Abstract
Neuroblastoma is a common cancer of childhood that often results in progressive minimal residual disease after primary tumor resection. Cytosine-phosphorothioate-guanine oligonucleotides (CpG ODN) have been reported to induce potent anti-tumor immune responses. In this paper, we report on the development of a CpG ODN loaded suture that can close up the wound following tumor excision and provide sustained localized delivery of CpG ODN to treat local disease recurrence. The suture was prepared by melt extruding a mixture of polylactic acid-co-glycolic acid (PLGA 75:25 0.47 dL/g) pellets and CpG ODN 1826. Scanning electron microscopy images showed that the sutures were free of defects and cracks. UV spectrophotometry measurements at 260 nm showed that sutures provide sustained release of CpG ODN over 35 days. Syngeneic female A/J mice were inoculated subcutaneously with 1×106 Neuro-2a murine neuroblastoma wild-type cells and tumors were grown between 5 to 10 mm before the tumors were excised. Wounds from the tumor resection were closed using CpG ODN loaded sutures and/or polyglycolic acid Vicryl suture. Suppression of neuroblastoma recurrence and mouse survival was significantly higher in mice where wounds were closed using the CpG ODN loaded sutures relative to all other groups.
Introduction
The optimal treatment of solid tumors is often surgical resection, but treatment failure is frequently marked by recurrence of local disease [1]. Neuroblastoma is the most common extracranial solid malignancy of infancy and is a high-risk disease that has a propensity for local recurrence following surgical resection [2]. Following excision of the tumor, the site of resection is closed with biodegradable sutures. Frequent intra-tumoral injections of immunostimulatory cytosine-phosphorothioate-guanine oligonucleotides (CpG ODN) has been shown to be effective in the treatment of neuroblastoma [3–4]. CpG ODN are potent immunostimulants that have shown potential as adjuvants in a wide variety of diseases including cancer [5–6]. Here, we report on the development of an immunostimulatory suture that provides sustained release of CpG ODN at the site of tumor excision, so that surgeons can effectively close the wound following removal of the tumor and simultaneously provide local therapy for control and elimination of minimal residual disease.
Methods and Materials
Suture Preparation
To prepare the immunostimulatory suture, we loaded ground up polylactic acid-co-glycolic acid (PLGA 75:25 0.47 dL/g, Absorbable Polymers International, Pelham, AL) pellets and CpG ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′, Coley Pharmaceutical Group, Wellesley, MA) that was endotoxin free (<0.03 Eu/mL; BioWhittaker, Walkersville, MD) into a Dynisco extruder hopper and sutures were extruded from a melted (≤70°C) mixture of PLGA pellets and lyophilized CpG ODN. The CpG ODN used in this study has a phosphorothioate-modified backbone that is more resistant to enzymantic and thermal degradation than non-modified CpG oligonucleotides.
Measuring release of CpG ODN from sutures
To characterize the release profiles of CpG ODN, 450 mgs of CpG ODN loaded sutures were placed into 10 mL of phosphate buffered saline (PBS) and placed on a plate shaker at 37°C. The supernatant was measured for release of CpG ODN at 260nm on a UV spectrophotometer (Spectramax M5 Microplate reader, Molecular Device) every day and fresh medium replaced.
Scanning Electron Microscopy
Samples for scanning electron microscopy (SEM, Hitachi S-4000) were prepared by coating suture samples on steel stubs with approximately 5 nm of gold by ion beam evaporation using a sputter coater (E550 Emitech sputter coater) set at 10 mA for 10 seconds prior to examination in the SEM operated at 5 kV accelerating voltage.
Murine Tumor Cell Lines
Neuro-2a (N2a), a murine neuroblastoma wild-type cell line, was purchased from American Type Culture Collection (Manassas, VA). The cells were cultured in vitro in Minimal Essential Medium (GIBCO, Grand Island, NY), supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 1% penicillin-streptomycin (10,000 U/mL), 10 mmol sodium pyruvate, 100 mmol nonessential amino acid (GIBCO), a 0.75g sodium bicarbonate (Fisher Scientific, Fair Lawn, NJ) and were free of Mycoplasma.
Anesthetic agents and animal care
Female A/J mice (6–8 weeks old; Harlan Laboratories, Indianapolis, Ind) were anesthetized using halothane inhalation (Halocarbon, Rive Edge, NJ) during inoculation. Mice undergoing tumor resection were anesthetized with intraperitoneal ketamine (87 mg/kg) and xylazine (13 mg/kg) mixture, and tumors were resected under sterile conditions. All of the animals were housed under standard conditions, which follows the US Public Health Services guide for the care and use of animals. Mice were euthanized if tumor size was greater than 2.5 cm in any dimension or if mice displayed a “sick mouse posture”.
Model of minimal residual disease
N2a (1 × 106) wild-type cells were inoculated subcutaneously on day 1. Caliper measurements of tumor development and growth were documented at least 3 to 4 times a week, and volumes were calculated as width2 × length × 0.5. At a specified time-point, when all the tumors had reached between 5 to 10 mm in any dimension, the tumors were resected without lymph node dissection. Mice were randomized before assigning to groups 1–4. The average maximum size of the tumors in any dimension for each group prior to resection were 1) 6.3 +/− 3.2 mm, 2) 8.1 +/− 1.6 mm, 3) 5.9 +/− 3.9 mm, and 4) 7.4 +/− 2.3 mm. The four groups tested included mice that had 1) wounds closed with polyglycolic acid Vicryl suture (Ethicon), 2) wounds closed with Vicryl at which time the site of resection was locally injected with 200 μg CpG ODN, 3) wounds closed with Vicryl and CpG ODN loaded suture implanted into the same mice remotely on the opposite side to the resection site and 4) wounds closed with Vicryl and CpG ODN loaded suture.
Statistical Analysis
The statistical analyses focused on different wound closure strategies following tumor resection and the effects on tumor recurrence and progression. The primary outcomes of interest were time to death and tumor growth over time. The log-rank test was used to compare the survival times between vaccination groups, and Kaplan-Meier survival functions. Tumor size (cm3) was regularly measured throughout the experiments, resulting in repeated measurements across time for each mouse. Mixed linear regression models were used to estimate and compare the group-specific tumor growth curves. Continuous first-order autoregressive structures were specified in the models to account for the within-subject correlation. In both the survival and growth curve analyses, statistically significant global tests of equality across groups were followed up with pairwise comparisons to identify specific group differences.
Results and Discussion
Immunostimulatory sutures were prepared by extruding a melted (≤70°C) mixture of PLGA (75:25 0.47 dL/g) pellets and lyophilized CpG ODN 1826 (Fig. 1a and Fig. 1b). Each piece of suture extrudate used in tumor suppression experiments was approximately 0.6 mm × 30 mm in dimensions with an average weight of 150 mg and loaded with 200 μg CpG ODN. Scanning electron microscopy images provided confirmation of the suture dimensions and showed that sutures had a solid morphology with no noticable fractures or cracks (Fig. 1c). In release studies, CpG ODN was found to be released over 35 days at a sustained rate (Fig. 1d). Release studies were carried out in PBS and we acknowledge that the presence of serum and enzymes found in vivo could alter the overal kinetics of CpG ODN release. However, we anticipate broadly similar sustained release profiles in vivo as the primary mechanism of PLGA degradation is hydrolytic [7–8]. By controlling the molecular weight and the chemical composition of the PLGA or other biodegradable polymers used to prepare the suture, we can control the release of CpG ODN from weeks to over a year [8–10]. We can control the dimensions of the suture by adjustment of the draw rate which provides a further mechanism for controlling the release profiles and mechanical properties of the suture. Increasing the draw rate decreases the mean diameter of the suture extrudate. The draw rate, composition, and molecular weight of PLGA used in the tumor suppression experiments in this study were carefully selected to closely match the duration of tumor growth measurements.
Figure 1.
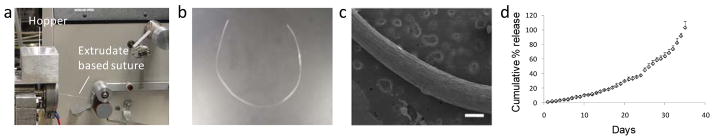
(a, b) Photograph of suture extrudate production. CpG ODN 1826 and ground PLGA pellets (75/25 0.47 dL/g) are placed into the hopper. At an operational temperature of 70°C for the rotor, flexible suture material is formed. (c) Scanning electron micrographs show the initial solid structure and surface morphology of the CpG ODN loaded sutures (scale bar = 0.4 mm) (d) Release profiles of CpG ODN measured using UV spectroscopy at 260 nm show sustained release of CpG ODN for over a month in PBS (n=3 +/− SD). Images and release profiles are representative of at least two repeats.
The murine subcutaneous neuroblastoma model is an ideal model for testing the potential use of the immunostimulatory suture as it reliably and aggressively recurs following resection [1]. To evaluate the use of immunostimulatory sutures in preventing recurrence of tumor in the minimal residual disease setting, we tested 4 groups of mice in which the primary tumor was resected. These groups (described in the materials and methods section) included mice in which the site of excision was then closed using either the immunostimulatory CpG ODN loaded sutures and/or Vicryl and tumor growth development monitored. All experiments were repeated at least once.
Syngeneic female A/J mice (6–8 weeks old; n=10 per group; Harlan Laboratories, Indianopolis, Ind) were anesthetized using halothane and inoculated subcutaneously with 1×106 Neuro-2a (N2a, ATCC, Manassas, VA) murine neuroblastoma wild-type cells on day 1. Tumor growth was measured with calipers and once the tumors had grown between 5 to 10 mm in any dimension, the mice were anesthetized with intraperitoneal injections of ketamine (87 mg/kg) and xylazine (13mg/kg) mixture and the tumors resected. Figure 2a shows that neuroblastoma recurrence and growth was suppressed most significantly in mice in which the excision site contained the immunostimulatory CpG ODN loaded suture. Prevention of neuroblastoma recurrence was achieved in all mice in this group upto day 35 and 8 out of 10 mice had survived by the study end-point (Fig. 2b). In contrast, all other groups had a proportion of mice that had died by day 19. The morphology, shape and consistency of tumors that recurred following resection were similar to the original excised tumors and consistent with our previous observations on neuroblastoma recurrence following resection [1]. Mouse survival and tumor growth suppression using the CpG ODN loaded sutures was remarkably better than in all other groups (P<0.05), including mice in which the site of tumor excision was locally injected with 200 μg of CpG ODN in solution. This data highlights the enhanced protection generated against progressive minimal residual disease by providing sustained release of CpG ODN at the site of resection over prolonged periods of time. The two mice that died in the group treated with CpG ODN loaded sutures had relatively small tumors. It is therefore possible that death in these two mice was due to metastases in the internal organs and this requires further investigation. Previous studies have shown that frequent intra-tumoral injections of CpG ODN can lead to the rejection of neuroblastoma [4] but high concentrations of CpG ODN still have the potential to induce inflammation and systemic septic shock-like systems [11]. This approach of using CpG ODN loaded sutures is very efficient at reducing residual disease that was otherwise deadly in the mice where wounds were closed with commercial Vicryl sutures. Of significant interest, mice that had the immunostimulatory CpG ODN loaded suture implanted remotely from the site of resection developed more aggressive tumor growth than control mice with tumor excision alone. This data clearly illustrates that the mechanism of CpG ODN action on tumor suppression is local and highlights the benefits of utilizing a suture or biodegradable implant to provide sustained local delivery of the CpG ODN. It is possible that remote delivery of the CpG ODN accelerates tumor growth because it delivers a danger signal that acts as a decoy for key immune cells otherwise needed at the site of resection but this theory requires further investigation. The CpG ODN released at the site of resection is believed to prevent tumor recurrence by stimulating activated macrophage and NK cell activity against any minimal residual disease present after resection [3–4, 12–14].
Figure 2.
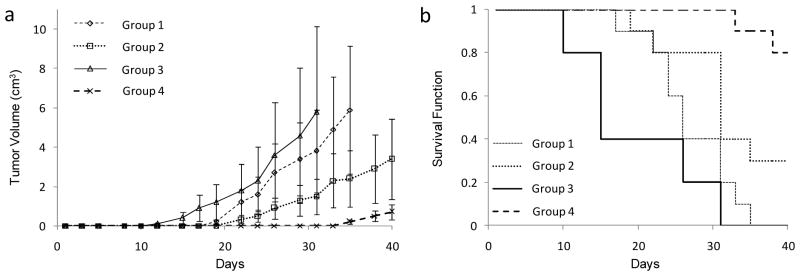
Tumor growth (a) and survival (b) of mice inoculated with 1 × 106 wild-type tumor cells. Subcutaneous tumors were grown between 5 to 10 mm following which mice underwent resection of the local disease. The wound at the site of resection was closed and four groups of mice were compared for recurrent tumor growth and survival. Group 1 had wounds that were closed using commercial polyglycolic acid Vicryl suture (n=10), group 2 was injected with CpG ODN locally and wounds closed using Vicryl (n=10), group 3 had wounds closed using Vicryl and sutures loaded with CpG ODN were implanted into the same mice remotely on the opposite side of the resection site (n=10), and group 4, the wounds were closed with Vicryl and CpG ODN loaded suture (n=10). Growth curves in each group were compared to each other and to controls. a) Tumor volume estimates from mixed linear models analysis of the 4 groups of mice. Tumor volume (cm3) is plotted as the mean +/− SD. Growth in group 4 was significantly different to all other groups (P<0.05) b) Kaplan-Meier plot of the estimated survival functions for the 4 groups of mice. Survival in group 4 was significantly different to all other groups (P<0.05).
Mice tolerated the CpG ODN loaded sutures and remained in good health as determined by the Body Condition Scoring Technique [15]. The mice appeared well conditioned. The vertebrae and dorsal pelvis were not prominent but palpable with slight pressure indicating a state of good health in the mice.
Conclusions
In summary, we have developed an immunostimulatory suture that could have the dual function of closing the site of tumor excision and providing sustained localized delivery of immunostimulatory ligands that prevents local tumor recurrence. Children with neuroblastoma frequently have a decent response to standard therapies where the tumor is reduced to a minimal residual disease, only to later fall victim to progressive residual disease [1]. These pre-clinical results suggest that closing the tumor excision wound site using CpG ODN loaded sutures has the potential to result in significant improvements in patient survival rates. Sutures prepared from PLGA are FDA approved and have been used effectively in patients for many years. CpG ODN as an adjuvant is demonstrated to be safe in primate models and in phase 2/3 human clinical trials [16]. The suture preparation uses an extrusion process that is simple, reproducible and adaptable for loading of a wide variety of other anti-tumor molecules, cytokines, antigens, and immunostimulants in combination or alone. Immunostimulatory sutures are therefore expected to have significant impact in a clinical setting.
Acknowledgments
We thank G.J. Weiner for critical discussions on the research and access to core cancer immunology resources. We thank D.R. Flanagan for use of the Dynisco Extruder.
References
- 1.Ohashi K, Kobayashi G, Fang S, Zhu XY, Antonia SJ, Krieg AM, et al. Surgical excision combined with autologous whole tumor cell vaccination is an effective therapy for murine neuroblastoma. Journal of Pediatric Surgery. 2006;41(8):1361–1368. doi: 10.1016/j.jpedsurg.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 2.Sandler AD, Chihara H, Kobayashi G, Zhu XY, Miller MA, Scott DL, et al. CpG oligonucleotides enhance the tumor antigen-specific immune response of a granulocyte macrophage colony-stimulating factor-based vaccine strategy in neuroblastoma. Cancer Research. 2003;63(2):394–399. [PubMed] [Google Scholar]
- 3.Auf G, Chen L, Fornes P, Le Clanche C, Delattre JY, Carpentier AF. CpG-oligodeoxynucleotide rejection of a neuroblastoma in A/J mice does not induce a paraneoplastic disease. Neuroscience Letters. 2002;327(3):189–192. doi: 10.1016/s0304-3940(02)00422-6. [DOI] [PubMed] [Google Scholar]
- 4.Carpentier AF, Chen L, Maltonti F, Delattre JY. Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer Research. 1999;59(21):5429–5432. [PubMed] [Google Scholar]
- 5.Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(20):10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartmann G, Weiner GJ, Krieg AM. CpG DNA: A potent signal for growth, activation, and maturation of human dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(16):9305–9310. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C, Ge Q, Ting D, Nguyen D, Shen HR, Chen JZ, et al. Molecularly engineered poly(ortho ester) microspheres for enhanced delivery of DNA vaccines. Nature Materials. 2004;3(3):190–196. doi: 10.1038/nmat1075. [DOI] [PubMed] [Google Scholar]
- 8.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chemical Reviews. 1999;99(11):3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 9.Intra J, Glasgow JM, Mai HQ, Salem AK. Pulsatile release of biomolecules from polydimethylsiloxane (PDMS) chips with hydrolytically degradable seals. Journal of Controlled Release. 2008;(127):280–287. doi: 10.1016/j.jconrel.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Zhang XQ, Dahle CE, Baman NK, Rich N, Weiner GJ, Salem AK. Potent antigen-specific immune responses stimulated by codelivery of CpG ODN and antigens in degradable microparticles. Journal of Immunotherapy. 2007;30(5):469–478. doi: 10.1097/CJI.0b013e31802fd8c6. [DOI] [PubMed] [Google Scholar]
- 11.Datta SK, Takabayashi K, Raz E. The therapeutic potential of antigen-oligonucleotide conjugates. Therapeutic Oligonucleotides. 2003:105–111. doi: 10.1196/annals.1281.022. [DOI] [PubMed] [Google Scholar]
- 12.Buhtoiarov IN, Sondel PM, Eickhoff JC, Rakhmilevich AL. Macrophages are essential for antitumour effects against weakly immunogenic murine tumours induced by class B CpG-oligodeoxynucleotides. Immunology. 2007;120(3):412–423. doi: 10.1111/j.1365-2567.2006.02517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnamachari Y, Salem AK. Innovative strategies for co-delivering antigens and CpG oligonucleotides. Advanced Drug Delivery Reviews. 2009;61(3):205–217. doi: 10.1016/j.addr.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miles SA, Sandler AD. CpG oligonucleotides for immunotherapeutic treatment of neuroblastoma. Advanced Drug Delivery Reviews. 2009;61(3):275–282. doi: 10.1016/j.addr.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Ullman-Cullere MH, Foltz CJ. Body condition scoring: A rapid and accurate method for assessing health status in mice. Laboratory Animal Science. 1999;49(3):319–323. [PubMed] [Google Scholar]
- 16.Jones TR, Obaldia N, Gramzinski RA, Charoenvit Y, Kolodny N, Kitov S, et al. Synthetic oligodeoxynucleotides containing CpG motifs enhance immunogenicity of a peptide malaria vaccine in Aotus monkeys. Vaccine. 1999;17(23–24):3065–3071. doi: 10.1016/s0264-410x(99)00145-0. [DOI] [PubMed] [Google Scholar]


