Abstract
We performed two controlled experiments to determine the amount of mass-dependent and mass-independent fractionation (MDF and MIF) of methylmercury (MeHg) during trophic transfer into fish. In Experiment 1, juvenile yellow perch (Perca flavescens) were raised in captivity on commercial food pellets and then their diet was either maintained on un-amended food pellets (0.1 µg/g MeHg), or was switched to food pellets with 1.0 µg/g or 4.0 µg/g of added MeHg, for a period of 2 months. The difference in δ202Hg (MDF) and Δ199Hg (MIF) between fish tissues and food pellets with added MeHg were within the analytical uncertainty (δ202Hg; 0.07 ‰, Δ199Hg; 0.06 ‰) indicating no isotope fractionation. In Experiment 2, lake trout (Salvelinus namaycush) were raised in captivity on food pellets, and then shifted to a diet of bloater (Coregonus hoyi) for 6 months. The δ202Hg and Δ199Hg of the lake trout equaled the isotopic composition of the bloater after 6 months, reflecting re-equilibration of the Hg isotopic composition of the fish to new food sources and a lack of isotope fractionation during trophic transfer. We suggest that the stable Hg isotope ratios in fish can be used to trace environmental sources of Hg in aquatic ecosystems.
Keywords: methylmercury, isotopic fractionation, trophic transfer, stable Hg isotope, fish
INTRODUCTION
Mercury (Hg) is a globally distributed toxic metal that bioaccumulates in aquatic organisms. Aquatic ecosystems provide favorable methylation conditions and this can lead to elevated levels of methylmercury (MeHg) in fish through biomagnification1. This ultimately poses serious health threats to humans and wildlife that depend on fish for their diet2. There has been a growing scientific effort to develop various means of identifying the environmental sources and exposure pathways of Hg in aquatic ecosystems. For instance, previous studies have indirectly identified Hg sources in aquatic food webs by coupling Hg concentrations in fish with potential sources3. Other studies have examined various ecological factors that promote bioaccumulation of Hg4. Ecological tracers such as stable carbon and nitrogen isotopes have been used in conjunction with Hg concentration measurements to allow comparisons of Hg concentrations in aquatic organisms with their feeding ecology5. The potential application of small fish as biosentinels of Hg contamination has been somewhat limited by the lack of ability to capture the complex processes involved in the bioaccumulation of Hg. Techniques that can illuminate Hg sources and exposure pathways and offer a more direct link between Hg sources and potential receptors are clearly needed.
Application of the variation in natural abundance of stable Hg isotopes has expanded rapidly in recent years6, with studies that now include atmospheric Hg cycling7, point source identification8, human exposure9, and determination of Hg sources to aquatic ecosystems10–13. The distinct Hg isotope ratios produced by fractionation and mixing through environmental processes provide insight into Hg sources and transformation pathways. Kinetic Hg isotope fractionation is a process where the different stable isotopes of Hg react at different rates in chemical reactions resulting in measurable differences in the Hg isotopic composition between reactants and products. This difference in the isotope ratios can become a proxy for tracing the sources of Hg. Depending on the reaction pathways, Hg isotope fractionation can occur as mass-dependent fractionation (MDF), similarly to common light-isotope fractionation, or by mass-independent fractionation (MIF), via the nuclear volume and magnetic isotope effects14,15. Differences in MDF are reported as δ202Hg, which is the difference in the 202Hg/198Hg ratio of a sample compared to the NIST-3133 standard in units of permil (‰). Differences in MIF are reported as Δ199Hg and Δ201Hg, which are the differences in the 199Hg/198Hg and 201Hg/198Hg ratios in ‰ from those predicted from the δ202Hg value based on MDF6.
Recently, Hg isotopes have been applied to aquatic ecosystems to establish links between sources and receptors of Hg. Gehrke et al.11 demonstrated that δ202Hg values in fish correlated strongly with δ202Hg values in intertidal sediments collected at a series of sites in the San Francisco Estuary (USA), with a nearly constant isotopic difference of 0.6 ‰. Unfortunately, the utility of using the MDF signature in fish as a tracer of the isotopic composition of a particular anthropogenic source of Hg is limited by a lack of understanding of Hg isotope fractionation between reservoirs within sediments and during trophic transfer to fish.
High positive Δ199Hg values have been observed in fish10, 12, 13, 16–19 and this has led to two contrasting explanations for the origin of MIF in fish. Bergquist and Blum16 documented large magnitude MIF (>0.5‰ change in Δ199Hg) during photochemical reduction of inorganic Hg (HgI) and photodemethylation of MeHg in aqueous medium, but not in dark abiotic control experiments. They suggested that MIF occurs in the odd-mass number isotopes (199Hg and 201Hg) via photochemical reactions and used the Δ199Hg values in fish collected from multiple lakes to estimate the extent of photodegradation of MeHg that took place prior to uptake by organisms at the base of the food web. Several subsequent studies have adopted this approach to estimate the extent of ecosystem-scale MeHg photodegradation and have assumed that Δ199Hg values are not modified by trophic transfer to fish10, 12, 16, 20. In contrast, Jackson et al.19 and Das et al.17 found a significant positive relationship between Δ199Hg and the trophic level (based on δ15N) along various freshwater food chains. Based on these observations, Das et al. 17 suggested that MIF of Hg isotopes occurs, at least in part, in vivo by internal metabolic processes within fish. The interpretation of Δ199Hg signatures in aquatic ecosystems is thus dependent on whether or not MIF of Hg isotopes occurs in vivo, and controlled experimental studies are needed to determine whether Δ199Hg values are changed by fractionation during trophic transfer to fish.
In the study reported here, we performed two sets of experiments to compare Hg isotopic compositions and concentrations in tissues of two freshwater fish species at different levels of MeHg exposure to test whether Hg isotopes are fractionated during trophic transfer to fish. In Experiment 1, we raised juvenile yellow perch (Perca flavescens) for three months on regular food pellets and then switched some of them for 2 months to a diet of food pellets amended with MeHg. The food pellets were spiked with varying concentrations of MeHg to allow determination of the isotope fractionation of MeHg. We chose to use juvenile yellow perch for Experiment 1 because it is an ideal biosentinel organism for studying Hg bioaccumulation in freshwater food webs due to its ubiquitous distribution in freshwater bodies21. Their omnivorous feeding behavior is also advantageous for raising them in a controlled environment. In a second experiment designed to mimic natural trophic transfer processes of Hg (Experiment 2), we raised lake trout (Salvelinus namaycush), a top predator in many freshwater food webs, on food pellets for 47 months until they reached an adult phase. We then switched their food for 6 months to bloater (Coregonus hoyi), which is their natural prey. The degree of fractionation was determined by comparing the Hg isotope values of the food fed to the fish with the Hg isotope values of the fish tissues.
MATERIALS AND METHODS
Experimental Design
Experiment 1: Yellow perch feeding experiments
Three-month-old yellow perch were raised at the Great lakes WATER Institute at the University of Wisconsin-Milwaukee during the summer of 2010. Twenty-four individuals were randomly selected to determine the average initial body length (47.6 ± 5.4 mm) and body weight (2.01 ± 0.62 g). In August, 2010, the fish were transferred into tanks that were maintained by a flow-through system at 8–10°C and in which water was filtered to remove suspended residues. They were divided into three different treatments (20 individuals in each tank and 4 replicate tanks for each level, totaling 240 fish) and fed with commercial food pellets (Zeigler Finfish Starter Diet, 2 mm) composed mainly of marine by-catch products, poultry by-products, hydrolyzed feather meal, fish oil, wheat, soy flour, and brewers dried yeast. The food pellets were manipulated to three different MeHg concentrations. To vary MeHg concentration in the food pellets, two batches of food pellets were soaked in synthetic MeHg solutions for two days at 1.0 µg/g and 5.0 µg/g (nominal concentrations) and then evaporated to dryness. Synthetic MeHg solution was prepared by diluting solid methylmercury (II) chloride (Sigma-Aldrich) in 100% ethanol. We achieved three different total-Hg (THg) concentration levels (actual concentrations): 0.076 µg/g dry wt. (background without added MeHg), 1.0 µg/g and 4.0 µg/g (pellets with added MeHg) and these are referred to as 0.1, 1.0 and 4.0 µg/g treatments hereafter. All food pellets were kept at minus 80°C prior to use.
Across all treatments, fish were fed twice a day with approximately 6% of their body weight in food pellets, for a total period of two months. Yellow perch that were fed with 0.1, 1.0 and 4.0 µg/g food pellets are referred to as YP-P0, YP-P1 and YP-P4 respectively. All fish achieved similar body length and weight after the experiment (average length= 76.8 ± 8.6 mm, average weight= 8.97 ± 2.69 g, n= 240), regardless of the MeHg levels in the food pellets. The fish were dissected to different organs, frozen and shipped to the University of Michigan, where subsamples of fish muscle and liver tissues were taken for THg concentration and stable Hg isotope analysis. We took either one or two fish from each of the 12 tanks for muscle tissues (n= 18) and pooled four to seven liver samples to achieve 2 liver analyses for each treatment (n= 6). The food pellets were analyzed in triplicate for each treatment level (n= 9).
Experiment 2: Lake trout feeding experiments
Lake trout were raised in the U.S. Fish and Wildlife Service Sullivan Creek National Fish Hatchery (Brimley, MI). Fish were fed with commercial fish food (Nelson Silver Cup extruded sinking feed), composed mainly of fish meal, soybean meal, blood meal, wheat flour, fish oil, hydrolyzed feather meal, and poultry by-products, for 47 months until they reached an average body mass of 570 g. A single individual was sacrificed and dissected for muscle tissue at this time (referred to as LT-P). Both the food pellets and the LT-P muscle tissues were sub-sampled to achieve 3 separate samples for analysis (LT-P, n= 3; pellets, n= 3). A total of 133 lake trout were sent to the U.S. Geological Survey Great Lakes Science Center (Ann Arbor, MI) in February 2010, distributed to 8 different tanks and switched to a diet of bloater. Tanks were maintained by a flow-through system at 8–10°C and water was filtered to remove suspended residues. The bloater fed to the lake trout were obtained from Lake Michigan in September 2009 and May 2010 and referred to as bloater-1 and bloater-2, respectively. The bloaters were caught at two different time periods because there was not enough of bloater-1 to complete the feeding experiment. The bloater diet was prepared by homogenizing whole fish in a stainless steel grinder and storing at minus 30°C prior to use. Bloater-1 and bloater-2 represent a composite of 3 and 6 bloaters respectively. Each bloater composite sample was analyzed twice for THg concentrations and isotopic composition (bloater-1, n= 2; bloater-2, n= 2).
We chose three tanks to analyze for THg concentrations and isotopic composition of the lake trout after 2 months (n= 3) and 6 months (n =3) of feeding with bloater. Lake trout were fed with bloater-1 for 2 months until they reached an average body mass of 694, 890 and 817 g in each tank (referred to as LT-B(2M)). From each tank, a total of 4 lake trout were sacrificed and homogenized as a composite sample. After an additional 3-months of feeding with bloater-1, the lake trout were switched to a diet of bloater-2 and fed for an additional 1-month period until they reached an average body mass of 1242, 1518 and 1566 g in each tank. The remaining 10 fish were sacrificed and homogenized as a composite sample representing 10 individuals from each tank (referred to as LT-B(6M)). Additional information on feeding procedures can be found in Madenjian et al.22. Individual fish were not analyzed after switching the food source, and the homogenized composite samples removed some of the possible effects of heterogeneity between individuals.
Mercury concentration analysis
All tissue and food pellet samples were analyzed for THg concentrations at the University of Michigan. The tissue samples were freeze-dried prior to analysis and concentrations are reported based on dry weight. Briefly, the samples were combusted at 800°C and THg was quantified using a Nippon Instruments MA-2000 AAS Hg analyzer. Calibration was obtained by a standard solution of NIST SRM 3133 and was checked between every three samples; the values were always within 5% of certified values. Two standard reference materials, ERM CE 464 (average measured THg = 4.71 µg/g, n=8) and NRCC DORM-2 (4.16 µg/g, n=7), were combusted along with samples, and always agreed within 10% of certified values.
MeHg concentrations were analyzed for the 0.1 µg/g food pellets from Experiment 1, as well as for the bloater-1 and bloater-2 and lake trout from Experiment 2, at the U. S. Geological Survey Mercury Research Lab in Middleton, Wisconsin, following the method described in Hammerschmidt and Fitzgerald23. Briefly, samples were digested in Teflon digestion tubes with dilute nitric acid (5M HNO3) at 60°C for 8 hours. 4.5M KOH and sodium tetraethylboarate (NaTEB) were added to form methylethylmercury (MeEtHg). Argon (Ar) gas was used to strip the MeEtHg from the liquid phase, and transferred into Tenex traps. MeEtHg was then desorbed back into the sample stream and different Hg species were separated using a gas chromatography column. MeHg and Hg2+ were converted to Hg0 and analyzed using a CVAFS detector. The detection limit was monitored on a daily basis and was 2 ng/g.
Mercury isotope analysis
Sample combustion and transfer
A two-stage combustion system was employed to thermally release and isolate Hg from fish tissue and fish food samples11. Briefly, a pre-fired ceramic boat was loaded with sample powder, covered with sodium carbonate and aluminum oxide and placed in a dual-stage quartz tube furnace. The combustion compartment was heated in a step-wise fashion to 750°C over a 6 hour period. Mercury-free oxygen was fed to carry released Hg0 from samples through the down-stream 1000°C decomposition compartment, and all Hg0 was subsequently oxidized in a “trap solution” of 1% KMnO4 in 10% H2SO4. To fully separate Hg from other combustion products, the sample trap solutions were partially neutralized by the addition of hydroxylamine, and then Hg2+ was reduced to Hg0 by addition of SnCl2. Hg0 was purged into another 1% KMnO4 trap solution using Ar gas, resulting in a matrix-free solution adequate for mass spectrometry analysis.
Recovery of Hg through combustion and transfer
Since the δ202Hg value of Hg in samples can be affected by incomplete recovery of Hg through the combustion and transfer processes, we monitored the THg concentrations in trap solutions after both combustion and transfer steps. THg recoveries were calculated using standard reference materials, ERM CE 464 (n= 9) and NRCC DORM-2 (n= 2) and ranged between 96 and 103% and between 93 to 99%, respectively. Procedural blanks contained negligible amounts of THg (average = 0.017 ng/g, n=5) when compared to sample concentration for isotopic measurements (i.e. 1–5 ng/g).
MC-ICP-MS analysis
Stable Hg isotope ratios were measured using a Nu Instruments multi-collector inductively coupled plasma mass spectrometer (MC-ICP-MS). Neutralized trap solutions (after the final purge and trap step) were diluted with the same matrix to achieve THg concentrations between 1 and 5 ng/g. Hg2+ in solution was reduced by SnCl2, and evolved Hg0 was separated from the solution using a frosted glass tip phase separator and introduced to the MC-ICP-MS. On-peak zero corrections were applied, and instrumental mass bias was corrected using an internal Tl standard (NIST SRM 997) and sample-standard (NIST SRM 3133) bracketing at uniform THg concentrations and matrix composition6. The bracketing standard was diluted using the same matrix and matched to the THg concentration of the samples within 5 %. MDF is reported as δ202Hg in permil (‰) referenced to NIST SRM 3133 and calculated as:
| (1) |
MIF represents the difference between the measured δxxxHg value and the value predicted based on MDF and the δ202Hg value16. MIF is thus reported as Δ199Hg and Δ201Hg in permil (‰). MIF is calculated as below based on an approximation valid for δ <10‰:
| (2) |
| (3) |
We use Δ199Hg as the default value to report MIF.
Analytical uncertainty of isotopic analysis
Analytical uncertainty at 2 s.d. was estimated as the larger of replicate measurements of the in-house standard (UM-Almáden) or of replicate analyses of procedural standards. UM-Almáden (n=81) had mean values (±2 s.d.) of δ202Hg= −0.57±0.06 ‰, Δ201Hg= −0.03±0.04 ‰, and Δ199Hg= −0.02±0.05 ‰. Standard reference material ERM CE 464 (freeze dried fish powder) was combusted, processed and analyzed in the same manner as samples (n=9) and its mean values were δ202Hg= 0.69±0.07 ‰, Δ201Hg= 1.97±0.03 ‰, and Δ199Hg= 2.39±0.06 ‰.
RESULTS AND DISCUSSION
Hg Concentrations and Bioaccumulation Factors in Fish
The THg concentrations in the muscle and liver tissues of the yellow perch (Experiment 1) increased with increasing MeHg concentration in the three different food pellet treatments after two months of feeding. This is consistent with other studies that have documented a positive relationship between the Hg concentrations in commercial food pellets and Hg concentrations in the muscle and liver tissues of other fish species24. The MeHg concentration analysis showed that the 0.1 μg/g food pellets were composed of 78% MeHg. The level of HgI in the 0.1 μg/g food pellets was used to calculate the % MeHg in the 1.0 µg/g and 4.0 µg/g pellets, which were 94% and 99% respectively. The average THg concentrations in the muscle tissues were 0.210±0.01 (n= 6, 1 s.d), 2.54±0.33 (n= 6), and 13.3±0.90 μg/g (n= 6) and in the liver tissues were 0.022±0.003 (n=2), 0.34±0.03 (n= 2), and 3.77±0.43 μg/g (n= 2) in YP-P0, YP-P1 and YP-P4, respectively (Supporting information Table S1).
The THg concentrations in the lake trout (Experiment 2) increased when their diet was switched to bloater, which had much higher THg concentrations than the food pellets. The average THg concentrations of the food pellets were 0.0267±0.001 µg/g (n=3, 1 s.d) and the bloater-1 and bloater-2 were 0.312±0.001 (n= 2) and 0.463±0.006 µg/g (n= 2) respectively. The LT-P, LT-B(2M) and LT-B(6M) had THg of 0.111±0.004 (n= 3), 0.138±0.02 (n= 3) and 0.316±0.02 µg/g (n= 3), respectively (Supporting information Table S2). The % MeHg in the LT-B(2M) and LT-B(6M) were 83% and 89% and the % MeHg in the bloater-1 and bloater-2 were 85% and 87%.
We calculated the bioaccumulation factors (BAFs) to determine the extent of Hg bioaccumulation at different levels of Hg exposure. In this study, the BAFs in Experiment 1 were calculated as the THg concentration in fish muscle divided by the average THg concentration in food pellets. Muscle tissues concentrations are often used for this purpose, as muscle is the major site of MeHg accumulation25. The BAFs were 2.8±0.2 (1 s.d), 2.7±0.3, and 3.1±0.2, for the 0.1, 1.0, and 4.0 μg/g treatments, respectively. The THg concentration of these small fish, which added over 400% body mass in the 2-month feeding period, appear to have risen to new Hg concentrations in equilibrium with their new diets and thus yielded constant BAF values across a >50-fold range in concentrations.
The BAF of LT-P in Experiment 2 was calculated as the THg concentration in muscle divided by the THg concentration in food pellets, and the LT-B(2M) and LT-B(6M) were calculated based on the THg concentration in whole fish divided by the THg concentration in the whole fish bloater diet. Due to the two batches of bloater that were used as food during the course of the experiment, we took the weighted average of the THg concentration of the bloater-1 (88%) and bloater-2 (12%) to calculate the BAF for the LT-B(6M) (bloater 1 and 2= 0.330 μg/g). The BAFs calculated for Experiment 2 are 4.1±0.2 (1 s.d), 0.44±0.1 and 0.96±0.1 for the food pellets (before the change to a bloater diet) and bloater diet after 2 and 6 months, respectively. Previous studies have reported BAFs of 3 to 5 between food and whole-body fish tissue concentrations of THg3, 26. The lower BAF values after 2 and 6 months on the bloater diet indicate that for these adult fish (which added only 39% and 100% body mass) THg concentrations had presumably not yet risen to values in equilibrium with their new diet.
Hg Isotopic Compositions in the Food
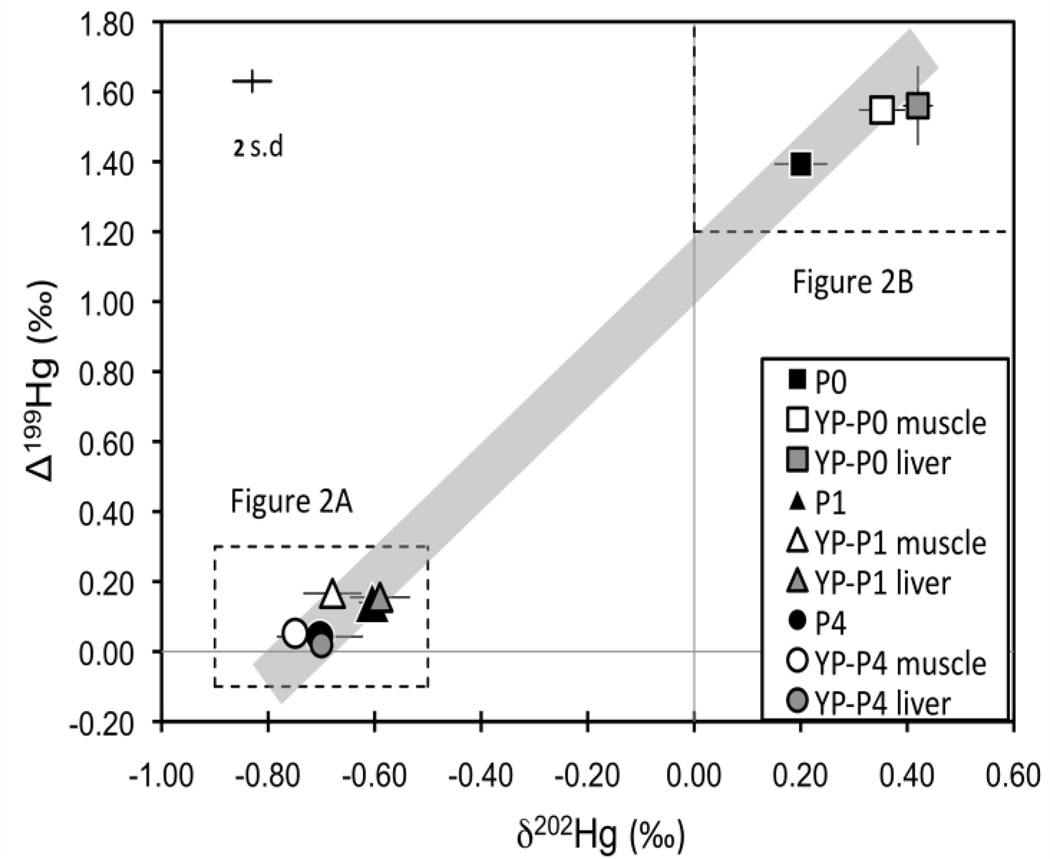
The food pellets with added synthetic MeHg used in Experiment 1 had much lower δ202Hg and Δ199Hg values than the food pellets without added MeHg (Fig 1; Fig 2; Supporting information Table S1). The isotopic composition of the un-amended food pellets reflects background Hg from marine by-catch products, poultry by-products and other materials from which they are composed. The isotopic composition of the food pellets with added MeHg reflects the synthetically prepared MeHg, which is derived from industrial sources of Hg and therefore has a Δ199Hg value near zero27. The synthetic MeHg showed similar δ202Hg as cinnabar (HgS)28–30 and metallic Hg (e.g., Almaden standard)16 from various sources. The Hg isotopic composition of the 0.1 µg/g and 4.0 µg/g food pellets are considered the “natural” pellet Hg and synthetic MeHg respectively, and as expected the isotopic composition of 1.0 µg/g food pellets fall on a mixing line between these end members (Fig 1).
Figure 1.
Plot of mean δ202Hg and Δ199Hg values from Experiment 1. The shaded line represents the mixing between 0.1 μg/g and 4.0 μg/g treatment pellets. Analytical uncertainly is indicated by the error bar (2 s.d.). Error bars on symbols are 1 s.d. of the mean of 3 food pellets, 2 liver tissues and 6 muscle tissues in each treatment. Dashed boxes show area of Figure 2A and 2B.
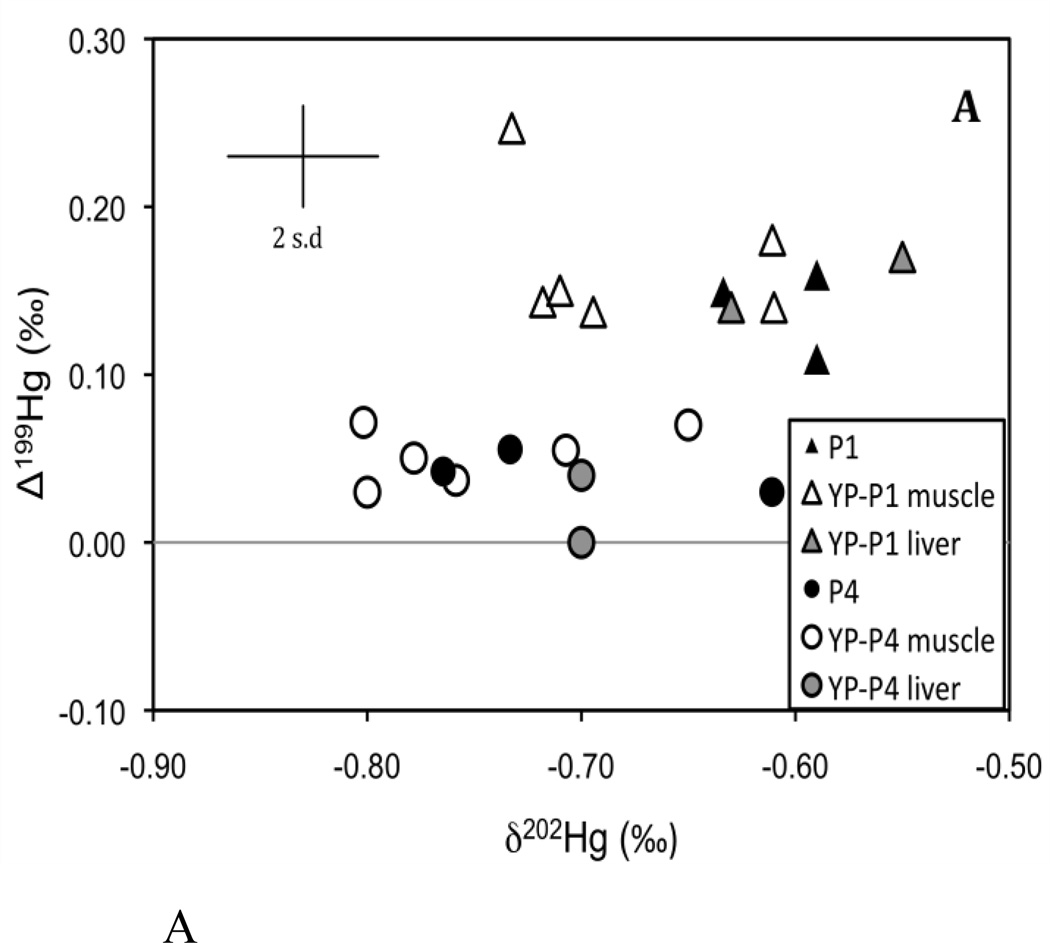
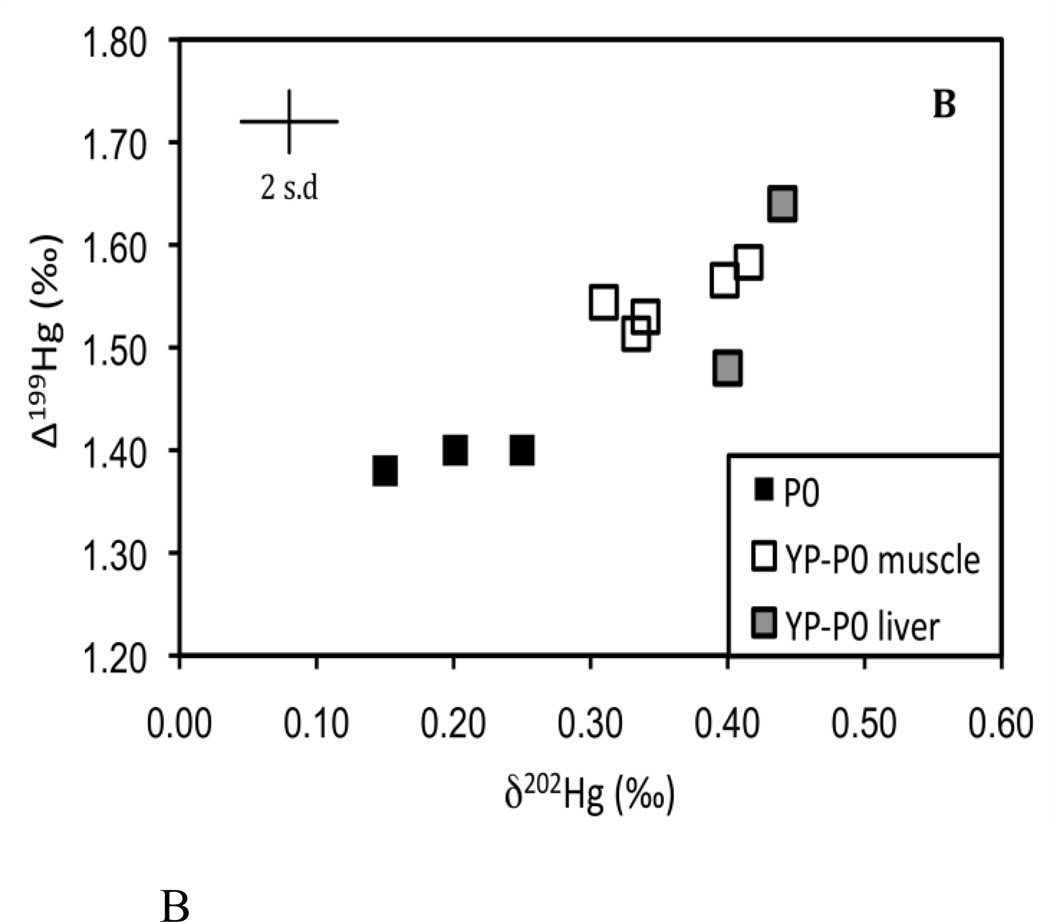
Figure 2.
Plot of individual δ202Hg and Δ199Hg values from Experiment 1. (A) represents the 0.1 µg/g treatment and (B) represents the 1.0 and the 4.0 µg/g treatment. Each figure corresponds to the dashed boxes shown in Figure 1. Analytical uncertainty is indicated by the error bar (2 s.d.).
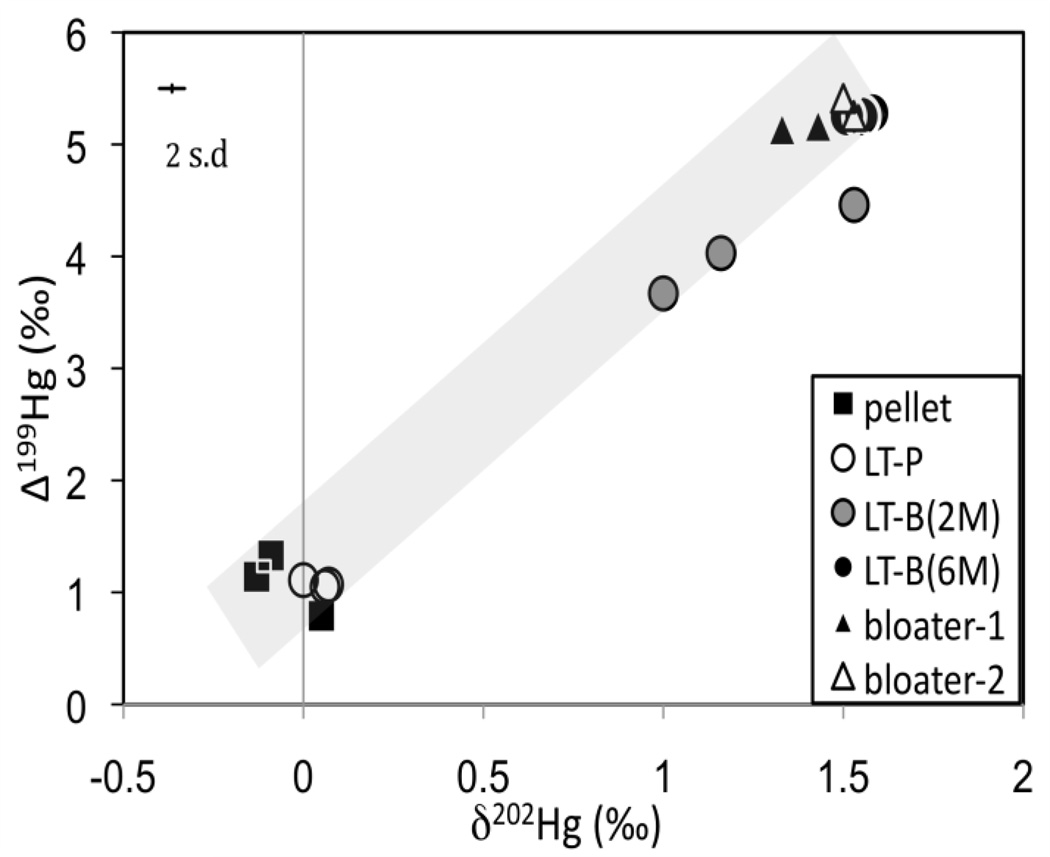
In Experiment 2, the food pellets and the new diet (i.e., bloater) were also markedly different in Hg isotopic composition, but in this case they were higher in δ202Hg and Δ199Hg compared to Experiment 1 (Fig 3; Supporting information Table S2). The bloater had a high proportion of MeHg and also naturally higher values of δ202Hg and Δ199Hg (without the use of synthetic MeHg). This allowed a comparison of possible isotopic fractionation during trophic transfer of naturally occurring MeHg, compared to the synthetic MeHg in Experiment 1. Significantly positive Δ199Hg values in the food pellets from both experiments suggest that Hg in the food may have originated from marine by-catch products, because of the similarity in measured isotopic compositions in various marine fish11, 13. The even higher Δ199Hg values in the bloater used in Experiment 2 were consistent with their origin as lake fish9, 10, 12, 16 (Supporting information Table S2). Moreover, the Δ199Hg/Δ201Hg ratio observed in the 0.1 µg/g food pellets (1.21±0.03, 1 s.d.), and the bloater (1.27±0.02, 1 s.d.) are consistent with the ratios previously reported for fish (~1.20)11–13, 16, 20.
Figure 3.
Plot of δ202Hg and Δ199Hg values from Experiment 2. The shaded line represents the mixing between the food pellets and bloater food source. Analytical uncertainty is indicated by the error bar (2 s.d).
Hg Isotopic Variation during Bioaccumulation and Trophic Transfer
After two months of feeding on the new food sources, and after an average body mass increase of over 400%, both δ202Hg and Δ199Hg in the muscle and liver tissues of yellow perch (Experiment 1) generally matched the respective food pellets used within each treatment (Fig. 1; Fig 2). The Hg isotopic compositions of the muscle tissues were indistinguishable from the liver tissues in each treatment despite considerable differences in the Hg concentrations. This indicates that Hg isotope fractionation does not occur under these conditions during the internal distribution of Hg between these tissues. To examine whether there were significant isotopic shifts in either δ202Hg or Δ199Hg during bioaccumulation and trophic transfer, we calculated the differences in measured δ202Hg and Δ199Hg between fish and their food (fish minus food) and found no difference in δ202Hg or Δ199Hg for either of the MeHg amended pellet treatments (Supporting information Fig S1A, B). This indicates that the Hg isotopic composition is directly transferred to the fish without isotope fractionation. In contrast, small isotopic differences in both δ202Hg and Δ199Hg (≤0.2‰) were observed between the un-amended 0.1 µg/g food pellets and the muscle and liver tissues of YP-P0 (Supporting information Fig S1A, B). It is important to clarify that the small differences in the isotopic composition of Hg between the fish and 0.1 µg/g food pellets are not the result of isotope fractionation that occurred during chemical reactions. Instead, we attribute this to the preferential uptake of MeHg in the muscle and liver tissues of YP-P0 compared to HgI, leading to a small Hg isotopic shift upon bioaccumulation (discussed below in further detail).
In the lake trout study (Experiment 2), in which the food pellets and the bloater had contrasting Hg isotopic compositions, we observed considerable, but incomplete, turnover of the Hg isotopic compositions in the LT-B(2M) 2 months after switching the diet to the bloater (and following an average weight increase of only 39%) (Fig 3). Lake trout from two of the three tanks sampled at 2 months (LT-B(2M)) fall on a mixing line, as expected, between the Hg isotopic values of the food pellets and the bloater. We do not have a clear explanation for why a third LT-B(2M) sample does not fall on this line, but we speculate that it is due to heterogeneity of the bloater food source. For lake trout fed bloater for 6 months (LT-B(6M)) (with an average weight increase of 100%) complete isotopic equilibration to the value of the bloater-2 food source appears to have occurred. This also corresponded with the increase in the THg concentrations in LT-B(6M) compared to LT-B(2M), indicating further accumulation and incorporation of Hg into the lake trout tissues.
To summarize the experimental results, we find no evidence for MDF or MIF during trophic transfer of Hg. In Experiment 1, when the proportion of MeHg to THg and the concentrations in the food pellets was high (1.0 µg/g and 4.0 µg/g treatments), there was no detectable difference in δ202Hg or Δ199Hg between the food pellets and yellow perch muscle or liver tissue. Similarly, in Experiment 2, Hg was transferred from the bloater food source to the lake trout, and the δ202Hg and Δ199Hg of the LT-B(6M) shifted to the value of the bloater after 6 months. In the experiment where yellow perch (Experiment 1) were fed with food pellets with low MeHg concentration and a significant proportion of HgI (22%), the fish were observed to have δ202Hg and Δ199Hg values that were slightly offset from the food pellets. We suggest that the fraction of HgI in the un-amended food pellets had a contrasting Hg isotopic composition compared to the MeHg, which seems reasonable given the widely contrasting materials (see above) that are used to make the food pellets and the observation that HgI and MeHg can have contrasting isotopic composition in individual tissues31. More efficient accumulation of MeHg, compared to HgI, in the fish could then account for the small discrepancy between the Hg isotopic composition of the un-amended pellets and the fish raised on them.
Das et al.17 previously reported a significant increase in Δ199Hg with trophic level (inferred from δ15N) and argued that in vivo processes in fish could cause MIF. It has, however, been suggested on theoretical grounds that internal metabolic processes are unlikely to cause the observed MIF of Hg isotopes32. The suggestion of in vivo MIF is not supported by our experiments, which demonstrate that MeHg, whether synthetically prepared or natural, is transferred without fractionation. The absence of isotope fractionation of MeHg observed in both juvenile (yellow perch) and adult (4-year old lake trout) fish and following different time periods after dietary changes (2 months vs 6 months) suggests that differences in age, growth rate, and Hg turnover explored in our experiments do not lead to significant isotope fractionation of MeHg. It is unclear, without additional studies, whether other metabolic processes or physiological states of fish could influence the isotope fractionation of MeHg in the wild. Nevertheless, our controlled experiments suggest that differing isotopic compositions between MeHg and HgI in aquatic organisms are most likely responsible for the differences in δ202Hg and Δ199Hg that have been observed during bioaccumulation and trophic transfer in some studies. Much higher bioaccumulation of MeHg compared to HgI has been well documented in a number of studies that have exposed fish to both Hg species through their diet33–35.
In contrast to Das et al.17, some studies have failed to observe any relationship between Hg isotope values and trophic levels in fish10,13. Instead, in those studies, the variations of MIF in fish were explained by the extent of photochemical demethylation of MeHg in the natural environment prior to uptake into the aquatic food web. If we assume that both HgI and MeHg are present in water and are equally exposed to light in the water column, we would expect HgI to impart a lesser degree of MIF compared to MeHg. The experimental study by Bergquist and Blum16 documented larger MIF of MeHg compared to HgI despite a similar rate of photoreduction. With bioaccumulation of two Hg species, HgI with lower δ202Hg and Δ199Hg values and MeHg with higher δ202Hg and Δ199Hg values, by low trophic level organisms such as phytoplankton36 and subsequent trophic transfer of predominantly MeHg, the pattern of increasing δ202Hg and Δ199Hg values with trophic position in aquatic food webs could be generated. As a side note we point out that the lack of Hg isotope fractionation during trophic transfer into fish does not imply that fractionation does not occur in other types of organisms. In fact, a recent study by Laffont et al.9 noted an average enrichment of δ202Hg by 2‰ in human hair compared to food sources (but no change in Δ199Hg) and suggested that in vivo processes in humans may cause MDF during transfer into hair.
Application of Hg Isotopes in Aquatic Ecosystems
The lack of isotope fractionation of Hg (both MDF and MIF) during trophic transfer into fish that we observe in this study suggests that the application of Hg isotope measurements can provide direct linkages between Hg sources and Hg in fish tissues. In the study by Gehrke et al.11, it was recognized that the use of young fish as biosentinels to detect spatial patterns of Hg sources was limited by a lack of understanding of Hg isotope fractionation between sediment and algae and during trophic transfer to fish. It was suggested that the observed constant offset of 0.6 ‰ in δ202Hg between the sediment and fish was the result of the combined effect of chemical transformations in sediment and trophic transfer. Based on our findings, we now suggest that the offset of 0.6 ‰ is not due to trophic transfer into fish and is instead the result of other processes. Our demonstration of a lack of Hg isotope fractionation upon trophic transfer focuses our attention towards understanding fractionation during processes that occur within sediments.
The rate at which the Hg isotopic composition re-equilibrated to the value of new food sources in our experiments yields insight into the timescale of Hg exposure recorded by the Hg isotopic composition of fish. The age-0 juvenile yellow perch in Experiment 1 and the mature age-4 lake trout in Experiment 2 reflected their new food sources after 2 and 6 months, respectively. After only 2 months however, the lake trout retained an isotopic signal of a mixture of their old and new food sources. In the case of input of new Hg sources to the environment, we expect that it would take longer than 2 months for older fish, depending on size and growth rate, to fully reflect the Hg isotopic composition of new Hg sources.
A number of studies have reported spatial patterns of Δ199Hg values both within and across aquatic systems10–13. Ganter et al.10 observed varying Δ199Hg values in Arctic char depending on the latitude of the Canadian lakes in which the fish were sampled. Moreover, their results exhibited species-specific variation in Δ199Hg values such that zooplankton occupying the water column had higher Δ199Hg values compared to benthic invertebrates. The interpretation of Δ199Hg values was previously hindered by uncertainties concerning the effect of potential biological fractionation versus fractionation by photochemical degradation of MeHg. Our experimental study clearly suggests that biological MIF is unimportant, and we can now more confidently estimate the proportion of MeHg lost through photodegradation10–13. Given the potentially diverse dietary sources, feeding behaviors and mobility of fish in natural ecosystems, there are clear limitations to using stable Hg isotopes in fish as tracers of Hg sources. We anticipate, however, that as we learn more about Hg isotope fractionation during specific processes in the environment, the application of δ202Hg and Δ199Hg in aquatic ecosystems will develop into a strong tracer of Hg sources in aquatic ecosystems.
Supplementary Material
ACKNOWLEDGEMENT
The authors would like to thank M Pickens for raising yellow perch, the U. S. Fish and Wildlife Service staff at the Sullivan Creek National Fish Hatchery for raising the lake trout, M Johnson for expert operation of the MC-ICP-MS, and D Krabbenhoft for generously providing MeHg analyses of fish food pellets. The USGS has not endorsed this publication and the views expressed should not be considered the views of the USGS. Funding was provided by grants from NSF (EAR-0952108) and US DOE Office of Science (BER) to JD Blum, NOAA pilot funds to JA Head, and a Great Lakes Fishery commission grant to CP Madenjian. This article is Contribution 000 of the U. S. Geological Survey Great Lakes Science Center.
Footnotes
SUPPORTING INFORMATION
Supporting figures (Fig S1A, B) and tables (Table S1, S2). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Gilmour CC, Henry EA, Mitchell EA. Sulfate stimulation of mercury methylation in freshwater sediments. Environ. Sci. Technol. 1992;26:2281–2287. [Google Scholar]
- 2.Mergler D, Anderson HA, Chan LHM, Mahaffey KR, Murray M, Sakamoto M, Stern AH. Methylmercury exposure and health effects in humans: A worldwide concern. Ambio. 2007;36:3–11. doi: 10.1579/0044-7447(2007)36[3:meahei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Mason RP, Sullivan KA. Mercury in Lake Michigan. Environ. Sci. Technol. 1997;31:942–947. [Google Scholar]
- 4.Chen CY, Stemberger RS, Kamman NC, Mayes BM, Folt CL. Patterns of Hg bioaccumulation and transfer in aquatic food webs across multi-lake studies in the northeast US. Ecotoxicol. 2005;14:135–147. doi: 10.1007/s10646-004-6265-y. [DOI] [PubMed] [Google Scholar]
- 5.Kwon SY, McIntyre PB, Flecker AS, Campbell LM. Mercury biomagnifications in the food web of a neotropical stream. Sci. Total. Environ. 2012:417–418. 92–97. doi: 10.1016/j.scitotenv.2011.11.060. [DOI] [PubMed] [Google Scholar]
- 6.Blum JD. In: Handbook of environmental isotope geochemistry. Applications to stable mercury isotope biogeochemistry. Baskaran Mark., editor. Berlin Germany: Springer; 2011. [Google Scholar]
- 7.Sherman LS, Blum JD, Keeler GJ, Demers JD, Dvonch T. Investigation of local mercury deposition from a coal-fired power plant using mercury isotope. Environ. Sci. Technol. 2012;46:382–390. doi: 10.1021/es202793c. [DOI] [PubMed] [Google Scholar]
- 8.Foucher D, Ogrinc N, Hintelmann H. Tracing mercury contamination from the Idrija mining region (Slovenia) to the Gulf of Trieste using Hg isotope ratio. Environ. Sci. Technol. 2009;43:33–39. doi: 10.1021/es801772b. [DOI] [PubMed] [Google Scholar]
- 9.Laffont L, Sonke JE, Maurice L, Hintelmann H, Pouilly M, Bacarreza YS, Perez T, Behra P. Anomalous mercury isotopic compositions of fish and human hair in the Bolivian Amazon. Environ. Sci. Technol. 2009;43:8985–8990. doi: 10.1021/es9019518. [DOI] [PubMed] [Google Scholar]
- 10.Gantner N, Hintelmann H, Zheng W, Muir DC. Variations in stable isotope fractionation of Hg in food webs of Arctic lakes. Environ. Sci. Technol. 2009;43:9148–9154. doi: 10.1021/es901771r. [DOI] [PubMed] [Google Scholar]
- 11.Gehrke GE, Blum JD, Slotton DG, Greenfield BK. Mercury isotope link mercury in San Francisco Bay forage fish to surface sediments. Environ. Sci. Technol. 2011;45:1264–1270. doi: 10.1021/es103053y. [DOI] [PubMed] [Google Scholar]
- 12.Perrot V, Epov VN, Pastukhov MV, Grebenshchikova VI, Zouiten C, Sonke JE, Husted S, Donard OFX, Amouroux D. Tracing sources and bioaccumulation of mercury in fish of Lake Baikal-Angara River using Hg isotopic composition. Environ. Sci. Technol. 2010;44:8030–8037. doi: 10.1021/es101898e. [DOI] [PubMed] [Google Scholar]
- 13.Senn DB, Chesney EJ, Blum JD, Bank MS, Maage A, Shine JP. Stable isotope (N, C, Hg) study of methylmercury sources and trophic transfer in the Northern Gulf of Mexico. Environ. Sci. Technol. 2010;44:1630–1637. doi: 10.1021/es902361j. [DOI] [PubMed] [Google Scholar]
- 14.Buchachenko AL, Ivanov VL, Roznyatovskii VA, Vorob’ev AK, Ustynyuk YA. Inversion of the sign of the magnetic isotope effect of mercury in photolysis of substituted dibenzylmercury. Dokl. Phys. Chem. 2008;420:85–87. [Google Scholar]
- 15.Schauble EA. Role of nuclear volume in driving equilibrium stable isotope fractionation of mercury, thallium, and other very heavy elements. Geochim. Cosmochim. Acta. 2007;71:2170–2189. [Google Scholar]
- 16.Bergquist BA, Blum JD. Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science. 2007;318:417–420. doi: 10.1126/science.1148050. [DOI] [PubMed] [Google Scholar]
- 17.Das R, Salters VJM, Odom AL. A case for in vivo mass-independent fractionation of mercury isotopes in fish. Geochem. Geophys. Geosyst. 2009;10:Q11012. [Google Scholar]
- 18.Epov VN, Rodriguez-Gonzalez P, Sonke JE, Tessier E, Amouroux D, Bourgoin LM, Donard OFX. Simultaneous determination of species-specific isotopic composition of Hg by gas chromatography coupled to multicollector ICPMS. Anal. Chem. 2008;80:3530–3538. doi: 10.1021/ac800384b. [DOI] [PubMed] [Google Scholar]
- 19.Jackson TA, Whittle DM, Evans MS, Muir DCG. Evidence for mass-independent and mass-dependent fractionation of the stable isotopes of mercury by natural processes in aquatic ecosystems. Appl. Geochem. 2008;23:547–571. [Google Scholar]
- 20.Point D, Sonke JE, Day RD, Roseneau DG, Hobson KA, Vander Pol SS, Moors AJ, Pugh RS, Donard OFX, Becker PR. Methylmercury photodegradation influenced by sea-ice cover in Arctic marine ecosystems. Nat. Geosci. 2011;4:188–194. [Google Scholar]
- 21.Greenfield BK, Hrabik TR, Harvey CJ, Carpenter SR. Predicting mercury levels in yellow perch: use of water chemistry, trophic ecology, and spatial traits. Can. J. Aquat. Sci. 2001;58:1419–1429. [Google Scholar]
- 22.Madenjian CP, David SR, Pothoven SA. Effects of activity and energy budget balancing algorithm on laboratory performance of a lake trout bioenergetics model. Trans. Am. Fish. Soc. 2012 in press. [Google Scholar]
- 23.Hammerschmidt CR, Fitzgerald WF. Methylmercury in freshwater fish linked to atmospheric mercury deposition. Environ. Sci. Tehcnol. 2006;40:7764–7770. doi: 10.1021/es061480i. [DOI] [PubMed] [Google Scholar]
- 24.Berntssen MHG, Hylland K, Julshamn K, Lundebye AK, Waagbo R. Maximum limits of organic and inorganic mercury in fish feed. Aquacult. Nutr. 2004;10:83–97. [Google Scholar]
- 25.Bloom NS. On the methylmercury content of fish tissue. Can. J. Fish. Aquat. Sci. 1992;49:1010–1017. [Google Scholar]
- 26.Watras CJ, Bloom NS. Mercury and methylmercury in individual zooplankton: Implications for bioaccumulation. Limol. Oceanogr. 1992;37:1313–1318. [Google Scholar]
- 27.Bergquist BA, Blum JD. The odds and evens of mercury isotope: Applications of mass-dependent and mass-independent isotope fractionation. Elements. 2009;5:353–357. [Google Scholar]
- 28.Hintelmann H, Lu SY. High precision isotope ratio measurements of mercury isotopes in cinnabar ores using multi-collector inductively coupled plasma mass spectrometry. Analyst. 2003;128:635–639. doi: 10.1039/b300451a. [DOI] [PubMed] [Google Scholar]
- 29.Smith CN, Kesler SE, Blum JD, Rytuba JJ. Isotope geochemistry of mercury in source rocks, mineral deposits and spring deposits of the California coast ranges, USA. Earth. Planet. Sc. Lett. 2008;269:399–407. [Google Scholar]
- 30.Stetson SJ, Gray JE, Wanty RB, Macalady DL. Isotopic variability of mercury in ore, mine-waste calcine, and leachates of mine-waste calcine from areas mined for mercury. Environ. Sci. Technol. 2009;43:7331–7336. doi: 10.1021/es9006993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epov VN, Berail S, Jimenez-Moreno M, Perrot P, Pecheyran C, Amouroux D, Donard OFX. Approach to measure isotopic ratios in species using multicollector-ICPMS coupled with chromatography. Anal. Chem. 2010;82:5652–5662. doi: 10.1021/ac100648f. [DOI] [PubMed] [Google Scholar]
- 32.Kritee K, Barkay T, Blum JD. Mass dependent stable isotope fractionation of mercury during mer mediated microbial degradation of monomethylmercury. Geochim. Cosmochim. Acta. 2009;73:1285–1296. [Google Scholar]
- 33.Boudou A, Ribeyre F. Experimental study of trophic contamination of Salmo gairdneri by two mercury compounds- HgCl2 and CH3HgCl- Analysis at the organism and organ levels. Water. Air. Soil. Pollut. 1985;26:137–148. [Google Scholar]
- 34.Pickhardt PC, Stepanova M, Fisher NS. Contrasting uptake routes and tissue distributions of inorganic and methylmercury in mosquitofish (Gambusia affinis) and redear sunfish (Lepomis microlophus) SETAC. 2006;25:2132–2142. doi: 10.1897/05-595r.1. [DOI] [PubMed] [Google Scholar]
- 35.Wang WX, Wong RSK. Bioaccumulation kinetics and exposure pathways of inorganic mercury and methylmercury in a marine fish, the sweetlips Plectorhinchus gibbosus. Mar. Ecol. Progr. Ser. 2003;261:257–268. [Google Scholar]
- 36.Mason RP, Reinfelder JR, Morel FMM. Bioaccumulation of mercury and methylmercury. Water. Air. Soil. Pollut. 1995;80:915–921. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.