Abstract
Background
Whereas low lung function is known to predict mortality in the general population, the prognostic significance of emphysema on computed tomography (CT) in persons without chronic obstructive pulmonary disease (COPD) remains uncertain.
Objective
To determine whether greater emphysema-like lung on CT is associated with all-cause mortality among persons without airflow obstruction or COPD in the general population.
Design
Prospective cohort study.
Setting
Population-based, multiethnic sample from 6 US communities.
Participants
2965 participants ages 45-84 years without airflow obstruction on spirometry.
Measurements
Emphysema-like lung was defined on cardiac CT as the number of lung voxels less than -950 Hounsfield Units, and was adjusted for the number of total imaged lung voxels.
Results
Among 2965 participants, 50.9% of whom never smoked, there were 186 deaths over a median of 6.2 years. Greater emphysema-like lung was independently associated with increased mortality (adjusted hazard ratio [HR]1.14 per one-half of the interquartile range, 95% CI 1.04-1.24, P=0.004), adjusting for potential confounders including cardiovascular risk factors and the forced expiratory volume in one second. Generalized additive models supported a linear association between emphysema-like lung and mortality without evidence for a threshold. The association was of greatest magnitude among smokers, although multiplicative interaction terms did not support effect modification by smoking status.
Limitations
Cardiac CT scans did not include lung apices. The number of deaths was limited among subgroup analyses.
Conclusions
Emphysema-like lung on CT was associated with all-cause mortality among persons without airflow obstruction or COPD in a general population sample, particularly among smokers. Recognition of the independent prognostic significance of emphysema on CT among patients without COPD on spirometry is warranted.
Primary Funding Source
NIH/NHLBI.
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the United States and globally (1, 2). COPD is defined physiologically by airflow obstruction on spirometry that does not completely reverse (3). Most medical therapies and almost all randomized clinical trials in COPD target the airways. Such therapies improve symptoms and reduce hospitalizations but have not been proven to affect disease progression or reduce mortality (4-7).
Pulmonary emphysema is defined anatomically as destruction of lung parenchyma and loss of intra-alveolar walls (8, 9). Emphysema was originally diagnosed on autopsy but can also be assessed via chest computed tomography (CT), which is now recommended as a screening tool for lung cancer (10-12).
Emphysema is common in the general population. Autopsy studies demonstrate that most smokers and up to 10% of never-smokers have some degree of emphysema (13). Emphysema on CT is a common “incidental” finding, occurring in 29% of smokers undergoing lung cancer screening (14) and 4% of healthy adults undergoing cardiac scanning (15). Furthermore, emphysema and COPD overlap less than previously thought: emphysema is frequently observed in the absence of COPD (16-18), and approximately half of COPD patients do not have substantial emphysema (19).
While it is known that reduced lung function is associated with increased all-cause mortality in the general population (20-22), and that emphysema on CT may portend a worse prognosis in COPD patients (16, 23) and in some but not all studies of selected smokers (14, 24), the prognostic importance of emphysema on CT among patients without COPD and in the broader population of smokers and non-smokers is unknown.
We therefore examined the associations between the extent of emphysema-like lung on CT and mortality among individuals free of airflow obstruction on spirometry (and therefore free of COPD) in a large, multiethnic, population-based cohort followed for 6 years after spirometry. We studied both smokers and never-smokers, since panlobular emphysema occurs with equal prevalence in individuals with and without a history of smoking (13, 17).
Methods
Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) enrolled 6814 participants aged 45 to 84 years, who self-reported White, African-American, Hispanic and/or Asian race/ethnicity in 2000-02 (25). Exclusion criteria were history of clinical cardiovascular disease, weight greater than 300 pounds (the maximum for CT scanners at the time) and impediments to long-term participation. Participants were recruited from Forsyth County, North Carolina; northern Manhattan and the Bronx, New York; Baltimore City and Baltimore County, Maryland; St Paul, Minnesota; Chicago, Illinois; and Los Angeles, California. Five participants were excluded from follow-up after discovery of pre-baseline cardiovascular events, and 12 participants were missing valid CT measurements (Appendix Figure 1).
Follow-up and Mortality
Interviewers contacted each MESA participant or a family member to inquire about vital status at intervals of 9 to 12 months. The National Death Index (NDI) was also regularly reviewed to assure complete follow-up for mortality through the most recent NDI update (December 31, 2010). Death from any cause was the primary endpoint.
Emphysema-like Lung
All MESA participants underwent cardiac CT at baseline using standardized protocols on either electron-beam CT or multidetector CT scanners (28) in 2000-02. For each participant, two scans were performed at suspended full inspiration from the carina to the lung bases with transverse fields-of-view that captured the whole lung field. These scans captured on average 65% of the total lung volume on full-lung scans acquired in a validation study (29) in MESA (see CT Appendix for details).
Image attenuation was assessed using a modified version of the Pulmonary Analysis Software Suite (30, 31) at a single reading center by trained readers without knowledge of other participant information. Emphysema-like lung was defined as the number of lung voxels with outside-air corrected attenuation less than -950 Hounsfield units (HU) based upon pathological comparisons (32) on the scan with higher air volume or, in the case of discordant quality scores, the higher quality scan (29). To correct for variations in scanner calibration and in the way different scanners handle scatter and beam hardening, we measured the attenuation of air outside the body, which should have a mean attenuation of -1000 HU, for each scan in a region distant from the body and scanner table. The outside-air corrected attenuation of each lung pixel was defined as measured pixel attenuation × (-1000/mean outside-air attenuation).
Regions of the lung with features suggestive of interstitial lung abnormalities (hereafter referred to as high attenuation areas [HAA]) were defined as the number of lung voxels with attenuation between -600 and -250 HU (33). All of these measures were previously validated against those obtained from full lung scans in MESA (r=0.93 for emphysema-like lung) (29, 33).
Spirometry
Spirometry was attempted between 2004 and 2006 for 3965 participants who had baseline measurements of endothelial function (99% of MESA), consented for genetic analyses (99% of MESA), and underwent an examination during the MESA Lung Study recruitment period (Appendix Figure 1). 3847 participants performed maneuvers in accordance with the American Thoracic Society/European Respiratory Society guidelines (34) on a dry rolling seal spirometer (Occupational Marketing); results were reviewed by a single investigator (35).
Airflow obstruction was defined as a ratio of the forced expiratory volume in one second (FEV1) to the forced vital capacity (FVC) less than 0.70, following current guidelines (3). Absence of airflow obstruction on pre-bronchodilator spirometry using this definition effectively excludes COPD, which is defined by post-bronchodilator FEV1/FVC < 0.70 (3). An FEV1/FVC ratio less than the lower limit of normal (36) was used to define airflow obstruction for a secondary analysis.
Covariates
Age, sex, race/ethnicity, educational attainment, cancer history, physician diagnoses of emphysema and asthma, intentional exercise per week, alcohol use, and tobacco use were self-reported at baseline. Never smoking was defined as a lifetime smoking history of less than 100 cigarettes, and current smoking as cigarette use within the past 30 days. Urinary cotinine was measured for a subset of 3929 participants; 78 participants (2%) who denied current smoking but had urinary cotinine levels greater than 100 nanograms per milliliter were reclassified as current smokers. Pack-years were calculated as (cigarettes per day/20) × years smoked.
Height, weight, systolic and diastolic blood pressure (BP), total cholesterol, high-density lipoprotein (HDL), creatinine, D-dimer, C-reactive protein (CRP), and fasting plasma glucose were measured using standard techniques (37). Medication use was assessed by validated medication inventory (38).
A phantom-adjusted coronary artery calcium Agatston score (39) was calculated from each cardiac CT and the mean of the two values was used, as previously described (26).
Statistical Analysis
Statistical tests were based upon multivariable-adjusted Cox proportional-hazards models and additive Cox models with penalized splines. The latter approach was used to test and account for any potential non-linearity in associations and to generate plots.
The study sample comprised participants with valid spirometry measures who did not have airflow obstruction. Survival time was calculated as age at death or, for non-deceased participants, age at last follow-up or the most recent NDI update, whichever was more recent, with left-truncation at age of spirometry.
The proportional-hazards assumption was confirmed via interaction terms with time (P>0.100). The number of emphysema-like voxels was first adjusted for total imaged lung voxels. Analyses were then sequentially adjusted for CT scanner type and tube current, site, baseline age, sex, race/ethnicity, weight, height, educational attainment, smoking status and pack-years, alcohol use, coronary artery calcium score, diabetes medication use, insulin use, fasting glucose, hypertension, antihypertensive medication use, systolic and diastolic BP, cholesterol medication use, total cholesterol, HDL, creatinine, history of cancer, and the FEV1. To account for potential confounding by subclinical interstitial abnormalities, models were adjusted for HAA. The full model was subsequently adjusted for potential mediators (exercise, CRP, and D-dimer). Multiple imputation was used to account for missing covariate data, which were infrequent (6% for pack-years, <1% for all other covariates). Effect estimates were calculated per one-half of the interquartile range for emphysematous voxels, since the distribution was skewed to the right.
All primary analyses were stratified by smoking status. For additional potential effect modifiers (age, race/ethnicity, body mass index [BMI], sex, scanner type, and site), multiplicative interaction terms and within-strata effect estimates were tested in fully-adjusted models. Sensitivity analyses were performed among participants without self-reported emphysema or asthma, and using alternative definitions of airflow obstruction.
The impact of CT emphysema measures on discrimination of incident all-cause mortality was tested in logistic models and assessed via improvements in the C-statistic.
All statistical analyses were performed in SAS version 9.2 (Cary, NC) or R (Vienna, Austria) (41).
Role of the Funding Source
MESA was funded by the National Heart, Lung and Blood Institute (NHLBI). The study was approved by NHLBI as well as the institutional review boards of all collaborating institutions. All participants gave written informed consent. Together with MESA investigators, the authors collected and analyzed the data, vouched for the data and analysis, and wrote and submitted this paper for publication. The NHLBI staff routinely monitored study performance and participated in the internal review of the manuscript before submission.
Results
The 2965 participants without airflow obstruction on valid spirometry had a mean age at CT scanning of 60 years, were 32.5% white, 25.6% African-American, 25.1% Hispanic and 16.8% Asian, and included 12.1% current-smokers, 37.0% former-smokers, and 50.9% never-smokers.
Participants with more emphysema-like voxels were predominantly male, more likely to be Caucasian, had higher educational attainment, and were more likely to have smoked cigarettes (Supplementary Tables 1 and 2).
Emphysema-like lung was only modestly correlated with the percent-predicted FEV1 (r=0.11) and the FEV1/FVC ratio (r=-0.08) among all participants with spirometry after controlling for total imaged lung volume, scanner characteristics, and site (P< 0.001 for both).
All-cause Mortality
There were 186 deaths among the 2965 participants over a median of 6.2 years after spirometry testing, corresponding to a mortality rate of 10.0 per 1000 person-years. Vital status was known for 100%.
All-cause Mortality among Participants without Airflow Obstruction
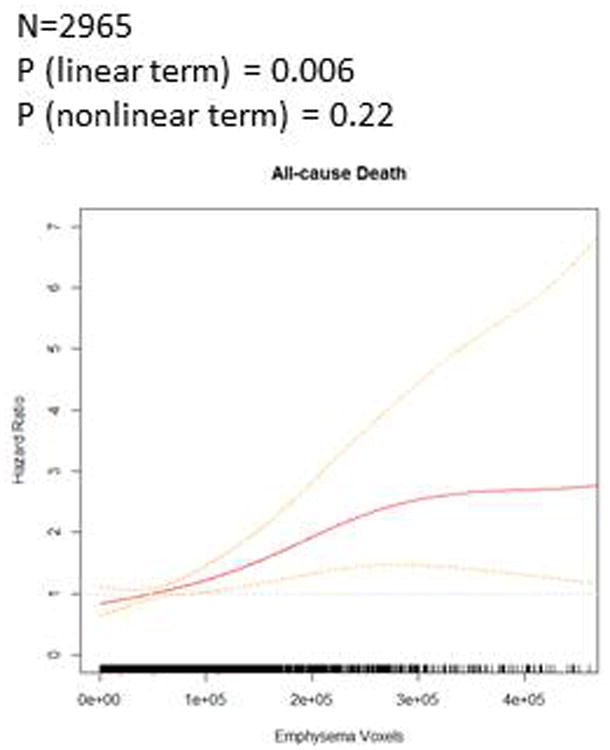
Emphysema-like lung was associated with all-cause mortality (Table 2). In the fully-adjusted linear model, an increase in emphysema-like voxels equivalent to one-half of the interquartile range was associated with a 14% greater mortality rate (95% confidence interval [CI] 4-24%, P=0.004). Additional adjustment for potential mediators only minimally attenuated the results (HR 1.13 per half-IQR of emphysematous voxels, 95% CI 1.04-1.24, P=0.006). Associations with mortality were strengthened after exclusion of participants below the 5th and above the 95th percentile for emphysematous voxels (HR 1.27 per half-IQR, 95% CI 1.09-1.48, P=0.002). The additive model showed little evidence for a non-linear relationship (P-value for linear term = 0.006, P-value for non-linearity = 0.22, Figure 1).
Table 2. Emphysema-like lung and all-cause mortality rates in persons without airflow obstruction over six years of follow-up, stratified by smoking status.
| Never smokers | Former smokers | Current smokers | Total | |
|---|---|---|---|---|
| Number of deaths | 88 | 69 | 29 | 186 |
| Total at risk | 1509 | 1097 | 359 | 2965 |
| Person-years | 9507 | 6860 | 2200 | 18567 |
| Mortality rate, per 1000 person-years | 9.26 | 10.06 | 13.18 | 10.02 |
| HR per ½ IQR of emphysema-like lung (95% CI) | ||||
| Volume-adjusted | 1.05 (0.91, 1.21) P = 0.49 |
1.09 (0.97, 1.21) P = 0.136 |
1.01 (0.77, 1.32) P = 0.94 |
1.04 (0.96, 1.13) P = 0.37 |
| + Scanner | 1.06 (0.90, 1.24) P = 0.52 |
1.13 (1.02, 1.25) P = 0.022 |
1.05 (0.77, 1.42) P = 0.76 |
1.07 (0.98, 1.17) P = 0.126 |
| + Demographics and body size | 1.04 (0.88, 1.24) P = 0.64 |
1.12 (1.02, 1.25) P = 0.024 |
1.07 (0.77, 1.48) P = 0.69 |
1.06 (0.97, 1.16) P = 0.178 |
| + Smoking history | 1.13 (1.02, 1.25) P = 0.023 |
1.10 (0.79, 1.53) P = 0.56 |
1.08 (0.99, 1.18) P = 0.068 |
|
| + Other risk factors | 1.06 (0.89, 1.26) P = 0.51 |
1.18 (1.06, 1.32) P = 0.004 |
1.21 (0.83, 1.73) P = 0.33 |
1.11 (1.01, 1.21) P = 0.023 |
| + FEV1 | 1.06 (0.89, 1.26) P = 0.49 |
1.20 (1.07, 1.35) P = 0.002 |
1.21 (0.82, 1.82) P = 0.32 |
1.12 (1.03, 1.23) P = 0.010 |
| + HAA | 1.09 (0.92, 1.29) P = 0.32 |
1.21 (1.07, 1.37) P = 0.002 |
1.32 (0.89, 1.99) P = 0.173 |
1.14 (1.04, 1.24) P = 0.004 |
Abbreviations: HR = hazard ratio. IQR = interquartile range. CI = confidence interval. FEV1 = forced expiratory volume in one second. FVC = forced vital capacity. CT = computed tomography. HAA = high attenuation areas. Emphysema-like lung is parameterized as emphysematous voxels. One interquartile range is equivalent to 91809 voxels. Airflow obstruction is defined as an FEV1/FVC ratio < 0.70 on spirometry. With the exception of the FEV1, covariates were measured at baseline. Demographic covariates are age, gender, race/ethnicity, and education. Body size covariates are height and weight. Other risk factors are coronary artery calcium, cardiac risk factors, creatinine, cancer history, and alcohol use.
Figure 1. Regions of emphysema-like lung and all-cause mortality among all participants without airflow obstruction.
All-cause Mortality by Smoking Status
Relationships between emphysema-like lung and all-cause mortality were of greatest magnitude among the relatively small subset of current smokers, statistically significant among former smokers, and positive but non-significant among never-smokers (Table 2). Evaluation of multiplicative interaction terms did not provide evidence for significant effect modification by smoking status (P=0.95). While additive models strongly supported a linear association between emphysematous voxels and all-cause mortality among smokers, non-linearity was a consideration among never-smokers (P-value for non-linearity 0.130).
Sensitivity Analyses and Mortality Prediction
Effect estimates were broadly consistent across strata of age, race/ethnicity, BMI, CT scanner type, and site, and the corresponding multiplicative interaction terms were not statistically significant (Table 3), although findings were attenuated in overweight and obese individuals.
Table 3. Emphysema-like lungand all-cause mortality rates by age group, race/ethnicity, BMI, sex, CT scanner type, and site, in persons without airflow obstruction over six years of follow-up.
| Strata | Deaths | At risk | Person years | Mortality rate | Emphysema-like lung | |
|---|---|---|---|---|---|---|
| Fully-adjusted HR per ½ IQR increase (95% CI) | P-value for interaction | |||||
| Age Group | ||||||
| First tertile (44-56) | 17 | 935 | 5927 | 2.87 | 1.11 (0.89, 1.37) | 0.183 |
| Second tertile (57-67) | 44 | 992 | 6215 | 7.08 | 1.25 (1.10, 1.41) | |
| Third tertile (68-84) | 125 | 1038 | 6424 | 19.46 | 1.10 (0.98, 1.22) | |
| Race/ethnicity | ||||||
| White | 52 | 963 | 5890 | 8.83 | 1.16 (1.03, 1.31) | 0.28 |
| Black | 63 | 759 | 4711 | 13.37 | 1.13 (1.01, 1.26) | |
| Hispanic | 45 | 745 | 4721 | 9.53 | 0.97 (0.76, 1.24) | |
| Chinese | 26 | 498 | 3244 | 8.01 | 1.33 (1.00, 1.73) | |
| BMI Group | ||||||
| <25 (Normal or Underweight) | 51 | 832 | 5224 | 9.76 | 1.24 (1.08, 1.41) | 0.27 |
| 25-30 (Overweight) | 63 | 1184 | 7441 | 8.47 | 1.11 (0.98.1.26) | |
| 30+ (Obese) | 72 | 941 | 5852 | 12.30 | 1.09 (0.96, 1.24) | |
| Sex | ||||||
| Female | 86 | 1609 | 10102 | 8.51 | 0.95 (0.75, 1.21) | 0.108 |
| Male | 100 | 1356 | 8465 | 11.81 | 1.16 (1.06, 1.26) | |
| CT scanner type | ||||||
| Electron Beam | 111 | 1782 | 11235 | 9.88 | 1.18 (1.06, 1.31) | 0.26 |
| Multi-detector | 75 | 1183 | 7332 | 10.23 | 1.09 (0.96, 1.23) | |
| Site | ||||||
| Forsyth County, North Carolina | 23 | 408 | 2584 | 8.90 | 1.10 (1.00, 1.28) | 0.39 |
| Northern Manhattan & Bronx, New York | 41 | 549 | 3489 | 11.75 | 1.24 (1.00, 1.45) | |
| Baltimore, Maryland | 36 | 344 | 1999 | 18.01 | 1.17 (1.00, 1.41) | |
| St Paul, Minnesota | 16 | 431 | 2749 | 5.82 | 0.76 (0.40, 1.30) | |
| Chicago, Illinois | 27 | 556 | 3504 | 7.71 | 1.09 (1.00, 1.28) | |
| Los Angeles, California | 43 | 677 | 4267 | 10.08 | 1.21 (1.00, 1.43) | |
Abbreviations: BMI = body mass index. HR = hazard ratio. IQR = Interquartile range. CI = Confidence Interval. CT=computed tomography. Mortality rate is reported per 1,000 person-years of observation. Numbers may not sum to totals due to rounding.
Emphysema-like lung is parameterized as emphysematous voxels. BMI values are expressed in kilograms/meters-squared. One interquartile range is equivalent to 91809 voxels. Airflow obstruction is defined as an FEV1/FVC ratio < 0.70 on spirometry. With the exception of the FEV1, covariates were measured at baseline.
Models are adjusted for a multiplicative interaction term (emphysematous voxels*stratum), total imaged lung volume, scanner type and milliamperes, site, age, gender, race/ethnicity, education, height, weight, coronary artery calcium, cardiac risk factors, creatinine, cancer history, alcohol use, forced expiratory volume in one second, and high attenuation areas.
There was also no evidence for interaction by gender (Table 3), although effect estimates were significant among men but close to the null among women, who comprised 65% of never-smokers (Supplementary Table 4), and 36% of whom were obese. Consistent results for men and women were obtained after excluding overweight and obese participants (for women, HR 1.41 per half-IQR, 95% CI 0.64-3.15; for men, HR 1.33 per half IQR, 95% CI 0.98-1.82).
Excluding participants with self-reported emphysema (N=13) and self-reported asthma (N=217) did not alter effect estimates (HR 1.13 per half-IQR, 95% CI 1.03-1.24, P=0.011).
Findings were also similar among participants without airflow obstruction defined by the lower limit of normal for the FEV1/FVC ratio (N=3378, HR 1.09 per half-IQR, 95% CI 1.01-1.18, P=0.035).In this sample, there was no evidence for effect modification by smoking status (HR per half-IQR 1.08 among both never-smokers and former smokers, p-value for interaction term = 0.35) or sex (HR per half-IQR 1.08 for women and 1.06 for men, p-value for interaction term = 0.24).
Emphysema-like lung non-significantly improved the prediction of all-cause mortality compared to a full model including demographic factors, body size, smoking, and other risk factors (c=0.791 versus c=0.786, P=0.30), as did the FEV1 (c=0.791, P=0.29). Addition of both emphysematous voxels and the FEV1 to the full model provided borderline incremental benefit (c=0.795, P=0.087).
Discussion
Emphysema-like lung assessed quantitatively on CT was associated with increased all-cause mortality among persons without airflow obstruction or COPD on spirometry in a large, population-based, multiethnic cohort. This association was independent of a large number of potential confounders including the FEV1, and persisted among participants without physician-diagnosed emphysema or asthma. Findings were of greater magnitude among former and current smokers compared to those among never-smokers. These findings suggest that “subclinical” emphysema among patients without spirometrically-defined COPD is clinically relevant.
The prognostic value of emphysema on CT is of general interest due to the relatively high population prevalence of emphysema (42), the paucity of research on the clinical significance of emphysema (as opposed to spirometrically-defined COPD), and the rapid growth in use of chest CT for lung cancer screening and assessment of coronary artery calcium (10, 12, 43). Yet there are no prior studies on the prognostic significance of emphysema on CT in a population-based sample, among patients without COPD, or among non-smokers, of which we are aware. Prior small studies of patients with alpha-1 antitrypsin deficiency (44), very severe COPD (24), and COPD more generally in Japan (23) and Norway (16) showed significant associations between emphysema-like lung on CT and all-cause mortality, where as an Italian lung cancer screening trial did not (45). None of these prior studies, however, were powered to examine emphysema-like lung on CT in participants without spirometrically-defined COPD.
Increased death rates from COPD and lung cancer have been associated with radiologist-scored emphysema on CT from a lung cancer screening cohort of heavy smokers (14), although covariate adjustment in that study was limited, pre-existing diagnoses were not considered, and spirometry was not performed. Furthermore, visual scoring can have low rates of agreement between radiologists (46), can be insensitive for panlobular emphysema (47), and is relatively time-consuming compared to quantitative approaches.
Mechanisms by which emphysema may contribute to premature death due to respiratory causes in patients both with and without COPD include direct physiologic effects of losing lung parenchyma and vasculature. This loss reduces the area available for gas exchange, which may be associated with hypoxemia, poor exercise capacity, and reduced functional status (48). Loss of alveolar walls also contributes to untethering of airways, loss of radial traction forces, and reduction in airway caliber (49), which may contribute to airflow obstruction (50, 51) that may be intermittent, producing functional limitation during exacerbations but not necessarily meeting the standard clinical definition of COPD (3). Indeed, emphysema-like lung on CT has recently been shown to be associated with respiratory exacerbations and hospitalizations among smokers independent of the FEV1 (52). Other mechanisms linking emphysema to all-cause mortality include increased susceptibility to pulmonary infections (53), impaired cardiac function (54) resulting in reduced cardiopulmonary reserve, and systemic inflammation (55).
Unlike prior studies, the present study, drawn from the general population, included a large number of participants who had never smoked cigarettes. Prior literature has indicated that emphysema – particularly panlobular emphysema – is only modestly associated with smoking, as shown on autopsy (13), on CT scan in COPD patients (17, 56), and in the current cohort (57). Nonetheless, compared to strong and consistent associations observed among smokers, findings among never-smokers were modest and did not attain statistical significance, although neither did the statistical test for effect modification by smoking history. Associations between emphysema-like lung and mortality were consistent across smoking strata among men, where as they were more variable among women. The latter results were likely due to chance given small numbers of events among women. Other considerations include measurement error due to reduced cardiac CT scan coverage among women (see CT Appendix) or underestimation of emphysema-like lung attributable to breast artifact, consistent with the finding of congruent effects for men and women after excluding overweight participants. Alternatively, the findings may reflect differences in emphysema prevalence and biology according to smoking or sex (13, 58,59).
It is unlikely that regions of emphysema-like lung are simply markers of increased cumulative tobacco exposure. In addition to the aforementioned – albeit tentative – findings in never-smokers, and the weak association of pack-years with emphysema on CT, analyses were adjusted for cotinine-confirmed smoking history, with little attenuation of results. Also, current smoking artifactually diminishes detection of emphysema-like voxels (60), which means that misspecification of smoking would bias results conservatively.
Spirometry was only available for a subset of participants, was acquired after baseline, and did not include post-bronchodilator measures. Nonetheless, we demonstrated statistically significant associations among participants without airflow obstruction – analyses that were conservative from a clinical perspective, since almost no patients with COPD have normal pre-bronchodilator spirometry, where as one third of patients with airflow obstruction on pre-bronchodilator spirometry do not meet clinical, post-bronchodilator criteria for COPD (61).
MESA cardiac CT scans did not image the lung apices, the main anatomical location of tobacco-associated centrilobular emphysema. Lack of imaging the apices is therefore likely to underestimate the quantity of emphysema-like lung, particularly among smokers, which may have led to an underestimation of risk, although the validation study sample (29) was not large enough to determine definitively the direction of this potential bias. Variability in cardiac CT scan coverage was slightly greater among racial minorities and obese participants (see CT Appendix), which may explain the somewhat larger effect estimates observed among Whites and normal-weight participants. Emphysema like lung on cardiac scans nevertheless showed high correlation with measures from full lung scans in MESA (29). As cardiac CT scans are performed for cardiovascular risk stratification, results from cardiac scans have clinical value in and of themselves.
We did not examine specific emphysema subtypes, which have shown associations with symptomatology and physiology among smokers (17). Automated methods for CT emphysema subtype determination are still in development (62, 63) and are not available for cardiac CT. Nonetheless, findings in never-smokers, the excellent visualization of the lower lungs, and results from a genome-wide association study of percent emphysema in this cohort demonstrating associations for variants in genes highly relevant to alpha-1 antitryps in metabolism (64), suggest that emphysema-like lung on cardiac CT scans may preferentially detect panlobular emphysema.
We performed statistical tests based on linear terms for percent emphysema since absolute values are currently dependent on scanner and protocol factors, making categorized results from these scans less generalizable. However, scanner and technology standardization are forthcoming (65), and results were not driven by outliers.
Neither emphysema-like voxels nor the FEV1 significantly improved the prediction of all-cause mortality. This is unsurprising given that age accounts for 94% of the variance in this important but non-specific endpoint.
We did not evaluate emphysema-like lung in comparison to the BODE index (66) as the index was specifically designed for patients with COPD, who were excluded. However, we adjusted for its major components: BMI (parameterized as height and weight), obstruction (the FEV1), and exercise tolerance (self-reported intentional exercise; 6-minute walk distance was not available). We did not adjust for its fourth component, dyspnea, as dyspnea is a likely consequence of emphysema.
In conclusion, greater emphysema-like lung on CT was independently associated with higher all-cause mortality among participants without airflow obstruction in a multiethnic population-based sample. Results persisted among participants without clinical disease. Associations were more consistent among smokers, however harmful associations among never-smokers could not be excluded. These findings suggest that emphysema confers excess risk independent of spirometrically-defined COPD. Clinical appreciation of the prognostic implications of emphysema on CT and investigation of therapies specifically targeting emphysema, of which there are none currently, are warranted.
Supplementary Material
Supplementary Table 1. Partial correlations between emphysema-like lung and covariates after accounting for total imaged lung voxels, CT scanner type, and milliamperes.
Supplementary Table 2. Baseline demographic characteristics and risk factors among 2,965 participants without airflow obstruction according to quartiles of emphysema-like lung.
Supplementary Table 3. Emphysema-like lung and all-cause mortality in persons without airflow obstruction, sequentially adjusted models, stratified by smoking status.
Supplementary Table 4. Emphysema-like lung and all-cause mortality rates in persons without airflow obstruction over six years of follow-up, stratified by smoking status and sex.
Figure 2. Regions of emphysema-like lung and all-cause mortality, stratified by smoking status.
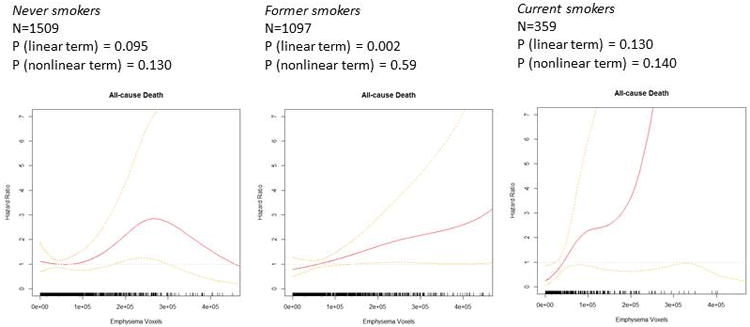
Table 1. Baseline demographic characteristics and risk factors among 2,965 participants without airflow obstruction.
| Never smokers | Former smokers | Current smokers | Total | |
|---|---|---|---|---|
| N | 1509 (50.9) | 1097 (37.0) | 359 (12.1) | 2695 (100.0) |
| Age, mean (SD), y | 60 (10) | 61 (10) | 56 (8) | 60 (10) |
| Male sex, n (%) | 530 (35.1) | 647 (59.0) | 179 (49.9) | 1356 (45.7) |
| Racial or ethnic group, n (%)* | ||||
| White | 437 (29.0) | 428 (39.0) | 98 (27.3) | 963 (32.5) |
| African-American | 327 (21.7) | 303 (27.6) | 129 (35.9) | 759 (25.6) |
| Hispanic | 377 (25.0) | 261 (23.8) | 107 (29.8) | 745 (25.1) |
| Asian | 368 (24.4) | 105 (9.6) | 25 (7.0) | 498 (16.8) |
| Educational attainment, n (%) | ||||
| Less than 9th grade | 183 (12.1) | 90 (8.2) | 34 (9.5) | 307 (10.4) |
| 9th-12th grade | 359 (23.8) | 270 (24.6) | 113 (31.5) | 742 (25.0) |
| Technical/associate degree or some college | 390 (25.8) | 318 (29.0) | 129 (35.9) | 837 (28.2) |
| Bachelor's degree | 287 (19.0) | 196 (17.9) | 40 (11.1) | 523 (17.6) |
| Graduate/professional school | 290 (19.2) | 223 (20.3) | 43 (12.0) | 556 (18.8) |
| Pack-years, median (IQR)† | - | 13 (4, 28) | 22 (11, 39) | 15 (5, 32) |
| Height, mean (SD), cm | 164 (10) | 168 (9) | 168 (10) | 166 (10) |
| Weight, mean (SD), lbs | 165 (37) | 181 (37) | 182 (41) | 173 (38) |
| Intentional exercise, median (IQR), MET-min/week | 770 (150, 1883) | 930 (210, 2205) | 630 (0, 1890) | 810 (158, 2040) |
| Alcohol use, n (%) | ||||
| Never drinker | 548 (36.3) | 97 (8.8) | 41 (11.4) | 686 (23.1) |
| Former drinker | 268 (17.8) | 322 (29.4) | 79 (22.0) | 669 (22.6) |
| Current drinker | 693 (45.9) | 678 (61.8) | 239 (66.6) | 1610 (54.3) |
| Diagnosis of emphysema, n (%) | 2 (0.1) | 8 (0.7) | 3 (0.8) | 13 (0.4) |
| Diagnosis of asthma, n (%) | 104 (6.9) | 83 (7.6) | 31 (8.6) | 218 (7.4) |
| History of cancer, n (%) | 70 (4.6) | 86 (7.8) | 15 (4.2) | 171 (5.8) |
| Diabetes | ||||
| Diabetes medication use, n (%) | 118 (7.8) | 108 (9.9) | 36 (10.0) | 262 (8.8) |
| Insulin use, n (%) | 18 (1.2) | 17 (1.6) | 4 (1.1) | 39 (1.3) |
| Fasting blood glucose, median (IQR), mg/dL | 89 (83, 98) | 90 (83, 99) | 90 (82, 100) | 89 (83, 98) |
| Hypertension | ||||
| Hypertension status, n (%)‡ | 614 (40.7) | 471 (42.9) | 133 (37.1) | 1218 (41.1) |
| Hypertension medication use, n (%) | 525 (34.8) | 395 (36.0) | 109 (30.4) | 1029 (34.7) |
| Systolic blood pressure, mean (SD), mmHg | 124 (19) | 125 (20) | 123 (21) | 124 (20) |
| Diastolic blood pressure, mean (SD), mmHg | 71 (10) | 73 (10) | 72 (10) | 72 (10) |
| Cholesterol | ||||
| Cholesterol medication use, n (%) | 225 (14.9) | 183 (16.7) | 36 (10.0) | 444 (15.0) |
| Total cholesterol, mean (SD), mg/dL | 197 (34) | 193 (34) | 195 (36) | 195 (34) |
| High-density lipoprotein cholesterol, mean (SD), mg/dL | 52 (15) | 50 (14) | 48 (15) | 51 (14) |
| Serum creatinine, mean (SD), mg/dL | 0.91 (0.21) | 0.97 (0.24) | 0.94 (0.25) | 0.94 (0.23) |
| C-reactive protein, median (IQR), mg/L | 1.74 (0.77, 3.92) | 1.78 (0.79, 4.07) | 2.37 (1.02, 5.35) | 1.8 (0.8, 4.1) |
| D-dimer, median (IQR), ug/mL | 0.20 (0.11, 0.35) | 0.20 (0.13, 0.35) | 0.18 (0.13, 0.30) | 0.2 (0.1, 0.4) |
| Spirometry | ||||
| Percent predicted FEV1, mean (SD) | 98 (16) | 98 (16) | 92 (16) | 97 (16) |
| FEV1/FVC ratio, mean (SD) | 0.79 (0.05) | 0.78 (0.05) | 0.78 (0.05) | 0.78 (0.05) |
| Site | ||||
| Forsyth County, North Carolina, n (%) | 178 (11.8) | 172 (15.7) | 58 (16.2) | 408 (13.8) |
| Northern Manhattan & Bronx, New York, n (%) | 277 (18.4) | 194 (17.7) | 78 (21.7) | 549 (18.5) |
| Baltimore, Maryland, n (%) | 158 (10.5) | 143 (13.0) | 43 (12.0) | 344 (11.6) |
| St Paul, Minnesota, n (%) | 179 (11.9) | 182 (16.7) | 70 (19.5) | 431 (14.5) |
| Chicago, Illinois, n (%) | 297 (19.7) | 211 (19.2) | 48 (13.4) | 556 (18.8) |
| Los Angeles, California, n (%) | 420 (27.8) | 195 (17.8) | 62 (17.3) | 677 (22.8) |
| CT scanner type, n (%) | ||||
| Electron beam CT | 994 (65.9) | 600 (54.7) | 188 (52.4) | 1782 (60.1) |
| Multidetector CT | 515 (34.1) | 497 (45.3) | 171 (47.6) | 1183 (39.9) |
| CT measures | ||||
| Total emphysema, median (IQR), % | 2.35 (0.98, 4.32) | 3.25 (1.37, 5.78) | 1.89 (0.78, 3.78) | 2.54 (1.08, 4.83) |
| High attenuation areas, median (IQR), % | 4.41 (3.68, 5.74) | 4.26 (3.62, 5.39) | 4.60 (3.94, 5.91) | 4.38 (3.67, 5.66) |
| Coronary artery calcium, median (IQR), Agatston score | 0 (0, 22) | 1 (0, 93) | 0 (0, 29) | 0 (0, 41) |
Abbreviations: N=number. SD = Standard Deviation. y = Years. IQR = inter-quartile range. mg = milligrams. dL = deciliters. mmHg = millimeters of mercury. L = liters. ug = microgram. FEV1 = forced expiratory volume in one second. FVC = forced vital capacity. CT = computed tomography. Total emphysema percent is calculated as the number of voxels with attenuation less than -950 Hounsfield Units (HU) divided by the total imaged lung voxels. High attenuation areas percent is calculated as the number of voxels with attenuation between -600 and -250 HU divided by the total imaged lung voxels.
Airflow obstruction is defined as an FEV1/FVC ratio < 0.70 on spirometry, which was performed on 3828 participants in 2004-2006. Besides spirometry measures, all other variables were measured at the baseline exam in 2000-2002.
Normally-distributed variables are presented as mean (standard deviation). Non-normally distributed variables are presented as median (interquartile range). With the exception of the first row (N), column percentages are presented, and may not total 100 because of rounding or missing data.
Racial or ethnic group was self-assessed.
Among ever-smokers.
Hypertension was defined as antihypertensive medication use, systolic blood pressure ≥140mmHg, or diastolic blood pressure ≥90mmHg.
Acknowledgments
Funding Support: MESA is supported by the National Heart, Lung, and Blood Institute (NHLBI) and was designed and conducted by the MESA investigators in collaboration with NHLBI staff.
Support for MESA is provided by contracts N01-HC-95159 through N01-HC-95169 and UL1-RR-024156 and UL1-RR-025005. The MESA Lung Study is funded by R01-HL077612, RC1-100543 and R01-93081 from the NHLBI.
NHLBI staff routinely monitored study performance and participated in the internal review of this manuscript prior to submission.
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Author Contributions: Dr Oelsner was primarily responsible for data analysis and drafting of the manuscript, and vouches for the integrity of the data and analyses presented.
All authors were involved in manuscript preparation, and all have approved the final manuscript.
Conflicts of interest: The authors report no conflicts of interest with the following exceptions: Dr. Hoffman is a founder of and owns shares in a company (Vida Diagnostics, Inc.) that has commercialized a version of the CT reading software used in this paper.
References
- 1.Minino AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59(10):1–126. [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 4.Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7:CD009285. doi: 10.1002/14651858.CD009285.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD009157. doi: 10.1002/14651858.CD009157.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7:CD002991. doi: 10.1002/14651858.CD002991.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karner C, Cates CJ. Long-acting beta(2)-agonist in addition to tiotropium versus either tiotropium or long-acting beta(2)-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;4:CD008989. doi: 10.1002/14651858.CD008989.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–51. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokohori N, Aoshiba K, Nagai A. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest. 2004;125(2):626–32. doi: 10.1378/chest.125.2.626. [DOI] [PubMed] [Google Scholar]
- 10.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(7):529–34. doi: 10.7326/0003-4819-148-7-200804010-00212. [DOI] [PubMed] [Google Scholar]
- 12.Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2013;159(6):411–20. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 13.Anderson AE, Jr, Hernandez JA, Eckert P, Foraker AG. Emphysema in Lung Macrosections Correlated with Smoking Habits. Science. 1964;144(3621):1025–6. doi: 10.1126/science.144.3621.1025. [DOI] [PubMed] [Google Scholar]
- 14.Zulueta JJ, Wisnivesky JP, Henschke CI, Yip R, Farooqi AO, McCauley DI, et al. Emphysema scores predict death from COPD and lung cancer. Chest. 2012;141(5):1216–23. doi: 10.1378/chest.11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burt JR, Iribarren C, Fair JM, Norton LC, Mahbouba M, Rubin GD, et al. Incidental findings on cardiac multidetector row computed tomography among healthy older adults: prevalence and clinical correlates. Arch Intern Med. 2008;168(7):756–61. doi: 10.1001/archinte.168.7.756. [DOI] [PubMed] [Google Scholar]
- 16.Johannessen A, Skorge TD, Bottai M, Grydeland TB, Nilsen RM, Coxson H, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187(6):602–8. doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- 17.Smith BM, Austin JHM, Newell JD, D'Souza BM, R A, Hoffman EA, et al. Pulmonary Emphysema Subtypes on Computed Tomography in Smokers. Am J Med. doi: 10.1016/j.amjmed.2013.09.020. epublished ahead of print October 9 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsushima K, Sone S, Fujimoto K, Kubo K, Morita S, Takegami M, et al. Identification of occult parechymal disease such as emphysema or airway disease using screening computed tomography. COPD. 2010;7(2):117–25. doi: 10.3109/15412551003631717. [DOI] [PubMed] [Google Scholar]
- 19.Marsh SE, Travers J, Weatherall M, Williams MV, Aldington S, Shirtcliffe PM, et al. Proportional classifications of COPD phenotypes. Thorax. 2008;63(9):761–7. doi: 10.1136/thx.2007.089193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannino DM, Diaz-Guzman E, Buist S. Pre- and post-bronchodilator lung function as predictors of mortality in the Lung Health Study. Respir Res. 2011;12:136. doi: 10.1186/1465-9921-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mannino DM, Diaz-Guzman E. Interpreting lung function data using 80% predicted and fixed thresholds identifies patients at increased risk of mortality. Chest. 2012;141(1):73–80. doi: 10.1378/chest.11-0797. [DOI] [PubMed] [Google Scholar]
- 22.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58(5):388–93. doi: 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haruna A, Muro S, Nakano Y, Ohara T, Hoshino Y, Ogawa E, et al. CT scan findings of emphysema predict mortality in COPD. Chest. 2010;138(3):635–40. doi: 10.1378/chest.09-2836. [DOI] [PubMed] [Google Scholar]
- 24.Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173(12):1326–34. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 26.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 27.International Classification of Diseases. 10th. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 28.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, et al. Reproducibility and validity of lung density measures from cardiac CT Scans--The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16(6):689–99. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo J, Reinhardt J, Kitaoka H, Zhang L, M G, Hoffman EA. Integrated system for CT-based assessment of parenchymal lung disease. Proceedings of IEEE International Symposium on Biomedical Imaging; New York. Institute of Electrical and Electronics Engineers; 2002. pp. 871–4. [Google Scholar]
- 31.Hoffman EA, Reinhardt JM, Sonka M, Simon BA, Guo J, Saba O, et al. Characterization of the interstitial lung diseases via density-based and texture-based analysis of computed tomography images of lung structure and function. Acad Radiol. 2003;10(10):1104–18. doi: 10.1016/s1076-6332(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 32.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152(2):653–7. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 33.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180(5):407–14. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 35.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest. 2010;137(1):138–45. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 37.Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013;145(1):6–23. doi: 10.1016/j.jtcvs.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Smith NL, Psaty BM, Heckbert SR, Tracy RP, Cornell ES. The reliability of medication inventory methods compared to serum levels of cardiovascular drugs in the elderly. J Clin Epidemiol. 1999;52(2):143–6. doi: 10.1016/s0895-4356(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 39.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 40.Kim HJ, Siegmund D. The likelihood ratio test for a change-point in simple linear regression. Biometrika. 1989;76:409–23. [Google Scholar]
- 41.R, Core, Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 42.Auerbach O, Hammond EC, Garfinkel L, Benante C. Relation of smoking and age to emphysema. Whole-lung section study. N Engl J Med. 1972;286(16):853–7. doi: 10.1056/NEJM197204202861601. [DOI] [PubMed] [Google Scholar]
- 43.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Dawkins PA, Dowson LJ, Guest PJ, Stockley RA. Predictors of mortality in alpha1-antitrypsin deficiency. Thorax. 2003;58(12):1020–6. doi: 10.1136/thorax.58.12.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sverzellati N, Cademartiri F, Bravi F, Martini C, Gira FA, Maffei E, et al. Relationship and prognostic value of modified coronary artery calcium score, FEV1, and emphysema in lung cancer screening population: the MILD trial. Radiology. 2012;262(2):460–7. doi: 10.1148/radiol.11110364. [DOI] [PubMed] [Google Scholar]
- 46.Mascalchi M, Diciotti S, Sverzellati N, Camiciottoli G, Ciccotosto C, Falaschi F, et al. Low agreement of visual rating for detailed quantification of pulmonary emphysema in whole-lung CT. Acta Radiol. 2012;53(1):53–60. doi: 10.1258/ar.2011.110419. [DOI] [PubMed] [Google Scholar]
- 47.Copley SJ, Wells AU, Muller NL, Rubens MB, Hollings NP, Cleverley JR, et al. Thin-section CT in obstructive pulmonary disease: discriminatory value. Radiology. 2002;223(3):812–9. doi: 10.1148/radiol.2233010760. [DOI] [PubMed] [Google Scholar]
- 48.Estepar RS, Kinney GL, Black-Shinn JL, Bowler RP, Kindlmann GL, Ross JC, et al. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188(2):231–9. doi: 10.1164/rccm.201301-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamid Q, Shannon J, Martin J. Physiologic Basis of Respiratory Disease. Hamilton, Ontario: BC Decker; 2005. [Google Scholar]
- 50.Saetta M, Finkelstein R, Cosio MG. Morphological and cellular basis for airflow limitation in smokers. Eur Respir J. 1994;7(8):1505–15. doi: 10.1183/09031936.94.07081505. [DOI] [PubMed] [Google Scholar]
- 51.Martin C, Frija J, Burgel PR. Dysfunctional lung anatomy and small airways degeneration in COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:7–13. doi: 10.2147/COPD.S28290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McAllister DA, Ahmed FS, Austin JH, Henschke CI, Keller BM, Lemeshow A, et al. Emphysema predicts hospitalisation and incident airflow obstruction among older smokers: a prospective cohort study. PLoS One. 2014;9(4):e93221. doi: 10.1371/journal.pone.0093221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beasley V, Joshi PV, Singanayagam A, Molyneaux PL, Johnston SL, Mallia P. Lung microbiology and exacerbations in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:555–69. doi: 10.2147/COPD.S28286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217–27. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(23):2445–54. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 56.D'Anna SE, Asnaghi R, Caramori G, Appendini L, Rizzo M, Cavallaro C, et al. High-resolution computed tomography quantitation of emphysema is correlated with selected lung function values in stable COPD. Respiration. 2012;83(5):383–90. doi: 10.1159/000329871. [DOI] [PubMed] [Google Scholar]
- 57.Powell R, Davidson D, Divers J, Manichaikul A, Carr JJ, Detrano R, et al. Genetic ancestry and the relationship of cigarette smoking to lung function and per cent emphysema in four race/ethnic groups: a cross-sectional study. Thorax. 2013 doi: 10.1136/thoraxjnl-2012-202116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dransfield MT, Washko GR, Foreman MG, Estepar RS, Reilly J, Bailey WC. Gender differences in the severity of CT emphysema in COPD. Chest. 2007;132(2):464–70. doi: 10.1378/chest.07-0863. [DOI] [PubMed] [Google Scholar]
- 59.Camp PG, Coxson HO, Levy RD, Pillai SG, Anderson W, Vestbo J, et al. Sex differences in emphysema and airway disease in smokers. Chest. 2009;136(6):1480–8. doi: 10.1378/chest.09-0676. [DOI] [PubMed] [Google Scholar]
- 60.Ashraf H, Lo P, Shaker SB, de Bruijne M, Dirksen A, Tonnesen P, et al. Short-term effect of changes in smoking behaviour on emphysema quantification by CT. Thorax. 2011;66(1):55–60. doi: 10.1136/thx.2009.132688. [DOI] [PubMed] [Google Scholar]
- 61.Tilert T, Dillon C, Paulose-Ram R, Hnizdo E, Doney B. Estimating the U.S. prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry: the National Health and Nutrition Examination Survey (NHANES) 2007--2010. Respir Res. 2013;14(1):103. doi: 10.1186/1465-9921-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dirksen A, MacNee W. The search for distinct and clinically useful phenotypes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188(9):1045–6. doi: 10.1164/rccm.201309-1649ED. [DOI] [PubMed] [Google Scholar]
- 63.Castaldi PJ, San Jose Estepar R, Mendoza CS, Hersh CP, Laird N, Crapo JD, et al. Distinct quantitative computed tomography emphysema patterns are associated with physiology and function in smokers. Am J Respir Crit Care Med. 2013;188(9):1083–90. doi: 10.1164/rccm.201305-0873OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manichaikul A, Hoffman EA, Smolonska J, Gao W, Cho MH, Baumhauer H, et al. Genome-wide study of percent emphysema on computed tomography in the general population. The Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource Study. Am J Respir Crit Care Med. 2014;189(4):408–18. doi: 10.1164/rccm.201306-1061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Newell JD, Jr, Sieren J, Hoffman EA. Development of quantitative computed tomography lung protocols. J Thorac Imaging. 2013;28(5):266–71. doi: 10.1097/RTI.0b013e31829f6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 67.Jensen HH, Godtfredsen NS, Lange P, Vestbo J. Potential misclassification of causes of death from COPD. Eur Respir J. 2006;28(4):781–5. doi: 10.1183/09031936.06.00152205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Partial correlations between emphysema-like lung and covariates after accounting for total imaged lung voxels, CT scanner type, and milliamperes.
Supplementary Table 2. Baseline demographic characteristics and risk factors among 2,965 participants without airflow obstruction according to quartiles of emphysema-like lung.
Supplementary Table 3. Emphysema-like lung and all-cause mortality in persons without airflow obstruction, sequentially adjusted models, stratified by smoking status.
Supplementary Table 4. Emphysema-like lung and all-cause mortality rates in persons without airflow obstruction over six years of follow-up, stratified by smoking status and sex.


