Summary
Cytomegalovirus gene expression in highly permissive, cultured fibroblasts occurs in three kinetic classes known as immediate early, early and late. Infection of these cells results in a predictable transcriptional program leading to high levels of virus production. Infection of other, so called, “non-permissive cell types” results in a transcriptional program that either fails to produce virus particles or production is substantially reduced compared to fibroblasts. We have found that CMV gene expression profiles in tissues from infected hosts differ greatly from those observed in infected tissue culture cells. The number of viral genes expressed in tissues is much more limited, and the number of highly active genes does not correlate with viral DNA load. Additionally, viral gene expression in vivo is tissue selective with no two tissues expressing the exact same viral gene profile. Thus, in vivo CMV gene expression appears to be governed by mechanisms that are still uncharacterized. Cytomegalovirus remains in a persistent phase for the lifetime of the host. During this phase only a limited number of host cells are infected, and it is very difficult to detect CMV gene expression in whole tissues without sub-fractionating infected vs. uninfected cells. Herein, we describe the development of a fluorescence-based laser capture microscopy technique coupled with small sample size microarray analysis to determine the viral gene expression in 50–100 infected cells isolated from frozen RCMV-infected tissue sections.
Keywords: Laser capture microscopy, cytomegalovirus, green fluorescence protein, microarray analysis
1. Introduction
Cytomegaloviruses (CMV) are ubiquitous β-herpesviruses that establish lifelong persistence following primary infection. In asymptomatic individuals, CMV persistence is typically maintained in a latent state, which is associated with a general lack of virus production and limited viral gene expression. However, CMV reactivation is considered to be the major source of virus in immunocompromised individuals, leading to significant disease in transplant patients or to congenital disease, such as deafness (1). CMV is the largest human herpesvirus containing over 200 potential open reading frames and many of these genes have been implicated in CMV disease (2, 3). During productive infections, CMV gene transcription is controlled temporally giving three kinetic categories of viral proteins: Immediate early (IE), Early (E), and Late (L). Expression of the IE genes does not require de novo protein synthesis and these viral proteins are potent transactivators of both viral and cellular gene transcription. The E class of viral proteins function in a number of different processes including cell cycle control, replication, and immune evasion. Expression of the E genes requires the synthesis of the IE proteins and is also dependent upon certain cellular factors. The L genes encode mostly structural proteins involved in virion assembly and egress, and expression of the L viral genes requires viral DNA production. Therefore, antiviral drugs that block viral DNA synthesis, such as ganciclovir, inhibit viral L gene expression but not the IE or E classes of viral genes. While much is known about viral gene transcription during lytic infections in vitro, very little is known about the specific gene transcription profiles that are associated with in vivo reactivation from latency or during persistence. It is now thought that CMV persistence may proceed via a non-classical gene expression profile that involves E and/or L gene expression without IE.
Typically, CMV gene expression studies have been limited to the analysis of only a few viral genes of interest. However, since the adoption of microarray technology several studies have reported the global transcription profiles associated with infection of cultured cells. Chambers et al., was the first to publish the viral transcriptional analysis from human-(H)CMV infected human foreskin fibroblasts utilizing microarrays, and was able to kinetically classify the HCMV AD169 transcriptome (4). Goodrum et al. used microarrays to study HCMV acute infection and latency transcription programs in CD34+ cells infected in vitro. They discovered that the pattern of viral gene expression in this progenitor cell type was different than that found in fibroblasts (5–7). In addition, the same group has reported that one of the genes expressed in the CD34+ stem cells, HCMV UL138, controls the ability of infected progenitor cells to become latently infected (8). Using microarray technology, we have compared rat CMV (RCMV) gene expression profiles of infected cultured endothelial cells, fibroblasts and smooth muscle cells to the viral transcriptomes detected in tissues derived from infected rats (9). In cultured cells, RCMV expresses about 95% of the known viral open reading frames (ORFs) with few differences between these three cell types. In contrast, we observed that RCMV gene expression in virus-infected rats is highly restricted with significantly lower expression of replication-essential viral genes (1×102 copies/gm tissue) and high expression (1×106 copies/gm tissue) of viral genes with unknown function or those with immune modulator phenotypes (9). In addition, we have also observed that the RCMV transcription profiles are tissue specific. Recently, next generation sequencing techniques have been employed to rapidly sequence whole CMV genomes, as well as to identify the complete HCMV transcriptome including the characterization of the viral non-coding RNAs (10, 11).
CMV infections persist for the life of the infected individual and are hypothesized to be involved in a number of chronic inflammatory diseases including atherosclerosis, graft rejection and cancer (12–16). However, the mechanisms and the viral genes involved in viral persistence and chronic disease are unknown. A major hurdle in determining which viral genes are expressed in tissues during persistence is the paucity of infected cells, making viral gene expression detection in whole tissue samples extremely difficult, to nearly impossible. Isolation of CMV infected cells from whole tissues is the only way to increase the viral to cellular gene signal to noise ratio and to reliably determine viral gene expression. We have thus developed a method to isolate RCMV infected cells from tissues utilizing a recombinant RCMV expressing green fluorescence protein (GFP) under the constitutive cellular EF1α promoter (17). This virus produces high levels of GFP even during non-productive infection conditions. Using fluorescence-based laser capture microscopy (LCM), we are able to isolate infected GFP+ cells from infected rat tissues and this process has greatly enhanced our ability to detect viral gene expression. We have tested a number of different tissue fixation conditions in order to optimize GFP retention and RNA extraction and these are described herein. We have also optimized the microarray conditions for these small sized total RNA samples. To identify viral gene expression, we have employed both printed glass-slide and semi-conductor microarrays containing oligonucleotide primers that represent the full complement of RCMV genes. While the techniques outlined here work well for isolating GFP+ cells from tissues, future directions include the characterization of HCMV gene expression in samples from human tissues using immuno-staining techniques to identify and capture HCMV infected cells.
2. Materials
2.1 Rat Cytomegalovirus Expressing GFP
2.2 RCMV Purification And Titration Components
Sorbitol cushion underlay: 20% D-sorbitol, 0.05M Tris-HCl pH 7.4, and 1mM MgCl2. Chemicals are from Sigma. Filter the reagent to sterilize.
Titration overlay: Minimal Essential Medium (MEM; Life Technologies) supplemented with 10% fetal bovine serum (FBS), non-essential amino acids, 100 U/ml penicillin, 100mg/ml streptomycin (pen/strep), 20mM L-Glutamine, and 0.5% (w/v) carboxymethylcellulose (CMC). Store at 4°C and preheat to 37°C prior to use.
Carboxymethyl cellulose (CMC): weigh 0.75g of low viscosity CMC and 0.75g of high viscosity CMC from Sigma. To a 500ml bottle add CMC and 60ml of PBS and 40ml of dH2O. Do not mix. Autoclave the liquid to solubilize CMC and sterilize. Store at room temperature. To each bottle add 200 ml of DMEM medium containing pen/strep and 5% Fetal bovine serum.
2.3 Laser Capture Microscopy
Microscope slides used for laser capture microscopy were 1.4 µm Pet-membrane slides (MMI-Membrane Slides #50102).
2.4 RCMV-Specific Microarrays And Reagents
Glass RCMV microarray slides were printed at the Spotted Microarray Core (SMC) at the Vaccine and Gene Therapy Institute, Oregon Health and Sciences University. Recent studies have also utilized semiconductor microarray slides (4×2k custom microarray chips) purchased from Custom Array Inc. (Bothell, WA).
Spotted RCMV microarray slides contain two unique 70mer antisense oligos for each of the 159 predicted viral ORFs [4] and an additional 2,925 rat cellular genes. The oligos were chosen with a 3’ bias and blasted against the NCBI database for alignment to the RCMV Maastricht strain and for possible cross hybridization to cellular sequences. Custom Array slides contain two unique 30–50mers antisense probes to each of the predicted RCMV ORFs. The Custom Array slides contain an additional 1400 unique cellular genes.
Custom Array’s Hybridization solution: 6× SSPE, 0.05% Tween-20, 20mM EDTA, 0.04% SDS, and 0.1µg/ml Salmon sperm DNA.
Prehybridization buffer: 6× SSPE, 0.05% Tween-20, 20mM EDTA, 5× Denhart’s solution, and 0.05% SDS.
Eukaryotic Total RNA Pico chip and RNA Ladder: Agilent RNA 6000 Pico Kit #5067-1573.
3. Methods
3.1 Production of Rat Cytomegalovirus RCMV Expressing GFP
Infect rat lung fibroblasts (RFL-6) with RCMV-GFP at multiplicity of infection (MOI) equal to 0.5. Incubate cells at 37°C until full cytopathic effect is observed.
Scrape infected cells, and then collect culture medium containing scraped cells into 250 ml bottles.
Centrifuge at 7,000 rpm (7,500 × g; Beckman JA-14 rotor) for 20 min to remove cell debris. Transfer cell-free supernatants to an additional clean tissue culture flask and set aside for further processing as described below in 3.1.5.
Lyse the infected cell pellets by freeze/thaw a total of three times in the 250 ml bottles.
Pellet lysed cell debris at 7,000 rpm (7,500 × g; Beckman JA-14 rotor) for 20 min and add the clarified supernatant to the culture supernatants collected above in 3.1.3.
Transfer cell free supernatants to SW28 centrifugation tubes containing a 5 ml sorbitol cushion underlay.
Centrifuge at 22,000 rpm (87,000 ×g; Beckman ultracentrifuge; rotor SW28) for 1 h at 8°C.
Resuspend the virus pellets in Minimal Essential Medium (18) culture media.
Store virus at –80°C.
3.2 Titration of RCMV-GFP
Prepare triplicate 10-fold serial dilutions of viral stocks in culture medium using at least two independent vials of stock virus.
Infect 24-well plates of RFL-6 rat fibroblasts with 0.2ml of 10-fold viral dilutions.
Incubate the infected cells at 37°C for 3 h.
Overlay wells with titration overlay material 1 ml MEM supplemented with 10% fetal bovine serum (FBS), non-essential amino acids, 100 U/ml penicillin, 100mg/ml streptomycin (pen/strep), 20mM L-Glutamine, and 5% carboxymethylcellulose (CMC).
Incubate for 7 days at 37°C.
Fix cells for 10 min at room temperature with 3.7% formalin prepared in 1× PBS.
Wash fixed cells with H2O.
Stain cells for 30 min with 0.05% aqueous methylene blue (Sigma).
Decant stain and wash with H2O.
Count virus plaques in triplicate wells.
Calculate the average virus titer as the number of plaque forming units per ml (pfu/ml) taking into account the dilution factor.
3.3 Infection of Rats With RCMV-GFP
Irradiate Lewis rats (γ-irradiated with 600 greys).
Infect irradiated rats with 5×105 pfu of RCMV-GFP within 24 hours of irradiation treatment. Mock-infected animals served as negative controls (see Note 2).
Euthanize rats at 3, 5, 7, 10, & 21 days post infection (dpi) via CO2 asphyxiation. Harvest tissues (heart, liver, kidney, spleen, bone marrow, and salivary glands) and blood.
Place a small portion of each tissue into a Cryo-safe tube and snap freeze in liquid nitrogen. Store at −80°C until used for nucleic acid analyses.
Embed a second portion of each tissue in Optimal cutting temperature (OCT) medium and snap freeze and stored at −80°C. This portion will be cut and utilized in microscopic analyses as described below (Section 3.6).
3.4 RCMV-GFP Infection of Cardiac Allograft Recipient Rats
We also infected a set of Lewis rats that received F344 rat donor heart allografts to study the effect that the immune environment present during allograft rejection plays on RCMV gene expression. Rat transplant operations were performed as previously described (19–23). Described first is the donor operation (steps 1–7) and then transplant operation (steps 8–12).
-
1
Anesthetize the rat with rat cocktail (1ml/kg, i.p.).
-
2
Open the abdomen midline.
-
3
Inject 100 units (0.3 ml) of aqueous heparin into the inferior vena cava.
-
4
Open the anterior chest wall via the sternum.
-
5
Place gauze containing iced saline on the heart.
-
6
Ligate the superior and inferior vena cava as well as the confluence of pulmonary veins and divide them.
-
7
Place heart in UW solution during recipient operation and euthanize the donor by exsanguination followed by bilateral thoracotomoy.
-
8
Anesthetize the recipient with inhaled isoflurane mixed with oxygen using a anesthesia machine.
-
9
Open the abdomen at the midline.
-
10
Isolate the infrarenal aorta and inferior vena cava.
-
11
Sew donor heart to the recipient aorta and donor pulmonary artery to the recipient inferior vena cava.
-
12
Irrigate the abdomen with warm saline with 1m/ml gentamicin sulfate. Provide analgesics as necessary.
-
13
Treat rats with low dose cyclosporine A (5mg/kg/day) for 10 days following transplantation to prevent acute cellular rejection.
-
14
Infect rats with 5×105 pfu of RCMV-GFP within 24 hours of transplantation. Mock-infected animals served as negative controls.
-
15Euthanize rats at 7 and 21 days post transplantation via CO2 asphyxiation. Harvest tissues (graft and native hearts, liver, kidney, spleen, bone marrow, and salivary glands) and blood.
-
3Place a small portion of each tissue into a Cryo-safe tube and snap freeze in liquid nitrogen. Store at −80°C until used for nucleic acid analyses.
-
4Embed a second portion of each tissue in Optimal cutting temperature (OCT) medium and snap freeze and stored at −80°C. This portion will be cut and utilized in microscopic analyses as described below (Section 3.6).
-
3
3.5 Isolation and Analysis of Rat PBMC and Bone Marrow Cells
At each of the time points described above in 3.2, whole blood and bone marrow cells were isolated and analyzed by flow cytometry for the presence of GFP positivity.
Flush rat femurs and tibias from the infected rats with a 23-gauge needle and syringe containing RPMI-1640 medium.
Strain bone marrow cells through a 70 µm filter.
Wash bone marrow cells twice with 50ml of RPMI-1640 medium containing 10% v/v fetal bovine serum, pen/strep and 2 mM L-glutamine.
Isolate rat peripheral blood cells (PBMC) from 5 ml of non-clotted whole blood.
Overlay whole blood over 5 ml of Ficoll hypaque in a 15 ml conical tube.
Centrifuge at 2,000 rpm (800×g) in a Beckman Table top centrifuge for 30 min.
Remove Buffy coat and place cells into a fresh 50 ml conical.
Wash cells twice with complete RPMI-1640 medium.
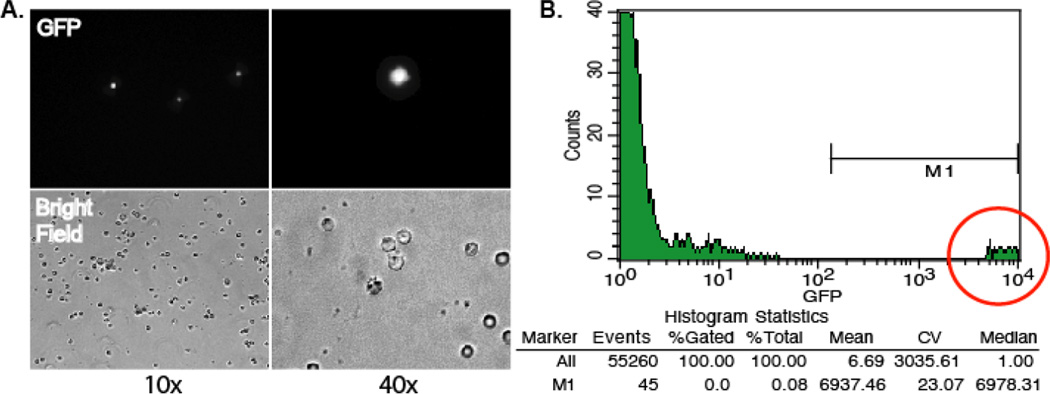
Analyze BM cells and PBMC for the presence of GFP on a FACS Calibur (Becton Dickinson) utilizing Cell Quest software. Analyze data using FlowJo software (Tree Star, Inc.). As shown in Figure 1, GFP expression is very high in the PBMC from RCMV-GFP infected rats. The data shown in Table 1 represents an analysis of BM and PBMC cells isolated from three infected rats at 3, 5, 7, and 10 days post infection. GFP+ cells are expressed as the percentage of total cells, which are typically present at 3, 5, and 7 dpi but peak at 5 dpi. Accordingly, there is approximately 1 infected cell per 1000 total PBMC and approximately 1 infected cell per 10,000 total bone marrow cells (Table 1). Thus, RCMV-GFP+ BM cells and PBMC can be sorted using a FACS Calibur flow cytometer making it possible to isolate the infected cell populations.
Figure 1. GFP detection in peripheral blood cells isolated from Lewis rats infected with RCMV-GFP at 10 dpi.
A. GFP expression in peripheral blood cells at 10× and 40× using fluorescence microscopy. B. Total peripheral blood cells were analyzed using a BD FACScan flow cytometer. The number of RCMV-GFP+ peripheral blood cells was determined to be about 1 in 1,000 cells.
Table 1.
Detection of RCMV-GFP in Infected Rat Tissues*
| Organ | Day 3 | Day 5 | Day 7 | Day 10 | Day 21 | Uninfected |
|---|---|---|---|---|---|---|
| Salivary Glands | − | + | + | ++ | ++ | − |
| Heart | + | +/− | +/− | + | − | − |
| Lung | ++ | ++ | + | ++ | + | − |
| Spleen | ++ | ++ | ++ | ++ | − | − |
| Liver | +/− | ++ | + | + | − | − |
| Kidney | +/− | ++ | + | +/− | − | − |
| PBMC** | 0.021 | 0.077 | 0.027 | 0 | 0 | 0 |
| BMC | 0.005 | 0.006 | 0 | 0 | 0 | 0 |
Groupings: no GFP+ foci in more than 5 tissue sections (−); 1–5 GFP+ foci per 5 tissue sections (+/−); multiple GFP+ foci per tissue section (+); and multiple GFP+ foci per 20× microscope field (++). n=5 rats
GFP positivity of PBMC and BMC was determined by flow cytometry. Listed are positive cells as a percent of total cells.
3.6 Cryosectioning of Frozen Rat Tissues
Cut 8 µm thick tissue sections from the OCT embedded RCMV-GFP infected rat tissues using a Leica CM3050S Cryostat.
Mount cut tissue sections for immunofluorescence microscopy onto glass microscope slides.
Mount cut tissue sections for Laser Capture Microscopy (LCM) onto 1.4 µm Pet-membrane slides (MMI-Membrane Slides #50102).
Store tissue sections frozen at −80°C until visualization or visualize immediately.
3.7 Fluorescence Microscopy
Tissue sections can be visualized immediately upon thawing or first fixed and then visualized by fluorescence microscopy (see Note 3). Herein we will outline the processing and visualization of fixed tissue sections, which was used to determine the level of infection (RCMV-GFP+ cells) in rat tissues at 3, 5, 7, 10 and 21 dpi (Table 1). The following fixing and washing steps can be performed in Coplin jars or in a humidified chamber.
Fix slides containing tissue sections from RCMV-GFP infected rats with 2% paraformaldahyde diluted in 1× PBS for 10 min at room temperature in the dark.
Wash twice with 1× PBS.
Visualize GFP fluorescence using a Delta Vision RT microscope by Applied Precision. Representative photomicrographs shown in Figure 2 were obtained at 60× magnification.
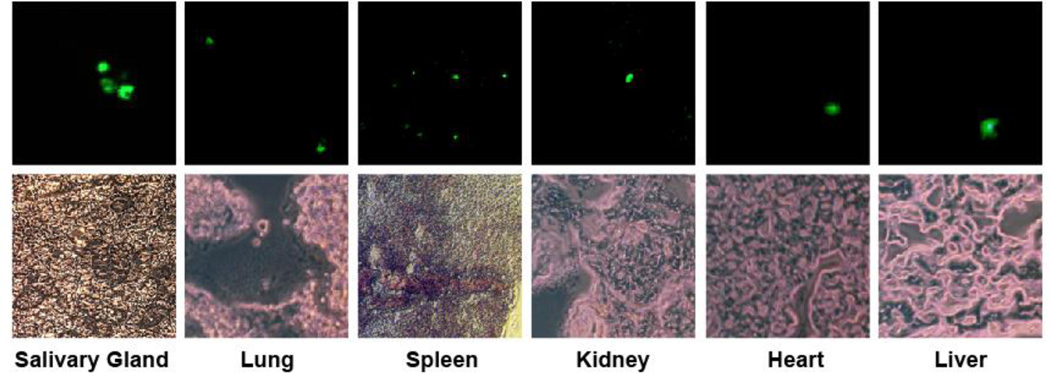
Count the RCMV-GFP+ cells from three infected rats per time point and tabulate data using excel, and display as shown in Table 1. Virus infected cells are readily detectable in the lung and spleen at 3 dpi. At 5, 7, and 10 dpi the virus was observed in multiple tissues including the heart, lung, spleen, liver and kidney. The peak of GFP detection for most tissues occurred at 5 dpi. However, the peak in salivary gland tissues was highest at day 21.
Figure 2. Fluorescence microscopic detection of RCMV-GFP infected tissues at 7 dpi.
Frozen tissues (salivary gland, lung, spleen, kidney, heart and liver) were cryosectioned (8 µm) and mounted on microscope slides. The thin sections were viewed using a Deltavision Deconvolution microscope and pictures were taken at 60× magnification. Fluorescence images are shown above their corresponding phase image.
3.8 Fluorescence-Based Laser Capture Microscopy
Our goal with LCM was to differentially collect RCMV-GFP infected vs. uninfected cells from tissue sections and extract intact viral and cellular RNA for expression analysis. LCM allows excision of samples as small as 100 µm2 by using a software directed microscope stage and ultraviolet cutting laser focused through the objective. Infected cells can be easily visualized by fluorescence of constitutively expressed GFP without permeabilizing or staining of cells. For our study, we employed two different laser capture microscopes. Initially, we used a Palm-LaserPressureCatapulting System. We then switched to a newer ArcturusXT LCM (see Figure 3 for a LCM workflow diagram). Both systems are controlled by an integrated computer system that allows accurate and efficient sample cutting and capture.
Fix tissue sections directly after cryo-sectioning with 100% Ethanol for 30 seconds or use fresh unfixed samples (see Note 4).
- Perform LCM microdissection with either a PALM or Arcturus LCM System.
- The PALM LaserPressureCatapulting (LPC) technique uses a pulsed nitrogen laser (337nm). The GFP positive cells in the tissue are visualized using a Zeiss Axiovert fluorescent research microscope. Cells are then micro-dissected by non-contact precise laser ablation using Robocut software (Microlaser Technologie, Bernried, Germany) and catapulted directly onto PALM adhesive caps (PALM Microlaser Technologies, Bernried, Germany) using the laser pressure catapulting technique of the instrument. PALM Adhesive caps contained RNA extraction buffer and were frozen at −80° until use.
- The ArcturusXT LCM system combines LCM and UV laser cutting methods. Tissue samples are collected by adherence to a plastic microtube cap. In general, the newer LCM systems have improved capabilities including the ability to visualize whole tissue sections in a grid pattern as shown in Figure 4. This feature makes it easier to quickly scan the tissue to identify RCMV-GFP+ cells. First, CapSure HS LCM Caps are lowered into place above the tissue sample. Then, an ultraviolet (UV) cut line is drawn around the GFP+ cells using the computer system, and infrared (IR) spots are automatically placed within the region to be cut. The IR laser activates the plastic cap above the site to be captured promoting adherence of the plastic cap to the target cells. Under direction of the computer, the UV laser performs the cutting releasing the cut zone from the remaining tissue. This system allows visualization of tissues after dissection to ensure the sample was extracted properly (Figure 5). Individual cells can also be acquired with simple adherence by placing individual IR spots over the cells of interest. After plastic adherence and cutting steps are complete for the entire sample, the plastic cap containing the adhered cells is lifted away from the tissue sample. The cap is then removed from the holder and placed on a tube containing RNA extraction buffer.
Scrape a portion of a tissue section directly into extraction buffer without LCM in order to obtain a control sample to determine the effect of the LCM procedure on RNA quality.
Store adhesive caps containing LCM sections in RNA Extraction buffer (Zymo Research Mini RNA isolation kit, catalogue #R1005) (see Note 5).
Figure 3. LCM Workflow Diagram.
Animals are infected and, at the time of harvest, the tissues are embedded in OCT until cryosectioned. The thin sections are placed on microscope slides as suggested by the LCM system manufacturer. LCM procedures are system specific: Arcturus LCM System uses a special plastic tube cap that is lowered just above the sample. A UV laser is used to cut the outline of the infected cell(s) and then an IR laser melts points of the cap to the tissue allowing the cut section to be pulled off of the microscope slide. After microdissection is complete the cap is placed on a microfuge tube containing RNA extraction buffer. PALM LCM System uses a UV laser for cutting around the GFP+ cell(s) and then the cut samples are catapulted by a laser push into a microfuge tube cap preloaded with RNA extraction buffer. RNA is purified, converted to cDNA and the cDNA is amplified and labeled. The labeled cDNA is hybridized to the microarray chips, scanned and analyzed.
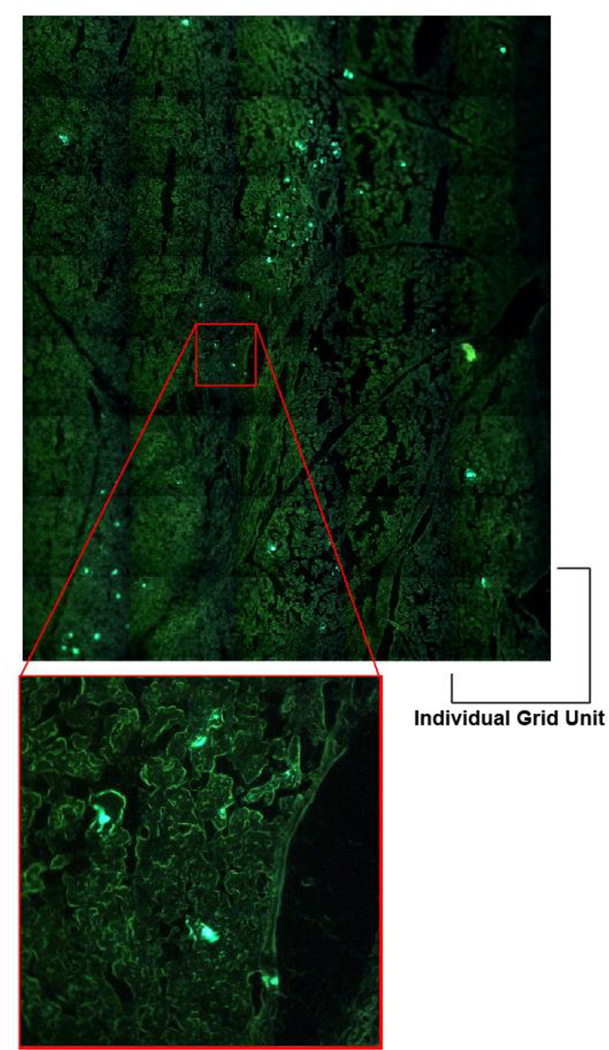
Figure 4. Detection of GFP+ cells in Salivary glands.
In order to identify GFP+ cells in salivary glands, we used the Arcturus LCM system to scan whole salivary gland tissue sections by fluorescence microscopy. The LCM system performs the scanning in a grid pattern containing multiple miniature scans, and then the system reassembles the complete tissue from the miniscans. This technique allowed us to visually locate GFP+ cells and then mark them for extraction.
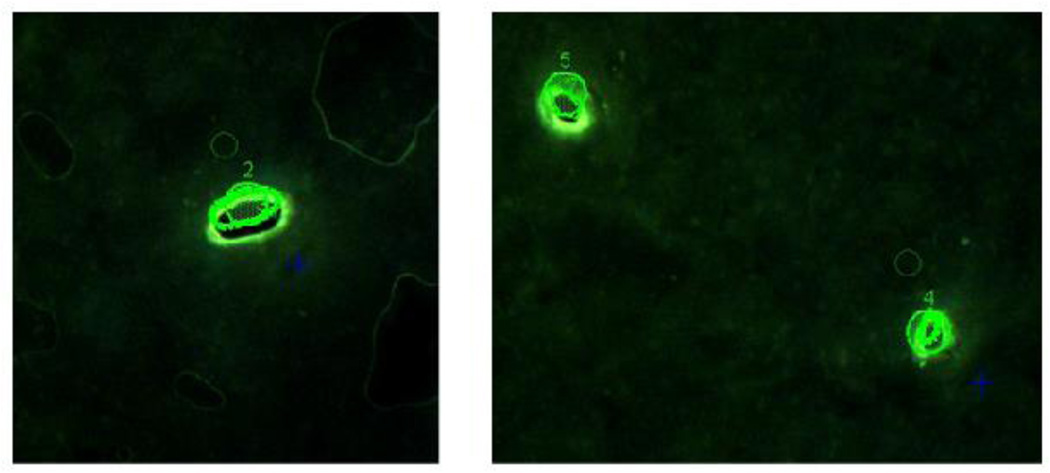
Figure 5. Visualization of LCM extracted samples.
Fluorescence microscopy was used to visualize removal of GFP+ cells from salivary gland tissues.
3.9 RNA isolation
Total RNA is isolated from laser captured tissue samples using Zymo Research’s Mini RNA isolation kit and the samples are analyzed for quality using an Agilent 2100 Bioanalyzer.
Digest samples in 200 µl of RNA Extraction buffer for 20 min on ice. Vortex samples for 10 min.
Add one volume of 100% Ethanol and incubate on ice for 10 min.
Transfer the solution to a Zymo-spin column and centrifuge for 1 min at 10,000 rpm in a microfuge.
Wash the column twice with 200 µl of Wash buffer and centrifuge for 1 min at 10,000 rpm in a microfuge to remove wash buffer.
Add 10 µl of RNAse free dH2O and centrifuge at 10,000 rpm for 1 min in a microfuge to elute RNA.
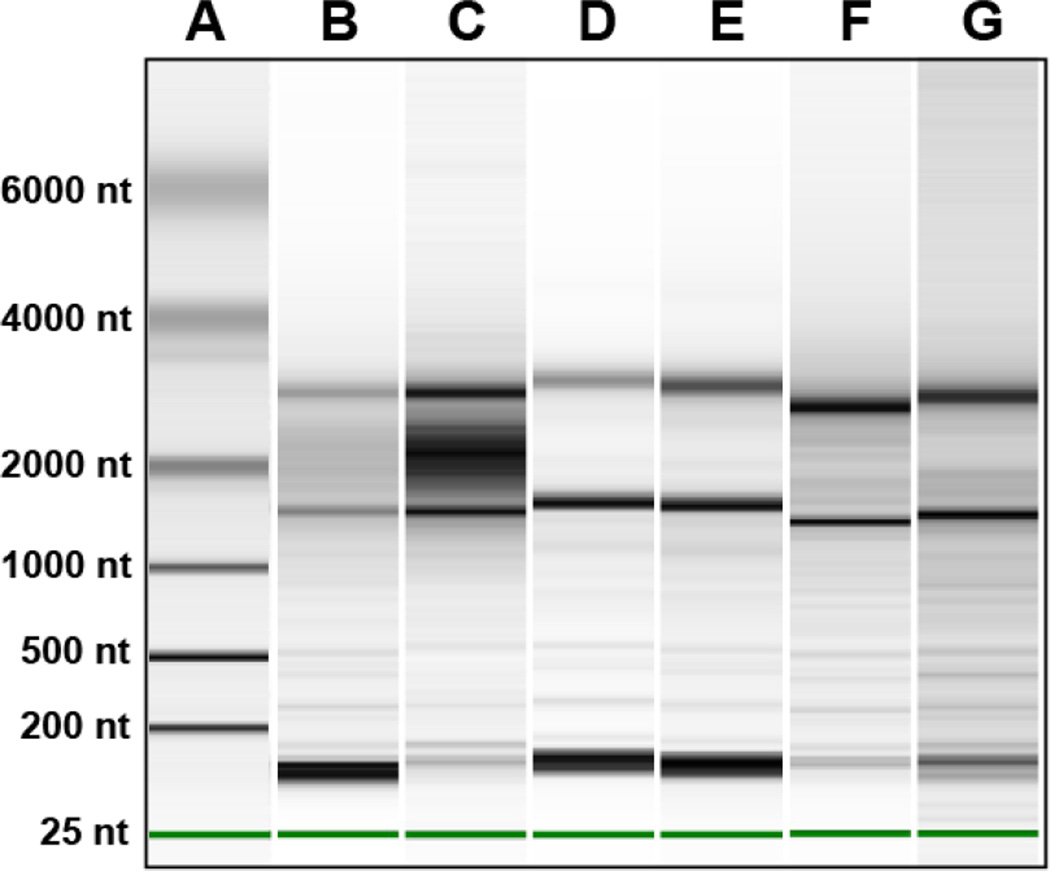
Analyze RNA quality using the Eukaryotic Total RNA Pico chip and a 2100 Bioanalyzer (Agilent). Total RNA extracted from LCM samples (1 µl) is compared to a control RNA sample and the quality of the LCM sample is evaluated based on the level and number of peaks present. Representative gel images of isolated RNA samples are shown in Figure 6.
Figure 6. Analysis of RNA Quality of LCM Captured GFP+ Cells.
Shown are representative gel images of isolated total RNA (1µl) that was analyzed with a 2100 Bioanalyzer (Agilent) using Eukaryotic Total RNA Pico chip. The quality of the LCM sample is evaluated based on the level and number of peaks present in the sample. Lane A is the RNA ladder and the band sizes are as labeled. Lane B, C and D are RNA harvested from GFP+ cells from spleen. The samples shown in Lanes B and C were not fixed and were on the microscope stage for 1 h and 2 h, respectively. Sample in Lane D was fixed in 100% ethanol for 30 seconds on the microscope slide prior to capture. Lane D sample was on the microscope stage for 1 h. Lanes E, F and G contain samples that were captured from GFP+ cells from native hearts infected for 7 dpi. The sample in Lane E was acquired by scraping a portion of the tissue section with a sterile razor blade. The sample was immediately placed into RNA extraction buffer. This sample represents the total RNA harvested from the complete tissue. Samples run in lanes F and G were fixed in 100% ethanol for 30 seconds on the slide and then LCM was used to capture GFP+ cells (time on stage was 1 h). These two samples demonstrate the reproducibility of this technique in extracting quality RNA from GFP+ cells captured by LCM.
3.10 Microarray analysis
To perform the microarray analysis, the RNA is first converted to dsDNA and then amplified by conversion to aRNA. The aRNA is converted to cDNA and then labeled with fluorescent dyes. The labeled cDNA is hybridized to the microarray chips.
Synthesize first strand cDNA from the entire RNA sample (100–200 ng) isolated above using 1.0 µM oligo dT-T7 primer (GGCCAGTGAATTGTAATACGACTCACTATAGGG(T)24), dNTP (0.5 mM), 40 units RNAse inhibitor, 1× first strand buffer (Invitrogen), and 200 units Superscript III reverse transcriptase (Invitrogen).
Generate doubled-stranded cDNA (dsDNA) by the addition of second strand buffer, dNTPs (0.3 mM), 40 units DNA polymerase, 10 units RNAse H, 10 units E. coli ligase (Invitrogen), and incubate at 16°C for 2 h.
Add 6 units of T4 DNA polymerase and incubate at 37°C for 10 min to polish 3’ overhangs
Heat inactivate samples at 70°C for 10 min.
Purify dsDNA by phenol:chloroform:isoamyl alcohol extraction.
Concentrate sample with Millipore YM100 columns by centrifugation.
Produce amplified RNA (aRNA) using the dsDNA sample as a template and the T7 Megascript kit (Ambion).
Purify aRNA with the RNeasy Mini kit (Qiagen).
Determine concentration by spectrophometry.
Incubate 5 µg of aRNA with 300 units Superscript III reverse transcriptase in the presence of 9 µg of random hexamers, 0.5 mM dNTPs, 1× first strand buffer (Invitrogen), and 60 units RNAse H inhibitor at 37°C for 2 h.
Hydrolyze aRNA by addition of 0.5 N NaOH and Reagent D from the Mirus Label IT Cy5 labeling kit and incubate the samples at 65°C for 30 min.
Add 100 µl of Neutralizing solution from the Mirus Label IT Cy5 labeling kit
Purify the cDNA sample using the Cyscribe GFX Purification kit (Amersham).
Florescent labeling is performed with the Mirus Label IT Cy5 labeling kit to chemically bind Cy5 to the synthesized cDNA. A total of 1 µg of purified cDNA is added to 1× labeling buffer M, 4 µl of Cy5 labeling reagent and dH2O to 100 µl. The samples are incubated in the dark at 37°C for 3 h.
Add 0.1 volume of Reagent D to the reaction mixture and incubate for 5 min on ice to stop the labeling reaction.
Add 1× Neutralization buffer and incubate for 5 min on ice.
Purify labeled cDNA using the Cyscribe GFX Purification Kit (Amersham)
Resuspend labeled cDNA in 35 µl of Custom Array’s Hybridization solution.
Incubate the CustomArray slides with prehybridization buffer for 60 min at 50°C.
Remove the pre-hybridization buffer from the slides, apply the labeled cDNA sample, and hybridize for 18 h at 50°C.
Wash the slide with 6× SSPE, 0.05% Tween-20 for 5 min at 50°C.
Wash for 1 min at room temperature with 3× SSPE: 0.05% Tween-20
Wash for 1 min at room temperature with 0.5× SSPE: 0.05% Tween-20
Wash for 1 min twice with 2× PBS with 0.1% Tween-20.
Wash for 1 min twice with 2× PBS.
Scan microarray slides using Bioscience GeneScan Lite laser scanner.
Analyze microarray images using Imagene digital processing software and Microarray Imager data analysis software (CustomArray Inc.).
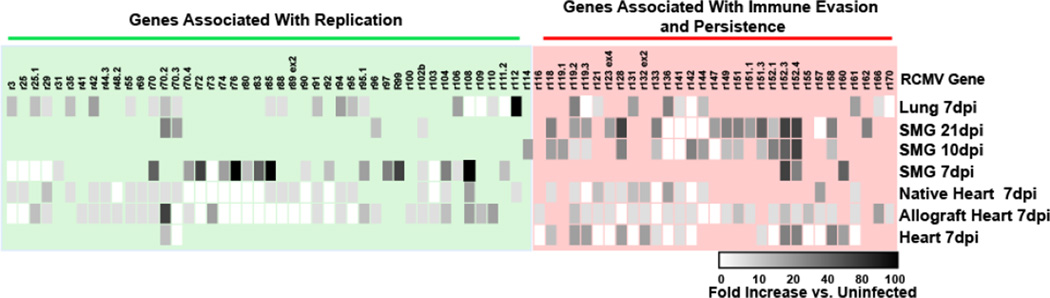
Data were subjected to analysis by student’s t-test. P values <0.05 were considered significant. Results from the microarray were compiled and displayed as a heat map (Figure 7).
Figure 7. Analysis of RCMV-GFP Gene Expression by LCM/Microarray.
Shown is a heat-map of LCM/microarray analysis of RCMV gene expression in GFP+ cell samples from infected hearts, allograft recipient native & graft hearts, lung (7 dpi), and SMG at 7, 10 & 21 dpi. Shown are the average fold increase values for three biological replicates compared to baseline (uninfected heart tissue).
4. Observations
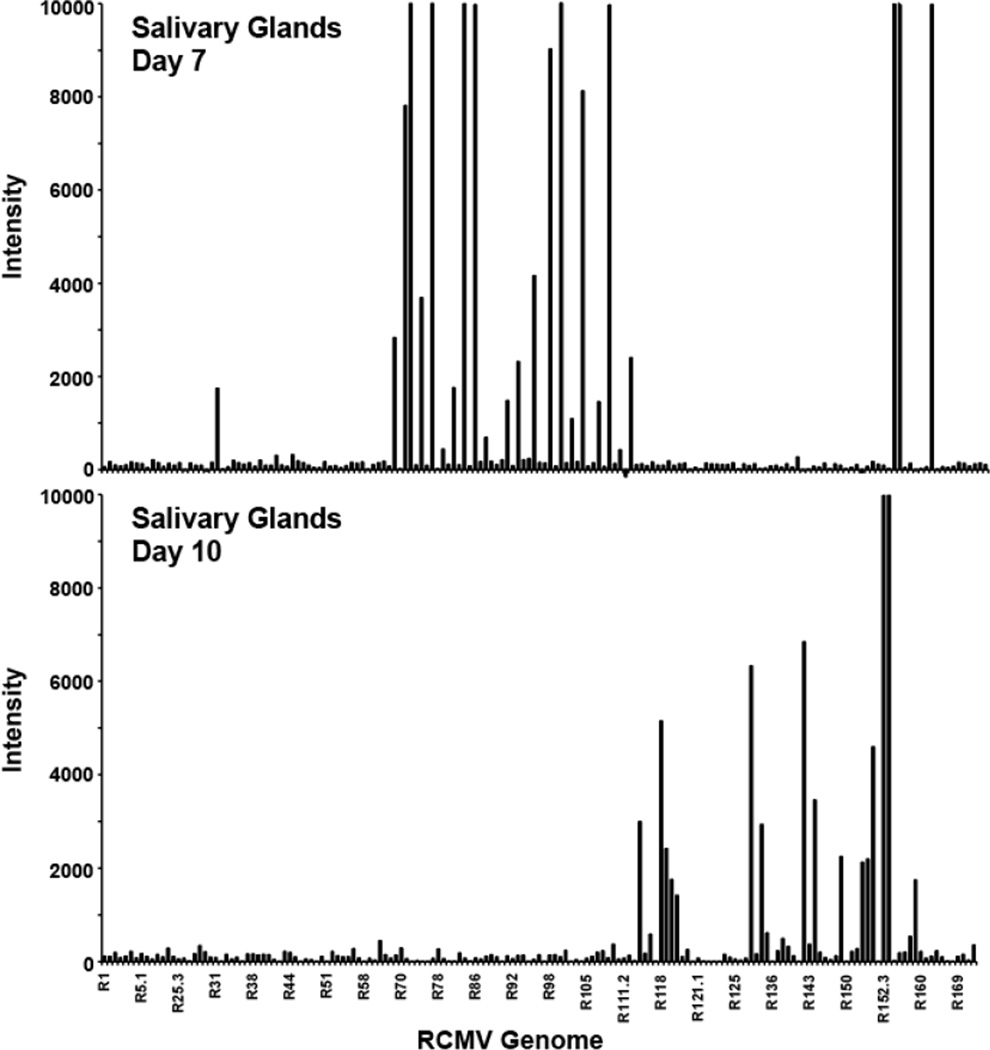
Laser capture microscopy has proven to be a powerful and valuable tool for examining questions regarding single cell metabolism, especially in whole tissue samples. We have developed LCM techniques to extract individual RCMV infected (GFP+) cells combined with viral transcriptome analysis via microarray. The RCMV-GFP virus used in this study expresses GFP under the constitutive cellular promoter EF-1α, which enables the expression and accumulation of GFP even during non-productive replication scenarios such as PBMCs and cells of the bone marrow (Table 1). Importantly, the course of infection and pathogenesis of this virus parallels the reported wild type RCMV infection patterns with an early involvement of lung and spleen followed by a widespread multi-organ distribution by day 5. This initial infection phase is followed by a long-term persistence phase that usually involves salivary gland tissues. The ability of RCMV-GFP to express even under non-productive infection conditions enabled us to detect and isolate single cells from several rat tissue types (salivary glands, heart, lung and spleen) using LCM. We discovered a number of important findings pertaining to in vivo CMV transcription (Figure 7). First, we have confirmed that CMV transcription is tissue specific, which parallels our previous findings using whole tissue preparations (9). Second, RCMV gene expression changes in the salivary gland from a profile that is associated with a productive infection at day 7 to one of persistence by day 10 (Figure 8). Interestingly, viral gene expression in infected cells captured from normal hearts resembles this salivary gland persistence phenotype. Lastly, RCMV gene expression is altered in rats during the inflammatory state produced by allograft rejection; even in tissues not undergoing active rejection (native heart in allograft recipients vs. native heart in non-transplant controls). This finding suggests a major viral reactivation event occurs during allograft rejection that stimulates CMV activation and promotes viral dissemination.
Figure 8. RCMV-GFP gene expression in salivary glands at 7 and 10 dpi.
Shown are the fluorescence intensities from microarray analysis of GFP+ cells isolated from RCMV-GFP infected rat salivary glands harvested at 7 and 10 dpi. LCM was used to capture GFP+ cells from 3 individual infected rats. The RNA was processed for microarray and analyzed on separate microarray chips.
Acknowledgements
The work presented in this manuscript was supported by grants from the National Institutes of Health (HL-083194 DNS) and (HL-66238-01 SLO). The Arcturus Laser Capture Microscope used for this study was supported by Award Number S10 RR027503 from the National Center For Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health. The Oregon National Primate Research Center Imaging Core is supported by NIH P51 RR000163. We thank Andrew Townsend for his assistance with graphics.
Footnotes
When constructing recombinant CMV expressing GFP, the choice of promoter type and placement of the cassette within the genome dramatically affects the level of GFP expression (24). The use of the EF-1α promoter in our RCMV-GFP construct caused the constitutive expression of the GFP protein and resulted in early and robust levels of expression. The GFP cassette was inserted into the R144–147 region of the RCMV genome and this may have increased the levels of GFP protein detected due to the availability of the EF-1α promoter to the transcription machinery. We have found that this region of the genome is highly expressed in tissues and during non-productive infection scenarios. It is possible that other regions of the RCMV genome may be sterically blocked under these same conditions, which would limit accessibility to the promoter (25).
Rodents were housed in the Association for Assessment and Accreditation of Laboratory Animals Care (AAALAC)-accredited Portland Veterans Affairs Medical Center animal facility in a specific-pathogen-free room, designated for CMV-infected rats, in compliance with guidelines provided by the United States Department of Agriculture/Department of Health and Human Services (USDA/HHS).
Since we cut our own tissue sections we always assess tissue section quality by microscopically visualizing GFP fluorescence immediately after thawing tissues on slides. This technique is used to ensure that the subsequent tissue sections are of good quality. We do not counterstain our tissues.
In general, GFP fluorescence tends to leak out of the fractured cells obtained during the cryosectioning process. We have employed a number of fixation conditions in order to prevent GFP leakage as well as optimize fluorescence stability and RNA quality and yield. By optimizing these conditions we determined that 100% ethanol fixation may stabilize RNA in some tissues but fixation is not necessary to retrieve high quality RNA. Freezing of tissue sections after cryosectioning negatively impacts the quality of recovered RNA. LCM directly after cryosectioning gave superior results to storing sections at −80°C between cryosectioning and LCM. Analysis of the RNA quality by Bioanalyzer is displayed in Figure 6. For best GFP retention we advise performing LCM immediately following thawing of the cut tissue sections.
Storing adhesive caps containing LCM tissue in Trizol appeared to give only slightly better results compared to RNA Extraction buffer. Overall, we obtained the best results when cryosections were taken directly to the LCM and microdissected within 1 h and caps containing tissue were then frozen in Trizol at −80°C until RNA processing.
When purifying RNA or cDNA we add an additional final centrifugation step prior to elution in order to make sure that the sample is completely free of contaminating Ethanol present in the wash buffers.
References
- 1.Britt W. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol. 2008;325:417–470. doi: 10.1007/978-3-540-77349-8_23. [DOI] [PubMed] [Google Scholar]
- 2.Chee MS, Bankier AT, beck S, Bohni R, Browne CM, Cerny R, Horsnell T, Hutchison CA, III, Kouzarides T, martignetti JA, Preddie E, satchwell SC, Tomlinson P, Weston KM, Barrell BG. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. In: McDougall JK, editor. Cytomegaloviruses. Berlin, Heidelberg, New yrok: Springer-Verlag; 1990. pp. 125–171. [DOI] [PubMed] [Google Scholar]
- 3.Davison AJ, Dolan A, Akter P, Addison C, Dargan DJ, Alcendor DJ, McGeoch DJ, Hayward GS. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J Gen Virol. 2003;84:17–28. doi: 10.1099/vir.0.18606-0. [DOI] [PubMed] [Google Scholar]
- 4.Chambers J, Angulo A, Amaratunga D, Guo H, Jiang Y, Wan JS, Bittner A, Frueh K, Jackson MR, Peterson PA, Erlander MG, Ghazal P. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J Virol. 1999;73:5757–5766. doi: 10.1128/jvi.73.7.5757-5766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodrum F, Jordan CT, Terhune SS, High K, Shenk T. Differential outcomes of human cytomegalovirus infection in primitive hematopoietic cell subpopulations. Blood. 2004;104:687–695. doi: 10.1182/blood-2003-12-4344. [DOI] [PubMed] [Google Scholar]
- 6.Goodrum FD, Jordan CT, High K, Shenk T. Human cytomegalovirus gene expression during infection of primary hematopoietic progenitor cells: A model for latency. Proc Natl Acad Sci U S A. 2002;99:16255–16260. doi: 10.1073/pnas.252630899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodrum F, Reeves M, Sinclair J, High K, Shenk T. Human cytomegalovirus sequences expressed in latently infected individuals promote a latent infection in vitro. Blood. 2007;110:937–945. doi: 10.1182/blood-2007-01-070078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrucelli A, Rak M, Grainger L, Goodrum F. Characterization of a novel Golgi apparatus-localized latency determinant encoded by human cytomegalovirus. J Virol. 2009;83:5615–5629. doi: 10.1128/JVI.01989-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Streblow DN, van Cleef KW, Kreklywich CN, Meyer C, Smith P, Defilippis V, Grey F, Fruh K, Searles R, Bruggeman C, Vink C, Nelson JA, Orloff SL. Rat cytomegalovirus gene expression in cardiac allograft recipients is tissue specific and does not parallel the profiles detected in vitro. J Virol. 2007;81:3816–3826. doi: 10.1128/JVI.02425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley AJ, Lurain NS, Ghazal P, Trivedi U, Cunningham C, Baluchova K, Gatherer D, Wilkinson GW, Dargan DJ, Davison AJ. High-throughput sequence analysis of variants of human cytomegalovirus strains Towne and AD169. J Gen Virol. 2009;90:2375–2380. doi: 10.1099/vir.0.013250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gatherer D, Seirafian S, Cunningham C, Holton M, Dargan DJ, Baluchova K, Hector RD, Galbraith J, Herzyk P, Wilkinson GW, Davison AJ. High-resolution human cytomegalovirus transcriptome. Proc Natl Acad Sci U S A. 108:19755–19760. doi: 10.1073/pnas.1115861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almond PS, Matas A, Gillingham K, Dunn DL, Payne WD, Gores P, Gruessner R, Najarian JS. Risk factors for chronic rejection in renal allograft recipients. Transplant. 1993;55:752–756. doi: 10.1097/00007890-199304000-00013. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 13.Grattan MT, Moreno-Cabral CE, Starnes VA, Oyer PE, Stinson EB, Shumway NE. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989;261:3561–3566. [PubMed] [Google Scholar]
- 14.Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, Nabors LB, Cobbs CG, Britt WJ. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347–3350. [PubMed] [Google Scholar]
- 15.Melnick JL, Adam E, Debakey ME. Cytomegalovirus and atherosclerosis. Eur Heart J. 1993;14(Suppl K):30–38. [PubMed] [Google Scholar]
- 16.Muhlestein JB, Horne BD, Carlquist JF, Madsen TE, Bair TL, Pearson RR, Anderson JL. Cytomegalovirus seropositivity and C-reactive protein have independent and combined predictive value for mortality in patients with angiographically demonstrated coronary artery disease. Circulation. 2000;102:1917–1923. doi: 10.1161/01.cir.102.16.1917. [DOI] [PubMed] [Google Scholar]
- 17.Baca Jones CC, Kreklywich CN, Messaoudi I, Vomaske J, McCartney E, Orloff SL, Nelson JA, Streblow DN. Rat cytomegalovirus infection depletes MHC II in bone marrow derived dendritic cells. Virology. 2009;388:78–90. doi: 10.1016/j.virol.2009.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minamishima I, Ueda K, Minematsu T, Minamishima Y, Umemoto M, Take H, Kuraya K. Role of breast milk in acquisition of cytomegalovirus infection. Microbiology and immunology. 1994;38:549–552. doi: 10.1111/j.1348-0421.1994.tb01821.x. [DOI] [PubMed] [Google Scholar]
- 19.Orloff SL, Hwee YK, Kreklywich C, Andoh TF, Hart E, Smith PA, Messaoudi I, Streblow DN. Cytomegalovirus latency promotes cardiac lymphoid neogenesis and accelerated allograft rejection in CMV naive recipients. Am J Transplant. 2011;11:45–55. doi: 10.1111/j.1600-6143.2010.03365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orloff SL, Streblow DN, Soderberg-Naucler C, Yin Q, Kreklywich C, Corless CL, Smith PA, Loomis CB, Mills LK, Cook JW, Bruggeman CA, Nelson JA, Wagner CR. Elimination of donor-specific alloreactivity prevents cytomegalovirus-accelerated chronic rejection in rat small bowel and heart transplants. Transplantation. 2002;73:679–688. doi: 10.1097/00007890-200203150-00005. [DOI] [PubMed] [Google Scholar]
- 21.Streblow DN, Kreklywich C, Yin Q, De La Melena VT, Corless CL, Smith PA, Brakebill C, Cook JW, Vink C, Bruggeman CA, Nelson JA, Orloff SL. Cytomegalovirus-mediated upregulation of chemokine expression correlates with the acceleration of chronic rejection in rat heart transplants. J Virol. 2003;77:2182–2194. doi: 10.1128/JVI.77.3.2182-2194.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Streblow DN, Kreklywich CN, Andoh T, Moses AV, Dumortier J, Smith PP, Defilippis V, Fruh K, Nelson JA, Orloff SL. The role of angiogenic and wound repair factors during CMV-accelerated transplant vascular sclerosis in rat cardiac transplants. Am J Transplant. 2008;8:277–287. doi: 10.1111/j.1600-6143.2007.02062.x. [DOI] [PubMed] [Google Scholar]
- 23.Streblow DN, Kreklywich CN, Smith P, Soule JL, Meyer C, Yin M, Beisser P, Vink C, Nelson JA, Orloff SL. Rat cytomegalovirus-accelerated transplant vascular sclerosis is reduced with mutation of the chemokine-receptor R33. Am J Transplant. 2005;5:436–442. doi: 10.1111/j.1600-6143.2004.00711.x. [DOI] [PubMed] [Google Scholar]
- 24.Qin JY, Zhang L, Clift KL, Hulur I, Xiang AP, Ren BZ, Lahn BT. Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PLoS One. 5:e10611. doi: 10.1371/journal.pone.0010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nevels M, Nitzsche A, Paulus C. How to control an infectious bead string: nucleosome-based regulation and targeting of herpesvirus chromatin. Rev Med Virol. 21:154–180. doi: 10.1002/rmv.690. [DOI] [PubMed] [Google Scholar]