Abstract
It is critical for cells to maintain a homeostatic balance of water and electrolytes because disturbances can disrupt cellular function, which can lead to profound effects on the physiology of an organism. Dehydration can be classified as either intra- or extracellular, and different mechanisms have developed to restore homeostasis in response to each. Whereas the renin-angiotensin system (RAS) is important for restoring homeostasis after dehydration, the pathways mediating the responses to intra- and extracellular dehydration may differ. Thirst responses mediated through the angiotensin type 1 receptor (AT1R) and angiotensin type 2 receptors (AT2R) respond to extracellular dehydration and intracellular dehydration, respectively. Intracellular signaling factors, such as protein kinase C (PKC), reactive oxygen species (ROS), and the mitogen-activated protein (MAP) kinase pathway, mediate the effects of central angiotensin II (ANG II). Experimental evidence also demonstrates the importance of the subfornical organ (SFO) in mediating some of the fluid intake effects of central ANG II. The purpose of this review is to highlight the importance of the SFO in mediating fluid intake responses to dehydration and ANG II.
Keywords: angiotensin, blood pressure, barin, fluid, renin
fluid balance is crucial for the homeostasis and survival of organisms because they have to properly maintain fluid and electrolyte concentrations for the normal function of all cells. Fluid balance can go awry in pathological states such as in chronic kidney disease and diabetes. Among its many roles in maintaining homeostasis, the renin-angiotensin system (RAS) is an important regulator of fluid balance. The RAS achieves this through the actions of its main effector peptide, angiotensin II (ANG II) through the ANG II type 1 (AT1R) and type 2 (AT2R) receptors. Activation of AT1R by blood-borne ANG II in target tissues causes increases in sodium and water retention, arginine vasopressin (AVP), and aldosterone release, vasoconstriction, increased sympathetic nervous system activity, and increased fluid intake. ANG II is produced by the consecutive enzymatic action of renin on its substrate angiotensinogen (AGT) and by angiotensin-converting enzyme (ACE) on its substrate angiotensin I (ANG I). The renin-AGT cleavage step is generally the rate-limiting step in the production of ANG II, and this is certainly true in the brain where the amount of renin is limiting. The RAS exists in two forms: a circulatory form where ANG II acts as an endocrine factor, and a tissue-specific form, where local production of ANG II acts in an autocrine or paracrine manner to induce AT1R signaling on ANG II producing or nearby cells (75). Intracrine, or intracellular forms of the RAS, have also been described (46, 109). Although the generation and action of other angiotensin peptides in the brain (such as ANG[1–7] by ACE2) has garnered considerable attention (147), this review will focus primarily on ANG II.
Localization of RAS Components Required for ANG II Production
In 1961, Bickerton and Buckley (5) demonstrated that angiotensin injections into the brain increased blood pressure. In 1968, Booth (7) demonstrated that injection of angiotensin amide into the hypothalamus caused a robust and specific drinking response. Finally in 1971, Ganten and colleagues (40) demonstrated that renin was expressed within the brain of nephrectomized dogs. Subsequent studies of primary neuronal and glial cultures, and from whole mouse and rat brains, identified that renin is mainly expressed in neurons, though it can also be found in glial cells (39, 50, 53). Several genetic strategies have also been useful to identify the cell types expressing renin in the brain. In the first, enhanced green fluorescent protein (eGFP) was expressed in transgenic mice (Ren-1c-GFP) using a region of the mouse Ren-1c promoter shown to accurately target renin expressing cells (65, 72, 73). In the second, knock-in mice expressing Cre-recombinase inserted into the Ren-1d locus were bred with mice that express β-galactosidase in response to Cre-recombinase (145). Another study took a similar strategy but employed the human renin promoter to drive expression of Cre-recombinase in mice also carrying a Cre-activatable reporter (1). The latter two approaches identify cells which at any time in their developmental history expressed renin. Renin was expressed mainly in neurons of nuclei important for cardiovascular regulation, such as the subfornical organ (SFO), parabrachial nucleus (PB), rostral ventrolateral medulla (RVLM), and paraventricular nucleus of the hypothalamus (PVN). Renin was also possibly found to be expressed in oligodendrocytes of the RVLM and hippocampus, but not in astrocytes.
AGT is expressed at much higher levels than renin in the brain and is easily detectable by immunohistochemistry and in situ hybridization. Both methods show that AGT is widely expressed in astrocytes (32, 131), and in those cells, was localized in the nucleus (122). Genetic deletion of glial-specific AGT lowers arterial pressure in double transgenic mice expressing both human renin and human AGT (121), and antisense inhibition of glial AGT in rats results is altered baroreflex regulation and increased exercise tolerance (43, 119). AGT is also expressed in neurons, specifically within nuclei of the brain important for cardiovascular (CV) regulation, such as the SFO, RVLM, PB, and PVN (58, 136, 150). ACE is widely expressed in areas important for CV regulation; e.g., the SFO, organum vasculosum of the lamina terminalis (OVLT), PVN, and supraoptic nucleus (SON) (14, 91, 110).
Using a dual transgenic reporter system, we showed that renin and AGT are coexpressed within cells of the SFO, PB, CA1–3 of the hippocampus, and the central nucleus of the amygdala (CeA) (72). The dual reporter model also revealed that renin and AGT are expressed in adjacent cells within the inferior olivary nucleus, reticular formation, ventromedial nucleus of the hypothalamus, CA1–3 of the hippocampus, RVLM, PVN, SCN, SFO, and the CeA. Consistent with a concept for coexpression of renin and AGT, angiotensin peptides were identified within cell bodies of the magnocellular parts of the SON and PVN, the cell bodies and fibers of the SFO, and the cell bodies of the BNST, CeA, and nucleus tractus solitarius (NTS) (81). The presence of intracellular angiotensin peptides is also consistent with molecular and functional evidence for the expression of a novel intracellular form of renin in the brain (74, 125), although genetic analysis revealed that the intracellular form of renin cannot compensate for a loss of renal-derived secreted renin (146). These studies support the hypothesis for local activation of the RAS within the brain and data supporting the concept that ANG II is synthesized within the SFO and acts in the PVN as a neurotransmitter (35, 78).
Increased expression of both the human renin and AGT genes in the brain results in increased blood pressure and water intake (96, 97) and depending on the cellular sites of production (glia vs. neurons) can differentially modulate baroreflex function (118). Moreover, increased fluid intake and blood pressure caused by increased production of ANG II in the brain is attenuated when the source of the ANG II substrate, AGT, is selectively deleted from the SFO (117, 126), and mice selectively expressing ANG II only in the SFO exhibit increased fluid intake (20). These studies convincingly show that ANG II production in the SFO plays an important role in both blood pressure and fluid balance. It is notable that unlike rats, mice appear to be resistant to the dipsogenic effects of peripheral ANG II, but will increase water intake after ANG II is directly injected into the brain, albeit with lower sensitivity (31, 114).
Localization of RAS Components Required for ANG II Action: Focus on ANG II Receptors
Mice and rats express two isoforms of AT1R termed AT1AR and AT1BR. AT1AR and AT1BR are differentially expressed (12), and in the brain are differentially regulated by dehydration and dietary sodium (18, 19). A comparison of AT1AR-deficient and AT1BR-deficient mice revealed that the pressor response to central ANG II infusion is mediated by AT1AR (24). On the contrary, AT1BR appears to be important for central ANG II-induced fluid intake since the polydipsia that occurs with intracerebroventricular ANG II is attenuated in AT1BR knockout mice (24). Mice deficient in AT2R exhibited an enhanced pressor response to intracerebroventricular ANG II, and the polydipsia caused by central ANG II infusion was abrogated in mice deficient in both AT1AR and AT2R (79). The difference between the effects of AT1AR and AT1BR, in particular, highlight a molecular divergence of pathways that mediate central ANG II phenotypes; i.e., fluid intake can be separated from blood pressure, at least in mice. Humans express only a single isoform of the AT1R, which is typically but incorrectly attributed only to the AT1AR. Studies on the role of these receptors in the dipsogenic response to ANG II will be discussed below.
The localization of AT1R expression in the brain and elsewhere has been reported in studies too numerous to cite here (reviewed in Ref. 2). Moreover, immunocytochemical detection of these receptors has been hampered by the recent recognition that antisera designed to specifically detect either AT1R (51) and AT2R (48) actually exhibit very poor specificity. Consequently, other approaches are required. Gonzalez et al. (41) used the NZ44 transgenic mouse (on the C57BL/6 genetic background) generated by the “Gene Expression Nervous System Atlas” (also known as GENSAT) where eGFP was placed under the control of the mouse AT1AR promoter in a large bacterial artificial chromosome containing the AT1AR locus. They reported that AT1AR is expressed in a variety of nuclei throughout the forebrain, midbrain, and hindbrain. Of importance for CV regulation, AT1AR was highly expressed in neurons of the SFO, OVLT, SFO, SON, PVN, CeA, RVLM, and NTS. In the SFO, both the perikarya and dendrites were intensely labeled. This was particularly true in the dorsolateral outer shell and ventromedial core of the SFO, which, as will be described below, appears to be important for blood pressure regulation. In the PVN, AT1AR-positive cells were densely packed together, were found in both perikarya and dendrites, but did not coexpress AVP. Astrocytes in the PVN also expressed AT1AR. There was light labeling for AT1AR in the caudal portion of the SON where AVP-producing cells are generally found. In the NTS, many cells were labeled in the medial and commissural subdivisions, and AT1AR was also expressed within astrocytes. Most of the cells in the RVLM expressed AT1AR on both their perikarya and dendrites, and these cells coexpressed tyrosine hydroxylase implying that they are serotonergic and part of the sympathetic nervous system.
Overexpressing AT1AR in all neurons of mice increases sympathetic tone, water and saline intake, and the pressor response to ANG II, responses that are AT1R dependent (76, 77). The first genetic studies probing the function of AT1AR in specific nuclei of the brain were performed using RNA interference (17). More recently, the importance of AT1AR in the SFO has been examined by genetic manipulation in mice carrying a conditional allele of the AT1AR (AT1ARFlox) gene. Intracerebroventricular injection of an adenovirus encoding Cre-recombinase (AdCRE) (127, 128) in AT1ARFlox mice results in an SFO-specific decrease in the expression of AT1AR mRNA, which attenuated the water and saline intake and blood pressure response to deoxycorticosterone acetate (DOCA) salt (44). Coupled with the discussion above, these studies highlight the importance of both ANG II production and action in the SFO in regulating blood pressure and fluid homeostasis. Details of the cellular mechanisms mediating these pathways in the SFO will be discussed later in this review.
Subfornical Organ: Anatomy, Connections, and Physiological Functions
The SFO can be anatomically subdivided into a core and peripheral portion. The core of the SFO is highly vascularized, and the local vasculature lacks a blood-brain barrier (BBB). Ultrastructural examination of the SFO has demonstrated that the capillaries have tongue-like projections and pinocytic vesicles in the endothelial cell layer. These projections and vesicles provide an anatomic basis for the SFO to be exposed to factors in the blood (28). Fluorescence was observed in the core portion of the SFO when a small-molecular-weight fluorescent marker was injected in the heart of mice (98). Thus the core of the SFO is anatomically situated to be permeated by blood-borne, low-molecular-weight molecules, such as ANG II, aldosterone, and AVP. The peripheral portion of the SFO is not as highly vascularized as the core, but it is located to respond to factors in the cerebrospinal fluid (CSF), such as ANG II and sodium (28).
The SFO protrudes ventrally from the fornix into the third ventricle just caudal to the foramen of Monroe at the confluence of the lateral to third ventricles. The lateral ventricles produce CSF and contain cilia to aid in the circulation of CSF through the ventricular system. The CSF has to converge and pass by the SFO located near the foramen of Monroe. Consequently, CSF bathes the SFO as it flows through the ventricular system. Ultrastructural examination of the SFO demonstrates that some SFO neurons project into the ventricles (29, 86). These projections have vesicles that indicate either release into or uptake of CSF from the ventricles. Ependymal cells located along the ventricular wall also form channels into the SFO. This may be another means for the SFO to secrete into or extract factors from the CSF. Furthermore, tanycytes lining the ventricles around the SFO project from the ventricles to neurons within the SFO. These tanycytes contain vesicles that could sample factors from the CSF. Thus the core portion of the SFO is anatomically situated to respond to blood-borne factors while the peripheral portion of the SFO is positioned to respond to factors in the CSF, making the SFO a unique sensory organ.
The SFO is also a distinctive nucleus in the brain in that its afferent and efferent projections are well placed to respond to and integrate both blood-borne and central nervous system (CNS) signals (Table 1). The rostral portion of the SFO mainly contains efferent axons while the caudal portion contains cell bodies (30). The SFO sends efferent axonal projections to the MnPO, OVLT, SON, the lateral preoptic area, the lateral hypothalamus adjacent to the SON, PVN, suprachiasmatic nucleus (SCN), and the medial habenular nucleus (82, 93, 94, 134) (Fig. 1). The connection to the MnPO appears to be especially dense since it has high terminal labeling (33, 82). After the MnPO, the OVLT followed by the SON appear to be the next nuclei, respectively, receiving the highest number of efferent projections from the SFO (94). In the SON, it is primarily the magnocellular (i.e., vasopressinergic) neurons that receive SFO input (13, 93, 133); and injection of a retrograde tracer into the SON demonstrated that it is mainly the peripheral portion of the SFO that projects to the SON (64). The connection of the SFO to the magnocellular PVN (mPVN) has also been demonstrated by an injection of the neuronal tracer, biotinylated dextran amine (BDA), into the peripheral portion of the SFO (68). In addition, injecting two different retrograde tracers into the MnPO and PVN revealed that a small proportion of SFO neurons in the periphery have collateral projections to both the MnPO and PVN that likely affect the vasopressin system (33). Neurons in the core portion of the SFO also project to the parvocellular PVN (pPVN), which synthesize corticotropin-releasing hormone, and to the BNST (133). The anatomic specificity for SFO efferent projections was confirmed by injecting BDA into the mPVN or pPVN and observing the tracer within the peripheral and core portions of the SFO, respectively (68). Thus the SFO is anatomically structured for a divergence of physiological effects; the peripheral portion being connected to areas important for fluid balance (i.e., the vasopressin system) while the core is connected to areas controlling blood pressure (i.e., the sympathetic system; Fig. 1). A detailed review of how the connection between the SFO and PVN is angiotensinergic and mediates an elevation of blood pressure can be found by Ferguson and colleagues (36).
Table 1.
Afferent and efferent connections of the SFO
| Afferent | Efferent |
|---|---|
| MnPO | MnPO (****) |
| SON | OVLT (***) |
| PVN (thalamus and hypothalamus) | SON (**) |
| MnPO | Lateral preoptic area |
| Anterior hypothalamic nucleus | Lateral hypothalamus |
| Substantia innominata | PVN |
| BNST | SCN |
| Rostral parts of the zona incerta | Medial habenular nucleus |
| NTS | |
| Infralimbic area of the prefortal cortex |
SFO, subfornical organ; OVLT, organum vasculosum of the lamina terminalis; SON, supraoptic nucleus; PVN, paraventricular nucleus of the hypothalamus; MnPO, median preoptic nucleus; BNST, basal nucleus of the stria terminalis; SCN, suprachiasmatic nucleus; NTS, nucleus tractus solitarius. The number of * indicates the relative density of efferent fibers from the SFO.
Fig. 1.
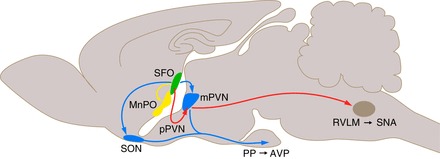
Neural circuitry: efferent projections from the SFO. The core of the subfornical organ (SFO) is highly vascularized and lacks a blood-brain barrier (BBB). Efferent projections (in red) from this region include those affecting sympathetic nerve activity (SNA) directly though projections to sympathetic neurons of the parvocellular PVN (pPVN) or indirectly through projecting to cells of the pPVN that project to sympathetic neurons of the rostroventral lateral medulla (RVLM). The periphery of the SFO can be affected by factors within the cerebrospinal fluid (CSF). Among its projections (in blue) are those regulating the vasopressin (AVP) axis through the supraoptic nucleus (SON) and magnocellular PVN (mPVN, blue). Projections (in yellow) are also sent to the median preoptic nucleus (MnPO). The cartoon is designed to illustrate the neural connections from the SFO but not their actual path.
In addition to sending projections elsewhere in the brain, the SFO receives projections from the MnPO, SON, PVN of the hypothalamus and thalamus, medial preoptic, anterior hypothalamic, substantia innominata, BNST, rostral parts of the zona incerta, NTS, and the infralimbic area of the prefrontal cortex (13, 33, 66–68, 133). Retrograde tracing from the SFO and anterograde tracing from the MnPO and PVN revealed that the MnPO sends the greatest number of projections to the SFO. Retrograde tracing also further highlights the anatomical segregation of the SFO since the substantia innominata, rostral parts of the zona incerta, and the infralimbic area of the prefrontal cortex project to the peripheral portion of the SFO while the BNST projects to the core of the SFO.
The SFO is also highly interconnected with the anteroventral third ventricle region (AV3V), which includes the OVLT, periventricular preoptic nucleus, and nucleus medianus. Electrolytic ablation of the AV3V initially makes rats adipsic to spontaneous water intake, blood-borne and CSF ANG II (9, 11, 62). During this acute postlesion period, the abolished fluid intake is specific for water and not generalized to all fluids or permanent. Rats will drink sugar-water while initially adipsic, and over time their spontaneous water intake eventually returns. Once spontaneous water intake returns, ANG II-induced and hypertonic saline-induced water intake is diminished, though hypovolemia-induced water intake is less affected. Obstructing ventricular flow with a plug around the AV3V blocks central ANG II-induced polydipsia but enhances peripheral ANG II-induced polydipsia (10). If the efferent projections from the SFO are severed, rats do not drink to peripheral ANG II, though they still increase fluid intake to intracerbroventricular injection of ANG II (80, 94). Electrolytic ablation of the SFO permanently abolishes fluid intake to sodium depletion and systemically administered ANG II (124, 129, 139). Overall the body of experimental evidence indicates that the dipsogenic effect of systemically administered ANG II is abolished by SFO lesions in the rat, although the involvement of structures located downstream of the SFO are likely to be involved. Ablation of ANG II-sensitive structures along the ventral part of the lamina terminalis (i.e., the MePO and OVLT) abolishes drinking to both systemically and centrally administered ANG II (61). So clearly, the SFO is a key sensory circumventricular organ involved in receiving systemically generated signals, integrating this information and relaying it to other areas involved in the generation of fluid intake (63).
Stimulators of the Brain RAS and Physiological Effects of SFO Activation
Dehydration can be classified as either extracellular (i.e., volumetric) or intracellular (i.e., osmotic). Extracellular dehydration induces drinking and an increase in salt preference and sodium appetite (137). Furosemide treatment, intraperitoneal ip polyethylene glycol (PEG), fluid restriction, or hemorrhage can all induce extracellular dehydration. Extracellular dehydration induced by fluid restriction or furosemide treatment increases the expression of AT1AR, activates ERK1/2, and increases the expression of AGT within the SFO (4, 19, 54). Similarly, elevating blood-borne ANG II either exogenously or indirectly by decreasing blood pressure via isoproterenol increases neuronal activity, the expression of AT1R within the SFO, and water intake (57, 69, 115). Fluid restriction also increases the expression of c-Fos (i.e., an indirect indicator of cellular activity) within the SFO, which remains elevated immediately after rehydration, whereas in other nuclei, c-Fos expression returns to normal (25). Table 2 summarizes stimuli that influence fluid intake and involve the SFO.
Table 2.
Stimuli affecting fluid intake involving the SFO
| Stimulus | Route | Molecular Effect | Abolition | Ref No. |
|---|---|---|---|---|
| Furosemide and captopril | iv | Increased p-p44/42 MAPK in OVLT/SFO and SON/PVN | SFO lesion (water and Na+ intake) | 34, 83, 99, 106, 140 |
| Increased ANG II in the SFO, OVLT, MnPO, PVN | MnPO lesion (Na+ intake) | |||
| Increased c-Fos in the SFO, OVLT, MnPO, mPVN, PB, AP, NTS | AT1R inhibition (icv or ip) | |||
| High dose iv Cap | ||||
| Polyethylene glycol | ip | Increased c-Fos in SFO, OVLT, SON | AGT knockout | 90, 114 |
| Increased PRA and plasma Aldo | ||||
| Fluid restriction | Increased SFO expression AGT, ANG II, AT1AR, ERK1/2, | AT2R inhibition | 4, 19, 25, 54, 100, 112 | |
| Increase SFO neuronal activity | ||||
| Increased expression of c-Fos OVLT, MnPO, SFO, SON | ||||
| Increased PRA | ||||
| Isoproterenol | iv | Increased SFO expression AT1R and neuronal activity | AT2R inhibition | 69, 112 |
| SFO AT1R inhibition | ||||
| ANG II | iv, sc | Increased SFO, SON, PVN neuronal activity | icv Aldosterone Synthase or Mineralocorticoid Receptor inhibition (hypertension and Fra activity in PVN) | 9, 57, 115, 144 |
| SFO AT1R | icv Losartan (polydipsia and neuronal activity) | |||
| SFO and PVN AT1R | SFO AT1R | |||
| Increased plasma, hypothalamus, and hippocampus Aldo | AV3V lesion | |||
| p-p44/42 MAPK inhibitor blocks upregulation of AT1R in SFO and PVN | ||||
| Hypertonic saline | iv, sc | Increased c-Fos in the SFO, OVLT, MnPO, mPVN, SON, NTS, RVLM, lateral PB | AV3V or lamina terminalis lesion | 9, 37, 56, 88–90, 112, 130 |
| AT2R inhibition | ||||
| SFO/OVLT lesion together or each separate | ||||
| Deoxycorticosterone acetate salt | sc | Increased AT1R in MnPO, SFO, PVN, NTS, AP | AV3V lesion | 8, 44, 47, 52, 60, 102 |
| Increased c-Fos OVLT, BNST, MnPO, PVN, SON, amygdala, preoptic area | AT1AR removal from SFO (polydipsia and hypertension) | |||
| Increased RAS-gene expression | icv Benzamil | |||
| Aldosterone | iv, sc | Increased c-Fos OVLT, BNST, MnPO, PVN, SON, amygdala, preoptic area | 148, 149 | |
| Increased RAS-gene expression | ||||
| Sodium depletion | Increased c-fos in SFO, MnPO, OVLT, PVN, SON | sc Captopril | 84, 129 | |
| Increased ANG I/II and Aldo in plasma and forebrain | SFO lesion | |||
| Decreased ACE and AT1R in PVN, SON, and OVLT | ||||
| Renin | icv | Increased c-Fos in SFO, MnPO, OVLT, SON, and PVN | icv Losartan | 113 |
| ANG II | icv | c-Fos in AT1R and AVP expressing SON and PVN | icv Captopril; icv ghrelin or TRPV4 agonist (blocks polydipsia) | 38, 49, 79, 92, 95, 107, 116, 123, 141, 154 |
| c-Jun in SON after repetitive icv ANG II | icv Ad-DN-Rac1or Ad-DN-Nox2 | |||
| c-Fos in SFO, SON, PVN, MnPO | hypothalamic disconnect acutely but not chronically decreases polydipsia induced by icv ANG II | |||
| PKC-a activity CAMKII in septum and hypothalamus | icv Chelerythrine, Go-6976, KN-93 | |||
| Increased c-fos in SFO, MnPO, OVLT, PVN, SON | AT1aR −/− or AT2R −/− | |||
| icv Losartan |
AGT-deficient mice (AGT−/−) exhibit an impaired dipsogenic response to acute extracellular dehydration suggesting a requirement for de novo synthesis of ANG II (90). In contrast, intracellular dehydration (discussed in more detail below) induced water intake in AGT−/− mice. This difference between extra- and intracellular dehydration was also seen when AT1R was knocked down in the SFO by antisense RNA (69). Thus production of ANG II by extracellular dehydration appears to increase fluid intake by acting through an AT1R-dependent mechanism located in the SFO.
Hypertonic saline is commonly used to increase osmolality thereby inducing intracellular dehydration. Intracellular dehydration increases fluid intake, decreases salt preference, and increases vasopressin (100, 137). Rats injected with intravenous hypertonic saline increase immunoreactivity for c-Fos within the SFO, OVLT, mPVN, SON, MnPO, NTS, RVLM, and lateral PB (56, 130). c-Fos immunoreactivity is selectively decreased in the MnPO, mPVN and SON, if the SFO, the ventral portion of the AV3V or these combined areas are lesioned (56). If the entire AV3V is lesioned, drinking to systemic hypertonic saline is abolished (9, 10). In sheep, lesion of the SFO alone does not alter water intake in response to intravenous hypertonic saline, whereas lesioning the OVLT or MnPO attenuates water intake and ablation of all three nuclei abolishes water intake in response to cellular dehydration (89).
Extracellular dehydration and sodium deficiency can be induced by the combined actions of furosemide (a diuretic/natriuretic agent). Rats with water and sodium deficit do not increase their water and sodium intakes if the SFO is lesioned (99, 129). If the cell bodies within, but not the fibers passing by the MnPO (a main efferent target of the SFO) are ablated, the increase in saline intake is reduced but water intake is not altered (26). Thus the SFO may also act as a sensor for intracellular dehydration by signaling to other nuclei, such as the OVLT and/or MnPO in the process of generating a sodium appetite.
It was reported that in rats, fluid intake induced by subcutaneous hypertonic saline is attenuated by a high dose of PD123319 (administered centrally), an AT2R antagonist, but not by an AT1R antagonist (112). Similarly, intracerbroventricular injection of an AT2R antagonist attenuates increased drinking after 24-h fluid restriction or intravenous hypertonic saline. It has been proposed that AT2R may be acting as a mechanosensor of cellular swelling in response to intracellular dehydration rather than being activated by angiotensin peptides. This is supported by data suggesting that angiotensin peptides were not needed for intracellular dehydration-induced polydipsia in AGT−/− mice (90). Thus fluid intake can occur centrally through different ANG II receptors. This may occur through the SFO since it is important in mediating fluid intake due to peripheral stimuli, such as ANG II and osmolality.
The SFO also responds to conditions in the CSF, such as osmolality and ANG II. The brain senses a change in osmolality since blood-borne hypertonic saline or mannitol induce water intake at doses only effective as an intracarotid rather than intravenous injection (120). Increasing the osmolality of the CSF increases the neural activity of the SFO which can stimulate the intake of water, release AVP, and elevate blood pressure through an AT1R-dependent mechanism (6, 55, 87, 111, 138). Adding NaCl to a hypertonic aCSF injection was a more potent dipsogen than if sucrose was used, so both osmosensitive and sodium-sensitive receptors may mediate polydipsia (87). Furthermore, ablation of the lamina terminalis, which includes the SFO, abolishes the induction of drinking and release of AVP that occurs upon intravenous hypertonic saline (88, 89). These studies demonstrate that an elevation in osmolality can induce water intake through central mechanisms, which may in part involve the SFO.
In addition to sodium in the CSF, the SFO can increase fluid intake due to ANG II within the brain. It has been known for decades that a central injection of ANG II is a potent dipsogen (124), but we will focus on the fluid balance effects of physiological and genetic elevation of the brain-RAS within areas of endogenous ANG II synthesis and action. First, ANG II can be elevated within the brain in response to acute application of the diuretic furosemide (furo) concomitant with a low dose of the ACE inhibitor captopril (cap), a model referred to as furo/cap (106). In rats, furo/cap increases fluid intake and sodium appetite within 2 h, which can be further enhanced by subsequent injections over a period of weeks. Furo/cap increases the activity of cells (as assayed by the number of c-Fos immunoreactive cells) within the SFO, OVLT, MnPO, both magnocellular and parvocellular PVN, PB, area postrema (AP), and NTS (105, 113, 140). Interestingly, a high dose of captopril blocks this increase in c-fos activity (140). Presumably this occurs because captopril passes into the brain where it blocks the de novo production of ANG II. Indeed, furo/cap induces ANG II production within the SFO, OVLT, MnPO, and PVN, and intracerebroventricular losartan attenuates the increase in fluid intake and sodium appetite due to furo/cap suggesting it is mediated by AT1R (83).
Second, DOCA-salt and aldosterone elevates brain RAS activity within endogenous areas, resulting in increased blood pressure and fluid intake through central activation of AT1R (44, 47, 60, 104, 148). DOCA-salt increases c-Fos expression within the OVLT, BNST, MnPO, PVN, SON, amygdala, and preoptic area in rodents (85, 140). DOCA-salt or aldosterone increases the expression of RAS genes (102, 149). Autoradiography for radiolabeled Sar1ANG II after DOCA treatment alone (i.e., without high salt fluid) demonstrates that AT1R binding activity increases within the MnPO, SFO, PVN, but not the SCN, whereas high salt alone increases binding selectively within the MnPO and SFO (27, 47). Treatment with both DOCA and salt further enhances expression of AT1R within the MnPO, SFO, PVN, NTS, and AP compared with either treatment alone. Cotreatment of DOCA-salt with aldosterone in rodents further increases AT1R expression only within the SFO (27). This DOCA-salt mediated increase of AT1R in areas of the brain important for cardiovascular regulation increases blood pressure, fluid intake, and sodium appetite through an ANG II and AT1R-dependent mechanism since both central (and region specific) losartan and captopril can attenuate these phenotypes (27, 60, 71, 104). The AV3V region and the anterior hypothalamic area (AHA) have been shown to be important for the DOCA-salt elevation of fluid intake, sodium appetite, and blood pressure (8, 71). Lesion of the AV3V region, which is highly interconnected with the AHA, indicates its importance in mediating the hypertensive effects of DOCA-salt. While lesion of just the SFO does not affect the DOCA-salt increase in blood pressure, fluid intake, or sodium appetite, genetic ablation of AT1AR from the SFO attenuates DOCA-salt hypertension and polydipsia (52, 103). Thus these and other data support a functional synergism between ANG II and aldosterone (42, 148, 149). These studies also highlight the importance of the SFO and its target nuclei to mediate central ANG II effects through AT1R.
Transgenic methods have also been used to specifically and supraphysiologically increase the RAS within endogenous areas of the brain. Transgenic mice expressing human renin in all neurons driven by the synapsin promoter (termed sR mice) have been bred to transgenic mice expressing human angiotensinogen driven by 1.5 kb of its endogenous promoter (termed A mice) (45, 97). This double transgenic model, termed the sRA mouse, has many properties in common with DOCA-salt mice. Moreover, expression of human renin in sR mice mimics endogenous expression, because renin is primarily expressed in neurons (73). Since AGT is driven by its endogenous promoter, and ANG II production can only occur in sites where AGT is made, the sRA model selectively increases the brain RAS in those areas where endogenous ANG II is produced (72). We have shown that ANG II production is upregulated particularly in the AV3V region and hypothalamus in sRA mice (45). This causes the same physiological effects, such as polydipsia, seen in models of elevated brain RAS activity, such as DOCA-salt. Plasma aldosterone is also increased in sRA mice. Peripheral treatment with spironolactone (an antagonist for the aldosterone receptor) attenuates the fluid and sodium phenotypes of sRA mice, whereas adrenalectomy completely corrects them, further highlighting the synergism between central ANG II and aldosterone.
Just as AT1AR in the SFO are necessary for the polydipsia and hypertension induced by DOCA-salt (52), we have also shown that de novo production of ANG II in the SFO is both necessary and sufficient for central ANG II-mediated fluid intake (20, 117, 126). We have shown this by using a unique extension of the sRA model called sRAFlox and by using a conditionally and spatially inducible model of ANG II production called sRARed (Fig. 2). sRAFlox are functionally identical to sRA mice except that the hAGT transgene contains loxP sites surrounding exon-II, providing a substrate for the selective deletion of AGT upon Cre-mediated recombination. An injection of AdCRE directly into the SFO of double transgenic sRAFlox mice selectively turns off the brain RAS within the SFO, which attenuates their polydipsia (117). Similarly, intracerebroventricular injection of AdCRE into single transgenic hAGTFlox mice selectively removes hAGT from the SFO, which blocks the pressor response to an intracerebroventricular injection of purified human renin (126). Like sRA mice, sRARed mice express human renin in all neurons; however, a stop sequence surrounded by loxP sites prohibits the expression of human AGT unless it is removed by Cre-mediated recombination. In this model, inducing the production of ANG II in the SFO in sRARed mice increases total fluid intake and increases the preference for a nonaversive concentration of NaCl (20). Surprisingly, the induction of ANG II in the SFO in sRARed mice was not sufficient to increase blood pressure or metabolism. This led us to suggest the possibility that the mechanisms controlling ANG II-dependent drinking and blood pressure in the SFO may differ in its dose response or the cellular specificity of where ANG II acts. ANG II may act separately on neurons projecting to nuclei controlling water and electrolyte homeostasis or to nuclei controlling the preganglionic neurons of the sympathetic nervous system, and this may be favored toward the former in the sRARed model.
Fig. 2.
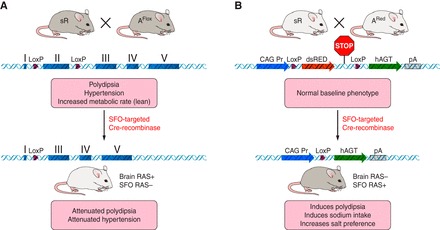
Schematic of mouse models. The figure schematically illustrates the sRAFlox and sRARed models described in the text. Brain-specific production of ANG II occurs in the double transgenic sRAFlox mice in and around cells expressing human renin under the control of a neuron-specific promoter and human angiotensinogen under the control of its own promoter (which is active in glial cells and certain neurons). These mice exhibit robust polydipsia, hypertension, and elevated resting metabolic rate. Expression of human angiotensinogen can be ablated in response to cre-recombinase. This cell-specific ablation can occur by breeding with an appropriate cre-driver or by injection of a virus expressing cre-recombinase. After SFO-targeted delivery of Cre-recombinase, the sRAFlox mice exhibited attenuated drinking and blood pressure. As an alternative, production of ANG II will only occur in response to cre-mediated recombination in the sRARED mouse model. This is because production of human angiotensinogen is blocked by a “STOP” signal that can be removed by cre-recombinase. Thus sRARED mice exhibit no changes in phenotype in the absence of a cre-driver. After SFO-targeted delivery of Cre-recombinase, the sRARED mice exhibited increased water and sodium intake and increased salt preference.
Downstream Mediators of Central Angiotensin Action: Role of Protein Kinase C and Mitogen-Activated Protein Kinases
AT1R is a G protein-coupled receptor (GPCR) linked to a Gq/11 α-subunit, which can increase diacylglyerol (DAG) and inositol trisphosphate (IP3) through activation of phospholipase C (PLC) (reviewed in Ref. 132). Classically, protein kinase C (PKC) can then be activated directly by DAG or indirectly through IP3 release of calcium. PKC isoforms are categorized according to their activators (101). Conventional PKCs (α, β, γ) are activated by DAG, calcium, and phospholipids; novel PKCs (δ, δ2, δ3, ϵ, η, θ) are activated by DAG, but not calcium; and atypical PKCs (ι, ζ, N1, N2, N3) require neither DAG nor calcium for activation. Intracerebroventricular injection of ANG II in rodents increases PKC-α activity in the MnPO, SFO, and PVN, whereas direct microinjection of ANG II into the RVLM increases the activation of all three conventional PKC isoforms (16, 38). Phorbol 12-myrstiate 13-acetate (PMA), an activator of PKC that bypasses receptor activation, induced PKC-α activity in primary cultures of cells derived from the rat SFO (21). PMA increased c-Fos expression and firing rate more in primary cultures of the AHA, brain stem, or hypothalamus from newborn spontaneously hypertensive rats (SHR) compared with similar cultures from Wistar-Kyoto rats (3, 70). The enhanced c-Fos expression in SHR is mediated by PKC-β, since knockdown of PKC-β prevented the induction (3). Moreover, pharmacologically blocking PKC with H-7 in ANG II-sensitive AHA neurons from SHR attenuates the enhanced c-Fos expression and neuronal firing rate more than control cultures (3, 70). Central injection of chelerythrine (a general inhibitor of all PKC isoforms) in rats blocks the increase in water intake due to intracerebroventricular ANG II (22, 38). Intracerebroventricular administration of Gö-6976, an inhibitor of the catalytic domains of PKC-α and PKC-β attenuated ANG II but not carbachol-induced water intake (38). Specifically decreasing PKC-α activity via intracerebroventricular injection of a dominant negative PKC-α attenuates the elevated water and 0.9% saline intake of sRA mice (21). Thus an elevation of the brain RAS activates PKC, and conventional isoforms of PKC mediate the dipsogenic effect of central ANG II.
Mitogen-activated protein kinase (MAPK) pathway has also been implicated to mediate the effects of central ANG II (23). This pathway may lie downstream of PKC because antisense inhibition of either PKC-α or PKC-β attenuates ANG II-induced phosphorylation of p38 MAPK and ERK1/2 in the RVLM of rats (16). Other data suggest that ERK1/2 activation in response to AT1R activation may occur independently of PKC through a β-arrestin pathway, although it remains unclear if this pathway is active in the brain (143). This AT1R-β-arrestin pathway may also be activated in response to membrane stretch (135). Inducing the brain RAS by furo/cap increases phosphorylation of ERK1/2 within the OVLT, SFO, SON, and PVN via AT1R; and its activation mediates the increase in sodium appetite (but not water intake) that occurs in furo/cap-treated rats (34). Activation of ERK1/2 has also been shown to regulate AT1R expression because blocking ERK1/2 inhibits the increase in AT1R expression within the SFO and PVN due to ANG II treatment (144, 152). These studies suggest that ANG II can activate ERK1/2, which upregulates AT1R, elevates blood pressure, and enhances sodium appetite, but not water intake.
Downstream Mediators of Central Angiotensin Action: Role of Reactive Oxygen Species and Endoplasmic Reticulum Stress
Peripheral infusion of ANG II increases reactive oxygen species (ROS) production within the SFO, and ANG II elevates ROS through AT1R in cultured cells (including the SFO) from the central nervous system (153, 155). Prior transfection of the SFO with either the intracellular or mitochondrial form of superoxide dismutase prevents polydipsia and hypertension induced by an intracerebroventricular injection of ANG II. However, a non-ANG II-dependent dipsogen and pressor agent carbachol is still able to increase fluid intake and blood pressure. Thus the production of ROS either in the cytosol or mitochondria of the SFO mediates the physiological effects of central ANG II action.
The source of ROS that mediates the effects of ANG II may occur through PKC-mediated activation of NADPH oxidase (NOX), specifically NOX2 and NOX4 (reviewed in Ref. 59). In the forebrain of mice, which includes the SFO, NOX2 is the main isoform expressed, though NOX1 and NOX4 are also expressed. In the midbrain, which includes the hypothalamus, NOX4 is the main isoform expressed, though the other isoforms are also expressed. In the hindbrain, which includes the NTS, RVLM, and AP, both NOX2 and NOX4 are highly expressed with much lower expression for NOX1. GF109203X (a pharmacological inhibitor of PKC-α or PKC-β) attenuates ANG II induction of ROS in NTS neurons, and NOX2-deficient NTS neurons do not increase ROS production in response to ANG II (142). Furthermore, chelerythrine blocks the phosphorylation of NOX2 in response to microinjection of ANG II into the RVLM (16). Downstream of PKC, pharmacologically blocking ROS in the RVLM prevents the phosphorylation of p38 MAPK due to microinjection of ANG II into the RVLM, and it prevents the phosphorylation of ERK1/2 due to chronic activation of AT1R (15). Thus central ANG II can increase the activity of PKC, production of ROS, and activation of MAPK pathways through NADPH oxidase-dependent mechanisms.
The importance of NADPH oxidase in mediating the physiological effects of central ANG II was demonstrated by blocking Rac-1 (part of the Rho family of GTPases) with adenoviral transfection of a dominant negative Rac-1 (AdDN-Rac1) in primary cultures derived from the lamina terminalis (154). Dominant negative Rac-1 virtually abolished NADPH oxidase activity, and when targeted to the SFO, AdDN-Rac1 abolished the increase in blood pressure and significantly attenuated the increase in fluid intake in response to central ANG II. Moreover, targeting NOX2 and NOX4 with RNA silencing in the SFO revealed that NOX2 mediates polydipsia, whereas either NOX2 or NOX4 can mediate the pressor effect of central ANG II (107). Neither had an effect on baseline fluid intake or blood pressure.
Endoplasmic reticulum (ER) stress has recently emerged as another mediator of the effects of ANG II. Injection of thapsigargin, a noncompetitive inhibitor of the ER calcium ATPase pump that blocks the uptake of calcium from the cytosol into the ER, centrally increases markers of ER stress such as phosphorylated PERK and phosphorylated IRE-1α (108). Thapsigargin-induced ER stress in the brain was recently shown to increase blood pressure and renal sympathetic nerve activity (151). ANG II treatment induced the expression or activity of several ER stress biomarkers (e.g., p58IPK, GRP78, and phosphorylated PERK) within the SFO. Central injection of tauroursodeoxycholic acid (TUDCA), a chemical chaperone, and adenoviral overexpression of GRP78, a molecular chaperone, both of which decrease ER stress, prevented the increase in blood pressure in response to peripheral ANG II. Blocking ER stress in the SFO also attenuated the ANG II-induced increase in ROS production. Thus ANG II can increase ER stress within the SFO to increase the production of ROS resulting in ANG II-induced hypertension.
Perspectives and Significance
The SFO is anatomically situated to respond to and integrate signals from both the periphery and CNS, and blood-borne or CSF ANG II can activate the SFO to increase fluid intake. Activation of AT1R within the SFO increases fluid intake through a PKC/ROS pathway and also mediates the increase in blood pressure due to ANG II through a PKC/ROS/ER Stress/ERK1/2 pathway.
GRANTS
This work was supported through from the National Institutes of Health Grants HL-048058, HL-061446, and HL-062984 to C. D. Sigmund; HL-084207 to C. D. Sigmund and J. L. Grobe; HL-014388, HL-098207, and AI-04586 to A. K. Johnson; and HL-098276 to J. L. Grobe. The authors also gratefully acknowledge the generous research support of the Roy J. Carver Trust.
REFERENCES
- 1.Allen AM, O'Callaghan EL, Hazelwood L, Germain S, Castrop H, Schnermann J, Bassi JK. Distribution of cells expressing human renin-promoter activity in the brain of a transgenic mouse. Brain Res 1243: 78–85, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Allen AM, Zhuo J, Mendelsohn FA. Localization and function of angiotensin AT1 receptors. Am J Hypertens 13: 31S-38S, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Amemiya T, Kambe T, Fukumori R, Kubo T. Role of protein kinase C beta in phorbol ester-induced c-fos gene expression in neurons of normotensive and spontaneously hypertensive rat brains. Brain Res 1040: 129–136, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Barth SW, Gerstberger R. Differential regulation of angiotensinogen and AT1A receptor mRNA within the rat subfornical organ during dehydration. Brain Res Mol Brain Res 64: 151–164, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Bickerton RK, Buckley JP. Evidence for a central mechanism in angiotensin-induced hypertension. Proc Soc Exp Biol Med 106: 834–837, 1961. [Google Scholar]
- 6.Blair-West JR, Burns P, Denton DA, Ferraro T, McBurnie MI, Tarjan E, Weisinger RS. Thirst induced by increasing brain sodium concentration is mediated by brain angiotensin. Brain Res 637: 335–338, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Booth DA. Mechanism of action of norepinephrine in eliciting an eating response on injection into the rat hypothalamus. J Pharmacol Exp Ther 160: 336–348, 1968. [PubMed] [Google Scholar]
- 8.Brody MJ, Johnson AK. Role of the anteroventral third ventricle region in fluid and electrolyte balance, arterial pressure regulation, and hypertension. F Neuroendo 6: 249–292, 1980. [Google Scholar]
- 9.Buggy J, Johnson AK. Anteroventral third ventricle periventricular ablation: Temporary adipsia and persisting thirst deficits. Neurosci Lett 5: 177–182, 1977. [DOI] [PubMed] [Google Scholar]
- 10.Buggy J, Johnson AK. Angiotensin-induced thirst: effects of third ventricle obstruction and periventricular ablation. Brain Res 149: 117–128, 1978. [DOI] [PubMed] [Google Scholar]
- 11.Buggy J, Jonhson AK. Preoptic-hypothalamic periventricular lesions: thirst deficits and hypernatremia. Am J Physiol Regul Integr Comp Physiol 233: R44–R52, 1977. [DOI] [PubMed] [Google Scholar]
- 12.Burson JM, Aguilera G, Gross KW, Sigmund CD. Differential expression of angiotensin receptor 1A and 1B in mouse. Am J Physiol Endocrinol Metab 267: E260–E267, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Carithers J, Bealer SL, Brody MJ, Johnson AK. Fine structural evidence of degeneration in supraoptic nucleus and subfornical organ of rats with lesions in the anteroventral third ventricle. Brain Res 201: 1–12, 1980. [DOI] [PubMed] [Google Scholar]
- 14.Chai SY, Mendelsohn FA, Paxinos G. Angiotensin converting enzyme in rat brain visualized by quantitative in vitro autoradiography. Neuroscience 20: 615–627, 1987. [DOI] [PubMed] [Google Scholar]
- 15.Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res 97: 772–780, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Chan SH, Wang LL, Tseng HL, Chan JY. Upregulation of AT1 receptor gene on activation of protein kinase Cbeta/nicotinamide adenine dinucleotide diphosphate oxidase/ERK1/2/c-fos signaling cascade mediates long-term pressor effect of angiotensin II in rostral ventrolateral medulla. J Hypertens 25: 1845–1861, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Chen H, Hoffman A, Cool D, Diz DI, Chappell MC, Chen A, Morris M. Adenovirus mediated small interferecne RNA for in vivo silencing of angiotensin AT1a receptors in mouse brain. Hypertension 47: 230–237, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Liu-Stratton Y, Hassanain H, Cool DR, Morris M. Dietary sodium regulates angiotensin AT1a and AT1b mRNA expression in mouse brain. Exp Neurol 188: 238–245, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Morris M. Differentiation of brain angiotensin type 1a and 1b receptor mRNAs: A specific effect of dehydration. Hypertension 37: 692–697, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Coble JP, Cassell MD, Davis DR, Grobe JL, Sigmund CD. Activation of the renin-angiotensin system specifically in the subfornical organ is sufficient to induce fluid intake. Am J Physiol Regul Integr Comp Physiol 307: R376–R386, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coble JP, Johnson RF, Cassell MD, Johnson AK, Grobe JL, Sigmund CD. Activity of PKC-alpha within the subfornical organ is necessary for fluid intake due to brain angiotensin. Hypertension 64: 141–148, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniels D, Mietlicki EG, Nowak EL, Fluharty SJ. Angiotensin II stimulates water and NaCl intake through separate cell signalling pathways in rats. Exp Physiol 94: 130–137, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniels D, Yee DK, Faulconbridge LF, Fluharty SJ. Divergent behavioral roles of angiotensin receptor intracellular signaling cascades. Endocrinology 146: 5552–5560, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Davisson RL, Oliverio MI, Coffman TM, Sigmund CD. Divergent functions of angiotensin II receptor isoforms in brain. J Clin Invest 106: 103–106, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Luca LAJ, Xu Z, Schoorlemmer GH, Thunhorst RL, Beltz TG, Menani JV, Johnson AK. Water deprivation-induced sodium appetite: humoral and cardiovascular mediators and immediate early genes. Am J Physiol Regul Integr Comp Physiol 282: R552–R559, 2002. [DOI] [PubMed] [Google Scholar]
- 26.de Lucca JW, Franci CR. Angiotensinergic pathway through the median preoptic nucleus in the control of oxytocin secretion and water and sodium intake. Brain Res 1014: 236–243, 2004. [DOI] [PubMed] [Google Scholar]
- 27.De Nicola AF, Seltzer A, Tsutsumi K, Saavedra JM. Effects of deoxycorticosterone acetate (DOCA) and aldosterone on Sar1-angiotensin II binding and angiotensin-converting enzyme binding sites in brain. Cell Mol Neurobiol 13: 529–539, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dellmann HD. Structure of the subfornical organ: a review. Microsc Res Tech 41: 85–97, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Dellmann HD, Linner JG. Ultrastructure of the subfornical organ of the chicken (Gallus domesticus). Cell Tissue Res 197: 137–153, 1979. [DOI] [PubMed] [Google Scholar]
- 30.Dellmann HD, Simpson JB. Regional differences in the morphology of the rat subfornical organ. Brain Res 116: 389–400, 1976. [DOI] [PubMed] [Google Scholar]
- 31.Denton DA, Blair-West JR, McBurnie M, Osborne PG, Tarjan E, Williams RM, Weisinger RS. Angiotensin and salt appetite of BALB/c mice. Am J Physiol Regul Integr Comp Physiol 259: R729–R735, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Deschepper CF, Bouhnik J, Ganong WF. Colocalization of angiotensinogen and glial fibrillary acidic protein in astrocytes in rat brain. Brain Res 374: 195–198, 1986. [DOI] [PubMed] [Google Scholar]
- 33.Duan PG, Kawano H, Masuko S. Collateral projections from the subfornical organ to the median preoptic nucleus and paraventricular hypothalamic nucleus in the rat. Brain Res 1198: 68–72, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Felgendreger LA, Fluharty SJ, Yee DK, Flanagan-Cato LM. Endogenous angiotensin II-induced p44/42 mitogen-activated protein kinase activation mediates sodium appetite but not thirst or neurohypophysial secretion in male rats. J Neuroendocrinol 25: 97–106, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferguson AV, Washburn DL. Angiotensin II: a peptidergic neurotransmitter in central autonomic pathways. Prog Neurobiol 54: 169–192, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson AV, Washburn DL, Latchford KJ. Hormonal and neurotransmitter roles for angiotensin in the regulation of central autonomic function. Exp Biol Med (Maywood) 226: 85–96, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Fitts DA, Freece JA, Van Bebber JE, Zierath DK, Bassett JE. Effects of forebrain circumventricular organ ablation on drinking or salt appetite after sodium depletion or hypernatremia. Am J Physiol Regul Integr Comp Physiol 287: R1325–R1334, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Fleegal MA, Sumners C. Drinking behavior elicited by central injection of angiotensin II: roles for protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Regul Integr Comp Physiol 285: R632–R640, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Fuxe K, Ganten D, Hokfelt T, Locatelli V, Poulsen K, Stock G, Rix E, Taugner R. Renin-like immunocytochemical activity in the rat and mouse brain. Neurosci Lett 18: 245–250, 1980. [DOI] [PubMed] [Google Scholar]
- 40.Ganten D, Marquez-Julio A, Granger P, Hayduk K, Karsunky KP, Boucher R, Genest J. Renin in dog brain. Am J Physiol 221: 1733–1737, 1971. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez AD, Wang G, Waters EM, Gonzales KL, Speth RC, Van Kempen TA, Marques-Lopes J, Young CN, Butler SD, Davisson RL, Iadecola C, Pickel VM, Pierce JP, Milner TA. Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience 226: 489–509, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grafe LA, Takacs AE, Yee DK, Flanagan-Cato LM. The role of the hypothalamic paraventricular nucleus and the organum vasculosum lateral terminalis in the control of sodium appetite in male rats. J Neurosci 34: 9249–9260, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groban L, Wang H, Machado FS, Trask AJ, Kritchevsky SB, Ferrario CM, Diz DI. Low glial angiotensinogen improves body habitus, diastolic function, and exercise tolerance in aging male rats. Cardiovasc Endocrinol 1: 49–58, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, Sigmund CD. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension 57: 600–607, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedans DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metabolism 12: 431–442, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology 23: 187–193, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutkind JS, Kurihara M, Saavedra JM. Increased angiotensin II receptors in brain nuclei of DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol 255: H646–H650, 1988. [DOI] [PubMed] [Google Scholar]
- 48.Hafko R, Villapol S, Nostramo R, Symes A, Sabban EL, Inagami T, Saavedra JM. Commercially available angiotensin II At(2) receptor antibodies are nonspecific. PLos One 8: e69234, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hashimoto H, Otsubo H, Fujihara H, Suzuki H, Ohbuchi T, Yokoyama T, Takei Y, Ueta Y. Centrally administered ghrelin potently inhibits water intake induced by angiotensin II and hypovolemia in rats. J Physiol Sci 60: 19–25, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hermann K, Raizada MK, Sumners C, Phillips MI. Presence of renin in primary neuronal and glial cells from rat brain. Brain Res 437: 205–213, 1987. [DOI] [PubMed] [Google Scholar]
- 51.Herrera M, Sparks MA, Alfonso-Pecchio AR, Harrison-Bernard LM, Coffman TM. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension 61: 253–258, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hilzendeger AM, Cassell MD, Davis DR, Stauss HM, Mark AL, Grobe JL, Sigmund CD. Angiotensin type 1a receptors in the subfornical organ are required for deoxycorticosterone acetate-salt hypertension. Hypertension 61: 716–722, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirose S, Yokosawa H, Inagami T. Immunochemical identification of renin in rat brain and distinction from acid proteases. Nature 274: 392–393, 1978. [DOI] [PubMed] [Google Scholar]
- 54.Hiyama TY, Yoshida M, Matsumoto M, Suzuki R, Matsuda T, Watanabe E, Noda M. Endothelin-3 expression in the subfornical organ enhances the sensitivity of Na(x), the brain sodium-level sensor, to suppress salt intake. Cell Metab 17: 507–519, 2013. [DOI] [PubMed] [Google Scholar]
- 55.Ho JM, Zierath DK, Savos AV, Femiano DJ, Bassett JE, McKinley MJ, Fitts DA. Differential effects of intravenous hyperosmotic solutes on drinking latency and c-Fos expression in the circumventricular organs and hypothalamus of the rat. Am J Physiol Regul Integr Comp Physiol 292: R1690–R1698, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Hochstenbach SL, Ciriello J. Effect of lesions of forebrain circumventricular organs on c-fos expression in the central nervous system to plasma hypernatremia. Brain Res 713: 17–28, 1996. [DOI] [PubMed] [Google Scholar]
- 57.Huang BS, Ahmadi S, Ahmad M, White RA, Leenen FH. Central neuronal activation and pressor responses induced by circulating ANG II: role of the brain aldosterone-“ouabain” pathway. Am J Physiol Heart Circ Physiol 299: H422–H430, 2010. [DOI] [PubMed] [Google Scholar]
- 58.Imboden H, Harding JW, Hilgenfeldt U, Celio MR, Felix D. Localization of angiotensinogen in multiple cell types of rat brain. Brain Res 410: 74–77, 1987. [DOI] [PubMed] [Google Scholar]
- 59.Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal 8: 1583–1596, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Itaya Y, Suzuki H, Matsukawa S, Kondo K, Saruta T. Central renin-angiotensin system and the pathogenesis of DOCA-salt hypertension in rats. Am J Physiol Heart Circ Physiol 251: H261–H268, 1986. [DOI] [PubMed] [Google Scholar]
- 61.Johnson AK. The periventricular anteroventral third ventricle (AV3V): its relationship with the subfornical organ and neural systems involved in maintaining body fluid homeostasis. Brain Res Bull 15: 595–601, 1985. [DOI] [PubMed] [Google Scholar]
- 62.Johnson AK, Buggy J. Periventricular preoptic-hypothalamus is vital for thirst and normal water economy. Am J Physiol Regul Integr Comp Physiol 234: R122–R129, 1978. [DOI] [PubMed] [Google Scholar]
- 63.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J 7: 678–686, 1993. [DOI] [PubMed] [Google Scholar]
- 64.Johnson RF, Beltz TG, Jurzak M, Wachtel RE, Johnson AK. Characterization of ionic currents of cells of the subfornical organ that project to the supraoptic nuclei. Brain Res 817: 226–231, 1999. [DOI] [PubMed] [Google Scholar]
- 65.Jones CA, Hurley MI, Black TA, Kane CM, Pan L, Pruitt SC, Gross KW. Expression of a renin- green fluorescent protein transgene in mouse embryonic, extra-embryonic and adult tissues. Physiol Genomics 4: 75–81, 2000. [DOI] [PubMed] [Google Scholar]
- 66.Kawano H, Masuko S. Tyrosine hydroxylase-immunoreactive projections from the caudal ventrolateral medulla to the subfornical organ in the rat. Brain Res 903: 154–161, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Kawano H, Masuko S. Peptidergic and catecholaminergic synaptic contacts onto nucleus preopticus medianus neurons projecting to the subfornical organ in the rat. Neurosci Res 55: 211–217, 2006. [DOI] [PubMed] [Google Scholar]
- 68.Kawano H, Masuko S. Region-specific projections from the subfornical organ to the paraventricular hypothalamic nucleus in the rat. Neuroscience 169: 1227–1234, 2010. [DOI] [PubMed] [Google Scholar]
- 69.Krause EG, Melhorn SJ, Davis JF, Scott KA, Ma LY, de Kloet AD, Benoit SC, Woods SC, Sakai RR. Angiotensin type 1 receptors in the subfornical organ mediate the drinking and hypothalamic-pituitary-adrenal response to systemic isoproterenol. Endocrinology 149: 6416–6424, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kubo T, Hagiwara Y. Protein kinase C activation-induced increases of neural activity are enhanced in the hypothalamus of spontaneously hypertensive rats. Brain Res 1033: 157–163, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Kubo T, Yamaguchi H, Tsujimura M, Hagiwara Y, Fukumori R. Blockade of angiotensin receptors in the anterior hypothalamic preoptic area lowers blood pressure in DOCA-salt hypertensive rats. Hypertens Res 23: 109–118, 2000. [DOI] [PubMed] [Google Scholar]
- 72.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Adjacent expression of renin and angiotensinogen in the rostral ventrolateral medulla using a dual-reporter transgenic model. Hypertension 43: 1116–1119, 2004. [DOI] [PubMed] [Google Scholar]
- 73.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Localization of Renin Expressing Cells in the Brain Using a REN-eGFP Transgenic Model. Physiol Genomics 16: 240–246, 2004. [DOI] [PubMed] [Google Scholar]
- 74.Lavoie JL, Liu X, Bianco RA, Beltz TG, Johnson AK, Sigmund CD. Evidence supporting a functional role for intracellular renin in the brain. Hypertension 47: 461–466, 2006. [DOI] [PubMed] [Google Scholar]
- 75.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system–an endocrine and paracrine system. Endocrinology 144: 2179–2183, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Lazartigues E, Dunlay SM, Loihl AK, Sinnayah P, Lang JA, Espelund JJ, Sigmund CD, Davisson RL. Brain-selective overexpression of angiotensin (AT1) receptors causes enhanced cardiovascular sensitivity in transgenic mice. Circ Res 90: 617–624, 2002. [DOI] [PubMed] [Google Scholar]
- 77.Lazartigues E, Sinnayah P, Augoyard G, Gharib C, Johnson AK, Davisson RL. Enhanced water and salt intake in transgenic mice with brain-restricted overexpression of angiotensin (AT1) receptors. Am J Physiol Regul Integr Comp Physiol 295: R1539–R1545, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Z, Ferguson AV. Subfornical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol Regul Integr Comp Physiol 265: R302–R309, 1993. [DOI] [PubMed] [Google Scholar]
- 79.Li Z, Iwai M, Wu L, Shiuchi T, Jinno T, Cui TX, Horiuchi M. Role of AT2 receptor in the brain in regulation of blood pressure and water intake. Am J Physiol Heart Circ Physiol 284: H116–H121, 2003. [DOI] [PubMed] [Google Scholar]
- 80.Lind RW, Johnson AK. Subfornical organ-median preoptic connections and drinking and pressor responses to angiotensin II. J Neurosci 2: 1043–1051, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lind RW, Swanson LW, Ganten D. Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system. An immunohistochemical study. Neuroendocrinology 40: 2–24, 1985. [DOI] [PubMed] [Google Scholar]
- 82.Lind RW, Van Hoesen GW, Johnson AK. An HRP study of the connections of the subfornical organ of the rat. J Comp Neurol 210: 265–277, 1982. [DOI] [PubMed] [Google Scholar]
- 83.Lu B, Yan J, Yang X, Li J, Chen K. Involvement of brain ANG II in acute sodium depletion induced salty taste changes. Regul Pept 179: 15–22, 2012. [DOI] [PubMed] [Google Scholar]
- 84.Lu B, Yang XJ, Chen K, Yang DJ, Yan JQ. Dietary sodium deprivation evokes activation of brain regional neurons and down-regulation of angiotensin II type 1 receptor and angiotensin-convertion enzyme mRNA expression. Neuroscience 164: 1303–1311, 2009. [DOI] [PubMed] [Google Scholar]
- 85.Mahon JM, Allen M, Herbert J, Fitzsimons JT. The association of thirst, sodium appetite and vasopressin release with c-fos expression in the forebrain of the rat after intracerebroventricular injection of angiotensin II, angiotensin-(1–7) or carbachol. Neuroscience 69: 199–208, 1995. [DOI] [PubMed] [Google Scholar]
- 86.Mark MH, Farmer PM. The human subfornical organ: an anatomic and ultrastructural study. Ann Clin Lab Sci 14: 427–442, 1984. [PubMed] [Google Scholar]
- 87.McKinley MJ, Blaine EH, Denton DA. Brain osmoreceptors, cerebrospinal fluid electrolyte composition and thirst. Brain Res 70: 532–537, 1974. [DOI] [PubMed] [Google Scholar]
- 88.McKinley MJ, Denton DA, Leksell LG, Mouw DR, Scoggins BA, Smith MH, Weisinger RS, Wright RD. Osmoregulatory thirst in sheep is disrupted by ablation of the anterior wall of the optic recess. Brain Res 236: 210–215, 1982. [DOI] [PubMed] [Google Scholar]
- 89.McKinley MJ, Mathai ML, Pennington G, Rundgren M, Vivas L. Effect of individual or combined ablation of the nuclear groups of the lamina terminalis on water drinking in sheep. Am J Physiol Regul Integr Comp Physiol 276: R673–R683, 1999. [DOI] [PubMed] [Google Scholar]
- 90.McKinley MJ, Walker LL, Alexiou T, Allen AM, Campbell DJ, Di NR, Oldfield BJ, Denton DA. Osmoregulatory fluid intake but not hypovolemic thirst is intact in mice lacking angiotensin. Am J Physiol Regul Integr Comp Physiol 294: R1533–R1543, 2008. [DOI] [PubMed] [Google Scholar]
- 91.Mendelsohn FA, Chai SY, Dunbar M. In vitro autoradiographic localization of angiotensin-converting enzyme in rat brain using 125I-labelled MK351A. J Hypertens Suppl 2: S41–S44, 1984. [PubMed] [Google Scholar]
- 92.Mietlicki EG, Nowak EL, Daniels D. The effect of ghrelin on water intake during dipsogenic conditions. Physiol Behav 96: 37–43, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miselis RR. The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural network subserving water balance. Brain Res 230: 1–23, 1981. [DOI] [PubMed] [Google Scholar]
- 94.Miselis RR. The subfornical organ's neural connections and their role in water balance. Peptides 3: 501–502, 1982. [DOI] [PubMed] [Google Scholar]
- 95.Moellenhoff E, Blume A, Culman J, Chatterjee B, Herdegen T, Lebrun CJ, Unger T. Effect of repetitive icv injections of ANG II on c-Fos and AT(1)-receptor expression in the rat brain. Am J Physiol Regul Integr Comp Physiol 280: R1095–R1104, 2001. [DOI] [PubMed] [Google Scholar]
- 96.Morimoto S, Cassell MD, Beltz TG, Johnson AK, Davisson RL, Sigmund CD. Elevated blood pressure in transgenic mice with brain-specific expression of human angiotensinogen driven by the glial fibrillary acidic protein promoter. Circ Res 89: 365–372, 2001. [DOI] [PubMed] [Google Scholar]
- 97.Morimoto S, Cassell MD, Sigmund CD. Glial- and neuronal-specific expression of the renin-angiotensin system in brain alters blood pressure, water intake, and salt preference. J Biol Chem 277: 33235–33241, 2002. [DOI] [PubMed] [Google Scholar]
- 98.Morita S, Miyata S. Different vascular permeability between the sensory and secretory circumventricular organs of adult mouse brain. Cell Tissue Res 349: 589–603, 2012. [DOI] [PubMed] [Google Scholar]
- 99.Morris MJ, Wilson WL, Starbuck EM, Fitts DA. Forebrain circumventricular organs mediate salt appetite induced by intravenous angiotensin II in rats. Brain Res 949: 42–50, 2002. [DOI] [PubMed] [Google Scholar]
- 100.Nagakura A, Hiyama TY, Noda M. Na(x)-deficient mice show normal vasopressin response to dehydration. Neurosci Lett 472: 161–165, 2010. [DOI] [PubMed] [Google Scholar]
- 101.Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem 272: 952–960, 1997. [DOI] [PubMed] [Google Scholar]
- 102.Nishimura M, Ohtsuka K, Iwai N, Takahashi H, Yoshimura M. Regulation of brain renin-angiotensin system by benzamil-blockable sodium channels. Am J Physiol Regul Integr Comp Physiol 276: R1416–R1424, 1999. [DOI] [PubMed] [Google Scholar]
- 103.Osborn JW, Jacob F, Hendel M, Collister JP, Clark L, Guzman PA. Effect of subfornical organ lesion on the development of mineralocorticoid-salt hypertension. Brain Res 1109: 74–82, 2006. [DOI] [PubMed] [Google Scholar]
- 104.Park CG, Leenen FH. Effects of centrally administered losartan on deoxycorticosterone-salt hypertension rats. J Korean Med Sci 16: 553–557, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pastuskovas CV, Cassell MD, Johnson AK, Thunhorst RL. Increased cellular activity in rat insular cortex after water and salt ingestion induced by fluid depletion. Am J Physiol Regul Integr Comp Physiol 284: R1119–R1125, 2003. [DOI] [PubMed] [Google Scholar]
- 106.Pereira DT, Menani JV, De Luca LAJ. FURO/CAP: a protocol for sodium intake sensitization. Physiol Behav 99: 472–481, 2010. [DOI] [PubMed] [Google Scholar]
- 107.Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension 54: 1106–1114, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Purkayastha S, Zhang H, Zhang G, Ahmed Z, Wang Y, Cai D. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc Natl Acad Sci USA 108: 2939–2944, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Redding KM, Chen BL, Singh A, Re RN, Navar LG, Seth DM, Sigmund CD, Tang WW, Cook JL. Transgenic mice expressing an intracellular fluorescent fusion of angiotensin II demonstrate renal thrombotic microangiopathy and elevated blood pressure. Am J Physiol Heart Circ Physiol 298: H1807–H1818, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rogerson FM, Schlawe I, Paxinos G, Chai SY, McKinley MJ, Mendelsohn FA. Localization of angiotensin converting enzyme by in vitro autoradiography in the rabbit brain. J Chem Neuroanat 8: 227–243, 1995. [DOI] [PubMed] [Google Scholar]
- 111.Rohmeiss P, Beyer C, Nagy E, Tschope C, Hohle S, Strauch M, Unger T. NaCl injections in brain induce natriuresis and blood pressure responses sensitive to ANG II AT1 receptors. Am J Physiol Renal Fluid Electrolyte Physiol 269: F282–F288, 1995. [DOI] [PubMed] [Google Scholar]
- 112.Rowland NE, Fregly MJ. Brain angiotensin AT-2 receptor antagonism and water intake. Brain Res Bull 32: 391–394, 1993. [DOI] [PubMed] [Google Scholar]
- 113.Rowland NE, Fregly MJ, Han L, Smith G. Expression of Fos in rat brain in relation to sodium appetite: furosemide and cerebroventricular renin. Brain Res 728: 90–96, 1996. [PubMed] [Google Scholar]
- 114.Rowland NE, Goldstein BE, Robertson KL. Role of angiotensin in body fluid homeostasis of mice: fluid intake, plasma hormones, and brain Fos. Am J Physiol Regul Integr Comp Physiol 284: R1586–R1594, 2003. [DOI] [PubMed] [Google Scholar]
- 115.Rowland NE, Li BH, Rozelle AK, Fregly MJ, Garcia M, Smith GC. Localization of changes in immediate early genes in brain in relation to hydromineral balance: intravenous angiotensin II. Brain Res Bull 33: 427–436, 1994. [DOI] [PubMed] [Google Scholar]
- 116.Rowland NE, Li BH, Rozelle AK, Smith GC. Comparison of fos-like immunoreactivity induced in rat brain by central injection of angiotensin II and carbachol. Am J Physiol Regul Integr Comp Physiol 267: R792–R798, 1994. [DOI] [PubMed] [Google Scholar]
- 117.Sakai K, Agassandian K, Morimoto S, Sinnayah P, Cassell MD, Davisson RL, Sigmund CD. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest 117: 1088–1095, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sakai K, Chapleau MW, Morimoto S, Cassell MD, Sigmund CD. Differential modulation of baroreflex control of heart rate by neuron- vs. glia-derived angiotensin II. Physiol Genomics 20: 66–72, 2004. [DOI] [PubMed] [Google Scholar]
- 119.Sakima A, Averill DB, Kasper SO, Jackson LM, Ganten D, Ferrario CM, Gallagher PE, Diz DI. Baroreceptor reflex regulation in anesthetized transgenic rats with low glial-derived angiotensinogen. Am J Physiol Heart Circ Physiol 292: H1412–H1419, 2007. [DOI] [PubMed] [Google Scholar]
- 120.Schoorlemmer GH, Johnson AK, Thunhorst RL. Effect of hyperosmotic solutions on salt excretion and thirst in rats. Am J Physiol Regul Integr Comp Physiol 278: R917–R923, 2000. [DOI] [PubMed] [Google Scholar]
- 121.Sherrod M, Davis DR, Zhou X, Cassell MD, Sigmund CD. Glial-specific ablation of angiotensinogen lowers arterial pressure in renin and angiotensinogen transgenic mice. Am J Physiol Regul Integr Comp Physiol 289: R1763–R1769, 2005. [DOI] [PubMed] [Google Scholar]
- 122.Sherrod M, Liu X, Zhang X, Sigmund CD. Nuclear localization of angiotensinogen in astrocytes. Am J Physiol Regul Integr Comp Physiol 288: R539–R546, 2005. [DOI] [PubMed] [Google Scholar]
- 123.Shi L, Mao C, Zeng F, Hou J, Zhang H, Xu Z. Central angiotensin I increases fetal AVP neuron activity and pressor responses. Am J Physiol Endocrinol Metab 298: E1274–E1282, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Simpson JB, Routtenberg A. Subfornical organ: site of drinking elicitation by angiotensin II. Science 181: 1772–1775, 1973. [DOI] [PubMed] [Google Scholar]
- 125.Sinn PL, Sigmund CD. Identification of three human renin mRNA isoforms resulting from alternative tissue-specific transcriptional initiation. Physiol Genomics 3: 25–31, 2000. [DOI] [PubMed] [Google Scholar]
- 126.Sinnayah P, Lazartigues E, Sakai K, Sharma RV, Sigmund CD, Davisson RL. Genetic ablation of angiotensinogen in the subfornical organ of the brain prevents the central angiotensinergic pressor response. Circ Res 99: 1125–1131, 2006. [DOI] [PubMed] [Google Scholar]
- 127.Sinnayah P, Lindley TE, Staber PD, Cassell MD, Davidson BL, Davisson RL. Selective gene transfer to key cardiovascular regions of the brain: comparison of two viral vector systems. Hypertension 39: 603–608, 2002. [DOI] [PubMed] [Google Scholar]
- 128.Sinnayah P, Lindley TE, Staber PD, Davidson BL, Cassell MD, Davisson RL. Targeted viral delivery of Cre recombinase induces conditional gene deletion in cardiovascular circuits of the mouse brain. Physiol Genomics 18: 25–32, 2004. [DOI] [PubMed] [Google Scholar]
- 129.Starbuck EM, Fitts DA. Influence of salt intake, ANG II synthesis and SFO lesion on thirst and blood pressure during sodium depletion. Subfornical organ. Appetite 31: 309–331, 1998. [DOI] [PubMed] [Google Scholar]
- 130.Starbuck EM, Wilson WL, Fitts DA. Fos-like immunoreactivity and thirst following hyperosmotic loading in rats with subdiaphragmatic vagotomy. Brain Res 931: 159–167, 2002. [DOI] [PubMed] [Google Scholar]
- 131.Stornetta RL, Hawelu Johnson CL, Guyenet PG, Lynch KR. Astrocytes synthesize angiotensinogen in brain. Science 242: 1444–1446, 1988. [DOI] [PubMed] [Google Scholar]
- 132.Sumners C, Fleegal MA, Zhu M. Angiotensin AT1 receptor signalling pathways in neurons. Clin Exp Pharmacol Physiol 29: 483–490, 2002. [DOI] [PubMed] [Google Scholar]
- 133.Swanson LW, Lind RW. Neural projections subserving the initiation of a specific motivated behavior in the rat: new projections from the subfornical organ. Brain Res 379: 399–403, 1986. [DOI] [PubMed] [Google Scholar]
- 134.Tanaka J, Kaba H, Saito H, Seto K. Subfornical organ neurons with efferent projections to the hypothalamic paraventricular nucleus: an electrophysiological study in the rat. Brain Res 346: 151–154, 1985. [DOI] [PubMed] [Google Scholar]
- 135.Tang W, Strachan RT, Lefkowitz RJ, Rockman HA. Allosteric modulation of beta-arrestin-biased angiotensin II type 1 receptor signaling by membrane stretch. J Biol Chem 289: 28271–28283, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thomas WG, Greenland KJ, Shinkel TA, Sernia C. Angiotensinogen is secreted by pure rat neuronal cell cultures. Brain Res 588: 191–200, 1992. [DOI] [PubMed] [Google Scholar]
- 137.Thornton SN. Thirst and hydration: physiology and consequences of dysfunction. Physiol Behav 100: 15–21, 2010. [DOI] [PubMed] [Google Scholar]
- 138.Thornton SN, Forsling ML, Baldwin BA, Delaney CE. Separate mechanisms for central osmotically-induced drinking and vasopressin release in minipigs. Physiol Behav 39: 541–545, 1987. [DOI] [PubMed] [Google Scholar]
- 139.Thrasher TN, Simpson JB, Ramsay DJ. Lesions of the subfornical organ block angiotensin-induced drinking in the dog. Neuroendocrinology 35: 68–72, 1982. [DOI] [PubMed] [Google Scholar]
- 140.Thunhorst RL, Xu Z, Cicha MZ, Zardetto-Smith AM, Johnson AK. Fos expression in rat brain during depletion-induced thirst and salt appetite. Am J Physiol Regul Integr Comp Physiol 274: R1807–R1814, 1998. [DOI] [PubMed] [Google Scholar]
- 141.Urzedo-Rodrigues LS, Depieri T, Cherobino AJ, Lopes OU, Menani JV, Colombari DS. Hypothalamic disconnection caudal to paraventricular nucleus affects cardiovascular and drinking responses to central angiotensin II and carbachol. Brain Res 1388: 100–108, 2011. [DOI] [PubMed] [Google Scholar]
- 142.Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, Iadecola C. Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension 48: 482–489, 2006. [DOI] [PubMed] [Google Scholar]
- 143.Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA 100: 10782–10787, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wei SG, Yu Y, Zhang ZH, Felder RB. Angiotensin II upregulates hypothalamic AT1 receptor expression in rats via the mitogen-activated protein kinase pathway. Am J Physiol Heart Circ Physiol 296: H1425–H1433, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu D, Borges GR, Davis DR, Agassandian K, Sequeira Lopez ML, Gomez RA, Cassell MD, Grobe JL, Sigmund CD. Neuron- or glial-specific ablation of secreted renin does not affect renal renin, baseline arterial pressure or metabolism. Physiol Genomics 43: 286–294, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu D, Borges GR, Grobe JL, Pelham CJ, Yang B, Sigmund CD. Preservation of intracellular renin expression is insufficient to compensate for genetic loss of secreted renin. Hypertension 54: 1240–1247, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1–7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol 300: R804–R817, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xue B, Beltz TG, Yu Y, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Central interactions of aldosterone and angiotensin II in aldosterone- and angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 300: H555–H564, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xue B, Zhang Z, Roncari CF, Guo F, Johnson AK. Aldosterone acting through the central nervous system sensitizes angiotensin II-induced hypertension. Hypertension 60: 1023–1030, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang G, Gray TS, Sigmund CD, Cassell MD. The angiotensinogen gene is expressed in both astrocytes and neurons in murine central nervous system. Brain Res 817: 123–131, 1999. [DOI] [PubMed] [Google Scholar]
- 151.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest 122: 3960–3964, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yu Y, Xue BJ, Zhang ZH, Wei SG, Beltz TG, Guo F, Johnson AK, Felder RB. Early interference with p44/42 mitogen-activated protein kinase signaling in hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension. Hypertension 61: 842–849, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Prog Biophys Mol Biol 84: 125–149, 2004. [DOI] [PubMed] [Google Scholar]
- 154.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res 95: 532–539, 2004. [DOI] [PubMed] [Google Scholar]
- 155.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004. [DOI] [PubMed] [Google Scholar]


