Abstract
Synthetic conjugation of a glucuronide to 1,25-dihydroxyvitamin D3 (1,25D3) to produce β-25-monoglucuronide-1,25D3 (βGluc-1,25D3) renders the hormone biologically inactive and resistant to mammalian digestive enzymes. However, β-glucuronidase produced by bacteria in the lower intestinal tract can cleave off the glucuronide, releasing the active hormone. In mice given a single oral dose of 1,25D3, 24-hydroxylase (Cyp24a1) gene expression was strongly enhanced in the duodenum, but not in the colon, despite circulating concentrations of 1,25D3 that peaked at ∼3.0 nmol/l. In contrast, in mice treated with an equimolar dose of βGluc-1,25D3, Cyp24a1 gene expression increased 700-fold in the colon but was significantly weaker in the duodenum compared with mice treated with 1,25D3. Similar results were observed with another vitamin D-dependent gene. When administered subcutaneously, 1,25D3 weakly stimulated colon Cyp24a1 gene expression while βGluc-1,25D3 again resulted in strong enhancement. Surgical ligation to block passage of ingesta beyond the upper intestinal tract abolished upregulation of colon Cyp24a1 gene expression by orally and subcutaneously administered βGluc-1,25D3. Feeding βGluc-1,25D3 for 5 days revealed a linear, dose-dependent increase in colon Cyp24a1 gene expression but did not significantly increase plasma 1,25D3 or calcium concentrations. This study indicates that the colon is relatively insensitive to circulating concentrations of 1,25D3 and that the strongest gene enhancement occurs when the hormone reaches the colon via the lumen of the intestinal tract. These findings have broad implications for the use of vitamin D compounds in colon disorders and set the stage for future therapeutic studies utilizing βGluc-1,25D3 in their treatment.
Keywords: transcription, RNA, intestine
in addition to its well-known endocrine actions on calcium metabolism, 1,25-dihydroxyvitamin D3 (1,25D3) can regulate cell proliferation and differentiation (3, 9, 47). Epidemiological evidence suggests that the prevalence of widespread vitamin D insufficiency may be linked to the development of several different types of cancer (15, 51), particularly colon cancer (12, 16, 18, 26, 31, 37, 40). Human colon cancer cell lines, such as HT-29 (55) and Caco-2 (5), and the mouse colon cancer cell line MC-26 (34) respond to the antiproliferative and prodifferentiating effects of 1,25D3 with reduced in vitro growth. Similarly, inflammatory bowel disease (IBD) has been shown to be linked to poor vitamin D status, and mouse models of IBD respond to 1,25D3 treatment (4, 20, 33, 43). Unfortunately, the doses of 1,25D3 required to effectively treat tumors or IBD in vivo result in strong stimulation of the vitamin's classical effects on calcium homeostasis and the development of life-threatening hypercalcemia (23, 45).
Since many cancerous cell lines respond to 1,25D3 treatment in vitro, a great effort is being made to develop “nonhypercalcemic” analogs of 1,25D3 to treat tumors (32, 41). Mice carrying xenografts of various cancer cell lines have been treated with some of these new analogs and have exhibited improved suppression of tumor growth, but even these drugs eventually caused hypercalcemia at doses that proved most effective at causing tumor regression (30, 46, 53). A phase I trial of the nonhypercalcemic analog EB1089 given to human colon cancer patients in the final phase of life (US Food and Drug Administration compassionate treatment lasting a few months) demonstrated some stabilization of tumor growth, but the study also indicated dose-dependent development of hypercalcemia (22). So, despite these promising results, there is still a need to find or develop compounds that can target vitamin D activity to the colon while avoiding hypercalcemia.
Glucuronide conjugates of vitamin D were recognized in bile secretions years ago and suggested a role for enterohepatic circulation in the metabolism of vitamin D (17, 36, 52). However, their importance in circulating vitamin D status and calcium homeostasis was generally dismissed (8). Nevertheless, on the basis of the earlier studies and our prior experience with glycoside derivatives of 1,25D3 (6), we synthesized a prodrug version of 1,25D3 by conjugating a glucuronide molecule in a β-linkage to form β-25-monoglucuronide-1,25D3 (βGluc-1,25D3). The glucuronide β-linkage limits binding of βGluc-1,25D3 to the vitamin D receptor (VDR) to ∼1% of that observed with the native hormone; therefore, βGluc-1,25D3 is biologically inactive (44). It is also too large and too water-soluble to be absorbed across the small intestine (39), and mammalian digestive enzymes of the upper intestinal tract are incapable of cleaving the glucuronide from 1,25D3 (20). However, once the prodrug reaches the lower intestinal tract, β-glucuronidase enzymes produced by bacteria residing in the lumen of the ileum and colon can cleave the glucuronide from the conjugate and liberate 1,25D3 to act on cells of the lower intestinal tract. Work from our laboratory demonstrated that orally administered βGluc-1,25D3 ameliorated the severity of colitis in mice with experimentally induced IBD (20). We found that the glucuronide compound also did not significantly raise blood concentrations of 1,25D3 and calcium (20). Furthermore, βGluc-1,25D3 appeared to act selectively to enhance vitamin D-dependent gene expression in the colon as opposed to the duodenum. In contrast, 1,25D3 treatment strongly stimulated target gene expression in the duodenum; however, it was unexpectedly much less effective at inducing expression in the colon, despite high circulating concentrations of the hormone. The present study sought to examine these initial findings in more detail and document the activity and selectivity of βGluc-1,25D3 vs. 1,25D3 in the intestinal tract as a prelude for the possible use of βGluc-1,25D3 in the treatment of colon cancer and IBD.
MATERIALS AND METHODS
Vitamin D compounds.
βGluc-1,25D3 (mol wt 592.76) was synthesized as described previously (20). It was purified by high-performance liquid chromatography to >97% purity, and the structure was confirmed by Fourier transform infrared spectroscopy and NMR. The 1,25D3 (mol wt 416.64) was purchased from Sigma Aldrich (St. Louis, MO) and was >98% pure. Quantitation of vitamin D compounds in ethanol solutions utilized to prepare each treatment was based on absorbance at 264 nm using a molar extinction coefficient of 18,200 absorbance units·mol−1·l−1.
Tissue culture.
LNCaP cells (CRL-1740, American Type Culture Collection, Manassas, VA) were maintained in RPMI 1640 medium containing 10% fetal bovine serum with penicillin (100 U/ml) and streptomycin (100 μg/ml) at 37°C. Cells were seeded into a six-well plate and treated with vehicle, 100 nM 1,25D3, or 100 nM βGluc-1,25D3 for 48 h. Medium was removed, and the cells were disrupted by direct treatment with 1.0 ml of TRIzol reagent (Invitrogen, Carlsbad, CA). RNA was processed and reverse-transcribed as described below, and PCR analysis of human 24-hydroxylase (Cyp24a1) and Gapdh genes was performed using the following primers: humCyp24 [5′-CAGGTGCCACGGGCAGAAGA (forward) and 5′-CCTGGATGTCGTATTTGCGGACAA (reverse)] and humGapdh [5′-CAGCCTCCCGCTTCGCTCTC (forward) and 5′-CCAGGCGCCCAATACGACCA (reverse)]. PCR analysis was performed in 25-μl reactions using GoTaq DNA polymerase (Promega, Madison, WI) under the following conditions: 95°C for 2 min followed by 35 cycles at 95°C for 20 s, 57°C for 30 s, and 72°C for 30 s. Aliquots (20 μl) of amplified products were removed and analyzed by agarose gel electrophoresis and ethidium bromide staining.
Animal experiments.
Rodents were housed individually or in groups in solid-bottom cages with contact bedding, in accordance with the accepted space requirements established by Laboratory Animal Resources at Iowa State University. Male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) and Sprague-Dawley rats (Harlan Laboratories, Madison, WI) were fed Teklad 2018 rodent diet (Harlan Laboratories) based on wheat, corn, and soybean meal and containing 1% calcium, 0.7% phosphorus, and vitamin D3 (1.5 IU/g) and were assumed to have normal intestinal biomes. The room was maintained at 24–26°C with a 12:12-h light-dark cycle. All procedures performed on the animals were submitted to and approved by the Iowa State University Institutional Animal Care and Use Committee.
Mice were treated with 24 pmol of 1,25D3 or βGluc-1,25D3, both orally and subcutaneously, in all experiments. Oral doses of 1,25D3 or βGluc-1,25D3 were prepared by suspension of the vitamin D compounds in 50 μl of a peanut oil-ethanol mixture (90:10) in the dosing trials or tie-off experiments. Subcutaneous injections of 1,25D3 or βGluc-1,25D3 were prepared in 100 μl of a propylene glycol-ethanol mixture (90:10). In the dosing trials, mice (3–5 per treatment) were returned to their cages and then euthanized 1, 3, 6, 9, 12, 18, and 24 h after treatment, and plasma and tissues were harvested. Mice were housed in groups of five animals in the feeding trial and fed 25 g of treated food daily for 5 consecutive days (5 g/mouse). Each 5 g of diet contained 7.9, 24, or 79 pmol of βGluc-1,25D3 mixed into the feed. Mice were euthanized 6 h after being offered food on day 5, and plasma and tissues were harvested.
In the tie-off surgical procedures, mice or rats were anesthetized with inhaled isoflurane, and local anesthesia/analgesia was induced with 0.075% bupivacaine injected to infiltrate subcutaneous and muscular tissue on either side of the abdominal incision site (200 μl/mouse and 1 ml/rat). The incision site was prepared for surgery, and a 2-cm midline longitudinal incision was made through the skin and linea alba to expose the abdominal cavity. A section of small intestine was exteriorized, and two ligating sutures were placed 1 cm apart to prevent aboral passage of material from the upper to the lower intestine. The intestine was returned to the abdominal cavity, and the incision was closed with interrupted sutures. Animals treated with subcutaneous doses of vitamin D compounds were injected in the paralumbar fossa while the animal was still under anesthesia. Animals receiving oral doses of the vitamin D compounds were treated after they recovered from anesthesia and were able to swallow the vitamin D compounds suspended in peanut oil (5–10 min after withdrawal of isoflurane). At 6 h after surgery, a second dose of bupivacaine was infiltrated into the site of the incision to provide analgesia from surgery. Sham-operated control animals were treated similarly, except the exteriorized intestine was returned to the abdominal cavity without being ligated. The mice were returned to their cages and euthanized 8 h after initial treatment with vitamin D compounds. Rats (3 per treatment) were similarly treated by subcutaneous injection with 120 pmol of 1,25D3 or βGluc-1,25D3 suspended in 200 μl of a propylene glycol-ethanol mixture (90:10) and euthanized 12 h after subcutaneous injection.
A 1-cm section of duodenum (2–3 cm from the pylorus, ∼50–75 mg wet tissue wt for mice and ∼75–100 mg wet tissue wt for rats) and a 1-cm section of colon (2–3 cm from the cecum, ∼25–50 mg wet tissue wt for mice and ∼50–75 mg wet tissue wt for rats) were obtained from each animal for mRNA analysis. Tissue samples were flushed with ice-cold phosphate-buffered saline and immediately homogenized in 1 ml of TRIzol reagent. Samples were kept frozen at −86°C prior to processing for RNA.
RNA processing.
Each TRIzol homogenate was thawed at room temperature, and 500 μl were placed in a clean microfuge tube, mixed thoroughly with 100 μl of chloroform for 15 s, and then centrifuged at 12,000 g for 15 min at 4°C. The upper aqueous phase was removed and mixed with 0.93 volume of 75% ethanol. The mixture was applied to an RNeasy spin column (Qiagen, Germantown, MD) and processed as described by the manufacturer, with an additional wash with 2 M NaCl-2 mM EDTA (pH 4.0) (11). RNA was eluted in 50 μl of water, and the concentration was obtained by UV spectrometry. Then 1 μg of RNA was used as a template for production of cDNA in a 20-μl reaction volume using random hexamers and SuperScript III (Invitrogen) or QuantiTect (Qiagen, Valencia, CA), as described by the manufacturers. The samples were diluted to a final volume of 100 μl with Tris-EDTA buffer and stored at −20°C prior to PCR analysis.
Quantitative real-time PCR.
Quantitative real-time PCR (qRT-PCR) analysis was performed using a thermal cycler (model Mx3005p, Stratagene, La Jolla, CA) and PerfeCTa SYBR Green FastMix, Low ROX reagent (Quanta Biosciences, Gaithersburg, MD). Amplification of murine target cDNAs was accomplished with the following primers (synthesized by Integrated DNA Technologies, Coralville, IA): mouse Cyp24 [5′-CACACGCTGGCCTGGGACAC (forward) and 5′-GGAGCTCCGTGACAGCAGCG (reverse)], mouse Gapdh [5′- GAAGGTCGGTGTGAACGGATTTGGC (forward) and 5′- TTGATGTTAGTGGGGTCTCGCTCCTG (reverse)], and mouse transient receptor potential cation channel subfamily V member 6 [Trpv6; 5′-GCCGAGACGAGCAGAACCTG (forward) and 5′-GCAGCTTGCTCAGAGCCTGGAC (reverse)]. Similarly, amplification of rat target cDNAs was accomplished with the following primers: rat Cyp24 [5′-AACCCTGCATCGACAACCGCC (forward) and 5′-TGTTCGCGGTCGTCTCCACT (reverse)] and rat Gapdh [5′-CCTGCACCACCAACTGCTTAGC (forward) and 5′-GCCAGTGAGCTTCCCGTTCAGC (reverse)]. Aliquots (8.3 ng) of cDNA were amplified under the following conditions: 95°C for 30 s followed by 45 cycles of 95°C for 1 s and 57°C for 30 s. All reactions were performed in duplicate, with three to five animals per treatment, and target gene expression was estimated using the cycle threshold (ΔCt) method normalized to Gapdh expression, as described previously (19, 20).
Plasma 1,25D3 analysis.
Mice and rats were euthanized by guillotine while under isoflurane anesthesia. Blood was collected from the cervical stump into heparinized tubes, and plasma was collected and frozen at −86°C until analysis. Because βGluc-1,25D3 is more water-soluble than 1,25D3 and, therefore, elutes with the methanol wash of the 0.5-g C18OH solid-phase extraction column (Varian, Lexington, MA), it is possible to measure only 1,25D3 in the samples. Plasma 1,25D3 concentrations were determined by radioimmunoassay on individual samples of plasma collected from the mice and rats (Heartland Assays) (27, 28). Calcium content was determined by colorimetric assay (Arsenazo III, Pointe Scientific, Canton, MI).
Statistical analysis.
Statistical analysis was performed on untransformed ΔCt data, and means were compared by Student's t-test using PSI-Plot (Pearl River, NY).
RESULTS
The Cyp24a1 gene codes for the 25-hydroxyvitamin D (25D3)-24-hydroxylase enzyme, which is widely expressed throughout the body and thought to be the first enzymatic step in the oxidative process leading to the inactivation of the hormone (1, 50). The protein is found in mucosal cells of the colon (29) and has been reported to be most strongly expressed at the base of the crypts, with diminished expression in the upper parts of the crypts (2). Expression of the gene is strongly enhanced by 1,25D3 and serves as a sensitive marker for ligands capable of binding to and activating the VDR (21), including LNCaP prostate cancer cells (14). To assess the stability and transcriptional activity of βGluc-1,25D3, LNCaP cells were incubated with 1,25D3 or βGluc-1,25D3 (Fig. 1, A and B), and Cyp24a1 gene expression was examined after 48 h of treatment. As seen in Fig. 1C, 1,25D3 was able to strongly stimulate Cyp24a1 mRNA relative to control cells (compare lanes 1 and 2), while incubation with βGluc-1,25D3 (lane 3) failed to enhance Cyp24a1 gene expression and was analogous to the control sample. Thus βGluc-1,25D3 appears to be stable and not to break down into the free hormone in culture medium for up to 48 h at 37°C, which confirms that the molecule is transcriptionally inert under these conditions (44).
Fig. 1.
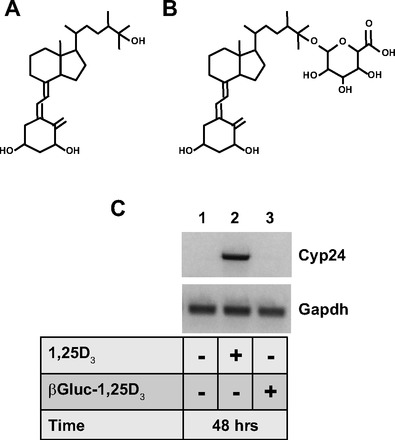
A and B: structures of 1,25-dihydroxyvitamin D3 (1,25D3) and β-25-monoglucuronide-1,25D3 (βGluc-1,25D3), respectively. C: agarose gel of PCR products obtained from LNCaP cells treated in culture as indicated for 48 h and analyzed for 24-hydroxylase (Cyp24a1) and Gapdh gene expression.
We then orally administered equimolar amounts (24 pmol) of 1,25D3 or βGluc-1,25D3 to C57BL/6 mice and assessed intestinal Cyp24a1 mRNA responses at various time points over the next 24 h. As seen in Fig. 2A, baseline plasma 1,25D3 concentrations were <0.2 nmol/l. Plasma concentrations of 1,25D3 rose dramatically 1 h after oral administration of 24 pmol of 1,25D3, peaking at >2.5 nmol/l; after 9 h, they fell quickly to baseline levels, where they remained. Blood concentrations of 1,25D3 following oral dosing with the same amount of βGluc-1,25D3 were modestly elevated through 6 h, peaking at ∼1 nmol/l, and then returned to baseline. In the colon (Fig. 2B), βGluc-1,25D3 profoundly stimulated Cyp24a1 gene expression, as determined by qRT-PCR analysis, with a maximum (∼700-fold) increase at 6 h. The same dose of 1,25D3 also increased colon Cyp24a1 gene expression, but only about fivefold above baseline. The opposite response was observed in the duodenums of these mice. Strong stimulation of this gene was observed after only 1 h with 1,25D3 treatment, reaching a maximum (>2,400-fold increase) at 6 h and then receding to control levels. Oral βGluc-1,25D3 increased Cyp24a1 expression in the duodenum, but the magnitude and duration of the gene enhancement were significantly reduced compared with 1,25D3 at various time points (Fig. 2C).
Fig. 2.
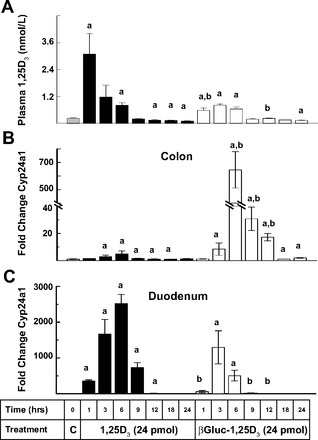
Time-course comparison of oral dosing with 1,25D3 or βGluc-1,25D3 on plasma 1,25D3 and Cyp24a1 colon and duodenum gene expression in mice. A: plasma 1,25D3 concentrations. B and C: quantitative real-time PCR (qRT-PCR) analysis of Cyp24a1 gene expression in colon and duodenum. Changes are relative to control (C) samples (mean fold change designated as 1-fold). aSignificantly different from control (P < 0.05). bSignificantly different from 1,25D3 treatment at the same time point (P < 0.05).
We also examined expression of the epithelial calcium channel transient receptor Trpv6 (42, 48, 54) induced by the vitamin D compounds in digestive tracts of these mice (Fig. 3). After oral administration of βGluc-1,25D3, stimulation of Trpv6 in the mouse colon was maximal at 9 and 12 h and remained modestly elevated through 24 h (Fig. 3A). Treatment with 1,25D3 stimulated colon Trpv6 gene expression at the same time points, but to levels that were less than one-half of those observed with βGluc-1,25D3. Again, the opposite response was observed in the duodenum, where treatment with 1,25D3 resulted in strong, statistically significant enhancement of Trpv6 at 3–12 h, while duodenal induction with the glucuronide was much weaker and only reached statistical significance at 9 and 12 h after treatment (Fig. 3B). These data are consistent with a model whereby the orally administered βGluc-1,25D3 passes through the upper intestinal tract largely intact before the active hormone is released in the lower gut through β-glucuronidase activity associated with resident bacterial populations.
Fig. 3.
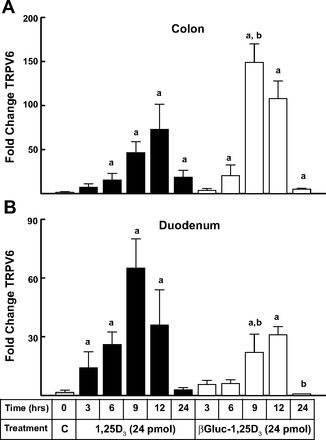
Quantitative real-time PCR analysis of time-course changes in transient receptor potential cation channel subfamily V member 6 (Trpv6) gene expression in the colon and duodenum of mice following oral dosing with 1,25D3 or βGluc-1,25D3. Changes are relative to control samples (see Fig. 2 legend). aSignificantly different from control (P < 0.05). bSignificantly different from 1,25D3 treatment at the same time point (P < 0.05).
While oral administration of βGluc-1,25D3 was expected to strongly impact gene expression in the lower intestinal tract, we wondered whether the compound would be able to exert activity on the colon if given subcutaneously. We hypothesized that βGluc-1,25D3 would be largely resistant to cleavage by mammalian enzymes while in circulation and, therefore, that its ability to reach the lumen of the colon, where the active hormone could be released by bacterial glucuronidase activity, might be restricted or even eliminated. As seen in Fig. 4A, blood concentrations of 1,25D3 were increased to ∼2.0 nmol/l by subcutaneous injection of 1,25D3 and then gradually returned to control levels after 18–24 h. In contrast, injection of βGluc-1,25D3 resulted in a later and smaller rise in serum concentrations of 1,25D3, which peaked at ∼0.5 nmol/l and then gradually fell to control levels after 24 h. There was a strong, ∼150-fold, increase in Cyp24a1 gene expression in the colon of mice receiving subcutaneous βGluc-1,25D3, which peaked at 9 h, while only weak stimulation of this gene was observed following subcutaneous injection with 1,25D3 (Fig. 4B). Subcutaneous treatment with 1,25D3 resulted in robust (>200-fold) Cyp24a1 induction in the duodenums of these mice after 6 h, while only weak, transitory stimulation of duodenal Cyp24a1 gene expression was observed with βGluc-1,25D3 (Fig. 4C). The data demonstrate that simply raising plasma concentrations of 1,25D3 did not correlate with enhanced gene expression in the colon and highlight the perplexing lack of responsiveness by the colon to elevated circulating concentrations of 1,25D3 independent of the mode of administration. The data also indicate that our hypothesis that subcutaneous βGluc-1,25D3 would not be active in the colon was incorrect and caused us to suspect that βGluc-1,25D3 was somehow reentering the intestinal tract to release the active hormone in the colon.
Fig. 4.
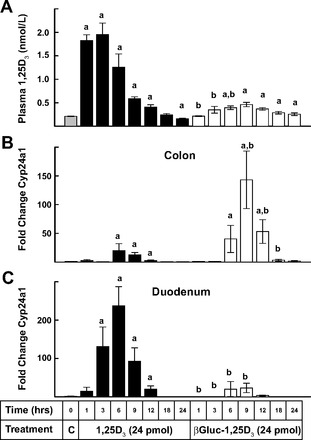
Time-course comparison of subcutaneous dosing of 1,25D3 and βGluc-1,25D3 on plasma 1,25D3 and colon and duodenum Cyp24a1 gene expression in mice. A: plasma 1,25D3 concentrations. B and C: qRT-PCR analysis of Cyp24a1 gene expression in the colon and duodenum. Changes are relative to control samples (see Fig. 2 legend). aSignificantly different from control (P < 0.05). bSignificantly different from 1,25D3 treatment at the same time point (P < 0.05).
In view of these prior results, we hypothesized that subcutaneously administered βGluc-1,25D3 was being removed from the circulation by the liver and being excreted back into the lumen of the upper intestine. To assess the likelihood of this scenario, mice were subjected to a surgical procedure whereby a section of the upper intestine (generally jejunum) was tied off with suture to prevent the passage of digestive contents into the lower colon. Sham-operated mice were included as controls. Mice were then treated with subcutaneous or oral administration of 24 pmol of βGluc-1,25D3 or 1,25D3, and the effects on Cyp24a1 gene expression in the colon were examined 8 h later. As seen in Fig. 5A, subcutaneously or orally administered βGluc-1,25D3 resulted in a significant decrease in Cyp24a1 gene expression, as measured by ΔCt, corresponding to strong induction of gene expression, in the colon of sham-operated mice. However, when the upper intestinal tract was tied off, the ability of βGluc-1,25D3 to stimulate Cyp24a1 gene expression, as evidenced by a decrease in ΔCt activity, was abolished, regardless of mode of administration. As previously observed, induction of Cyp24a1 mRNA following 1,25D3 treatment was greatly muted compared with the βGluc-1,25D3 response in sham-operated mice. Nonetheless, even the weak effect attributable to 1,25D3 treatment, particularly the response to subcutaneous administration, was eliminated when the upper intestine was tied off. Interestingly, surgically tied-off untreated mice also exhibited a significant increase in Cyp24a1 ΔCt activity compared with sham controls. No overt signs of tissue decay were observed over the time period of the experiment for any of the mice, and ΔCt values for the mouse Gapdh gene used in normalizing Cyp24a1 gene expression were statistically the same for all the samples (20.06 ± 0.19 and 19.93 ± 0.17 for sham-operated and surgically tied-off animals, respectively).
Fig. 5.
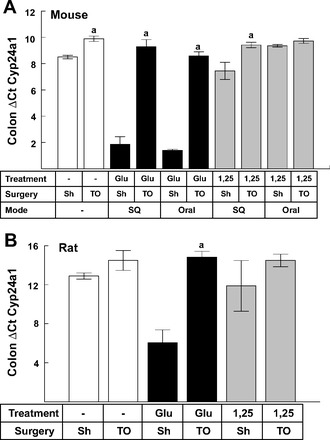
Effect of surgical blockade of digesta passage on colon Cyp24a1 gene expression in mice and rats. A: changes in Cyp24a1 gene expression (ΔCt) in mice dosed orally and subcutaneously (SQ) with 24 pmol of βGluc-1,25D3 (Glu) or 1,25D3 (1,25). B: changes in Cyp24a1 gene expression (ΔCt) in rats dosed subcutaneously with 120 pmol of βGluc-1,25D3 or 1,25D3. aSignificantly different from corresponding sham-operated (Sh) group receiving the same vitamin D treatment (P < 0.05). TO, tied-off.
To determine whether this response extended to other species, we performed a similar experiment using rats, but the analysis was limited to subcutaneous injections of βGluc-1,25D3 or 1,25D3. As depicted in Fig. 5B, Cyp24a1 gene expression was again strongly enhanced in the colon of sham-operated rats treated with βGluc-1,25D3, as evidenced by the sharp drop in ΔCt values. As in mice, no enhancement of Cyp24a1 gene expression was observed with subcutaneous βGluc-1,25D3 in rat colon when the upper intestinal tract was surgically blocked. Furthermore, treatment with 1,25D3 again produced a very modest increase in gene expression in the colon, which was reduced to control levels when the upper gut was surgically tied off. These data in mice and rats suggest that 1,25D3 and βGluc-1,25D3 are taken up from the circulation and that both compounds are excreted into liver bile.
Finally, Cyp24a1 gene expression was determined in mice fed three different amounts of βGluc-1,25D3 (7.9, 24, and 79 pmol/day) added to the diet for 5 days. As seen in Fig. 6A, at the end of the 5-day treatment period, there was no significant change in plasma 1,25D or calcium concentrations in any animals receiving βGluc-1,25D3 in their diet compared with controls. In contrast, when the results were plotted (Fig. 6B), there was a highly correlative linear relationship (R2 = 0.99) between the amount of βGluc-1,25D3 fed to the mice and the response in Cyp24a1 gene expression in the colon of these mice. No statistically significant enhancement of Cyp24a1 gene expression was seen in the corresponding duodenums of the mice (data not shown).
Fig. 6.
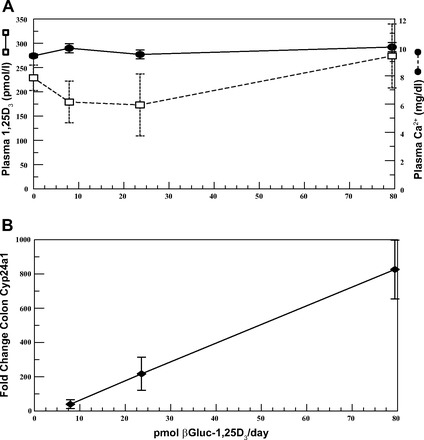
Plasma 1,25D3 and colon Cyp24a1 gene expression in mice fed 10–80 pmol of βGluc-1,25D3 per day for 5 days. A: 1,25D3 and calcium concentrations in plasma at the end of 5 days. B: plot and best-fit line (R2 = 0.99) of βGluc-1,25D3 dose vs. fold change in Cyp24a1 gene expression. Colon Cyp24a1 gene expression in treated mice was analyzed by qRT-PCR and compared with control animals not fed βGluc-1,25D3 (mean fold change in control mice designated as 1-fold).
DISCUSSION
An important aspect of vitamin D metabolism within the colon is the mechanism whereby vitamin D and its metabolites reach this tissue. Classical work on the endocrine action of vitamin D suggests that the active hormone, 1,25D3, is produced in the kidney during negative calcium balance and secreted into the general circulation. The circulating 1,25D3 is then carried to the intestine and thought to cross the basolateral membrane of enterocytes to bind to VDRs. In the duodenum, this results in enhanced calcium and phosphorus transport (7). In the colon, it may affect the propensity for tumor development or inflammatory responses by regulating tight cell junction integrity and local immune function (15, 35).
Another mechanism whereby vitamin D and its metabolites might reach intestinal tissues is excretion of vitamin D metabolites into the bile (25). Both 1,25D3 and 25D3 are known to enter the enterohepatic circulation; however, the importance of the enterohepatic circulation of vitamin D compounds to vitamin D deficiency and calcium homeostasis is arguable. Malabsorptive syndromes prevent absorption of vitamin D from the diet but can also lead to loss of endogenous metabolites of vitamin D, leading to osteomalacia associated with bowel disease (49). Clements et al. (8) administered oral and intravenous doses of radioactively labeled vitamin D to patients with T-tube biliary drainage after cholecystectomy. The vitamin was mainly excreted as highly polar inactivation products, and <4% of the metabolites in bile was present as 25D3 or its glucuronide conjugate. Clements et al. concluded that there was insufficient vitamin D or 25D3 in the bile for the reabsorption of these metabolites to make a significant contribution to normal vitamin D status. Wiesner et al. (52) administered radiolabeled 1,25D3 to five normal vitamin D-replete human subjects and found that 15.6% of the injected dose appeared in bile as more polar metabolites of 1,25D3, most likely in a glucuronidated form. Of the injected dose, 27% and 7.5% appeared in the feces and urine, respectively, at 24 h. In another two subjects, biliary radioactivity was sampled at two jejunal sites separated by a distance of 40 cm; a 24.8% decrease in radioactivity over this segment of bowel suggested that the 1,25D3 was being reabsorbed as the more polar metabolite or following conversion to the free 1,25D3 in the intestine. Hashizume et al. (24) also recently demonstrated that the human UDP-glucuronosyltransferase UGT1A4 could efficiently catalyze glucuronidation of 1,25D3, with the 25-O-glucuronide identified as the major product. Collectively, these data demonstrate that products of vitamin D can be modified by liver cells and excreted in normal human bile and that these products are present in the enterohepatic circulation in normal humans.
The present study establishes the selective effects of βGluc-1,25D3 on vitamin D-dependent gene expression in the lower colon. Whether the compound was administered orally or subcutaneously, βGluc-1,25D3 targeted gene expression to the colon of mice and rats, as evidenced by strong stimulation of Cyp24a1 and Trpv6 genes. This was achieved despite much smaller increases in circulating 1,25D3 concentrations in comparison to treatment with the natural hormone. In addition, Cyp24a1 and Trpv6 gene expression in the duodenum were impacted less by βGluc-1,25D3 than 1,25D3 administration. This suggests that the majority of the βGluc-1,25D3 reached the lumen of the lower intestinal tract intact, where it was cleaved by resident bacterial β-glucuronidase enzyme activity to release the active hormone. It also suggests that germ-free animals lacking bacterial cell populations in the intestinal tract should be unresponsive to βGluc-1,25D3 and presumably demonstrate little or no stimulation of vitamin D-dependent gene expression in the colon.
The current experiments reveal that the bulk of 1,25D3 given to mice acted on upper intestinal cells and that only minute amounts appeared to reach the colon. Administration of 24 pmol of 1,25D3 produced robust stimulatory responses for Cyp24a1 and Trpv6 gene products in the duodenum. However, the colon was relatively unaffected by treatment with the natural hormone, as very little stimulation of these gene products was observed, despite significantly higher circulating concentrations of 1,25D3. Our findings suggest that 1,25D3 reaching the basolateral surface of the colonic epithelium via the circulation is less able to stimulate vitamin D-dependent gene expression than apically delivered hormone. The data further question the concept that circulating 1,25D3 is free to diffuse into and out of cells and suggest that cell polarity and possible active transport systems may be involved in the hormone's cellular entry. Moreover, the data highlight the difficulties in trying to establish doses of 1,25D3 that inhibit in vivo colon tumor formation or growth. Our findings suggest that it will be virtually impossible to administer doses of 1,25D3 that are high enough to produce concentrations of hormone in the colon of test subjects sufficient to inhibit tumorigenesis without also causing severe hypercalcemia. Our data also imply that, in many of the previous studies examining 1,25D3 or its analogs as colon cancer therapeutic agents, only the effects of a small fraction of the administered compounds that escaped absorption or reabsorption to reach the colon were seen (13, 30, 38, 53).
The inability of βGluc-1,25D3 or 1,25D3 to alter gene expression when passage of digestive contents to the colon was blocked confirms these concepts and leads us to the following conclusions. 1) Orally administered βGluc-1,25D3 reaches the lumen of the lower intestinal tract, where it is cleaved and delivers 1,25D3 to the apical membrane of colon cells, resulting in strong upregulation of vitamin D-dependent genes. 2) Subcutaneously administered βGluc-1,25D3 in mice and rats only reaches the colon via passage of ingesta from the upper to the lower intestinal tract. This observation is consistent with the hypothesis that the liver may be removing βGluc-1,25D3 from the circulation and excreting it, most likely still in the β-glucuronide form, into the duodenum, where it flows with the ingesta to the colon and is cleaved. 3) While oral 1,25D3 failed to significantly upregulate colon Cyp24a1 in the mouse study, subcutaneous administration of the same dose caused a slight upregulation of colon gene expression in mice and rats. However, when the flow of digesta to the colon was blocked by tying off the small intestine of mice and rats, the weak Cyp24a1 response to subcutaneous 1,25D3 treatment was also abolished, although the change was statistically significant only for mice (Fig. 5). This suggests that even the low level of activity attributable to subcutaneously delivered 1,25D3 is mediated by entry into the lumen of the gut and its crossing of the apical membrane of colon cells. Furthermore, basal Cyp24a1 activity in the untreated mice also dropped significantly when the upper digestive tract was blocked, and a similar trend was exhibited in rats (Fig. 5). Whether endogenous 1,25D3 is excreted into the bile to reach the lower colon or some portion of the circulating hormone is taken up and modified by the liver to a glucuronide form prior to excretion into bile, which is then liberated in the colonic lumen, remains to be elucidated. However, the x-intercept of the best-fit line from the dose-response data (Fig. 6) suggests that basal expression of Cyp24a1 in the colon of untreated, vitamin D-replete control mice corresponds to exposure to the equivalent of ∼2–4 pmol/day of 1,25D3 activity. Other factors could be involved in determining the weak basal expression of Cyp24a1 in the colon of these mice, but this estimate is consistent with the observations from untreated animals in the surgical tie-off experiments (Fig. 5) and other reports that liver cells can modify 1,25D3 and excrete a glucuronidated form into bile (24, 36).
In prior work from our laboratory, it was noted that feeding of βGluc-1,25D3 for up to 8 days produced only a slight rise in blood calcium concentrations, while the same dose of 1,25D3 yielded a significant rise in plasma calcium (20). It is clear that βGluc-1,25D3 does not raise blood 1,25D3 concentrations as much as the native hormone. It is also has a far less potent effect on duodenal gene expression and would be less likely to activate calcium absorption mechanisms than the native hormone. This would seem to be borne out by observations in our earlier report (20) and in the current study, which found that feeding variable amounts of βGluc-1,25D3 caused no significant rise in blood calcium concentrations. Accordingly, βGluc-1,25D3 appears to satisfy two of the requirements that would warrant further study for the treatment of colon tumors or IBD: 1) it selectively targets the colon, as evidenced by gene expression, and 2) it does not elicit a significant rise in blood calcium concentrations.
The importance of hepatic excretion of vitamin D compounds and the enterohepatic circulation of vitamin D compounds, particularly 1,25D3, to normal vitamin D physiology was largely dismissed, because duodenal activity and calcium metabolism did not seem to be greatly affected by the hepatic excretion of 1,25D3. Our study suggests that this phenomenon is more than a physiological curiosity or simple way of disposing of vitamin D from the body; it is likely the predominant means whereby active forms of vitamin D are delivered to the colonic epithelium. Moreover, our study suggests a commensal relationship with resident bacterial populations in the lower gut that liberate vitamin D metabolites passing down the lumen of the colon. In this way, the apparently more responsive apical membrane of host colonic epithelia or immune cells can absorb the freed metabolites to carry out their cellular functions. This may be a critical pathway in maintaining overall colon health in an area of the digestive tract that would otherwise be lacking ready access to the vitamin. More recent discoveries focused on the autocrine action of vitamin D (3, 10) suggest that the 1,25D3 important to colon function could also arise from colon tissue 1α-hydroxylase action on 25D3. Therefore, further investigation is also warranted to determine whether the known glucuronidation of 25D3 in the liver and its secretion into bile could be a means of targeting release of 25D3 in the colon to provide substrate for local 1,25D3 production. This scenario could provide a further link between the vitamin D status of an individual, as determined by circulating 25D3 concentrations, and colon health, as suggested by numerous epidemiological studies (12, 16, 18, 26, 31, 37, 40).
GRANTS
This work was supported in part by grants from the Grow Iowa Values Fund.
DISCLOSURES
Glycomyr (Ames, IA) supplied the βGluc-1,25D3 used in the studies. J. P. Goff and R. L. Horst are president and vice-president, respectively, of this company, which is working to develop commercial uses of glycosides of vitamin D.
AUTHOR CONTRIBUTIONS
N.J.K., R.L.H., and J.P.G. are responsible for conception and design of the research; N.J.K., R.L.H., and J.P.G. performed the experiments; N.J.K., R.L.H., and J.P.G. analyzed the data; N.J.K., R.L.H., and J.P.G. interpreted the results of the experiments; N.J.K. prepared the figures; N.J.K. drafted the manuscript; N.J.K., R.L.H., and J.P.G. edited and revised the manuscript; N.J.K., R.L.H., and J.P.G. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Cathy Martens, Julia Gloviczki, and Chad Clancy for excellent technical assistance. The authors also acknowledge the support of Heartland Assays, Inc., for determining 1,25D3 concentrations in our samples.
REFERENCES
- 1. Akeno N, Saikatsu S, Kawane T, Horiuchi N. Mouse vitamin D-24-hydroxylase: molecular cloning, tissue distribution, and transcriptional regulation by 1α,25-dihydroxyvitamin D3. Endocrinology 138: 2233– 2240, 1997. [DOI] [PubMed] [Google Scholar]
- 2. Baeke F, van Etten E, Gysemans C, Overbergh L, Mathieu C. Vitamin D signaling in immune-mediated disorders: evolving insights and therapeutic opportunities. Mol Aspects Med 29: 376– 387, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Bises G, Kallay E, Weiland T, Wrba F, Wenzl E, Bonner E, Kriwanek S, Obrist P, Cross HS. 25-Hydroxyvitamin D3-1α-hydroxylase expression in normal and malignant human colon. J Histochem Cytochem 52: 985– 989, 2004. [DOI] [PubMed] [Google Scholar]
- 4. Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr 130: 2648– 2652, 2000. [DOI] [PubMed] [Google Scholar]
- 5. Chen A, Davis BH, Bissonnette M, Scaglione-Sewell B, Brasitus TA. 1,25-Dihydroxyvitamin D3 stimulates activator protein-1-dependent Caco-2 cell differentiation. J Biol Chem 274: 35505– 35513, 1999. [DOI] [PubMed] [Google Scholar]
- 6. Cheng YH, Goff JP, Sell JL, Dallorso ME, Gil S, Pawlak SE, Horst RL. Utilizing Solanum glaucophyllum alone or with phytase to improve phosphorus utilization in broilers. Poult Sci 83: 406– 413, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol 347: 25– 29, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clements MR, Chalmers TM, Fraser DR. Enterohepatic circulation of vitamin D: a reappraisal of the hypothesis. Lancet 1: 1376– 1379, 1984. [DOI] [PubMed] [Google Scholar]
- 9. Cross HS, Kallay E, Khorchide M, Lechner D. Regulation of extrarenal synthesis of 1,25-dihydroxyvitamin D3—relevance for colonic cancer prevention and therapy. Mol Aspects Med 24: 459– 465, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Cross HS, Nittke T, Peterlik M. Modulation of vitamin D synthesis and catabolism in colorectal mucosa: a new target for cancer prevention. Anticancer Res 29: 3705– 3712, 2009. [PubMed] [Google Scholar]
- 11. Das A, Spackman E, Pantin-Jackwood MJ, Suarez DL. Removal of real-time reverse transcription polymerase chain reaction (RT-PCR) inhibitors associated with cloacal swab samples and tissues for improved diagnosis of avian influenza virus by RT-PCR. J Vet Diagn Invest 21: 771– 778, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Fedirko V, Bostick RM, Goodman M, Flanders WD, Gross MD. Blood 25-hydroxyvitamin D3 concentrations and incident sporadic colorectal adenoma risk: a pooled case-control study. Am J Epidemiol 172: 489– 500, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fichera A, Little N, Dougherty U, Mustafi R, Cerda S, Li YC, Delgado J, Arora A, Campbell LK, Joseph L, Hart J, Noffsinger A, Bissonnette M. A vitamin D analogue inhibits colonic carcinogenesis in the AOM/DSS model. J Surg Res 142: 239– 245, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Flanagan JN, Zheng S, Chiang KC, Kittaka A, Sakaki T, Nakabayashi S, Zhao X, Spanjaard RA, Persons KS, Mathieu JS, Holick MF, Chen TC. Evaluation of 19-nor-2α-(3-hydroxypropyl)-1α,25-dihydroxyvitamin D3 as a therapeutic agent for androgen-dependent prostate cancer. Anticancer Res 29: 3547– 3553, 2009. [PubMed] [Google Scholar]
- 15. Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J 441: 61– 76, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol 9: 227– 231, 1980. [DOI] [PubMed] [Google Scholar]
- 17. Gascon-Barre M. Biliary excretion of [3H]-25-hydroxyvitamin D3 in the vitamin D-depleted rat. Am J Physiol Gastrointest Liver Physiol 242: G522– G532, 1982. [DOI] [PubMed] [Google Scholar]
- 18. Giardina C, Madigan JP, Tierney CA, Brenner BM, Rosenberg DW. Vitamin D resistance and colon cancer prevention. Carcinogenesis 33: 475– 482, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25: 386– 401, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Goff JP, Koszewski NJ, Haynes JS, Horst RL. Targeted delivery of vitamin D to the colon using β-glucuronides of vitamin D: therapeutic effects in a murine model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 302: G460– G469, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goff JP, Reinhardt TA, Engstrom GW, Horst RL. Effect of dietary calcium or phosphorus restriction and 1,25-dihydroxyvitamin D administration on rat intestinal 24-hydroxylase. Endocrinology 131: 101– 104, 1992. [DOI] [PubMed] [Google Scholar]
- 22. Gulliford T, English J, Colston KW, Menday P, Moller S, Coombes RC. A phase I study of the vitamin D analogue EB 1089 in patients with advanced breast and colorectal cancer. Br J Cancer 78: 6– 13, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris DM, Go VL. Vitamin D and colon carcinogenesis. J Nutr 134: 3463S– 3471S, 2004. [DOI] [PubMed] [Google Scholar]
- 24. Hashizume T, Xu Y, Mohutsky MA, Alberts J, Hadden C, Kalhorn TF, Isoherranen N, Shuhart MC, Thummel KE. Identification of human UDP-glucuronosyltransferases catalyzing hepatic 1α,25-dihydroxyvitamin D3 conjugation. Biochem Pharmacol 75: 1240– 1250, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higashi T, Miura K, Kikuchi R, Shimada K, Hiyamizu H, Ooi H, Iwabuchi Y, Hatakeyama S, Kubodera N. Characterization of new conjugated metabolites in bile of rats administered 24,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3. Steroids 65: 281– 294, 2000. [DOI] [PubMed] [Google Scholar]
- 26. Holick MF. Vitamin D and sunlight: strategies for cancer prevention and other health benefits. Clin J Am Soc Nephrol 3: 1548– 1554, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol 282: 174– 186, 1997. [DOI] [PubMed] [Google Scholar]
- 28. Hollis BW, Horst RL. The assessment of circulating 25(OH)D and 1,25(OH)2D: where we are and where we are going. J Steroid Biochem Mol Biol 103: 473– 476, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horvath HC, Lakatos P, Kosa JP, Bacsi K, Borka K, Bises G, Nittke T, Hershberger PA, Speer G, Kallay E. The candidate oncogene CYP24A1: a potential biomarker for colorectal tumorigenesis. J Histochem Cytochem 58: 277– 285, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huerta S, Irwin RW, Heber D, Go VL, Koeffler HP, Uskokovic MR, Harris DM. 1α,25-(OH)2-D3 and its synthetic analogue decrease tumor load in the Apcmin mouse. Cancer Res 62: 741– 746, 2002. [PubMed] [Google Scholar]
- 31. Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, Jansen EH, Slimani N, Byrnes G, Rinaldi S, Tjonneland A, Olsen A, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, Morois S, Kaaks R, Linseisen J, Boeing H, Bergmann MM, Trichopoulou A, Misirli G, Trichopoulos D, Berrino F, Vineis P, Panico S, Palli D, Tumino R, Ros MM, van Gils CH, Peeters PH, Brustad M, Lund E, Tormo MJ, Ardanaz E, Rodriguez L, Sanchez MJ, Dorronsoro M, Gonzalez CA, Hallmans G, Palmqvist R, Roddam A, Key TJ, Khaw KT, Autier P, Hainaut P, Riboli E. Association between prediagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. BMJ 340: b5500, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jimenez-Lara AM. Colorectal cancer: potential therapeutic benefits of vitamin D. Int J Biochem Cell Biol 39: 672– 677, 2007. [DOI] [PubMed] [Google Scholar]
- 33. Jorgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, Bartels LE, Kelsen J, Christensen LA, Dahlerup JF. Clinical trial: vitamin D3 treatment in Crohn's disease—a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther 32: 377– 383, 2010. [DOI] [PubMed] [Google Scholar]
- 34. Kane KF, Langman MJ, Williams GR. Antiproliferative responses to two human colon cancer cell lines to vitamin D3 are differently modified by 9-cis-retinoic acid. Cancer Res 56: 623– 632, 1996. [PubMed] [Google Scholar]
- 35. Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294: G208– G216, 2008. [DOI] [PubMed] [Google Scholar]
- 36. Kumar R, Nagubandi S, Mattox VR, Londowski JM. Enterohepatic physiology of 1,25-dihydroxyvitamin D3. J Clin Invest 65: 277– 284, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol 29: 3775– 3782, 2011. [DOI] [PubMed] [Google Scholar]
- 38. Murillo G, Nagpal V, Tiwari N, Benya RV, Mehta RG. Actions of vitamin D are mediated by the TLR4 pathway in inflammation-induced colon cancer. J Steroid Biochem Mol Biol 121: 403– 407, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nagubandi S, Kumar R, Londowski JM, Corradino RA, Tietz PS. Role of vitamin-D glucosiduronate in calcium homeostasis. J Clin Invest 66: 1274– 1280, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis 30: 88– 92, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Norman AW. The vitamin D endocrine system: manipulation of structure-function relationships to provide opportunities for development of new cancer chemopreventive and immunosuppressive agents. J Cell Biochem Suppl 22: 218– 225, 1995. [DOI] [PubMed] [Google Scholar]
- 42. Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem 274: 22739– 22746, 1999. [DOI] [PubMed] [Google Scholar]
- 43. Peyrin-Biroulet L, Oussalah A, Bigard MA. Crohn's disease: the hot hypothesis. Med Hypotheses 73: 94– 96, 2009. [DOI] [PubMed] [Google Scholar]
- 44. Rambeck WA, Weiser H, Meier W, Labler L, Zucker H. Biological activity of the 3 mono-β-d-glucopyranosides of 1,25-dihydroxycholecalciferol. Int J Vitam Nutr Res 55: 263– 267, 1985. [PubMed] [Google Scholar]
- 45. Smith DC, Johnson CS, Freeman CC, Muindi J, Wilson JW, Trump DL. A phase I trial of calcitriol (1,25-dihydroxycholecalciferol) in patients with advanced malignancy. Clin Cancer Res 5: 1339– 1345, 1999. [PubMed] [Google Scholar]
- 46. Spina C, Tangpricha V, Yao M, Zhou W, Wolfe MM, Maehr H, Uskokovic M, Adorini L, Holick MF. Colon cancer and solar ultraviolet B radiation and prevention and treatment of colon cancer in mice with vitamin D and its Gemini analogs. J Steroid Biochem Mol Biol 97: 111– 120, 2005. [DOI] [PubMed] [Google Scholar]
- 47. Tangpricha V, Flanagan JN, Whitlatch LW, Tseng CC, Chen TC, Holt PR, Lipkin MS, Holick MF. 25-Hydroxyvitamin D-1α-hydroxylase in normal and malignant colon tissue. Lancet 357: 1673– 1674, 2001. [DOI] [PubMed] [Google Scholar]
- 48. Taparia S, Fleet JC, Peng JB, Wang XD, Wood RJ. 1,25-Dihydroxyvitamin D and 25-hydroxyvitamin D-mediated regulation of TRPV6 (a putative epithelial calcium channel) mRNA expression in Caco-2 cells. Eur J Nutr 45: 196– 204, 2006. [DOI] [PubMed] [Google Scholar]
- 49. Thieler S, Scholmerich J. [Gastrointestinal diseases and osteomalacia]. Internist (Berl) 49: 1197– 1198, 1200, 1202–1195, 2008. [DOI] [PubMed] [Google Scholar]
- 50. Tomon M, Tenenhouse HS, Jones G. Expression of 25-hydroxyvitamin D3-24-hydroxylase activity in Caco-2 cells. An in vitro model of intestinal vitamin D catabolism. Endocrinology 126: 2868– 2875, 1990. [DOI] [PubMed] [Google Scholar]
- 51. Welsh J. Cellular and molecular effects of vitamin D on carcinogenesis. Arch Biochem Biophys 523: 107– 114, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wiesner RH, Kumar R, Seeman E, Go VL. Enterohepatic physiology of 1,25-dihydroxyvitamin D3 metabolites in normal man. J Lab Clin Med 96: 1094– 1100, 1980. [PubMed] [Google Scholar]
- 53. Xu H, Posner GH, Stevenson M, Campbell FC. ApcMIN modulation of vitamin D secosteroid growth control. Carcinogenesis 31: 1434– 1441, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology 136: 1317– 1327, e1311–e1312, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhao X, Feldman D. Regulation of vitamin D receptor abundance and responsiveness during differentiation of HT-29 human colon cancer cells. Endocrinology 132: 1808– 1814, 1993. [DOI] [PubMed] [Google Scholar]


