Abstract
Beryllium is commonly used in the dental industry. This study investigates the association between particle size and shape in induced sputum (IS) with beryllium exposure and oxidative stress in 83 dental technicians. Particle size and shape were defined by laser and video, whereas beryllium exposure data came from self-reports and beryllium lymphocyte proliferation test (BeLPT) results. Heme oxygenase-1 (HO1) gene expression in IS was evaluated by quantitative polymerase chain reaction. A high content of particles (92%) in IS > 5 µ in size is correlated to a positive BeLPT risk (odds ratio [OR] = 3.4, 95% confidence interval [CI]: 0.9–13). Use of masks, hoods, and type of exposure yielded differences in the transparency of IS particles (gray level) and modulate HO1 levels. These results indicate that parameters of size and shape of particles in IS are sensitive to workplace hygiene, affect the level of oxidative stress, and may be potential markers for monitoring hazardous dust exposures.
Keywords: beryllium, bio monitoring, dental technician, occupational exposure, particles exposure, sputum induction
Chronic beryllium disease (CBD) is an occupational health problem of workers involved in the manufacturing and processing of beryllium-containing materials. Beryllium sensitization (BeS) is an immune response to the metallic element without evidence of disease. In an effort to reduce the incidence of CBD and BeS, the US Department of Energy developed an occupational exposure limit of 0.2 µg/m3 for workers and an air emission limit of 10 g/24 hours (Agency for Toxic Substances and Disease Registry; http://www.atsdr.cdc.gov/csem/csem.html).
Lower levels of exposure, however, were reported as posing a risk of sensitization in individuals directly and indirectly exposed to beryllium.1 Although there have been efforts to protect Be-exposed workers, the current occupational health standards for Be do not provide adequate protection against the development of CBD or sensitization.2
The mass of beryllium in a beryllium-containing aerosol is dictated by the particle size distribution. Additionally, the deposition of particles in various regions of the respiratory tract, and therefore bioavailability, is determined by the particle size distribution.
Slower clearance rates of aerosols deposited in the distal airways may result in greater beryllium retention, potentially resulting in increased sensitization and disease rate. In addition, it was shown that respirable beryllium oxide particles dissolved by macrophages over a period of time may be released in the pulmonary alveolar environment, providing the necessary input to the immune system.3 Moreover, particle chemical composition, size, number, and surface area may influence bioavailability of beryllium and may be used in future epidemiology models to more adequately explain the risks associated with BeS and CBD.4–6
Accumulated data suggest that exposure to particulate matter (PM) may lead to pulmonary inflammation7 and that beryllium stimulates the formation of reactive oxygen species (ROS).8 Dobis et al9 have shown that beryllium induced oxidative stress through its ability to deplete endogenous thiol antioxidants and increase ROS levels. Heme oxygenase-1 (HO1) plays a key role in the detoxifying pathways.10 HO1 products, together with enzyme activity, leads to the reduction of oxidation and inflammation.11 We previously showed that the duration of exposure to welding fumes affects particle burden, the number of inflammatory cells, and the level of oxidative stress.12 In the present study, we focus on the biological monitoring exposure metrics resulting from particle size distribution analysis and from particle shape delineation, together with the oxidative stress mechanism as defined by HO1-related gene expression.
METHODS
Study population
The selected workers were all actively practicing dental technicians. Recruitment of the study population was done from the Israeli Trade and Labor Ministry Registry for Dental Technicians Laboratories. We telephone-contacted 38 of the 53 laboratories (71.7%), but only 25 of them employing a total of 115 dental technicians agreed to participate in the study. The final response rate was 83/115 (72.1%) individuals.
Ethical approval was granted by the Tel Aviv Sourasky Medical Center Institutional Ethics Committee. All the subjects gave written informed consent and made one visit for clinical assessment by means of pulmonary function testing (PFT), induction of sputum, and beryllium lymphocyte proliferation testing (BeLPT). All the participants completed a demographic and clinical questionnaire ncluding smoking habits, self-reported diseases, and respiratory symptoms. CBD diagnosis was made by a positive biopsy (noncaseating granuloma) and positive blood test, whereas BeS was diagnosed by a positive blood test but no evidence of beryllium-related lung disease.
Exposure assessment
Exposure assessment was done by a self-reported questionnaire followed by an interview with an occupational physician in order to verify the reported information according to well-established guidelines.13,14 All of the dental technicians worked 8 to 10 hours/day and could be exposed to metals and nonmetal materials (ie, acrylics, monomers, silicates). Some of them were exposed to fumes (ie, burn out furnaces, casting procedures, cleaning chemicals, etc) or dust by-products (ie, polishing grinding sandblasting, mixing, etc) during the processing of the different prosthetic devices, and some were exposed to both due to the limited manpower in small laboratories. The reliability of the self-reported data on fumes and dust was .743 (Cronbach’s alpha test). The dental technicians were exposed before 2001 because the use of beryllium for dental tools was banned in Israel in 2001 by the Israeli Ministry of Health and Ministry of Industry, Trade and Labor.
PFTs, induction of sputum, and processing
PFTs were performed by a Masterlab spirometer (Master-lab E. Jaeger, Würzburg, Germany). The measurement was performed using standard protocols according to American Thoracic Society guidelines.15 Sputum induction was performed during the morning of the last day of the 5-day work week with an aerosol of hypertonic saline generated by an ultrasonic nebulizer (Omron U1; Omron Health Care, Kyoto, Japan). Briefly, subjects inhaled nebulized 3% saline by an ultrasonic nebulizer for up to 20 minutes and were encouraged to cough and expectorate sputum into a sterile plastic container after spitting out the saliva into a different container. We accepted the first sample that became available within 20 minutes of induction. Some patients could “produce” within 10 minutes of inhaling hypertonic saline and others needed more time (never more than 20 minutes according to study protocol). The sputum was processed by the method of Popov et al.16 Flow-cytometric analysis was performed on a dual FACS 440 equipped with an Ar-Kr laser (Becton-Dickinson; Mountain View, CA, USA). Lymphocytic subsets were identified by monoclonal antibodies as follows: CD3 = total T cells, CD4 = T-helper cells, and CD8 = T-suppressor cytotoxic cells.
BeLPT
BeLPT was performed in all participating dental technicians according the method of Mroz et al.17 The normal ranges of the stimulation index (SI) are laboratory dependent and are based on the mean peak SI plus 3 standard deviations (SDs) for unexposed subjects. SIs higher than these values were considered to be elevated. An abnormal BeLPT was required to have 2 or more elevated SIs occurring at any of the BeSO4 concentrations tested. An SI > 2.5 was considered abnormal.
RNA extraction and real-time polymerase chain reaction (RT-PCR)
RNA extraction was done on the sputum cell by TRI reagent–chloroform solutions (Peq gold Trifast, Erlangen, Germany). RT-PCR was carried out with 0.5 µg of total RNA that was extracted from the sputum cells as described elsewhere.18 Quantitative RT-PCR (qRT-PCR) was carried out using Applied Biosystems AB7500 SYBER Green PCR Mix (Applied Biosystems Inc., Carlsbad, CA, USA). Primers for HO1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were design by Primer Express software (v 3.0.1) (Applied Biosystems Inc., Carlsbad, CA, USA).
Particle analyses (size and shape)
Particle size analyses were performed according to a laser technique based on the time of transition theory using an DIPA 2000 analyzer (Donner Technologies, xx, Israel).19 DIPA 2000 is a computerized inspection system for PS analysis (PSA) in the range of 0.5 to 3600 µ employing laser and video measurement. The laser system is based on principles of the laser obscuration/time of transition (TOT) theory. The TOT is directly related to the particle diameter: D = v × t, where D = particle diameter, v = trajectory velocity of the laser beam, t = time. The video system is based on principles of image analysis, and the moving particles are pictured with a sensitive charge-couple device (CCD). Since each aspect ratio (AR) of the pixel array in the CCD camera is known, the software constrains the analysis to define a particle by consist of a minimum number of pixels. We used 5 parameters to characterize the particle shape. The AR represents the ratio of the minimum and maximum ferret diameters to determine round versus long particles. Convexity (Co) determines surface roughness, ranging between 0 (multiple potholes) to 1 (smooth). Circularity (Ci) is a measure of the closeness to a perfect circle and it is sensitive to both changes in overall form and surface roughness, ranging between 0 (nonspherical) and 1 (spherical). Average concavity (AC) is used to characterize the cavity on particles surface. The gray level (GL) characterizes the opaqueness of particles, ranging between 0 (opaque) to 255 (translucid). The measurements were done by adding of 3 drops of induced sputum (IS) suspension into a cuvette containing 3 mL of stirred distilled water. Particle measurement was performed long enough to reach 95% confidence interval [CI]. The results of the particle size distribution were an average of 3 sequential measurements.
Statistical methods
Parameters with normal distribution (shape, carbon monoxide diffusion capacity [DLCO]) were analyzed by parametric tests, whereas those with nonparametric distribution (PFTs and IS cells) were transformed to logarithmic values and then analyzed by a general linear model. Some of the parameters were directly assessed by a nonparametric test (HO1). Associations between the presence of positive BeLPT or exposure (yes/no) and various covariates (type of exposure and protection) were tested by the Fisher exact test for categorical variables. The percentage of particles was examined as a possible predictor for a positive BeLPT test. First, the size of particles was eliminated to particles <5 µ, since only these particles are respirable. Second, a cutoff point for the percentage of particles under 5 µ was determined using a receiver operating characteristic (ROC) curve. Sensitivity and specificity for predicting a positive BeLPT test were calculated and the cutoff point of 92% was chosen, since it yielded the highest sensitivity and specificity (64% and 63%, respectively).
The association between this cutoff and a positive BeLPT was evaluated by logistic regression. Models were evaluated by using the change in −2 log likelihood and the p values of individual covariates to determine their significance to the model. All statistical analyses were performed using the SPSS software version 15.0 for Windows (SPSS, Chicago, IL, USA). All p values are 2-sided, and a p value <.05 was considered significant.
RESULTS
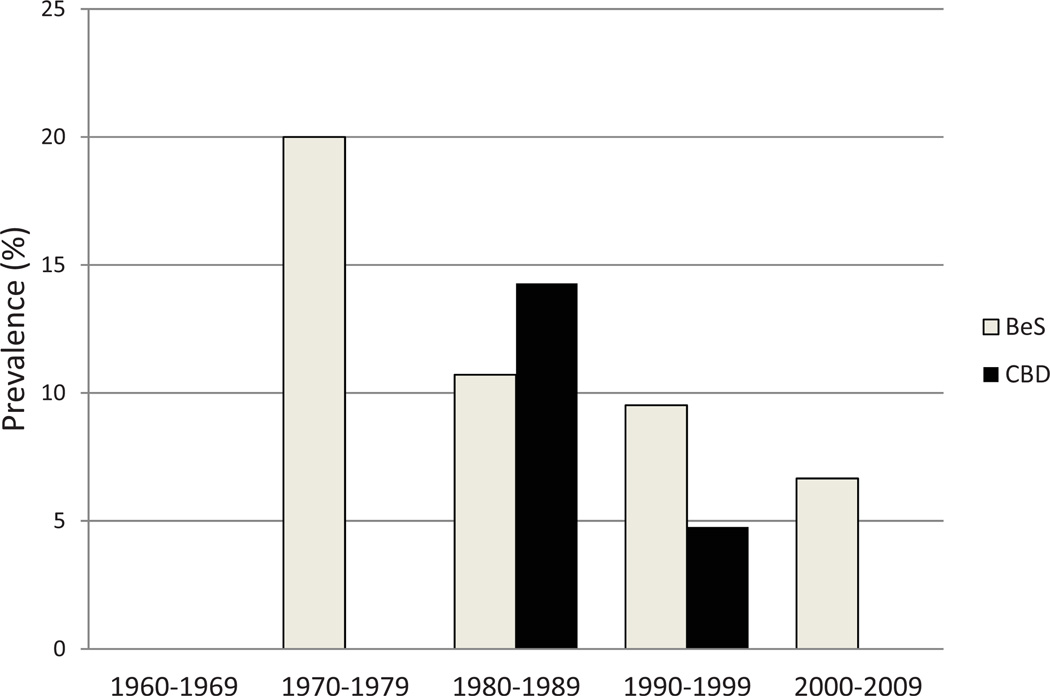
Eighty-three dental technicians were included in the study. There were 69 males (83%) and 14 females (17%). There were no significant gender-based differences in average age, smoking habits or exposure, and extent of exposure (Table 1). The 83-member study group included 68 with negative BeLPT findings and 15 with positive BeLPT findings (9 were newly identified as having BeS individuals and were 6 known as having CBD). Self-reported questionnaire data were fulfilled by all 83 dental technicians, but 69/83 provided information on hygienic conditions (mask and hoods) and 76/83 for type of exposure (fumes vs fumes and dust). Differential cell counts were done in 80/83 samples, T-cell subsets were tested in 69/83, and particle shape and size analyses were carried out in 81/83 IS samples. The prevalence of CBD and BeS was calculated from the first reported exposure decade (1970–1979) to the last one (2000–2009): although it showed a decrease in CBD, there were new sensitization cases (Figure 1).
Table 1.
Subject Characteristics (N = 83)
| Males n = 69 (83%) |
Females n = 14 (17%) |
Total N = 83 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | M | SD | % | M | SD | % | M | SD | % | n |
| Age, years | 41.6 | 11.8 | 37.8 | 10.5 | 41 | 11.6 | ||||
| Exposure, years | 19.3 | 10.8 | 16 | 10.7 | 18.8 | 10.8 | ||||
| Current smoking (yes) | 29%* | 14.2%* | 26% | 22 | ||||||
| Working with beryllium (yes) | 72%* | 77%* | 73% | 61 | ||||||
Percentage of all “Yes” responses from the total according to gender.
Fig. 1.
Prevalence of BeS and CBD in dental technician cohort (N = 83) between years 1960 and 2009. Classification to CBD and BeS was done according BeLPT test and clinical criteria x axis: years in decades (beginning of work by self-report); y axis: point of prevalence (%) was calculated from the number of workers tested by BeLPT in this decade. BeLPT = beryllium lymphocyte proliferation tests; CBD = chronic beryllium disease; BeS = beryllium sensitization.
The exposed group was significantly older than the non-exposed group (43 ± 11 vs 36 ± 9 years, p = .015), and had significantly higher cumulative exposure years (20.4 ± 10 vs 14.2 ± 10, p = .024), but no differences were found regarding other types of exposures. Exposure to beryllium was strongly associated with positive BeLPT findings (odds ratio [OR] = 3.2, 95% CI: 0.97–10.6, p = .06) (Table 2).
Table 2.
Exposure Assessment of the Study Population (N = 83)*
| Exposed to beryllium |
n | Age (years) |
Working years (years) |
Positive BeLPT† (n = 15, 18.1%) % |
|||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Yes | 61 | 43 | 11.9 | 20.4 | 10.6 | 25%‡ | |
| No | 22 | 36 | 9 | 14.2 | 10.1 | 4.8%‡ | |
| p = .015§ | p = .024§ | OR: 3.2 CI: 0.97–10.6 p = .06 |
|||||
Note. BeLPT = beryllium lymphocyte proliferation tests; OR= odds ratio; CI = confidence interval.
Self-reported on a questionnaire.
Positive BeLPT: 2 or more concentration above the SI in BeLPT test (CBD or BeS).
Percentage of positive BeLPT (4.8% represent 1 worker that was unaware of his exposure).
t test analysis: p = .015 for age and p = .024 for cumulative years of exposure.
Chi-square analysis.
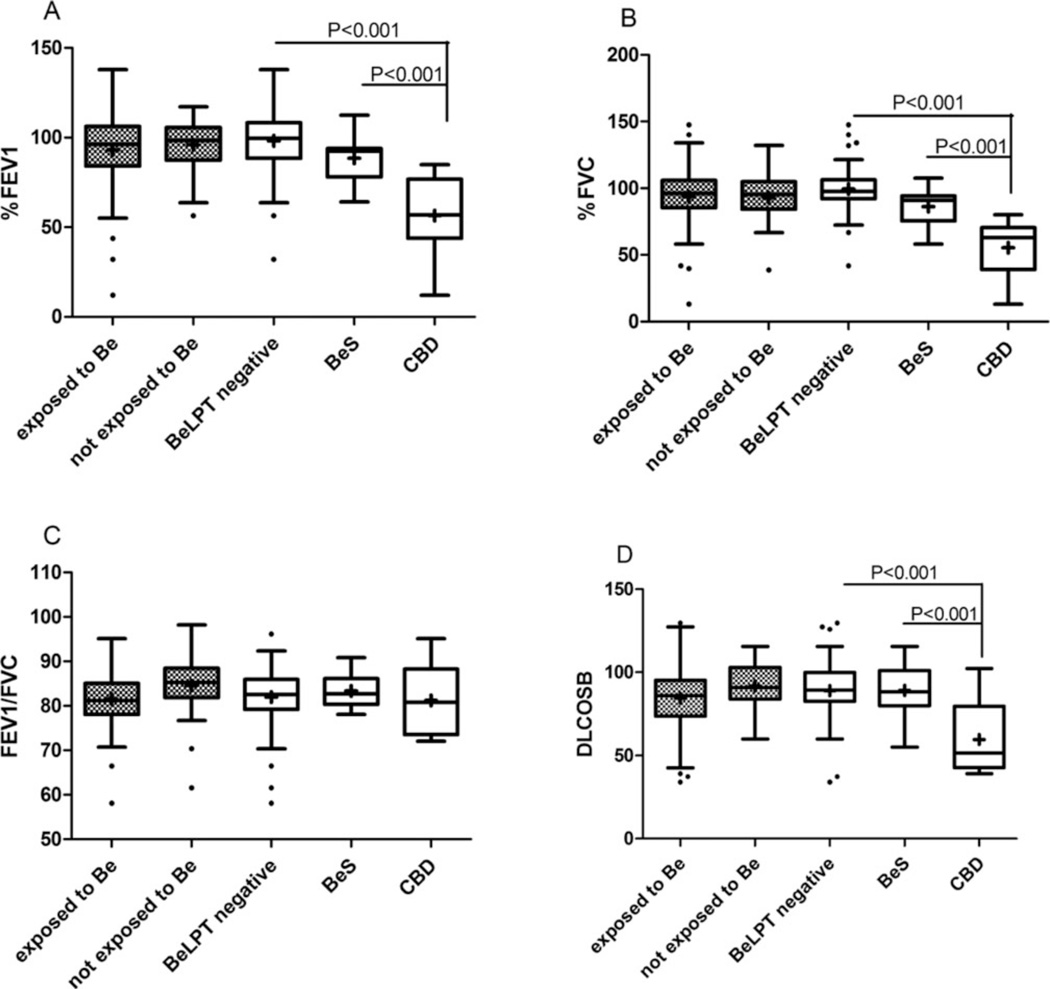
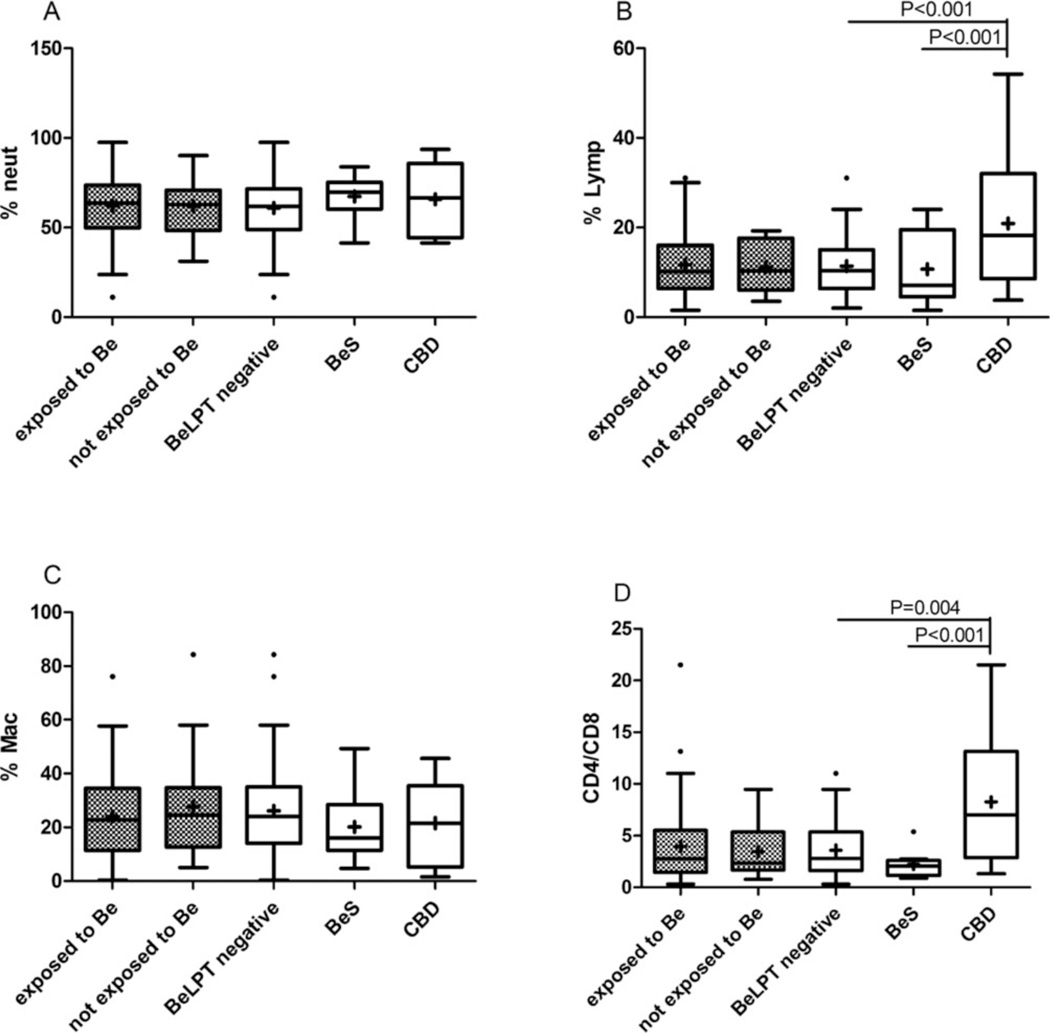
All study participants’ PFTs were within normal values for both exposed and unexposed individuals (Figure 2). However, a significant decline was observed in all of the PFT parameters of those diagnosed as having CBD compared with the technicians with BeS and those with a negative BeLPT (percent forced expiratory volume in 1 second [FEV1%]: 54 ± 24 vs 90 ± 12 and 97.3 ± 16.4, p < .001; percent forced vital capacity [FVC%]: 56.7 ± 23.1 vs 88.5 ± 11.6 and 98.7 ± 15.9, p < .001; 56 ± 16 vs 93.5 ± 13.8 and 89.8 ± 16.7, p < .001, with the exception of the FEV1/FVC values) (Figure 2). Cellular parameters in the IS samples were tested by differential cell counts and CD4/CD8 analysis. The CBD group had a significantly higher CD4/CD8 ratio and higher percentages of lymphocytes compared with both the BeS group and the negative BeLPT workers (9.4 ± 6.86 vs 2.2 ± 1.4 and 3.7 ± 2.6, p < .001; 21.4 ± 16 vs 12.4 ± 7.8 and 11.2 ± 6.2, p < .001; respectively) (Figure 3).
Fig. 2.
Pulmonary function test of study population (N = 83). Box plot diagrams representing medians and interquartile ranges. Gray dots boxes: workers exposed (n = 61) and nonexposed (n = 22) to Be by self-report. White boxes: workers classified by BeLPT result p < 05 was found to be significant using GLM model after logarithmic transformation (negative BeLPT: n = 68; BeS: n = 9; CBD n = 6). (A) FEV1 = forced expiratory volume in 1 second; (B) FVC = forced vital capacity; (C) TLC = total lung capacity; (D): DLcoSB = diffusion capacity of lung CO in a single breath.
Fig. 3.
Induced sputum (IS) differential cell count (DCC) in the study population (N = 80). (A, B, C) Differential cell counts were performed by counting 400 cells in Giemsa-stained cytopreps. White boxes: workers classified by BeLPT results (negative BeLPT: n = 66; BeS: n = 8; CBD: n = 6). Gray dots boxes: workers exposed (n = 59) and nonexposed (n = 21) to Be by self-report. (D) T-cell subpopulations CD4 and CD8 were done by FACS analysis. White boxes: workers classified by BeLPT result (negative BeLPT: n = 56; BeS: n = 8; CBD: n = 5). Gray dots boxes: workers exposed (n = 52) and nonexposed (n = 17) to Be by self-report p < 05 was considered significant using GLM model after logarithmic transformation. (A) Neut = neutrophils; (B) Lym = lymphocytes; (C) Mac = macrophages; (D) CD4 = helper T cells; CD8 = T cells.
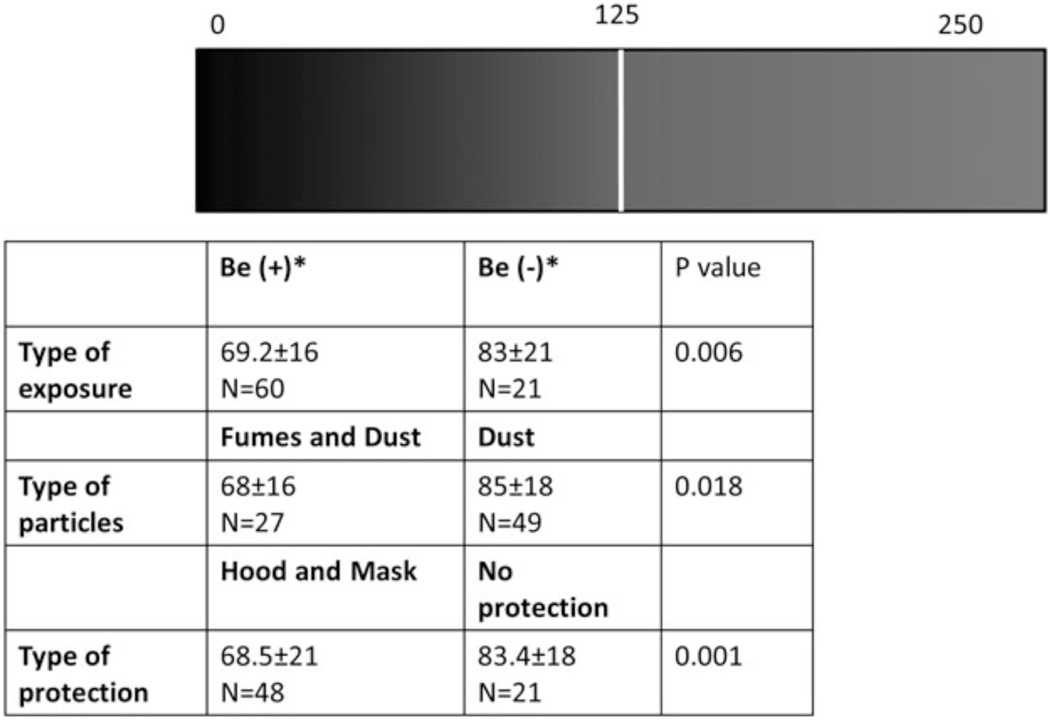
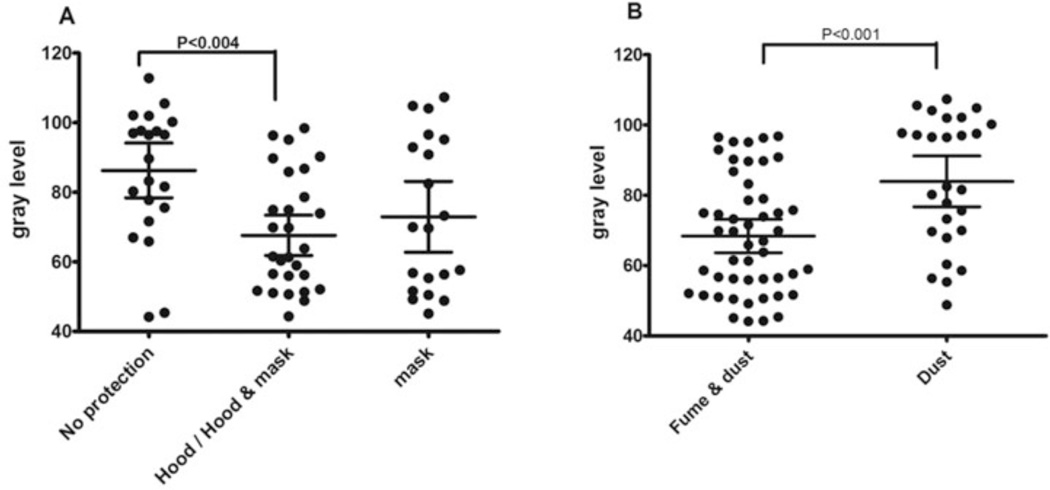
We analyzed the size and shape of particles present in the IS of 81/83 of the participating dental technicians, first according to beryllium exposure, then according to their BeLPT test results, and finally according to the type of protection used at the workplace (Table 3 and Figure 4). We found that the transparency of the particles measured by image analysis (the GL) was significantly lower in the exposed group compared with the nonexposed group (69.2 ± 16 vs 83 ± 21, p = .006), but that the BeLPT results had no significantly influence on the analysis of the particle size and shape (Table 3). Further analysis of GL in terms of the type of hygienic protection revealed that the GL index of particle mixtures in the airways of unprotected technicians (83.4 ± 18) was significantly higher than the GL index of workers who used any kind of hygienic protection at the workplace (68.5 ± 21, p = .001) (Figures 4 and 5A). In terms of the type of exposure, the GL index of transparency was significantly higher in workers exposed to dust (85 ± 18) compared with those exposed to fumes (68 ± 16, p = .018) (Figures 4 and 5B).
Table 3.
Particle Shape in Induced Sputum Samples of Dental Technicians Who Were and Were Not Exposed to Beryllium According to Questionnaire and BeLPT Test (N = 81)
| Particle shape |
Particle size % Particle <5 µ |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AR |
CO |
Ci |
AC |
GL |
||||||||
| Characteristic | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD |
| Exposed* (n = 60) | 0.6 | 0.1 | 0.9 | 0.01 | 0.59 | 0.05 | 0.88 | 0.01 | 69.2 | 16 | 87.4 | 10 |
| Nonexposed* (n = 21) | 0.62 | 0.07 | 0.9 | 0.03 | 0.56 | 0.1 | 0.88 | 0.03 | 83 | 21 | 88.9 | 10 |
| p = .5 | p = .6 | p = .2 | p = .7 | p = .006 | p = .3 | |||||||
| Negative BeLPT† (n = 66) | 0.62 | 0.08 | 0.9 | 0.02 | 0.57 | 0.09 | 0.88 | 0.02 | 73.8 | 19 | 87.7 | 11 |
| BeS (n = 9) | 0.57 | 0.1 | 0.9 | 0.01 | 0.61 | 0.05 | 0.88 | 0.01 | 71.2 | 18 | 89.3 | 8.2 |
| CBD (n = 6) | 0.6 | 0.03 | 0.89 | 0.01 | 0.58 | 0.04 | 0.88 | 0.01 | 69.3 | 20 | 93.3 | 4 |
| p = .3 | p = .6 | p = .9 | p = .7 | p = .5 | p = .2 | |||||||
By self-report.
Classification based on BeLPT test and clinical findings.
BeLPT = beryllium lymphocyte proliferation tests; CBD = chronic beryllium disease; BeS = beryllium sensitization. AR = aspect ratio; Co = convexity; Ci = circularity; AC = average concavity; GL = gray level.
Fig. 4.
Gray level (GL) ranges according type of exposure, particles, and protection. *GL ranges were measured by image analysis as described in Methods. The index ranged from 0 (opaque) to 255 (transparent). The use of protection means was evaluated by self-report and confirmed by an occupational physician p < 05 was found to be significant by ANOVA analysis and multiple comparisons. Fume = produced by condensation of metal vapors; dust = generated by activities.
Fig. 5.
Gray level (GL) of induced sputum (IS) particles of study population. (A) Working in different hygienic conditions: no protection (n = 21); hood and mask protection (n = 28); mask protection (n = 20). (B) Exposed to Be fumes and dust. GL was evaluated by an Eyetech scanner, and the index ranged from 0 (opaque) to 255 (fume and dust: n = 49; dust: n = 27) (transparent). Box plot diagrams representing means and interquartile ranges p < 05 was found to be significant by ANOVA analysis and multiple comparisons. Fume = produced by condensation of metal vapors; dust = generated by activities such as cutting, crushing, and detonation of metal.
We sought a cutoff for percent of particles in an IS sample that would confer a risk of having a positive BeLPT. All measurements of particles <5 µ were divided into binary parameters by using the interquartile range (IQR): 43 patients were classified as being under the IQR and 38 above it. Logistic regression showed that dental technicians who were classified in the upper quartile range of 92% of particles <5 µ were at greater risk to develop a positive BeLPT (OR = 3.16, 95% CI: 0.96–10.51, p = .05) (Table 4). Moreover, this accumulation of particles <5 µ induced a higher percent of lymphocytes in IS samples (12.75 ± 9.35 vs 10.8 ± 6.11, p = .06), with no change in the CD4/CD8 ratio (Table 5).
Table 4.
A Interquartile Range (IQR) and Cutoff of Particle Size Distribution (PSD) < 5 in the Study Population (N = 81)
| Parameter | Coefficient estimate |
OR | 95% Confidence interval |
p value |
|
|---|---|---|---|---|---|
| Particle 0–5 µ | 1.15 | 3.16 | 0.96 | 10.51 | .05 |
| Age | 0.26 | 1.02 | 0.97 | 1.082 | .33 |
| Smoking | −0.57 | 0.56 | 0.13 | 2.41 | .43 |
| No protection mean | −0.63 | 0.53 | 0.11 | 2.45 | .53 |
| Hood protection | −0.78 | 0.41 | 0.066 | 2.62 | .41 |
| Hood and personal masks protection | −1 | 0.37 | 0.068 | 2 | .37 |
Particles 0–5 µ were evaluated by Eyetech laser scanner and divided to dichotomy parameter as describe in Methods. The use of protection means was evaluated by self-report and confirmed by an occupational physician. The “no protection” group was used as reference group. p value and confidence intervals was calculated by logistic regression model.
Table 5.
Deferential Cell Count and Particle < 5 µ in Induced Sputum Samples of Dental Technicians (N = 80)
| Macrophages |
Lymphocytes |
Neutrophils |
CD4/CD8 |
HO1 gene expression |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Particles | M | SD | M | SD | M | SD | M | SD | M | SD |
| Particles <5 µ (<92%) | 23.03 | 16 | 10.8 | 6.11 | 63.07 | 18.75 | 4.05 | 3.66 | 0.07 | 0.03 |
| Particles <5 µ (>92%) | 27.61 | 18.28 | 12.75 | 9.35 | 58.4 | 20.65 | 3.5 | 3.08 | 0.06 | 0.02 |
| p value | .17 | .06 | .06 | .12 | .89 | |||||
Note. Particles <5 µ was evaluated by Eyetech laser scanner and divided into dichotomy parameters as describe in Statistical Methods. CD4 = helper T cells; CD8 = T cells.
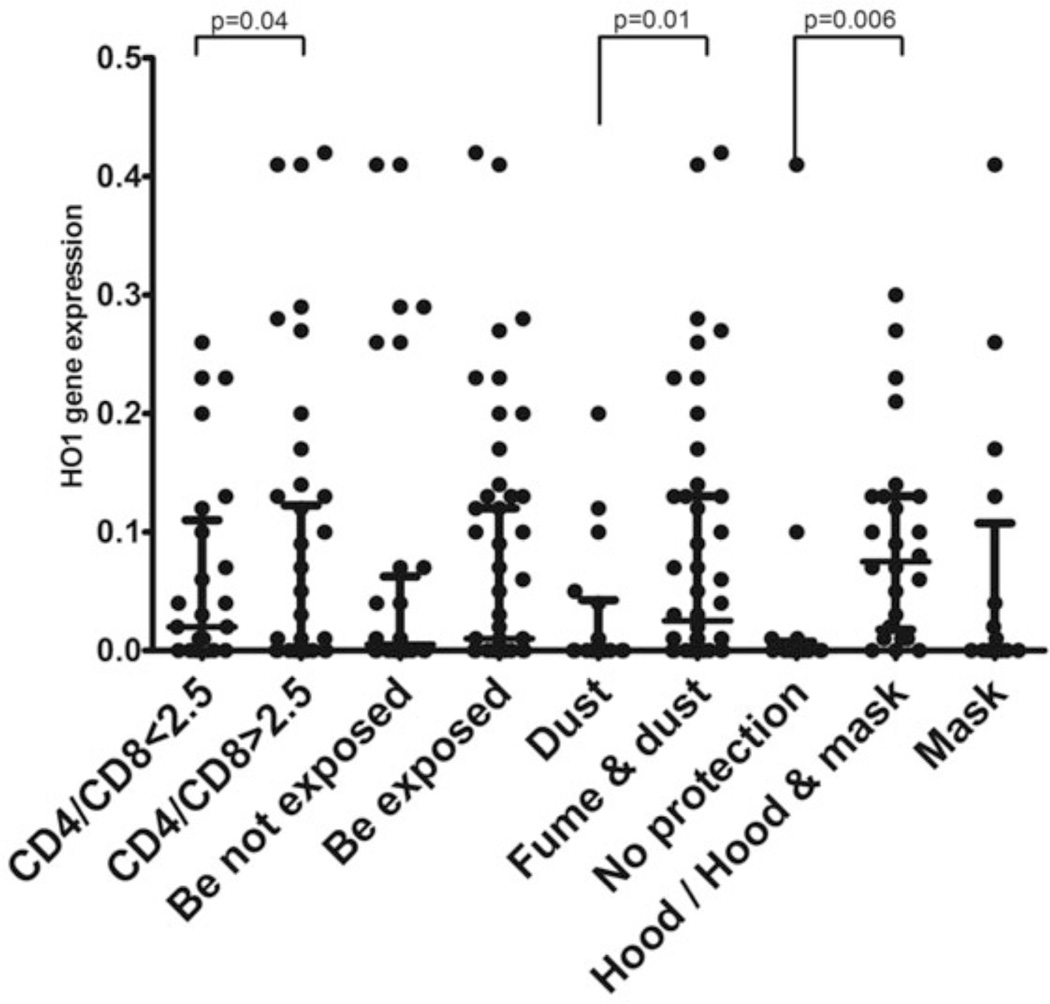
The oxidative study analysis focused on HO1 gene expression. A high expression of the HO1 gene was associated with a normal (ie, <2.5) CD4/CD8 ratio (0.065 ± 0.08 abnormal ratio vs 0.05 ± 0.09 normal ratio, p = .04). This HO1 gene expression was more likely to be higher in the combined fume- and dust-exposed group compared with the group exposed solely to dust (0.08 ± 0.1 vs 0.04 ± 0.07, respectively, p = .015). The group that did not use any protection had significant lower expression of HO1 compared with those who did use hood/hood and mask protection (0.035 ± 0.1 vs 0.07 ± 0.008, respectively, p = .006) (Figure 6).
Fig. 6.
HO1 gene expression in dental technicians exposed to beryllium dust and fumes. Gene expression was evaluated by qRT-PCR. The medians and interquartile ranges are shown. (A) CD4/CD8 subpopulation (< 2.5: n = 27; >2.5:n = 34). (B) Beryllium exposure by self-report (exposed: n = 56; not exposed: n = 18). (C) Dust- (n = 24) vs fume- (n = 44) exposed subjects. (D) Different hygienic conditions: no protection (n = 16); hood and mask protection (n = 27); mask protection (n = 18).
COMMENT
We showed here for the first time that hygienic conditions in the workplace may affect particles in the airways and that these changes are directly correlated to the oxidative stress mechanism. According to the US Occupational Safety and Health Administration, workplace exposure limits for beryllium are 8-hour time-weighted average of 2 µg/m3. This standard was taken as being protective against acute beryllium pneumonitis and CBD. The standard method for sampling beryllium content in the ambient air is by cellulose acetate filters at an airflow rate of 1 L/min, and this is analyzed using atomic absorption spectrophotometry. Several studies have indicated that the current 2 µg/m3 exposure limit for beryllium in the workplace is grossly inadequate to prevent disease occurrence.20,21 Furthermore, CBD and BeS have been identified in workers whose average beryllium exposure levels were 20–100 times lower than the current permissible limit.20 It is clear that the current method does not take into account the internal exposure, which is affected by the hygienic condition and by the cumulative exposure. Moreover, aerosol concentration does not reflect the internal particulate matter burden. Little is known about the physicochemical properties of beryllium aerosols associated with increased risk of CBD and BeS. It is assumed that chemical composition, size, number, and surface area may influence the bioavailability of beryllium and contribute to the risk of CBD by the slow release of beryllium ions (Be2+) produced by particles that dissolve in the lung over time.5,6
The present study focused on the internal exposure assessment of particles in IS samples of dental technicians who had been exposed to hazardous dust and fumes by characterizing the shape and size of the particles. The first case of CBD in a dental technician in Israel was reported by us in 2001.22 A subsequent study on a cohort of dental technicians involved in casting, cutting, grinding, polishing, and finishing of dental alloys containing beryllium revealed that one half of them were suffering from CBD.23 In both of those studies, the analysis of the chemical composition of the particles was done by scanning electron microscope analysis, which found a wide range of metals (Cr, Au, Fe, Zr, Pb, Ni, etc) but not beryllium. We do not have the technology to directly determine the presence of beryllium in the lungs. However, in 1996, Newman et al reported that beryllium can be inferred to be present if the BeLPT results are positive.24
Although the use of beryllium for dental tools has been banned in Israel by the Israeli Ministry of Health and Ministry of Industry, Trade and Labor—noting that most of CBD patients were dental technicians—new cases continue to be diagnosed due to past exposures or to disregarding the prohibition. Lack of awareness of the potentially dangerous effect of beryllium exposure or inadequate supervision in workplaces may result in morbidity, with PFT deterioration and respiratory symptom complaints. Indeed, although three quarters of our cohort (61/83, 73%) were aware of past exposure to beryllium, the others were not clear about the issue or about the ramifications of past exposure, and 6 of them already had CBD at the time of study enrollment. One pitfall of self-reported questionnaires is the accuracy of recall. In this context, the validity of our retrieved data was confirmed first by the correlation of BeLPT results to self-reported beryllium exposure and second by the correlation to age (ie, young dental technicians were more aware of the health effects of beryllium exposure). Moreover, it was demonstrated that data recovered by occupational physicians was less likely to be distorted or inaccurate.13,25
Our results that showed deterioration of functional parameters (eg, FEV1, FVC) in BeS and CBD were compatible with those of several other studies that reported a correlation between functional and clinical status.26,27 However, we found no significant differences in PFT findings between exposed and nonexposed workers, indicating that genetic factors may have a crucial role in the development of BeS and CBD.28
Cellular lymphocytic parameters in IS samples also correlated with disease activity. Elevated percentage of lymphocytes and CD4/CD8 ratio in sputum samples were studied intensively in interstitial lung diseases (including occupational lung diseases): the results demonstrated that this ratio can effectively identify CD4+ inflammation and that it correlates with the severity of the disease.18 Another study23 that focused on 17 different workplaces with possible exposure to beryllium demonstrated that the CD4/CD8 ratios in IS are highly suggestive for supporting the diagnosis of CBD.
The GL, which is a shape parameter indicating the grade of transparency of the particles, was demonstrated here as being significantly associated with type of exposure, type of particles (ie, fumes and dust), and type of protection at work (ie, masks and hood). There are few data on the physiochemical properties of beryllium aerosols associated with increased risk of beryllium sensitization and CBD. Such information is needed to evaluate whether airborne mass of beryllium is the appropriate metric of exposure or, alternatively, to provide a scientific basis for using information on particle size, surface area, and chemistry to support an improved exposure limit based on bioavailability through the inhalation and dermal routes of exposure.5
It is known that spectral characteristics of the transparency and polarization of scattered and emitted light of particles may be a marker of physical properties (ie, size distribution, composition, and structure).29 We believe that shape parameters (ie, GL) depend upon the composition of chemical elements, which was shown here to be modulated by the type of exposure to particles and the protective modules that were used. The precise influence of taking precautionary measures on particulate deposition in the lung remains undetermined. The use of protective equipment could result in modifying the particle size of the deposited fraction in the lung. Moreover, the small particles that pass through the protective equipment may be altered in terms of the level of transparency as well.30 It must be noted that although the differences of GLs in the negative BeLPT, the BeS and the CBD groups are not statistically significant, probably due to the small numbers in each group, there is nevertheless a clear decreasing trend in the index of transparency towards the CBD. It is known that particle deposition in various regions of the lungs has been shown to vary with airway diseases. The global prevalence of obstructive airways diseases (asthma, chronic obstructive pulmonary disease, and emphysema) is significant, and each of these diseases results in particle deposition that is substantially different from the norm. However, there are only limited data on the various deposition efficiencies associated with beryllium exposure-related workers. The gray level, novel shape parameter presented herein, may contribute new perspectives on these issues.
We used the analysis of particulate size distribution in IS to predict the risk for developing sensitization to beryllium. It emerged that a cutoff at 92% of particles under 5 µ in size is associated with the risk for a positive BeLPT result. It is known that large particles (>5 µ) are removed from the lungs by physical mechanisms (eg, cilia epithelium) and coughing, whereas small particles invade the alveoli and can remain there for as long as 1 year after exposure.31 Indeed, our group found that particles inhaled during the collapse of the World Trade Center can be measured in the subjects’ IS 10 months after the event and that they were smaller than particles in the air.32 Moreover, this accumulation seems to induce lymphocytic inflammation, as was previously shown in a longitudinal study for exposed workers.33
Our analysis of the correlation between the molecular pathways of HO1 and exposure assessment revealed that the fume- and dust-exposed group had a higher HO1 gene expression than the group exposed to dust alone. This is compatible with previous evidence that inorganic dust and metal fumes induce inflammation and oxidative stress in the airways34 and that fume exposure promoted oxidative stress as a defense response expressed by HO1 gene expression.8 Moreover, HO1-positive alveolar macrophages during immunostaining in the lungs of rats exposed to crocidolite asbestos35 and increased expression of pulmonary HO1 in silicosis mediated the suppression of reactive oxygen species activity and led to subsequent pathological changes, thereby attenuating disease progression. The higher levels of HO1 in dust- and fume-exposed dental technicians represent an additive effect compared with exposure to dust alone and show a key role of this enzyme in the control of oxidative stress as a vital immune protective effect.36
On the other hand, the workers who did not use any protection had the lowest HO1 gene expression. This raises the possibility that the information regarding respirators as retrieved from the questionnaire is not sensitive enough to clearly distinguish those who are provided protection from those who are not, and/or whether the respirators were actually used by those performing jobs with the highest potential for exposure. It is also not entirely clear whether any inappropriate or inconsistent use of respirators may result in higher exposure than when respirators are not used. In addition, the findings with regard to protection may be correlated to the type of particles deposited in the airways of those workers, given that induction of different immune pathways were shown to be associated with the different kinds of mineral particles that were induced.37
Our results showed that HO1 gene expression correlated negatively with the CD4/CD8 ratio in our subjects’ IS. It is known that CD4+ T cells play a critical role in the immunopathogenesis of CBD,38 and that HO1 plays an important role in modulating immune reactions induced by various T-cell subpopulations.36 We assume that an elevated HO1 gene expression reduces the risk of developing pathology characterized by CD4 inflammation, whereas failure of this protective role of HO1 is characterized by an elevated CD4/CD8 ratio. The main limitation of the current study is the use of a novel parameter (the GL) in the exposure metrics of exposed workers in general and dental technicians in particular. This is a pioneer study in the field of biological monitoring; therefore, it cannot be compared with findings on various types of exposure in different populations. However, we believe that the addition of the shape parameter to the already well-established size index may be used to assess early airway damage at an individual level.
In conclusion, this is the first study to demonstrate that dental technicians exposed to particles in the workplace have a typical pattern of particle morphology in their lungs. This morphology, as demonstrated by the new metric, the gray level, is affected by the hygienic condition of the workplaces and by-products of the working process (fumes and dust), and that it is associated with oxidative stress in the molecular pathway.
Acknowledgments
The research was funded partly the USA-Israel Bi-National Science Foundation.
This work was performed in partial fulfillment of the requirements for a PhD degree of Moshe Stark, Sackler Faculty of Medicine, Tel Aviv University, Israel.
Footnotes
Publisher's Disclaimer: Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information. Taylor and Francis shall not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of the Content.
This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden. Terms & Conditions of access and use can be found at http://www.tandfonline.com/page/terms-and-conditions
Contributor Information
Moshe Stark, Institute of Pulmonary Diseases, National Laboratory Service for ILD, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; and the Department of Epidemiology and Preventive Medicine, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Yehuda Lerman, Department of Occupational Health, Clalit Medical Services, Tel Aviv, Israel; and the Department of Epidemiology and Preventive Medicine, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Arik Kapel, Department of Occupational Health, Clalit Medical Services, Tel Aviv, Israel.
Asher Pardo, Department of Environmental and Occupational Health, School of Public Health, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Yehuda Schwarz, Institute of Pulmonary Diseases, National Laboratory Service for ILD, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
Lee Newman, Department of Environmental Occupational Health, Colorado School of Public Health, and Department of Medicine, School of Medicine, University of Colorado, Aurora, Colorado, USA.
Lisa Maier, Department of Environmental and Occupational Health Sciences, National Jewish Medical and Research Center, Denver, Colorado, USA.
Elizabeth Fireman, Institute of Pulmonary Diseases, National Laboratory Service for ILD, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; and the Department of Epidemiology and Preventive Medicine, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
References
- 1.Schuler CR, Virji MA, Deubner DC, et al. Sensitization and chronic beryllium disease at a primary manufacturing facility, part 3: exposure–response among short-term workers. Scand J Work Environ Health. 2012;38(3):270–281. doi: 10.5271/sjweh.3192. [DOI] [PubMed] [Google Scholar]
- 2.Rosenman K, V. Hertzberg C, Rice MJ, et al. Chronic beryllium disease and sensitization at a beryllium processing facility. Environ Health Perspect. 2005;113:1366–1372. doi: 10.1289/ehp.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefaniak AB, Virji MA, Day GA. Dissolution of beryllium in artificial lung alveolar macrophage phagolysosomal fluid. Chemosphere. 2011;83:1181–1187. doi: 10.1016/j.chemosphere.2010.12.088. [DOI] [PubMed] [Google Scholar]
- 4.Stefaniak AB, Hoover MD, Dickerson RM, et al. Surface area of respirable beryllium metal, oxide, and copper alloy aerosols and implications for assessment of exposure risk of chronic beryllium disease. AIHA J. 2003;64:297–305. doi: 10.1080/15428110308984820. [DOI] [PubMed] [Google Scholar]
- 5.Stefaniak AB, Hoover MD, Day GA, et al. Characterization of physicochemical properties of beryllium aerosols associated with prevalence of chronic beryllium disease. J Environ Monit. 2004;6:523–532. doi: 10.1039/b316256g. [DOI] [PubMed] [Google Scholar]
- 6.Day GA, Hoover MD, Stefaniak AB, et al. Bioavailability of beryllium oxide particles: an in vitro study in the murine J774A.1 macrophage cell line model. Exp Lung Res. 2005;31:341–360. doi: 10.1080/01902140590918731. [DOI] [PubMed] [Google Scholar]
- 7.Salvi S, Blomberg A, Rudell B, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999;159:702–709. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- 8.Sawyer RT, Dobis DR, Goldstein M, et al. Beryllium-stimulated reactive oxygen species and macrophage apoptosis. Free Radic Biol Med. 2005;38:928–937. doi: 10.1016/j.freeradbiomed.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Dobis DR, Sawyer RT, Gillespie MM, et al. Modulation of lymphocyte proliferation by antioxidants in chronic beryllium disease. Am J Respir Crit Care Med. 2008;177:1002–1011. doi: 10.1164/rccm.200707-1021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morse D. The role of heme oxygenase-1 in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29(3 Suppl):S82–S86. [PubMed] [Google Scholar]
- 11.Eisenstein RS, Garcia-Mayol D, Pettingell W, Munro HN. Regulation of ferritin and heme oxygenase synthesis in rat fibroblasts by different forms of iron. Proc Nat Acad Sci U S A. 1991;88:688–692. doi: 10.1073/pnas.88.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stark M, Zubareb J, Jacovovitz R, et al. HO-1 and VEGF gene expressions are time dependant during exposure to welding fumes. Cytokine. 2009;46:290–295. doi: 10.1016/j.cyto.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Rybicki BA, Johnson CC, Peterson EL, Kortsha GX, Gorell JM. Comparability of different methods of retrospective exposure assessment of metals in manufacturing industries. Am J Ind Med. 1997;31:36–43. doi: 10.1002/(sici)1097-0274(199701)31:1<36::aid-ajim6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Benke G, Sim M, Fritschi L, Aldred G, Forbes A, Kauppinen T. Comparison of occupational exposure using three different methods: hygiene panel, job exposure matrix (JEM), and self reports. Appl Occup Environ Hyg. 2001;16:84–91. doi: 10.1080/104732201456168. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.Popov TA, Pizzichini MM, Pizzichini E, et al. Some technical factors influencing the induction of sputum for cell analysis. Eur Respir J. 1995;8:559–565. [PubMed] [Google Scholar]
- 17.Mroz MM, Kreiss K, Lezotte DC, Campbell PA, Newman LS. Reeblood lymphocyte transformation test in the diagnosis of chronic beryllium disease. J Allergy Clin Immunol. 1991;88:54–60. doi: 10.1016/0091-6749(91)90300-d. [DOI] [PubMed] [Google Scholar]
- 18.Fireman E, Topilsky I, Greif J, et al. Induced sputum compared to bronchoalveolar lavage for evaluating patients with sarcoidosis and non-granulomatous interstitial lung disease. Respir Med. 1999;93:827–834. doi: 10.1016/s0954-6111(99)90269-x. [DOI] [PubMed] [Google Scholar]
- 19.Lerman Y, Segal B, Rochvarger M, Weinberg D, Kivity O, Fireman E. Induced-sputum particle size distribution and pulmonary function in foundry workers. Arch Environ Health. 2003;58:565–571. doi: 10.3200/AEOH.58.9.565-571. [DOI] [PubMed] [Google Scholar]
- 20.Kelleher PC, Martyny JW, Mroz MM, Maier LA, Ruttenber AJ, Young DA, Newman LS. Beryllium particulate exposure and disease relations in a beryllium machining plant. J Occup Environ Med. 2001;43:238–249. doi: 10.1097/00043764-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Newman LS, Mroz MM, Maier LA, Daniloff EM, Balkissoon R. Efficacy of serial medical surveillance for chronic beryllium disease in a beryllium machining plant. J Occup Environ Med. 2001;43:231–237. doi: 10.1097/00043764-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Fireman E, Kramer MR, Kaufman N, Muller-Quernheim J, Lerman Y. Beryllium disease: first case reported in Israel. Isr Med Assoc J. 2001;3:224–225. [PubMed] [Google Scholar]
- 23.Fireman EM, Mazor O, Kramer M, Priel I, Lerman Y. Noninvasive diagnosis of chronic beryllium disease in workers exposed to hazardous dust in Israel. Occup Environ Med. 2010;67:631–635. doi: 10.1136/oem.2009.050039. [DOI] [PubMed] [Google Scholar]
- 24.Newman LS. Significance of the blood beryllium lymphocyte proliferation test. Environ Health Perspect. 1996;104(Suppl 5):953–956. doi: 10.1289/ehp.96104s5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakke PS, Hanoa R, Gulsvik A. Relation of occupational exposure to respiratory symptoms and asthma in a general population sample: self-reported versus interview-based exposure data. Am J Epidemiol. 2001;154:477–483. doi: 10.1093/aje/154.5.477. [DOI] [PubMed] [Google Scholar]
- 26.Richeldi L. Chronic beryllium disease: a model for pulmonary sarcoidosis? Acta Biomed. 2005;76(Suppl 2):11–14. [PubMed] [Google Scholar]
- 27.Rodrigues EG, McClean MD, Weinberg J, Pepper LD. Beryllium sensitization and lung function among former workers at the Nevada Test Site. Am J Ind Med. 2008;51:512–523. doi: 10.1002/ajim.20585. [DOI] [PubMed] [Google Scholar]
- 28.McCleskey TM, Buchner V, Field RW, Scott BL. Recent advances in understanding the biomolecular basis of chronic beryllium disease: a review. Rev Environ Health. 2009;24:75–115. doi: 10.1515/reveh.2009.24.2.75. [DOI] [PubMed] [Google Scholar]
- 29.Kolokolova L, Gustafson BÅ. Scattering by inhomogeneous particles: microwave analog experiment comparison to effective medium theories. J Quant Spectrosc Radiat Transfer. 2001;70:611–625. [Google Scholar]
- 30.Truce R, Crynach R, Blair R. The problem of fine particles: POWER. 2008 Sep 30; Available at: http://www.coalpowermag.com/environmental/the-problem-of-fine-particles156.html. [Google Scholar]
- 31.Carvalhoa TC, Petersb JI, Williams RO. Influence of particle size on regional lung deposition: what evidence is there? Int J Pharm. 2011;406:1–10. doi: 10.1016/j.ijpharm.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 32.Fireman EM, Lerman Y, Ganor E, et al. Induced sputum assessment in New York City firefighters exposed to World Trade Center dust. Environ Health Perspect. 2004;112:1564–1569. doi: 10.1289/ehp.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman LS, Mroz MM, Balkissoon Rand Maier LA. Beryllium sensitization progresses to chronic beryllium disease a longitudinal study of disease risk. Am J Respir Crit Care Med. 2005;171:54–60. doi: 10.1164/rccm.200402-190OC. [DOI] [PubMed] [Google Scholar]
- 34.Castranova V, Frazer DG, Manley LK, Dey RD. Pulmonary alterations associated with inhalation of occupational and environmental irritants. Immunopharmacology. 2004;2:163–172. doi: 10.1016/s1567-5769(01)00169-2. [DOI] [PubMed] [Google Scholar]
- 35.Nagatomo H, Morimoto Y, Oyabu T, et al. Expression of heme oxygenase-1 in the lungs of rats exposed to crocidolite asbestos. Inhal Toxicol. 2005;17:293–296. doi: 10.1080/08958370590922580. [DOI] [PubMed] [Google Scholar]
- 36.Camara NO, Soares MP. Heme oxygenase-1 (HO-1), a protective gene that prevents chronic graft dysfunction. Free Radic Biol Med. 2005;38:426–435. doi: 10.1016/j.freeradbiomed.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 37.Ovrevik J, Myran TT, Refesnes M, Becher RG, Hetland RB, Schwarze PE. Mineral particles of varying composition induce differential chemokine release from epithelial lung cells: importance of physicochemical characteristics. Ann Occup Hyg. 2005;93:219–231. doi: 10.1093/annhyg/meh087. [DOI] [PubMed] [Google Scholar]
- 38.Fontenot AP, Canavera SJ, Gharavi L, Newman LS, Kotzin BL. Target organ localization of memory CD4(+) T cells in patients with chronic beryllium disease. J Clin Invest. 2002;110:1473–1482. doi: 10.1172/JCI15846. [DOI] [PMC free article] [PubMed] [Google Scholar]