natl Acad Sci USA 88: 10042–10044, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somlyo AP, Somlyo AV. From pharmacomechanical coupling to G-proteins and myosin phosphatase. Acta Physiol Scand 164: 437–448, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Tonini M, Spelta V, De Ponti F, De Giorgio R, D'Agostino G, Stanghellini V, Corinaldesi R, Sternini C, Crema F. Tachykinin-dependent and -independent components of peristalsis in the guinea pig isolated distal colon. Gastroenterology 120: 938–945, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Vittal H, Pasricha PJ. Botulinum toxin for gastrointestinal disorders: therapy and mechanisms. Neurotox Res 9: 149–159, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Yau WM. Effect of substance P on intestinal muscle. Gastroenterology 74: 228–231, 1978. [PubMed] [Google Scholar]
- 32.Wang ZY, Håkanson R. (+/−)-CP-96,345, a selective tachykinin NK1 receptor antagonist, has non-specific actions on neurotransmission. Br J Pharmacol 107: 762–765, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X, Pasricha PJ. Botulinum toxin for spastic GI disorders: a systematic review. Gastrointest Endosc 57: 219–235, 2003. [DOI] [PubMed] [Google Scholar]


