Abstract
Background
The beryllium lymphocyte proliferation test (BeLPT) identifies persons sensitized to beryllium (BeS) and thus at risk for chronic beryllium disease (CBD). BeLPT test results are abnormal (AB), borderline (BL), or normal (NL). This manuscript addresses the predictive value and interpretation of BL BeLPT results.
Methods
The various three-result combinations that meet or exceed a nominal referral criteria of 1 AB + 1 BL are assessed with probability modeling and compared.
Results
At 2% prevalence, the three-result combinations that meet or exceed this referral criteria and associated probabilities of BeS are: (a) 1 AB + 1 BL + 1 NL (72%); (b) 3 BL (91%); (c) 2 AB + 1 NL (95%); (d) 1 AB + 2 BL (99%); (e) 2 AB + 1 BL (100%); and (f) 3 AB (100%).
Conclusion
These results suggest that BL results are meaningful and that three BL results predict BeS across a broad range of population prevalences. An analysis of longitudinal BeLPT results and clinical findings from an actual surveillance program is warranted to confirm the model’s predictions.
Keywords: BeLPT, borderline, beryllium sensitization, BeS, chronic beryllium disease, CBD
INTRODUCTION
The beryllium lymphocyte proliferation test (BeLPT) is used to identify persons who are sensitized to beryllium (BeS). These sensitized individuals either have or are at risk for developing chronic beryllium disease (CBD), which manifests primarily as a granulomatous inflammation of the lungs [Rossman et al., 1988; Newman et al., 1992; Kreiss et al., 2007]. This manuscript addresses the interpretation of borderline (BL) BeLPT results collected during the medical surveillance of persons exposed to beryllium.
The BeLPT
The methodology and characteristics of the BeLPT have been described in numerous publications [Kreiss et al., 1989; Mroz et al., 1991; Maier, 2001]. Briefly, mononuclear cells are isolated from peripheral blood and cultured with and without beryllium in media that contain a radioactive DNA-precursor (tritiated thymidine). Six test conditions are created by culturing with three different beryllium concentrations over two different time periods. Afterwards, the radioactivity of each stimulated culture is measured and compared to that of unstimulated cultures to determine six stimulation indices (SIs). Test results are considered abnormal (AB), BL, or normal (NL), respectively, for two (or more), one, or no elevated SIs.
The database used by Stange et al. [2004] contained 19,396 serial BeLPT results collected over 10 years for 7,820 current and former employees. The BeLPT is quite specific, with a false positive rate for single tests of approximately 1% [Stange et al., 2004]. The major limitation of the BeLPT pertains to its sensitivity. The proportion of false negative test results for a single BeLPT has been found to be approximately 25% [Stange et al., 2004]. While there is no true gold standard, Stange’s approach to identifying false positives and false negatives can be summarized as follows: persons truly sensitized to Be will have more than 1 AB during serial testing, while persons truly not sensitized will have no AB or (rarely) 1 AB result.
Sensitization Criteria
Typically, more than one test is conducted before confirming BeS. While clinicians often define BeS as 2 AB test results [Kreiss et al., 1993; Stange et al., 1996], all medical testing is imperfect; that is, false positive and false negative results do occur. Using data from the Stange et al. paper [2004], Middleton et al. [2006, 2008] estimated the epidemiologic characteristics of three testing algorithms and the associated nominal criteria used to select persons for medical evaluation. These nominal criteria are: 1 AB result, 1 AB + 1 BL result, and 2 AB results. Each of these nominal criteria identifies a group of result combinations that meet or exceed a minimum criteria. For example, for the nominal criteria 1 AB + 1 BL, the minimum three-result combination is actually 1 AB + 1 BL + 1 NL.
Only one algorithm and criteria (1 AB + 1 BL) accepts BL as a meaningful final result. The other two algorithms and criteria (1 AB and 2 AB) simply repeat BL results until an AB or a NL is obtained in its place.
While a single AB is often insufficient for establishing BeS, the predictive value of the nominal criteria 1 AB + 1 BL approaches that of 2 AB over a range of BeS prevalences [Middleton et al., 2008]. For example, at a 2% prevalence of BeS, the overall positive predictive value for the nominal criteria 2 AB was 98.4% and that of 1 AB + 1BL was 94.4%. CBD has also been diagnosed in exposed workers with only 1 AB (or 1 AB + 1 BL) [Kreiss et al., 1989, 2007; Deubner et al., 2001; Newman et al., 2001]. This shows that cases of CBD could be missed if diagnostic evaluations are always limited to workers with at least 2 AB results.
In most BeLPT surveillance programs, an initial AB or BL is followed by a blood sample split and sent to two different laboratories for analysis (Fig. 1). We note that the algorithm based on the nominal criteria 1 AB + 1 BL actually requires a minimum of three tests for confirmation. That is, when a single test in the first round is followed by a split sample in the second round (Fig. 1), 1 AB and 1 BL must be included among the three results generated. Positive predictive values can be calculated for the group of three-result combinations known to meet or exceed this nominal criteria (i.e., 1 AB + 1 BL + 1 NL, 2 AB + 1 NL, 1 AB + 2 BL, 2 AB + 1 BL, and 3 AB) [Middleton et al., 2008], or for the individual three-result combinations specified.
FIGURE 1.
One abnormal BeLPT and one borderline BeLPT meet nominal criteria for diagnostic evaluation.
Post-Test Probability of BeS (PTPBeS)
To assess the individual three-result combinations, we define the post-test probability of BeS (PTPBeS) to be the positive predictive value for BeS of any single combination of three results. We define this new parameter to distinguish the predictive value of three-result combinations from PPV calculations that include all outcomes meeting or exceeding a nominal criteria (e.g., 1 AB + 1 BL). Like any positive predictive value, the value of the PTPBeS depends on both the algorithm followed and the prevalence of BeS in the group tested.
Goals and Objectives
The purpose of this analysis is to clarify the role of BL results, especially the result combination 3 BL. Assuming the testing algorithm specified in Figure 1 and a population prevalence of BeS from 1% to 10%, we set out to complete the following:
calculate the PTPBeS values for each three-result combination known to meet or exceed the nominal criteria 1 AB + 1 BL (i.e., 1 AB + 1 BL + 1 NL, 2 AB + 1 NL, 1 AB + 2 BL, 2 AB + 1 BL, and 3 AB); and,
calculate PTPBeS for the result combination 3 BL and compare it to that of the other three-result combinations specified.
METHODS
This project included a reanalysis of existing de-identified and published data; therefore, approval and oversight by an institutional review board was not needed.
Figure 1 shows the algorithm for BeLPT testing which (given an initial BL or AB) yields the three-result combinations. The PTPBeS of each three-result combination is used to rank the combination. While the relative PTPBeS is intuitive for most combinations, the relative PTPBeS for 3 BL is not intuitive. It will be estimated here and compared to that of the other result combinations. The general formula for PPV is [Fleiss et al., 2003]:
This formula is applied in the Results Section to estimate the PTPBeS for the various three-result combinations.
The probability of any specific single test result is different for persons truly sensitized than for persons not truly sensitized. For persons truly BeS, the single test probabilities are PAB = 0.5970, PBL = 0.1260, and PNL = 0.2770. For persons not truly BeS, the single test probabilities are PAB = 0.0109, PBL = 0.0158, and PNL = 0.9733 [Stange et al., 2004; Middleton et al., 2006, 2008]. These single test probabilities are used to estimate the likelihood of having a specific three-result combination occur and then to calculate the combination’s ability to predict BeS (PTPBeS) for population prevalences from 1% to 10%.
RESULTS
Equation (1): we define,
For example, the probability of 3 AB, given true beryllium sensitization, is:
Similarly, the probability of 3 AB for a participant not truly BeS is:
Equation (2): This information is used to calculate each combination’s positive predictive value for BeS as follows:
Because it is a positive predictive value, the PTPBeS for specific three-result combinations depends on the prevalence of BeS in the population tested [Fleiss et al., 2003]. Using Equation (2) for 3 AB results and a 2% prevalence of BeS, the PTPBeS is:
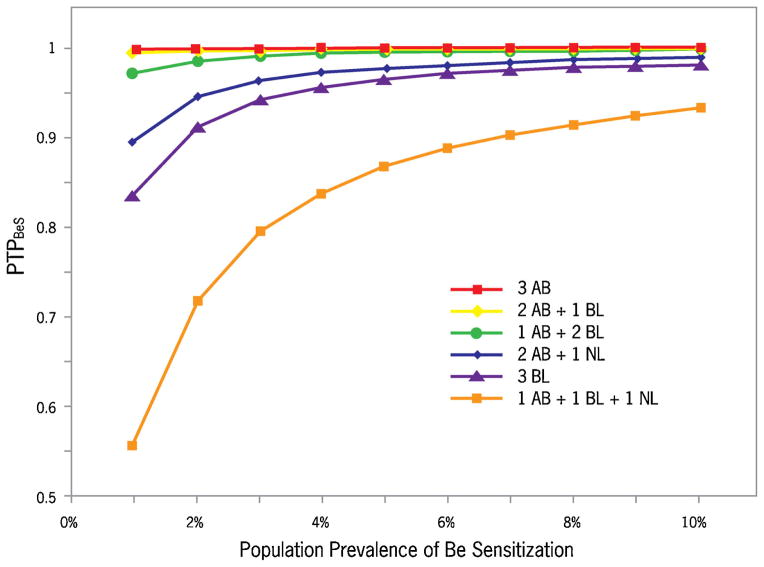
The PTPBeS for the various three-result combinations at 2% prevalence, in order of descending probability of BeS are (Table I): 3 AB (100%); 2 AB + 1 BL (100%); 1 AB + 2 BL (99%); 2 AB + 1 NL (95%); 3 BL (91%); 1 AB + 1 BL + 1 NL (72%). For each three-result combination, the likelihood of BeS rises as the prevalence increases (Fig. 2).
TABLE I.
Post-Test Probability of BeS (PTPBeS) at Various Prevalences, by Result Combination
| Results (three-tests)a | Post-test probability of BeS (PTP-BeS)b
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Population prevalence
| ||||||||||
| 1% | 2% | 3% | 4% | 5% | 6% | 7% | 8% | 9% | 10% | |
| 3 AB | 0.999 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| 2 AB,1BL | 0.996 | 0.998 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 1.000 | 1.000 | 1.000 |
| 1AB, 2 BL | 0.972 | 0.986 | 0.991 | 0.993 | 0.995 | 0.996 | 0.996 | 0.997 | 0.997 | 0.997 |
| 2 AB,1NL | 0.896 | 0.946 | 0.964 | 0.973 | 0.978 | 0.982 | 0.985 | 0.987 | 0.988 | 0.989 |
| 3 BL | 0.837 | 0.912 | 0.940 | 0.955 | 0.964 | 0.970 | 0.974 | 0.978 | 0.980 | 0.983 |
| 1AB,1BL,1NL | 0.557 | 0.717 | 0.794 | 0.838 | 0.867 | 0.888 | 0.903 | 0.915 | 0.925 | 0.932 |
Single test first round, split sample second round if AB or BL.
If PTPBeS ≥ 0.9995, then PTPBeS ~1.000.
FIGURE 2.
Post-test probabilities of BeS (PTPBeS) at various prevalences, by specific three-result combinations.
DISCUSSION
Using the common clinical algorithm for BeLPT testing shown in Figure 1, AB and BL results are followed by a sample split and sent to two laboratories to confirm the initial test result. Across a range of prevalences, we provided model-based calculations for the positive predictive values of various three-result combinations and referred to them as PTPBeS.
The results suggest that BL results do have meaning and that the combination 3 BL has a sufficiently high predictive value in many situations to form an appropriate basis to refer the patient for a diagnostic evaluation. In fact, the predictive value of 3 BL results was higher than that for the minimum three-result combination: 1 AB + 1 BL + 1 NL.
The use of BL results in combination with other results to predict BeS has rarely been evaluated. The best insight into the BeLPT comes from a medical surveillance program conducted over a 10-year period that involved current and former workers at United States Department of Energy sites [Stange et al., 2004]. Stange et al. [2004] found 355 initial BL BeLPT results among 19,396 tests performed on 7,820 workers during serial medical surveillance. The most common follow up test result after a BL was a NL (76.3%; 271/355). Only 9.2% (25/271) of these workers were eventually found to have BeS and 3.3% (9/271) to have CBD. Among 47 workers with an initial BL followed by an AB in the same laboratory, 51.1% (24/47) were found to have BeS and 23.4% (11/47) to have CBD. Among 37 workers with a BL followed by another BL, 35.2% (13/37) were eventually found to have BeS and 8.1% (3/37) to have CBD.
The modeling in our analysis predicts a relatively high positive predictive value for 3 BL, exceeding that of 1 AB + 1 BL + 1 NL. This is not particularly surprising, considering the way BeLPTs are performed and interpreted. One elevated SI (out of six) defines a BL, while two (or more) are required for an AB. Therefore, the number of elevated SIs required for 3 BL (1 + 1 + 1 = 3) is the same as the minimum required for 1 AB + 1 BL + 1 NL (2 + 1 + 0 = 3).
Limitations
We modeled predictive values for BeS prevalences from 1% to 10% based on a single large serial database with a BeS prevalence of 4%. While our testing algorithm is clearly specified, real screening programs are rarely so well-defined or so closely followed. These model-based calculations do not precisely replicate the actual testing performed in a medical surveillance program. This limits the application of model results to real world screening programs.
Further, there are a number of assumptions implicit to the models by Middleton et al. [2006, 2008]. BeLPT results from various laboratories are considered interchangeable in these calculations. BeS is considered to be a constant state and the probabilities for various outcomes are assumed to remain constant over the testing period. The immune systems of all sensitized persons are assumed to respond similarly to in vitro challenges by beryllium sulfate, as are the immune systems of all persons not sensitized. These assumptions are not always consistently met.
Finally, it is also important to note that the analysis presented here only attempts to model screening or surveillance situations. Other factors are sometimes present, such as respiratory signs or symptoms that increase the likelihood of CBD. Such considerations are often critically important when deciding whether or not to conduct a medical evaluation.
Next Steps
The best way to test the utility of the models is to compare them to the actual BeLPT results and clinical evaluations. It might also be possible to identify BeLPT patterns that are more common among those who progress to CBD than among those who do not. Such information would be useful in counseling patients about their risk for CBD to allow doctors and patients to make more informed choices regarding medical evaluations and interventions.
CONCLUSION
The BeLPT is highly specific and moderately sensitive for BeS. BL results can increase the sensitivity of testing and appear to be worth considering. For example, the result combination 3 BL appears to be a fairly good predictor of BeS in many situations. Information about exposure levels, the prevalence of BeS in the group tested, and respiratory signs and symptoms provides a context for decision-making.
We believe that analyses of actual longitudinal BeLPT results and clinical outcomes in another exposed population are warranted. Such analyses should be designed to confirm these findings and the validity of the models in general.
Acknowledgments
The work for this manuscript was performed by staff at the Agency for Toxic Substances and Disease Registry (ATSDR) in Atlanta, GA, and at National Jewish Health, Department of Medicine, Division of Occupational & Environmental Health Sciences in Denver, Colorado. The salaries of the authors were covered by their respective organizations. No other funding was provided for this work.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Agency for Toxic Substances and Disease Registry.
References
- Deubner DC, Goodman M, Iannuzzi J. Variability, predictive value, and uses of the beryllium blood lymphocyte proliferation test (BLPT): Preliminary analysis of the ongoing workforce survey. Appl Occup Environ Hyg. 2001;16:521–526. doi: 10.1080/10473220120220. [DOI] [PubMed] [Google Scholar]
- Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3. New York: John Wiley & Sons; 2003. [Google Scholar]
- Kreiss K, Newman LS, Mroz MM, Campbell PA. Screening blood test identifies subclinical beryllium disease. J Occup Med. 1989;31:603–608. doi: 10.1097/00043764-198907000-00011. [DOI] [PubMed] [Google Scholar]
- Kreiss K, Mroz MM, Zhen B, Martyny J, Newman LS. Epidemiology of beryllium sensitization and disease in nuclear workers. Am Rev Respir Dis. 1993;148:985–991. doi: 10.1164/ajrccm/148.4_Pt_1.985. [DOI] [PubMed] [Google Scholar]
- Kreiss K, Day GA, Schuler CR. Beryllium: A modern industrial hazard. Annu Rev Public Health. 2007;28:259–277. doi: 10.1146/annurev.publhealth.28.021406.144011. [DOI] [PubMed] [Google Scholar]
- Maier LA. Beryllium health effects in the era of the beryllium lymphocyte proliferation test. Appl Occup Environ Hyg. 2001;16:514–520. doi: 10.1080/104732201750169570. [DOI] [PubMed] [Google Scholar]
- Middleton DC, Lewin MD, Kowalski PJ, Cox SS. The BeLPT: Algorithms and implications. Am J Ind Med. 2006;49:36–44. doi: 10.1002/ajim.20241. [DOI] [PubMed] [Google Scholar]
- Middleton DC, Fink J, Kowalski PJ, Lewin MD, Sinks T. Optimizing BeLPT criteria for beryllium sensitization. Am J Ind Med. 2008;51:166–172. doi: 10.1002/ajim.20548. [DOI] [PubMed] [Google Scholar]
- Mroz MM, Kreiss K, Lezotte DC, Campbell PA, Newman LS. Reexamination of the blood lymphocyte transformation test in the diagnosis of chronic beryllium disease. J Allergy Clin Immunol. 1991;88:54–60. doi: 10.1016/0091-6749(91)90300-d. [DOI] [PubMed] [Google Scholar]
- Newman LS, Mroz MM, Schumacher B, Daniloff E, Kreiss K. Beryllium sensitization precedes chronic beryllium disease. Am Rev Respir Dis (Suppl) 1992;145:A324. [Google Scholar]
- Newman LS, Mroz MM, Maier LA, Daniloff EM, Balkissoon R. Efficacy of serial medical surveillance for chronic beryllium disease in a beryllium machining plant. J Occup Environ Med. 2001;43:231–237. doi: 10.1097/00043764-200103000-00011. [DOI] [PubMed] [Google Scholar]
- Rossman MD, Kern JA, Elias JA, Cullen MR, Epstein PE, Preuss OP, Markham TN, Daniele RP. Proliferative response of bronchoalveolar lymphocytes to beryllium: A test for chronic beryllium disease. Ann Intern Med. 1988;108:687–693. doi: 10.7326/0003-4819-108-5-687. [DOI] [PubMed] [Google Scholar]
- Stange AW, Furman FJ, Hilmas DE. Rocky flats beryllium health surveillance. Env Health Perspect. 1996;104:981–986. doi: 10.1289/ehp.96104s5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange AW, Furman FJ, Hilmas DE. The beryllium lymphocyte proliferation test: Relevant issues in beryllium health surveillance. Am J Ind Med. 2004;46:453–462. doi: 10.1002/ajim.20082. [DOI] [PubMed] [Google Scholar]