Abstract
The kidney is a major site of arginine synthesis where citrulline is converted to arginine via argininosuccinate synthase (ASS) and lyase (ASL). The rate-limiting step in arginine synthesis by the normal kidney is the rate of citrulline delivery and uptake to the renal cortex. We tested whether with chronic kidney disease (CKD) renal arginine synthesis may be compromised. Using the renal ablation/infarction (A/I) injury model, we measured renal citrulline delivery and uptake as well as arginine release at early, moderate, and severe stages of CKD vs. healthy controls. The renal plasma flow (RPF) and arterial-renal venous difference was measured at baseline and during citrulline infusion. Citrulline delivery was reduced at all stages of disease due to marked reductions in RPF and despite moderately increased plasma citrulline. Early after A/I, the kidney demonstrated a compensatory increase in citrulline uptake while at moderate and severe injury baseline citrulline uptake fell. At all stages of CKD, renal arginine release was markedly reduced. Citrulline infusion increased plasma citrulline in all groups, resulting in increased renal delivery vs. baseline. In healthy kidneys and early injury, citrulline uptake increased with the infusion, but only in the normal kidney did arginine production increase in parallel with the increased citrulline uptake. At moderate and severe injury, there was no increase in citrulline uptake or arginine production. The fall in arginine production in A/I was due to an early loss of ASS and ASL conversion of citrulline, which combined with a later reduction in citrulline uptake.
Keywords: argininosuccinate synthase, and nitric oxide
there is a net nitric oxide (NO) deficiency in patients and animals with chronic kidney disease (CKD) and end-stage renal disease (ESRD) (1, 3). There are multiple possible causes of NO deficiency in renal disease, one of which is a reduced availability of the rate-limiting NO synthase (NOS) substrate l-arginine (l-Arg), since the kidney is an important site of l-Arg synthesis (10, 35). Renal l-Arg synthesis requires citrulline, made primarily in the small intestine, which is taken up into the kidney and converted by argininosuccinate synthase (ASS) and argininosuccinate lyase (ASL) (10, 35) into l-Arg. The l-Arg is then released into the circulation via the renal vein, taken up into cells, and utilized in several pathways including NO synthesis (35).
In normal healthy adults, l-Arg is a nonessential amino acid since endogenous production is sufficient for metabolic needs (35). A significant fraction (∼40%) of ingested l-Arg undergoes catabolism (35); thus, the majority of circulating l-Arg used for other organ metabolic processes is provided by renal production and protein turnover (35). Citrulline does not undergo significant hepatic uptake and is therefore available for renal l-Arg synthesis (34). In normal individuals, citrulline is rate limiting for renal l-Arg synthesis (10), and citrulline supplementation stimulates l-Arg synthesis, leading to increases in plasma l-Arg (13).
Humans and animals with renal disease show increases in plasma citrulline correlating with decreased renal function (8). This is probably caused by a reduction in renal uptake and utilization of citrulline rather than increased citrulline production or intake. However, the effect of renal disease on renal l-Arg production is controversial, with reductions in renal l-Arg production being reported in humans and rats with CKD (9, 31) as well as maintained l-Arg production in the rat with CKD and in humans with ESRD (4, 17).
In this study, we have undertaken a systematic evaluation of renal citrulline uptake and l-Arg synthesis during the evolution of the ablation-infarction (A/I) model of CKD. We have previously reported that total and renal NO production are significantly reduced in this CKD model(12). In in vivo studies, we measured the renal uptake of citrulline and release of l-Arg in controls and in early, moderate, and severe stages of CKD studied under baseline conditions and during acute citrulline supplementation. We also measured the abundance of various enzymes that influence l-Arg synthesis and breakdown.
MATERIALS AND METHODS
Male Harlan Sprague-Dawley rats were housed in conventional cages and given free access to normal chow and water. Urine was collected overnight (16 h) in metabolic cages with animals given free access to water only. Urine protein excretion (UpV) was measured by the Bradford method (Bio-Rad, Hercules, CA). After baseline UpV was measured, the rats were fasted overnight and underwent A/I surgery. Under full sterile conditions, the animals were anesthetized with isoflurane, and the left and right flank were shaved. An incision was made under the floating rib, and the left kidney was exposed. Using 6-0 silk, branches of the renal artery were ligated to achieve about a two-third infarction of the left kidney. The right kidney was removed after ligation of the renal vein and artery. Sham animals had both kidneys exposed, but no ligation or ablation was performed. The animals were separated into four groups: sham (n = 11); 1–2 wk postinjury (early; n = 11); 4–5 wk postinjury (moderate; n = 7); and 10–12 wk postinjury (severe; n = 17). UpV and body weight (BW) were measured every 2 wk, starting 1 wk after A/I injury. All experiments were approved by the University of Florida Institutional Animal Care and Use Committee.
In vivo renal uptake of citrulline.
Animals were anesthetized with inactin injection (120 mg/kg BW ip). Body temperature was monitored through a rectal probe and regulated by a heated surgical table and lamp. The left femoral artery was cannulated, and baseline arterial blood was taken and spun for plasma. Red blood cells were reconstituted with artificial plasma (2.5% bovine serum albumin and 2.5% γ-globulin in lactated Ringer solution) at a volume equal to plasma removed and restored to the rat via a femoral vein catheter. Blood pressure (BP) was monitored via the femoral artery catheter. The trachea was cannulated, and a constant flow of oxygen was streamed across to maintain BP. The bladder was catheterized, and a timed baseline urine sample was collected following a brief recovery time. Sham animals had the right ureter ligated and cut upstream of the ligation to allow for single kidney urine collection via the bladder. The left renal vein was cannulated without obstruction of normal blood flow. Artificial plasma was infused into the femoral vein at 1% BW/h for the first 20 min of surgery then reduced to 0.15% BW/h for the remainder of the experiment. Baseline renal plasma flow (RPF) was measured by infusing p-aminohippuric acid (PAH; 1.3 mg/ml for sham, 0.22 mg/ml for all A/I groups) in 0.9% saline at 1.2 ml·100 g BW−1·h−1 into the femoral vein following a 0.5-ml bolus PAH injection. After 40-min equilibration, a 20-min urine sample was taken with midpoint blood taken from the femoral artery and renal vein to determine renal extraction of PAH. A bolus of 100 mM citrulline was given, and the infusion was switched to contain 30 mM citrulline, while the saline concentration was adjusted to maintain osmolarity. After 40-min equilibration, urine was collected for 20 min with midpoint blood samples from the femoral artery and renal vein. Hematocrit was measured and plasma taken for analysis of PAH, l-Arg, and citrulline; red blood cells were then reconstituted with artificial plasma and returned to the rat. Following the final blood draw, the kidneys were perfused blood free with PBS, and the kidney was harvested. Part of the kidney was fixed for histology (see below), and the remainder was divided into cortex and medulla and flash frozen then stored at −80°C for later analysis (see below).
Renal function.
PAH concentration was established using a colorimetric assay previously described (25) to calculate left kidney RPF. Total creatinine clearance (CCr) was calculated using timed urine samples, and plasma collected at the beginning of the acute surgery was analyzed for creatinine by HPLC using the method of Tsikas et al. (32) with modifications as described by us previously (15).
Calculations.
Total renal uptake of citrulline was calculated as renal citrulline delivery (single kidney RPF × arterial citrulline concentration) − citrulline outflow (RPF × venous citrulline concentration) − urinary citrulline excretion, and then multiplied by number of kidneys (2 for sham, 1 for CKD). Total renal release of l-Arg was calculated as renal vein l-Arg outflow (RPF × venous l-Arg concentration) − renal l-Arg delivery (RPF × arterial l-Arg concentration) − urinary l-Arg excretion, and then multiplied by number of kidneys (2 for sham, 1 for CKD).
Citrulline.
Renal cortex homogenate, plasma, and urine citrulline were measured by a colorimetric assay employing acidic diacetylmonoxime (23) modified by us and described previously (29).
l-Arg measurements.
Plasma and urine l-Arg were measured using reverse-phase HPLC with a Waters AccQ-Fluor fluorescent reagent kit (Waters, Milford, MA). Samples were prepared by mixing 60 μl of plasma or 10 μl of urine with 350 μl of borate buffer (pH = 9). This was placed on an unconditioned Oasis MCX column (Waters) then washed with 1 ml borate buffer, 3 × 1 ml H2O, and 1 ml MEOH. The sample was eluted with 1 ml NH4OH/H2O/MEOH (10:40:50), dried under nitrogen gas, and then reconstituted with 30 μl H2O. Recovery was ∼85%. Twenty microliters of the samples was mixed with 60 μl borate buffer and 20 μl of AccQ Fluor reagent (Waters). Fifty microliters of mixture was injected onto a Luna 150 × 3-mm C18 reversed-phase column (Phenomenex, Torrance, CA). A flow rate of 1.3 ml/min was attained with a PerkinElmer Series 200 Pump, and fluorescence intensity was measured using a series 200 fluorescent detector, EX 250/EM 395 (PerkinElmer Life and Analytical Sciences, Shelton, CT). Standards contained concentrations of l-Arg in the range of 200–3.12 μM. This method was adapted from Heresztyn et al. (14).
Western blotting.
Western blot analysis was made as described previously (30). Briefly, a measurement was conducted using kidney cortex (100 μg total protein) loaded on 12% polyacrylamide gels. Rabbit polyclonal antibodies (developed by Dr. Masataka Mori, Kumamoto University, Kumamoto, Japan) (37) against ASS and ASL were used at 1:2,000 dilutions (overnight incubations). A goat anti-rabbit IgG-HRP secondary antibody (1:3,000 dilution, 1-h incubation, Bio-Rad) was used for detection. Bands of interest were visualized using SuperSignal West Pico reagent (Pierce, Rockford, IL) and quantified by densitometry (VersaDoc imaging system and Quantity One Analysis software, Bio-Rad), as integrated optical density (IOD) after subtraction of background. The IOD was factored for Ponceau red staining to correct for any variations in total protein loading and for an internal standard (rat liver). The protein abundance was represented as IOD/Ponceau Red/Int Std.
Histology.
The left kidney was cut along the transverse axis and fixed in 10% formalin for 48 h and then paraffin embedded. Sections were cut at 5 μm onto Superfrost/Plus microscope slides (Fisher Scientific). Slides were deparaffinized and rehydrated in xylene and serial alcohol incubations and stained with periodic acid-Schiff (PAS; Sigma, St. Louis, MO) followed by hematoxylin as the secondary stain. Up to 100 glomeruli were scored blindly based on the following scale: 0 = healthy glomeruli, +1 = <25% damage, +2 = 25–50% damage, +3 = 51–75% damage, and +4 = >75% damage. A glomerulosclerosis index score (GSI) was calculated using the following equation: (number of +1) + 2 (number of +2) + 3 (number of +3) + 4 (number of +4)/total glomeruli observed.
Statistics.
One-way ANOVA combined with a Newman-Keuls post hoc test was used for statistical evaluation of mean values across groups. A paired t-test with a two-tailed P value was used to compare baseline and citrulline infusions. Linear regression was used to determine the relationship between two parameters; P values and goodness of fit (r2) are shown. All graphs and statistics were done with Prism 4 software (GraphPad Software, San Diego, CA). Values are reported as means ± SE, with P < 0.05 considered statistically significant.
RESULTS
As shown in Table 1, both BW and functional renal mass fell with ⅚ A/I, and there was a progressive increase in proteinuria and glomerular damage with increasing time after surgery. At death, all CKD groups had elevated BP and reduced CCr.
Table 1.
Physiological data and renal function
| Sham (n = 11) | 1–2 Wk (n = 11) | 4–5 Wk (n = 7) | 10–12 Wk (n = 17) | |
|---|---|---|---|---|
| BW, g | 447±21 | 372±7† | 378±8† | 406±11* |
| UpV, mg·24 h−1·100 g BW−1 | 8±2 | 21±3† | 40±4‡ | 93±24‡ |
| MAP, mmHg | 114±3 | 132±9† | 148±3‡ | 154±4‡ |
| Functional renal mass, g | 3.32±0.09 | 1.88±0.12‡ | 1.76±0.16‡ | 2.12±0.05‡ |
| GSI | 0.09±0.01 | 0.25±0.05 | 0.76±0.25* | 1.31±0.16‡ |
| Total CCr, ml·min−1·100 g BW−1 | 0.72±0.08 | 0.37±0.05‡ | 0.23±0.05‡ | 0.21±0.04‡ |
Values are means ± SE. n = No. of rats; BW, body wt; CCr, creatinine clearance. Urine protein excretion (UpV) is based on 16-h overnight collection. Mean arterial pressure (MAP) was taken at time of acute surgery via femoral artery catheter. Functional renal mass is based on 2 kidney weights in sham and single kidney minus scar tissue in ablation-infarction (A/I) rats. The glomerulosclerosis index (GSI) was measured in representative tissue sections stained with periodic acid-Schiff.
P < 0.05,
P < 0.01,
P < 0.001 vs. sham, all by 1-way ANOVA.
Baseline plasma citrulline values were significantly and progressively increased in CKD rats compared with sham (Table 2). Citrulline infusion increased plasma concentrations of citrulline in all animals and most markedly in CKD rats. Baseline plasma l-Arg concentrations were not different in any group, and citrulline infusion only increased plasma l-Arg in sham and 1- to 2-wk animals (Table 2). At the termination of the study, after citrulline infusion, we found that the renal cortex content of citrulline was increased at all stages of CKD.
Table 2.
Citrulline and L-Arg concentrations in plasma and renal cortex citrulline
| Sham (n = 7) | 1–2 Wk (n = 11) | 4–5 Wk (n = 7) | 10–12 Wk (n = 17) | |
|---|---|---|---|---|
| Plasma citrulline, μM | ||||
| Baseline | 61±3 | 107±8‡ | 120±3‡ | 153±5‡ |
| Citrulline infusion | 145±8 | 349±24‡ | 393±18‡ | 478±64‡ |
| P < 0.01 | P < 0.001 | P < 0.001 | P < 0.001 | |
| Plasma L-Arg, μM | ||||
| Baseline | 65±6 | 71±5 | 62±8 | 70±5 |
| Citrulline infusion | 114±10 | 110±10 | 66±8* | 73±6* |
| P < 0.001 | P < 0.01 | NS | NS | |
| Renal cortex, μM/g protein | ||||
| Citrulline infusion | 8.5±1 | 26±7* | 37±7† | 27±5† |
Values are means ± SE. n = No. of rats; NS, no significant difference. Citrulline is measured in plasma and tissue using a colorimetric assay. L-Arg concentrations are measured via HPLC. Statistics comparing each injury with sham were done with one-way ANOVA. Statistics comparing baseline vs. infusion were done with a paired t-test.
P < 0.05.
P < 0.01.
P < 0.001.
Baseline RPF was reduced in all CKD groups (Table 3). Citrulline infusion resulted in an increased RPF to the sham rats but had no effect on RPF in any CKD group. The decline in RPF due to CKD resulted in reduced delivery of citrulline to the kidneys at baseline, despite increased plasma citrulline concentrations. With citrulline infusion there were increases in renal delivery of citrulline in all groups, although the magnitude of the increase was less in all CKD groups vs. sham.
Table 3.
Effect of citrulline infusion on renal plasma flow and resulting renal citrulline delivery
| Sham (n = 7) | 1–2 Wk (n = 11) | 4–5 Wk (n = 7) | 10–12 Wk (n = 17) | |
|---|---|---|---|---|
| Total RPF, ml/min | ||||
| Baseline | 9.4±0.7 | 3.1±0.3† | 1.3±0.3† | 2.2±0.3† |
| Citrulline infusion | 14.2±0.9 | 2.6±0.7† | 1.9±0.5† | 2.0±0.3† |
| P < 0.01 | NS | NS | NS | |
| Total renal citrulline delivery, nmol/min | ||||
| Baseline | 632±52 | 324±36* | 150±31† | 331±53* |
| Citrulline infusion | 2,170±271 | 816±182† | 586±166† | 630±104† |
Values are means ± SE. n = No. of rats. Renal plasma flow (RPF) was measured by p-aminohippuric acid (PAH) clearance at baseline and during citrulline infusion. Sham RPF is for 2 kidneys. Renal citrulline delivery was determined by RPF × plasma citrulline concentrations. Statistics comparing each injury group with sham were done with 1-way ANOVA. Statistics comparing baseline vs. infusion were done with a paired t-test.
P < 0.01,
P < 0.001 vs. sham.
At baseline, total renal uptake of citrulline at 1–2 wk postinjury was slightly higher than in sham, but as CKD developed renal citrulline uptake fell below sham values (Fig. 1A). Citrulline infusion increased the total renal citrulline uptake in sham (∼13-fold) and to a lesser extent in 1- 2-wk animals (∼2-fold) but had no impact on citrulline uptake as CKD developed. The majority of citrulline uptake was via reabsorption of filtered citrulline in the sham animals (Table 4). At early injury, the filtration fraction was reduced and the increased citrulline uptake seen at this stage was via peritubular transport (Table 4), which was lost at more severe CKD stages. At baseline, total renal release of l-Arg was diminished (Fig. 1B) at all stages of renal injury. Citrulline infusion significantly increased the total renal l-Arg release in sham but did not improve l-Arg release in the injured kidney.
Fig. 1.
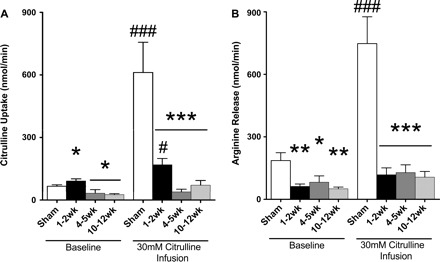
Renal uptake of citrulline and release of l-arginine (l-Arg) at baseline and during citrulline infusion. A: total renal uptake of citrulline. B: total renal l-Arg release. Statistics comparing each injury to sham were done with 1-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001 vs. sham. Statistics comparing baseline vs. infusion were done with a paired t-test. #P < 0.05, ### P < 0.001 vs. group baseline (n = 7–17/group).
Table 4.
Citrulline uptake by the proximal tubule via filtration vs. peritubular transport
| Sham (n = 7) | 1–2 Wk (n = 11) | 4–5 Wk (n = 7) | 10–12 Wk (n = 17) | |
|---|---|---|---|---|
| Renal uptake, nmol/min | 66±7 | 91±11* | 32±16* | 26±5* |
| Filtered, nmol/min | 50±4 | 28±4* | 16±3† | 11±2† |
| Peritubular uptake, nmol/min | 11±8 | 55±16* | 17±15 | 7±5 |
| % Filtered uptake | 89±10 | 36±10† | 71±13 | 65±15 |
Values are means ± SE. n = No. of rats. Filtered citrulline was calculated by citrulline delivery multiplied by filtration fraction. Peritubular uptake was calculated by total renal citrulline uptake subtracted from filtered citrulline minus excreted citrulline. Statistics comparing each injury group with sham were done with one-way ANOVA.
P < 0.05,
P < 0.01 vs. sham.
Baseline urinary citrulline excretion was low and similar, apart from a transient increase at 1–2 wk of injury vs. sham (Table 5). Citrulline infusion had no effect on urinary citrulline excretion compared with baseline in any group. Baseline l-Arg excretion was slightly higher only at 10–12 wk CKD vs. sham. Citrulline infusion increased l-Arg excretion in sham but had no impact at any CKD stage (Table 5).
Table 5.
Urinary excretion of citrulline and L-Arg factored for body weight at baseline and during citrulline infusion
| Sham (n = 7) | 1–2 Wk (n = 11) | 4–5 Wk (n = 7) | 10–12 Wk (n = 17) | |
|---|---|---|---|---|
| Citrulline excretion, pg·min−1·100 g BW−1 | ||||
| Baseline | 3.7±1.1 | 12.7±3.8† | 1.3±0.1 | 4.8±1.1 |
| Citrulline infusion | 4.2±1.1 | 10.0±2.7† | 1.5±0.3 | 4.0±0.7 |
| NS | NS | NS | NS | |
| Arginine excretion, pg·min−1·100 g BW−1 | ||||
| Baseline | 0.7±0.2 | 0.6±0.1 | 0.3±0.1 | 1.2±0.3* |
| Citrulline infusion | 1.4±0.3 | 0.8±0.2 | 0.5±0.1 | 1.0±0.2 |
| P < 0.01 | NS | NS | NS |
Values are means ± SE. n = No. of rats. Statistics comparing each injury group with sham were done with 1-way ANOVA. Statistics comparing baseline vs. infusion were done with paired t-test.
P < 0.01,
P < 0.001 vs. sham.
As shown in Fig. 2A, there is an ∼1:1 correlation between renal citrulline uptake and l-Arg output in the sham rats. At 1–2 wk postinjury, the relationship is blunted (∼4:1, Fig. 2B) and there is no relationship between citrulline uptake and l-Arg production at the later stages of CKD (Fig. 2, C and D).
Fig. 2.
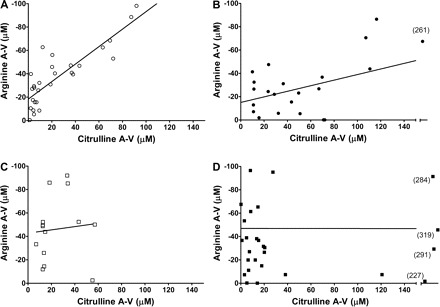
l-Arg and citrulline arterial-renal venous (A-V) correlations with the l-Arg A-V difference shown as a negative number reflecting net l-Arg release by the kidney into the renal vein. Shown is the correlation between the citrulline A-V difference and l-Arg A-V difference in sham (r2 = 0.8143, P < 0.0001; A), 1–2 wk postinjury (r2 = 0.4925, P = 0.0026; B), 4–5 wk postinjury [r2 = 0.1336, not significant (NS); C], and 10–12 wk postinjury (r2 = 0.00007, NS; D). Scale is set from 0 to 150 on the x-axis, and samples lying outside this range are shown on the extended axis, with exact values in parenthesis. Linear regression analysis was performed on each correlation to obtain the r2 and P values shown.
Renal ASS abundance was reduced at all stages of CKD vs. sham (Fig. 3A). When factored for remaining functional renal mass, there is a 60–80% reduction in total renal ASS abundance at all stages of CKD (P < 0.001 vs. sham). ASL density was unchanged at 1–2 wk after injury, was elevated at 4–5 wk, and then was restored to sham values at 10–12 wk (Fig. 3B). When factored for remaining functional renal mass, there is a 60% reduction in total renal ASL abundance at 1–2 wk after injury (P < 0.05) and no change in more severe CKD.
Fig. 3.
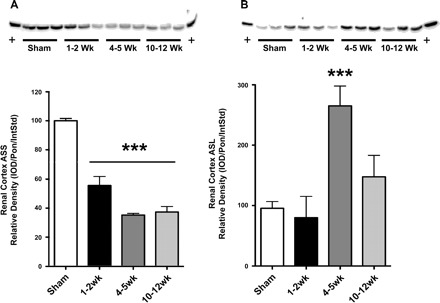
Argininosuccinate synthase (ASS) and lyase (ASL) protein expression. Shown is renal cortex ASS (A) and ASL (B) protein expression per milligram total protein with density analysis and representative blot. Protein blots are shown with equal protein loading in each lane, and densitometry is represented in graphs. Statistics comparing each injury to sham were done with 1-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001 vs. sham (n = 6/group).
DISCUSSION
Using the ⅚ A/I model of CKD, a model with reductions in total and renal NO production (12), we found that in the presence of substantial injury both renal citrulline uptake and renal citrulline to l-Arg conversion were impaired compared with rats with normal kidney function. In fact, the renal l-Arg production was impaired quite early, before significant structural damage was evident. Thus the renal production of l-Arg was markedly reduced in this CKD model over a wide range of injury.
Renal l-Arg synthesis requires the delivery of an adequate supply of citrulline, and we observed a reduction in renal citrulline delivery at all stages of injury progression. This was due to such large reductions in RPF that the increases in plasma citrulline (routinely seen in CKD and also seen here) were unable to compensate to maintain citrulline delivery. Not surprisingly, renal citrulline uptake was below sham levels at moderate and severe injury, although we observed mildly increased citrulline uptake early after ⅚ A/I. Exogenous citrulline infusion resulted in increased renal delivery of citrulline in all groups. However, while renal uptake of citrulline was greatly increased in sham animals, there was only a slightly increase in early CKD and no stimulation of uptake at moderate and severe injury.
Cellular uptake of citrulline is accomplished by a number of l-amino acid carrier systems. There are sodium-independent transporters in the nervous system (26) and sodium-dependent transporters in rat endothelial cells (33). In the kidney, Mitsuoka et al. (19) identified B(0)AT1 and b(0,+)AT, sodium-independent and -dependent transporters of citrulline on the apical membrane of cultured rat renal proximal tubular cells. On the basolateral side of the proximal tubules, sodium-independent transporters are present and OAT1 is the primary basolateral transporter (22). Giusto et al. (11) demonstrated a reduction in basolateral OAT1 following renal ischemia-reperfusion injury in rats. As shown in Table 4, the majority of the citrulline taken up into the normal (sham) kidney is delivered by filtration, as also reported by Munger et al. (21). With the ⅚ A/I surgery, there is an immediate and persistent fall in glomerular filtration rate, leading to declines in citrulline delivery by filtration. Our findings are consistent with an initial increase in basolateral citrulline transport capacity by the hypertrophying remnant at 1–2 wk post-⅚ A/I, which is rapidly lost as injury progresses. In the normal kidney, we found that exogenous citrulline infusion caused a huge increase in renal uptake of citrulline, suggesting a large reserve capacity of citrulline transport, possibly on both apical and basolateral membranes. The failure of administered citrulline to boost renal citrulline uptake in CKD is suggestive of widespread loss of citrulline transport capacity.
Our findings are different from those of Bouby et al. (4), who, using the same injury model, found no change in citrulline uptake at 4–6 wk postinjury. In their study, the delivery of citrulline was well maintained by a threefold increase in plasma citrulline with only an ∼30% reduction in RPF, whereas our animals showed >85% reduction in RPF and only a twofold increase in plasma citrulline 4–5 wk postinjury. Obviously, the rate of CKD progression was much greater in our study, for reasons still unknown.
According to Brosnan et al. (10) and Munger et al. (21), citrulline delivery is the limiting determinant of the rate of renal l-Arg synthesis in the normal rat kidney. Our findings support this since we observed a ∼1:1 relationship between citrulline uptake and l-Arg production in the sham animals both under baseline conditions and after exogenous citrulline. However, exogenous citrulline did not significantly improve l-Arg release at any stage of CKD, despite increased citrulline uptake at 1–2 wk (Fig. 1). This implicates a defect in the renal machinery for citrulline-to-l-Arg conversion, i.e., in the ASS and ASL located in the proximal convoluted tubules of the rat kidney (18). This is supported by our finding of reduced renal cortical ASS protein abundance at all stages of CKD, which coupled with loss of functional renal mass means a substantial overall loss of total renal ASS abundance and suggests reduced activity. The renal cortical ASL protein abundance was unchanged in early injury but increased above sham values at moderate injury. Given the loss of functional renal mass, this means a fall or little change in total renal ASL abundance and activity. Moradi et al. (20) showed that 6 wk after the milder ⅚ nephrectomy CKD model, no change in ASS/ASL protein abundance was seen in whole kidney, but when factored for viable renal mass there was a reduction in total abundance of ASS/ASL. Although ASS and ASL protein abundance may not always reflect activity, our present observations suggest that there is a direct correlation in the ⅚ A/I model. Our findings provide evidence for an association between ASS protein abundance and a loss of renal l-Arg synthesis, although further studies need to be conducted to determine the mechanisms regulating ASS expression.
Again, our findings conflict with those of Bouby et al. (4), who reported unchanged renal l-Arg production at 4–6 wk post-⅚ A/I and maintained l-Arg-to-citrulline conversion in isolated proximal convoluted tubules. These differences may reflect the milder level of injury in this earlier study. However, we also observed reduced renal l-Arg production, despite small increases in baseline citrulline uptake 1–2 wk post-⅚ A/I when injury was mild, and this finding agrees with a report after unilateral ischemia-reperfusion injury (24). Studies by Chan et al. (9) agree with our findings with a report of reduction in ASS/ASL activity in rat kidney cortex homogenates from rats with advanced CKD (8–10 wk) due to ⅚ A/I. Finally, in clinical studies in patients with nondiabetic CKD with ∼25% residual renal function, there was an ∼65% reduction in both citrulline uptake and l-Arg release vs. hypertensive patients with normal renal function (31).
Consistent with our findings regarding renal l-Arg release, citrulline infusion increased RPF only in sham animals. Although indirect, this suggests that the increased renal l-Arg production seen in sham animals resulted in a functional vasodilation, presumably through increased NO synthesis. Studies with perinatal citrulline supplementation in female spontaneous hypertensive rats (SHRs) showed increased renal NO levels compared with untreated SHRs (16). Additionally, l-Arg infusion increases RPF in humans, an effect which is lost in older patients with essential hypertension (5).
Renal excretion of citrulline and l-Arg was minimal in all animals at baseline states. Furthermore, citrulline infusion did not increase citrulline excretion in sham rats or at any stage of CKD. This either reflects reduced filtration of citrulline by the injured kidneys or implies that even the injured kidney has the capacity to reabsorb filtered amino acids. Citrulline infusion increases excretion of l-Arg only in the sham animals, perhaps reflecting increased proximal tubule levels of l-Arg via increased de novo synthesis, which may result in reduced luminal reabsorption in healthy kidneys.
Despite clear evidence of impaired l-Arg release by the damaged ⅚ A/I kidney, there is no reduction in circulating l-Arg values. A similar result was reported by Chan et al. (9) in rats with CKD and by Tizianello et al. (31) in humans with CKD. Furthermore, plasma l-Arg levels in CKD and end-stage patients are relatively normal (2, 27, 28). This maintenance of a normal plasma l-Arg in the face of significant renal disease could reflect 1) that circulating l-Arg is not determined by renal l-Arg synthesis; 2) CKD loss of renal l-Arg production is compensated for by increased l-Arg synthesis in other locations (e.g., endothelium); 3) compensation by alternate l-Arg sources such as dietary intake/changes in gastrointestinal absorption and/or increased protein turnover (studies need to be conducted under strict dietary control of l-Arg); 4) reduced endothelial uptake creates intracellular l-Arg deficiency but leaves a deceptively normal plasma l-Arg (36); 5) a compensatory decrease in whole-body l-Arg catabolism (6, 7); or 6) reduced metabolism by arginase due to increased levels of urea, which inhibit the enzymatic activity of arginase (20). Resolution of these important questions will improve our ability to manage CKD.
In conclusion, this study demonstrates that renal l-Arg release is reduced over a range of CKD severity in the ⅚ A/I rat, due both to reduced citrulline uptake and reduced ASS/ASL activity in the renal cortex. Our acute citrulline infusion data suggest that citrulline supplementation will not increase renal l-Arg release in the presence of established renal injury. Despite evidence of marked falls in renal l-Arg production, plasma l-Arg is relatively maintained in CKD, which seems to contradict the current dogma that the kidney is the primary source for endogenous l-Arg production. However, it is more likely that the impact of decreased renal l-Arg production is camouflaged by an impaired uptake of l-Arg into vascular endothelium. We thus suggest that the combination of loss of renal l-Arg synthesis together with decreased endothelial l-Arg uptake contributes to the net NO deficiency of CKD.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Harold Snellen for technical assistance on HPLC and Dr. Sidney Morris (University of Pittsburgh) for providing the ASS and ASL antibodies and helpful discussions.
REFERENCES
- 1. Aiello S, Noris M, Todeschini M, Zappella S, Foglieni C, Benigni A, Corna D, Zoja C, Cavallotti D, Remuzzi G. Renal and systemic nitric oxide synthesis in rats with renal mass reduction. Kidney Int 52: 171–181, 1997. [DOI] [PubMed] [Google Scholar]
- 2. Bergstrom J, Alvestrand A, Furst P. Plasma and muscle free amino acids in maintenance hemodialysis patients without protein malnutrition. Kidney Int 38: 108–114, 1990. [DOI] [PubMed] [Google Scholar]
- 3. Blum M, Yachnin T, Wollman Y, Chernihovsky T, Peer G, Grosskopf I, Kaplan E, Silverberg D, Cabili S, Iaina A. Low nitric oxide production in patients with chronic renal failure. Nephron 79: 265–268, 1998. [DOI] [PubMed] [Google Scholar]
- 4. Bouby N, Hassler C, Parvy P, Bankir L. Renal synthesis of arginine in chronic renal failure: in vivo and in vitro studies in rats with 5/6 nephrectomy. Kidney Int 44: 676–683, 1993. [DOI] [PubMed] [Google Scholar]
- 5. Campo C, Lahera V, Garcia-Robles R, Cachofeiro V, Alcazar JM, Andres A, Rodicio JL, Ruilope LM. Aging abolishes the renal response to l-arginine infusion in essential hypertension. Kidney Int Suppl 55: S126–S128, 1996. [PubMed] [Google Scholar]
- 6. Castillo L, Chapman TE, Sanchez M, Yu YM, Burke JF, Ajami AM, Vogt J, Young VR. Plasma arginine and citrulline kinetics in adults given adequate and arginine-free diets. Proc Natl Acad Sci USA 90: 7749–7753, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castillo L, Sanchez M, Chapman TE, Ajami A, Burke JF, Young VR. The plasma flux and oxidation rate of ornithine adaptively decline with restricted arginine intake. Proc Natl Acad Sci USA 91: 6393–6397, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ceballos I, Chauveau P, Guerin V, Bardet J, Parvy P, Kamoun P, Jungers P. Early alterations of plasma free amino acids in chronic renal failure. Clin Chim Acta 188: 101–108, 1990. [DOI] [PubMed] [Google Scholar]
- 9. Chan W, Wang M, Kopple JD, Swendseid ME. Citrulline levels and urea cycle enzymes in uremic rats. J Nutr 104: 678–683, 1974. [DOI] [PubMed] [Google Scholar]
- 10. Dhanakoti SN, Brosnan JT, Herzberg GR, Brosnan ME. Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol Endocrinol Metab 259: E437–E442, 1990. [DOI] [PubMed] [Google Scholar]
- 11. Di Giusto G, Anzai N, Endou H, Torres AM. Elimination of organic anions in response to an early stage of renal ischemia-reperfusion in the rat: role of basolateral plasma membrane transporters and cortical renal blood flow. Pharmacology 81: 127–136, 2008. [DOI] [PubMed] [Google Scholar]
- 12. Erdely A, Wagner L, Muller V, Szabo A, Baylis C. Protection of Wistar-Furth rats from chronic renal disease is associated with maintained renal nitric oxide synthase. J Am Soc Nephrol 14: 2526–2533, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartman WJ, Torre PM, Prior RL. Dietary citrulline but not ornithine counteracts dietary arginine deficiency in rats by increasing splanchnic release of citrulline. J Nutr 124: 1950–1960, 1994. [DOI] [PubMed] [Google Scholar]
- 14. Heresztyn T, Worthley MI, Horowitz JD. Determination of l-arginine and NG, NG - and NG, NG′ -dimethyl-l-arginine in plasma by liquid chromatography as AccQ-Fluor fluorescent derivatives. J Chromatogr 805: 325–329, 2004. [DOI] [PubMed] [Google Scholar]
- 15. Kim YH, Pham TD, Zheng W, Hong S, Baylis C, Pech V, Beierwaltes WH, Farley DB, Braverman L, Verlander JW, Wall SM. Role of pendrin in iodide balance: going with the flow. Am J Physiol Renal Physiol 297: F1069–F1079, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koeners MP, van Faassen EE, Wesseling S, de Sain-van der Velden M, Koomans HA, Braam B, Joles JA. Maternal supplementation with citrulline increases renal nitric oxide in young spontaneously hypertensive rats and has long-term antihypertensive effects. Hypertension 50: 1077–1084, 2007. [DOI] [PubMed] [Google Scholar]
- 17. Lau T, Owen W, Yu YM, Noviski N, Lyons J, Zurakowski D, Tsay R, Ajami A, Young VR, Castillo L. Arginine, citrulline, and nitric oxide metabolism in end-stage renal disease patients. J Clin Invest 105: 1217–1225, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levillain O, Hus-Citharel A, Morel F, Bankir L. Localization of arginine synthesis along rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 259: F916–F923, 1990. [DOI] [PubMed] [Google Scholar]
- 19. Mitsuoka K, Shirasaka Y, Fukushi A, Sato M, Nakamura T, Nakanishi T, Tamai I. Transport characteristics of l-citrulline in renal apical membrane of proximal tubular cells. Biopharm Drug Dispos 30: 126–137, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Moradi H, Kwok V, Vaziri ND. Effect of chronic renal failure on arginase and argininosuccinate synthetase expression. Am J Nephrol 26: 310–318, 2006. [DOI] [PubMed] [Google Scholar]
- 21. Munger KA, Blantz RC, Lortie MJ. Acute renal response to LPS: impaired arginine production and inducible nitric oxide synthase activity. Am J Physiol Regul Integr Comp Physiol 291: R684–R691, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Nakakariya M, Shima Y, Shirasaka Y, Mitsuoka K, Nakanishi T, Tamai I. Organic anion transporter OAT1 is involved in renal handling of citrulline. Am J Physiol Renal Physiol 297: F71–F79, 2009. [DOI] [PubMed] [Google Scholar]
- 23. Prescott LM, Jones ME. Modified methods for the determination of carbamyl aspartate. Anal Biochem 32: 408–419, 1969. [DOI] [PubMed] [Google Scholar]
- 24. Prins HA, Nijveldt RJ, Gasselt DV, van Kemenade F, Teerlink T, van Lambalgen AA, Rauwerda JA, van Leeuwen PA. The flux of arginine after ischemia-reperfusion in the rat kidney. Kidney Int 62: 86–93, 2002. [DOI] [PubMed] [Google Scholar]
- 25. Sasser JM, Baylis C. The natriuretic and diuretic response to dopamine is maintained during rat pregnancy. Am J Physiol Renal Physiol 294: F1342–F1344, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmidlin A, Fischer S, Wiesinger H. Transport of l-citrulline in neural cell cultures. Dev Neurosci 22: 393–398, 2000. [DOI] [PubMed] [Google Scholar]
- 27. Schmidt RJ, Baylis C. Total nitric oxide production is low in patients with chronic renal disease. Kidney Int 58: 1261–1266, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmidt RJ, Domico J, Samsell LS, Yokota S, Tracy TS, Sorkin MI, Engels K, Baylis C. Indices of activity of the nitric oxide system in hemodialysis patients. Am J Kidney Dis 34: 228–234, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tain YL, Baylis C. Determination of dimethylarginine dimethylaminohydrolase activity in the kidney. Kidney Int 72: 886–889, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tain YL, Freshour G, Dikalova A, Griendling K, Baylis C. Vitamin E reduces glomerulosclerosis, restores renal neuronal NOS, and suppresses oxidative stress in the ⅚ nephrectomized rat. Am J Physiol Renal Physiol 292: F1404–F1410, 2007. [DOI] [PubMed] [Google Scholar]
- 31. Tizianello A, De Ferrari G, Garibotto G, Gurreri G, Robaudo C. Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. J Clin Invest 65: 1162–1173, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsikas D, Wolf A, Frolich JC. Simplified HPLC method for urinary and circulating creatinine. Clin Chem 50: 201–203, 2004. [DOI] [PubMed] [Google Scholar]
- 33. Wileman SM, Mann GE, Pearson JD, Baydoun AR. Role of l-citrulline transport in nitric oxide synthesis in rat aortic smooth muscle cells activated with LPS and interferon-gamma. Br J Pharmacol 140: 179–185, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am J Physiol Endocrinol Metab 241: E473–E480, 1981. [DOI] [PubMed] [Google Scholar]
- 35. Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J 336: 1–17, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xiao S, Schmidt RJ, Baylis C. Plasma from ESRD patients inhibits nitric oxide synthase activity in cultured human and bovine endothelial cells. Acta Physiol Scand 168: 175–179, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu Y, Terada K, Nagasaki A, Takiguchi M, Mori M. Preparation of recombinant argininosuccinate synthetase and argininosuccinate lyase: expression of the enzymes in rat tissues. J Biochem 117: 952–957, 1995. [DOI] [PubMed] [Google Scholar]


