Abstract
We have previously shown that early treatment with fresh frozen plasma (FFP) is neuroprotective in a swine model of hemorrhagic shock (HS) and traumatic brain injury (TBI). However, it remains unknown whether this strategy would be beneficial in a more clinical polytrauma model. Yorkshire swine (42–50 kg) were instrumented to measure hemodynamic parameters, brain oxygenation, and intracranial pressure (ICP) and subjected to computer-controlled TBI and multi-system trauma (rib fracture, soft-tissue damage, and liver injury) as well as combined free and controlled hemorrhage (40% blood volume). After 2 h of shock (mean arterial pressure, 30–35 mm Hg), animals were resuscitated with normal saline (NS; 3×volume) or FFP (1×volume; n=6/group). Six hours postresuscitation, brains were harvested and lesion size and swelling were evaluated. Levels of endothelial-derived vasodilator endothelial nitric oxide synthase (eNOS) and vasoconstrictor endothelin-1 (ET-1) were also measured. FFP resuscitation was associated with reduced brain lesion size (1005.8 vs. 2081.9 mm3; p=0.01) as well as swelling (11.5% vs. 19.4%; p=0.02). Further, FFP-resuscitated animals had higher brain oxygenation as well as cerebral perfusion pressures. Levels of cerebral eNOS were higher in the FFP-treated group (852.9 vs. 816.4 ng/mL; p=0.03), but no differences in brain levels of ET-1 were observed. Early administration of FFP is neuroprotective in a complex, large animal model of polytrauma, hemorrhage, and TBI. This is associated with a favorable brain oxygenation and cerebral perfusion pressure profile as well as higher levels of endothelial-derived vasodilator eNOS, compared to normal saline resuscitation.
Key words: : brain, hemorrhage, plasma, resuscitation, trauma
Introduction
Despite recent treatment advances, traumatic brain injury (TBI) remains a leading cause of morbidity and mortality, directly contributing to up to one third of all injury-related civilian deaths1 and more than 40% of battlefield deaths.2 Given that the primary brain injury is only amendable to prevention, current guidelines place emphasis on minimizing the secondary brain injury through maintaining adequate cerebral oxygenation and perfusion.3 In practice, management includes the avoidance of hypotension, which is particularly detrimental in TBI patients.4 In contrast, the current paradigm of low-volume or hypotensive resuscitation in patients suffering from uncontrolled hemorrhagic shock (HS) advocates permissive hypotension preceding operative control.5 Owing to the high-energy nature often involved in TBI, this injury modality is often complicated by extracerebral injuries, such as organ damage and hemorrhage,2,6 and it is thus clear that resuscitation priorities often will be at odds in this complicated clinical scenario. Few guidelines address resuscitation priorities in these combined injuries, and, further, there is conflicting evidence regarding the optimal fluid, with clinical reports indicating no major advantages of artificial colloids over crystalloids,7 whereas use of albumin may even be detrimental.8
In the general trauma population, accumulating evidence suggests that resuscitation with high ratios of fresh frozen plasma (FFP) to packed red blood cells (PRBCs) confers a survival advantage to patients requiring massive transfusion.9,10 This effect may, in part, be owing to the avoidance of large-volume crystalloid resuscitation, a strategy that has previously been associated with adverse outcome.11 Recent evidence does, however, suggest that FFP may exert a protective effect on the endothelium.12 Though little is known of the effects of FFP resuscitation on TBI, this endothelial-sparing effect could potentially be associated with an improvement in endothelial barrier function and subsequent decreased cerebral edema formation. Given that fluid extravasation in the brain is particularly detrimental, this could, in turn, translate into better cerebral perfusion and oxygenation, compared with artificial fluid choices with no endothelial protective effects.
Using a large animal model of combined TBI and HS, we have recently reported that resuscitation with FFP reduced brain lesion size, compared to 0.9% normal saline (NS) and 6% hetastarch (Hextend), and also reduced post-traumatic edema compared to NS.13 This model, however, used a controlled model of hemorrhage without any associated extracranial injuries, which is not very clinically realistic. Whether early administration of FFP would also provide neuroprotection in a clinically relevant model (TBI in conjunction with HS, fractures, and soft-tissue injury) is unknown and constitutes the focus of this study. We hypothesized that resuscitation with FFP compared to NS would decrease brain lesion size and swelling in such a model. The primary end point was brain lesion size 8 h following TBI.
Methods
The study was conducted in accord with the Animal Welfare act. All experiments were performed under veterinarian supervision and were approved by the institutional animal care and use committee.
Group allocation
Before surgery, 12 female Yorkshire swine (weight, 42–50 kg; Tufts Veterinary School, Grafton, MA) were randomized to resuscitation with either FFP (n=6) or 0.9% NS (n=6).
Preparation and monitoring phase
Animal preparation was performed according to our previously published protocols.13,14 Briefly, on the day of the experiment, animals were sedated with 5 mg/kg of Telazol intramuscularly (Pfizer, New York, NY) and anesthetized by mask inhalation using 4% isoflurane in 100% oxygen. Upon attaining sufficient depth of anesthesia, intubation was performed using a direct laryngoscopy technique with a 7.0-mm endotracheal tube. Upon confirmation of tracheal intubation, levels of inhaled isoflurane were adjusted down to 1–3% inspired fraction in room air. Cannulations of the right external jugular vein, right and left femoral arteries, and right femoral vein were then performed by a cut-down technique. The external jugular vein was used for the placement of a pulmonary artery catheter. The right femoral artery was used for continuous monitoring of the mean arterial pressure (MAP), which was recorded every 5 min. The left femoral artery was used for blood draw, and the femoral vein was used for fluid administration.
Injury phase
After cannulations, a mid-line laparotomy was used to gain access to the abdominal cavity. The distal 2 inches of the right medial liver lobe were sharply transected and allowed to bleed freely. Volume of blood loss was measured by continued suctioning into a canister. Further, laparotomy pads were weighed. Upon reaching 250 mL of free hemorrhage, bleeding was controlled by packing and cauterization of small vessels. Larger vessels were sutured. Final hemostasis was secured by an interlocking running suture across the cut surface of the liver. During hemorrhage, one of the free floating ribs (overlying the liver) was manually fractured, while soft-tissue injury was created by crushing the rectus muscle along the incision line with a Kelly clamp. The abdomen was then rapidly closed with #1 nylon sutures.
Animals were then carefully flipped to a prone position in preparation for the creation of TBI, as previously described.13 Briefly, a 20-mm burr hole was made on the right side of the skull over the frontal lobe in order to expose the dura. A catheter for intracranial pressure (ICP) monitoring and monitoring of cerebral oxygenation (Integra LifeSciences, Plainsboro, NJ) was inserted through a 2-mm burr hole on the left side of the skull, 10 mm lateral and 10 mm anterior to the bregma. ICP was continuously measured and recorded every 5 min. TBI was created through the 20-mm burr hole using a computer-controlled cortical impact device,13,15 and the hole was subsequently sealed using bone and bone wax in order to prevent leakage of cerebrospinal fluid (CSF).
Concurrent with the TBI, controlled hemorrhage was started using the femoral arterial line, as previously described.13 Before the experiment, 40% blood volume had been calculated and the previously hemorrhaged 250 mL was deducted from this for the purpose of the calculation of controlled blood-volume draw. Total blood loss equaled 40% of the estimated blood volume.
Resuscitation and observation phase
Upon completion of the controlled hemorrhage, animals were left hypotensive for 2 h. During this phase, we targeted an MAP of 30–35 mm Hg, adjusted by titrating the inhaled fraction of isoflurane.
Upon completion of the shock phase, animals were resuscitated according to their randomized groupings, either FFP (n=6) or NS (n=6; Hospira Inc., Lake Forest, IL). Volume of FFP matched the volume of shed blood, whereas volume of NS was 3 times the volume of blood loss to simulate the resuscitation volume used in clinical settings. FFP had been obtained from healthy porcine donors and was thawed in the morning of the experiment, as previously described.16 Upon completion of resuscitation, animals were observed for 6 h under continued anesthesia before killing. During the observation phase, blood gases were obtained hourly and electrolytes were corrected as needed. Immediately following the termination of the study, brain and other organs were harvested for the purpose of subsequent tissue analysis.
Preparation of brain samples
Preparation of these samples has been described in detail.17 Briefly, brains were sliced into 5-mm coronal sections beginning 10 mm anterior and ending 30 mm posterior to the lesion. These slices were incubated in 2% 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich, St. Louis, MO) to demonstrate the presence of viable tissue. Lesion size and swelling were subsequently calculated as previously described.17 Briefly, volume of lesion size was measured from the stained coronal sections using ImageJ image analysis software (National Institutes of Health, Bethesda, MD). For each coronal section, the surface area was measured and converted from pixels to mm2, and all sections were combined to obtain a total volume of lesion size. Brain swelling was quantified by comparing the injured and uninjured hemispheres: [(ipsilateral hemisphere's volume/contralateral hemisphere's volume) – 1]×100.
Thirty milligrams of ipsilateral cortical brain tissue samples from the border zone of the TBI were subjected to whole-cell lysis for the purpose of tissue analysis, as previously described.17 Briefly, brain tissue samples were sonicated by an ultrasonic cell disruptor and extracted using a whole-cell lysis extraction kit (Chemicon International, Temecula, CA). The homogenate was centrifuged at 8000g for 20 min at 4°C, and the supernatant was used for enzyme-linked immunosorbent assay (ELISA) analysis.
Enzyme-linked immunosorbent assay
All assays were performed according to the manufacturer's instructions. The ELISA technique was used to assay brain whole-cell lysis samples for endothelial-derived vasodilator nitric oxide synthase (eNOS; Novatein Bio, Cambridge, MA) and vasoconstrictor endothelin-1 (ET-1; Novatein Bio).
Statistical analysis
Data are presented as medians with interquartile range. Differences between groups at distinct time points were compared at equal time points using Mann-Whitney's U test whereas linear mixed modeling was used to compare differences in correlated data over time (hemodynamics, brain oxygenation, and ICPs). A p value of 0.05 was considered significant. All statistical analysis was performed using SPSS statistical software (IBM Corp., Armonk, NY).
Results
Hemodynamics, intracranial pressure, and brain oxygenation
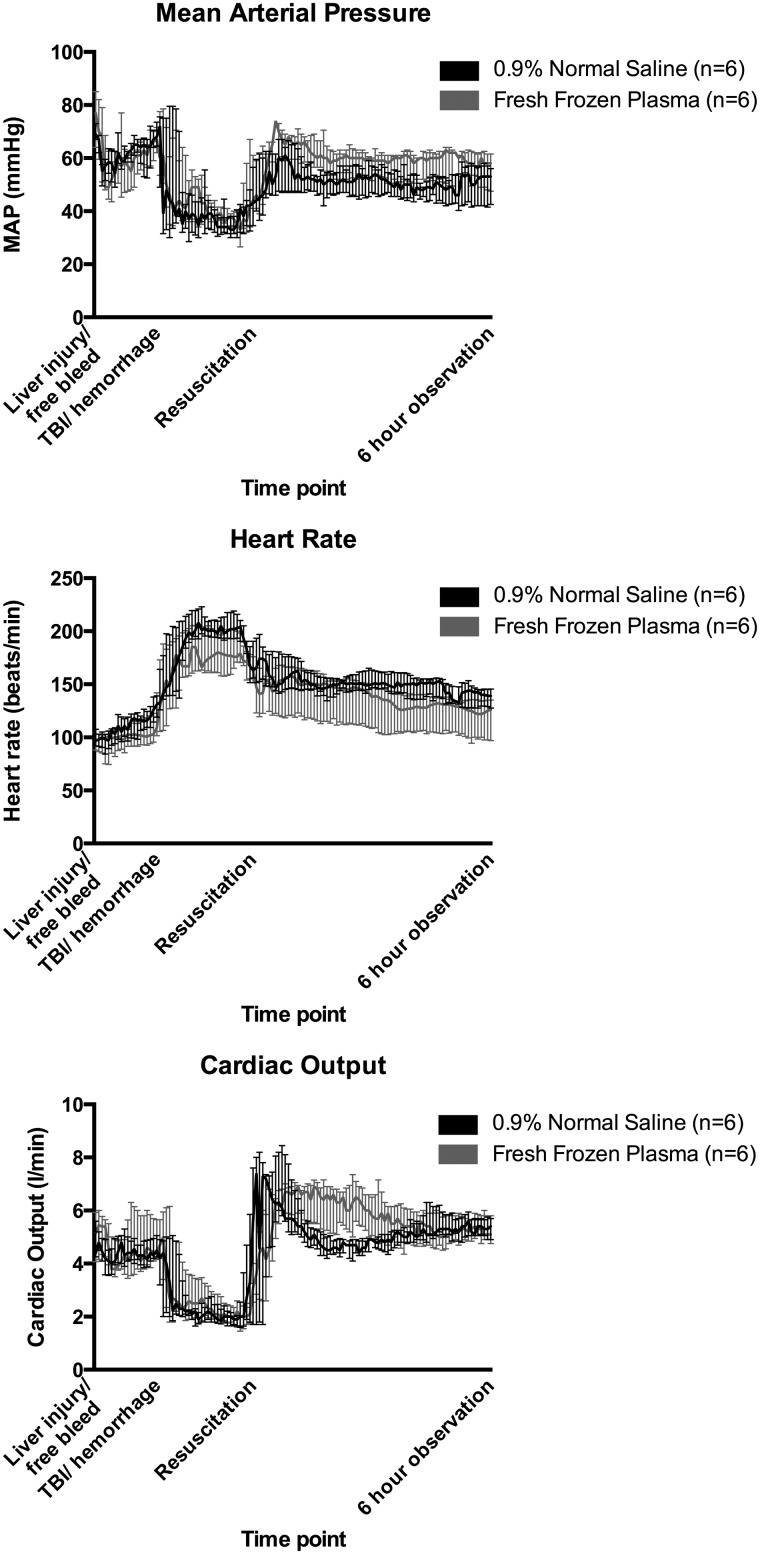
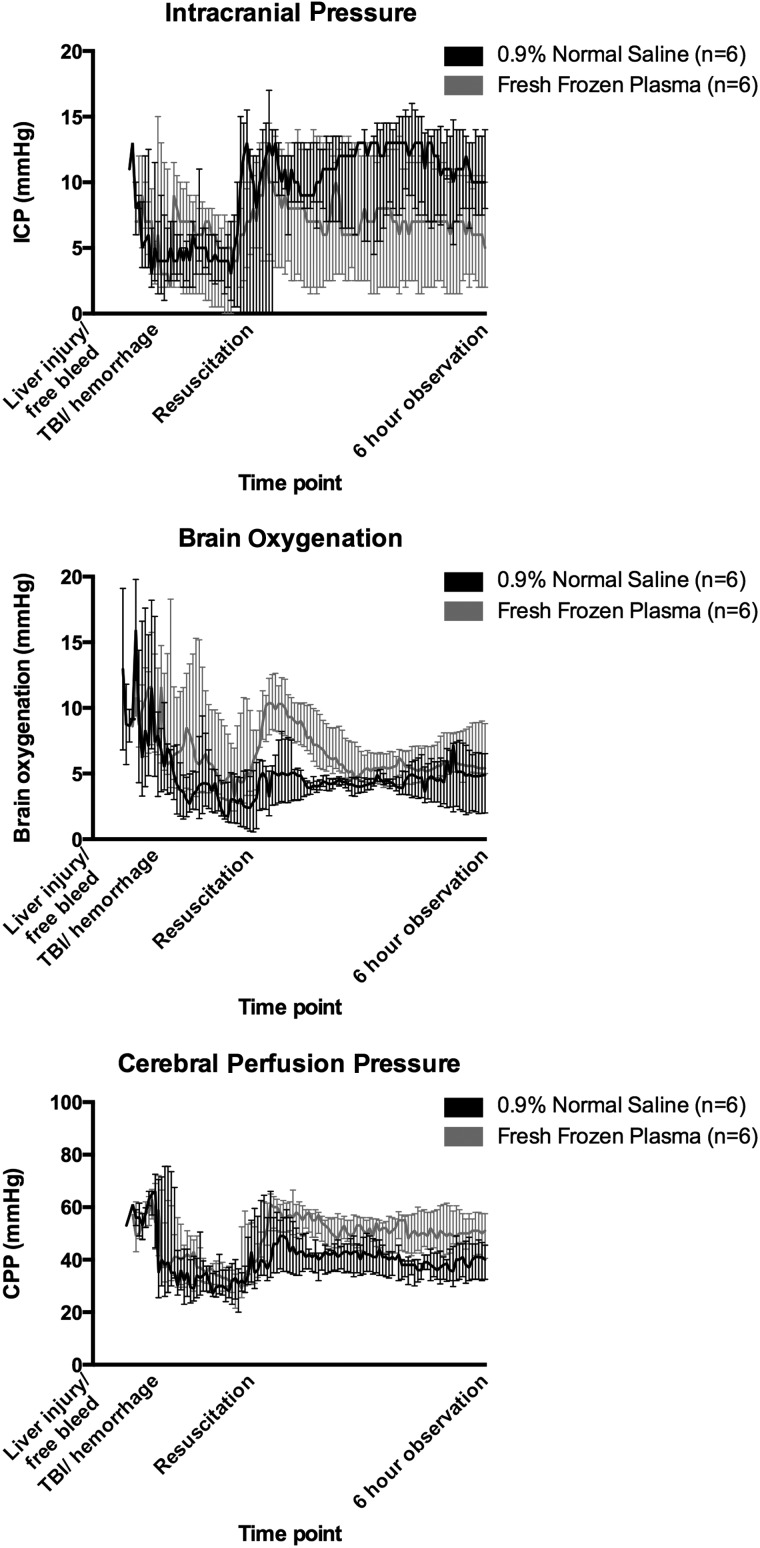
Values are presented in Table 1. Selected hemodynamic values are depicted in Figure 1 while ICP, brain oxygenation, and cerebral perfusion pressure (CPP) are shown in Figure 2. No differences in MAP, heart rate (HR) or cardiac output (CO) were identified before resuscitation. After resuscitation, FFP-resuscitated animals had overall higher median MAP (60.0 vs. 51.0 mm Hg; p=0.03). No differences in HR or CO between groups were observed after resuscitation. CPP values were comparable between groups before resuscitation, but were higher in FFP-treated animals after resuscitation (52.0 vs. 40.0 mm Hg; p=0.01).
Table 1.
Hemodynamic Values following Resuscitation
| Value | Normal saline (n=6) | Fresh frozen plasma (n=6) | p value | |
|---|---|---|---|---|
| Baseline | MAP (mm Hg) | 68.5 (66.5–73.5) | 77.0 (64.0–85.0) | 0.29† |
| Heart rate (beats/min) | 102.0 (92.5–107.8) | 97.0 (88.0–107.5) | 0.73† | |
| Cardiac output (L/min) | 4.6 (4.3–6.1) | 5.2 (4.1–5.9) | 0.89† | |
| Cerebral perfusion pressure (mm Hg) | 63.0 (60.5–66.0) | 58.0 (53.0–64.0) | 0.76† | |
| Intracranial pressure (mm Hg) | 3.0 (2.0–6.5) | 7.0 (3.0–9.5) | 0.15† | |
| Brain oxygenation (mm Hg) | 7.5 (6.0–17.3) | 11.6 (7.2–15.8) | 0.84† | |
| Shock phase | MAP (mm Hg) | 38.0 (33.8–43.3) | 41.0 (36.0–50.0) | 0.54* |
| Heart rate (beats/min) | 194.5 (179.3–202.0) | 171.0 (160.8–186.3) | 0.49* | |
| Cardiac output (L/min) | 2.1 (1.8–2.5) | 2.4 (2.0–3.3) | 0.87* | |
| Cerebral perfusion pressure (mm Hg) | 32.5 (28.0–41.0) | 36.0 (31.0–43.0) | 0.70 | |
| Intracranial pressure (mm Hg) | 4.0 (2.0–6.0) | 6.0 (2.0–8.0) | 0.43* | |
| Brain oxygenation (mm Hg) | 3.0 (1.8–5.3) | 5.9 (4.1–9.8) | 0.09* | |
| Resuscitation/6-h phase | MAP (mm Hg) | 51.0 (44.0–55.0) | 60.0 (55.0–63.0) | 0.03* |
| Heart rate (beats/min) | 150.0 (142.0–156.0) | 138.0 (125.0–149.0) | 0.27* | |
| Cardiac output (L/min) | 5.0 (4.5–5.4) | 5.7 (5.0–6.4) | 0.90* | |
| Cerebral perfusion pressure (mm Hg) | 40.0 (34.0–45.0) | 52.0 (46.0–57.0) | <0.01* | |
| Intracranial pressure (mm Hg) | 11.0 (8.0–13.0) | 7.0 (3.0–12.0) | 0.23* | |
| Brain oxygenation (mm Hg) | 4.4 (3.6–5.5) | 6.2 (5.1–8.3) | 0.05 |
Data presented as medians with interquartile ranges.
Median values over the 2-h shock phase or the 6-h observation phase, respectively. Actual values compared using linear mixed modeling to account for correlated data.
Single-time-point median values compared using Mann-Whitney's U test.
MAP, mean arterial pressure.
FIG. 1.
Mean arterial pressure (MAP), heart rate, and cardiac output throughout the experiment. Medians with interquartile range. Levels were comparable throughout the shockphase, but fresh frozen plasma–resuscitated animals exhibited overall higher MAP following resuscitation (p=0.03). TBI, traumatic brain injury.
FIG. 2.
Intracranial pressures (ICP), brain oxygenation, and cerebral perfusion pressures (CPP) throughout the experiment. Medians with interquartile range. ICP levels were comparable following resuscitation between groups, but fresh frozen plasma–resuscitated animals had higher brain oxygenation (p=0.05) and CPP (p<0.01) following resuscitation. TBI, traumatic brain injury.
Brain oxygenation was also comparable between groups before resuscitation, but was higher in FFP-treated animals after resuscitation (6.2 vs. 4.4 mm Hg; p=0.05). No significant differences were noted in ICP levels at any time point.
Lesion size and brain swelling
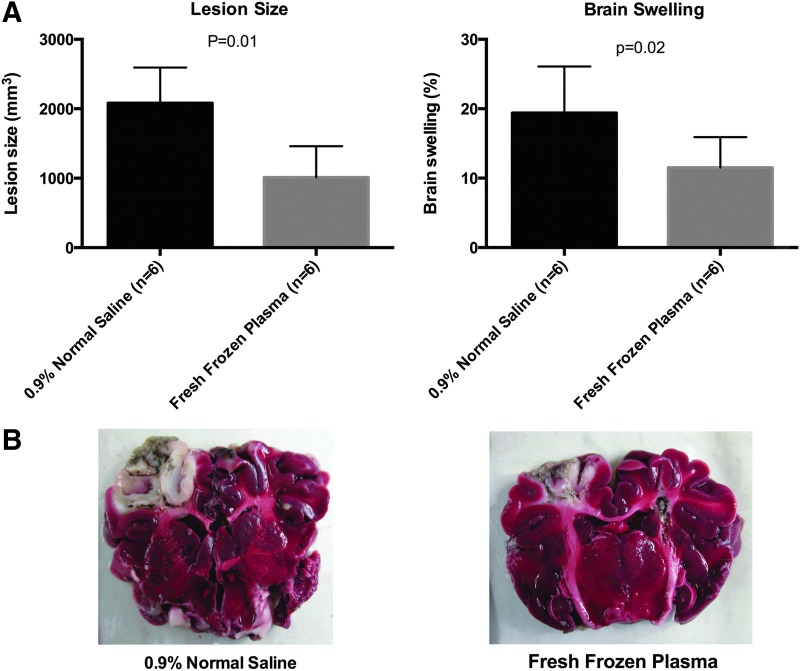
Values are presented in Table 2 and graphically depicted in Figure 3. FFP-resuscitated animals had significantly smaller lesion sizes (1005.8 vs. 2081.9 mm3; p=0.01) as well as brain swelling (11.5% vs. 19.4%; p=0.02), compared with NS-resuscitated animals.
Table 2.
Blood Gas, Brain Lesion Size and Swelling, and Markers of Endothelial Activation in Brain and Circulation
| Value | Normal saline (n=6) | Fresh frozen plasma (n=6) | p value | ||
|---|---|---|---|---|---|
| Baseline | Blood count | Hematocrit (%) | 27.8 (26.7–33-9) | 25.6 (24.7–37.8) | 0.54 |
| Blood gas | pH | 7.47 (7.45–7-49) | 7.47 (7.44–7.48) | 0.59 | |
| Lactate (mmol/L) | 1.9 (1.4–2.0) | 1.7 (1.3–2.3) | 0.94 | ||
| pO2 (mm Hg) | 101.2 (93.8–114.7) | 110.5 (98.5–111.4) | 0.39 | ||
| pCO2 (mm Hg) | 34.0 (32.5–38.5) | 37.3 (35.7–38.5) | 0.24 | ||
| Postresuscitation | Blood count | Hematocrit (%) | 16.5 (14.8–19.4) | 20.7 (18.6–21.8) | 0.04 |
| Blood gas | pH | 7.37 (7.31–7.39) | 7.42 (7.37–7.43) | 0.08 | |
| Lactate (mmol/L) | 2.9 (2.5–6.7) | 5.4 (4.4–5.8) | 0.33 | ||
| pO2 (mm Hg) | 101.8 (91.9–123.2) | 84.2 (74.5–117.5) | 0.33 | ||
| pCO2 (mm Hg) | 37.1 (26.1–39.2) | 42.6 (40.6–46.7) | 0.02 | ||
| Six-hour observation | Blood count | Hematocrit (%) | 18.2 (16.2–20-6) | 17.7 (16.0–19.8) | 0.82 |
| Blood gas | pH | 7.43 (7.39–7.45) | 7.49 (7.48–7.49) | <0.01 | |
| Lactate (mmol/L) | 1.5 (0.8–2.5) | 2.1 (1.4–2.4) | 0.24 | ||
| pO2 (mmHg) | 101.9 (95.1–107.3) | 103.4 (90.3–111.7) | 0.94 | ||
| pCO2 (mmHg) | 34.5 (28.7–36.1) | 41.7 (28.7–45.0) | 0.09 | ||
| Brain | Lesion size and swelling | Lesion size (mm3) | 2081.9 (1370.4–2592.6) | 1005.8 (762.0–1461.1) | 0.01 |
| Swelling (%) | 19.4 (13.7–26.1) | 11.5 (6.2–15.9) | 0.02 | ||
| Biomarkers | eNOS (ng/mL) | 816.4 (779.5–838.6) | 852.9 (846.9–859.3) | 0.03 | |
| ET-1 (ng/mL) | 373.7 (364.2–378.3) | 394.5 (371.3–405.8) | 0.09 |
Data presented as medians with interquartile range.
eNOS, endothelial nitric oxide synthase; ET-1, endothelin-1.
FIG. 3.
Brain lesion size and swelling (A) and representative images of brain slices from fresh frozen plasma– and normal saline–resuscitated animals (B). Viable tissues are stained red, and the area of necrosis appears as gray/white. Color image is available online at www.liebertpub.com/neu
Markers of endothelial activation and blood gas analysis
Values are presented in Table 2. FFP resuscitation resulted in higher levels of eNOS (852.9 vs. 816.4 ng/mL; p=0.03), without a significant difference in levels of ET-1. Blood-gas analysis revealed higher pH levels in FFP-resuscitated animals after the 6-h observation phase (7.49 vs. 7.43; p<0.01).
Discussion
In this large animal model of combined TBI and polytrauma, we have shown that resuscitation with FFP attenuates brain lesion size and swelling compared to NS. This was associated with improvements in CPPs and brain oxygenation as well as increased serum levels of eNOS. Collectively, these results are in line with our previous findings, indicating a neuroprotective effect of early FFP infusion, compared to resuscitation with NS, following combined TBI and hemorrhagic shock.13 As such, these findings not only validate our previous report, but also indicate that the neuroprotective effect of FFP may extend to more clinically realistic settings where the TBI occurs in combination with multi-system trauma. Further, our results suggest that the observed neuroprotection may be associated with optimized cerebral perfusion and brain oxygenation profiles. This is likely primarily owing to superior plasma volume expansion and reduced cerebral edema in the FFP group, contrasted with rapid fluid extravasation into the brain parenchyma in the NS group. Alternatively, the observed favorable brain oxygenation profile in the FFP group may, in part, be associated with an endothelial preservation resulting in better vasomotor control through mediators such as eNOS,18 with subsequent improvements in cerebral perfusion pressures and brain oxygenation.
Vasospasm after TBI has been reported in as many as 60% of patients and is a well-known predictor of adverse neurological outcome.19 This phenomenon may, in part, be attributed to an injury-induced up-regulation of ET-1.20 High CSF levels of ET-1 have thus been shown to predict adverse outcome after TBI.21 Counteracting this vasoconstrictor effect are factors such as eNOS, an endothelial-derived enzyme generating nitric oxide (NO) and thus an important aspect of maintaining vasodilation and cerebral perfusion. Several converging lines of evidence have thus collectively identified an important role of eNOS and NO in regulation of cerebral blood flow after TBI. In patients, brain microdialysate levels of NO have been shown to correlate with regional cerebral blood flow,22 suggesting an important role of this metabolite in regulating local vessel tone. Further, reports from murine models have indicated that inhalation of NO after TBI is neuroprotective and reduces cerebral inflammation,23,24 whereas other reports have indicated that eNOS-deficient mice have lower cerebral blood flow than wild-type mice after cortical impact.18 In contrast to this, other reports have linked high brain microdialysate levels of NO to adverse outcome after TBI,25 whereas results from a murine cortical impact model reported reduced brain edema formation in eNOS-deficient mice.24 Although these results may seem conflicting, it is likely that TBI-induced dysregulation of the balance between vasoconstrictors, such as ET-1, and vasodilators, such as eNOS and NO, promotes development of the secondary brain injury by inducing local hypoxia through compromised cerebral perfusion. Although we did not identify differences in levels of ET-1, it is likely that our findings of increased eNOS levels in FFP-resuscitated animals could contribute to the better cerebral perfusion pressures and brain oxygenation observed in this study.
Several limitations in this study deserve recognition. First, this is a short-term study observing the neuroprotective effects of FFP resuscitation in the early hours after TBI. It is thus likely that the secondary brain injury is not fully developed following this short observation, and we cannot conclude on the long-term effects of FFP resuscitation. The fact that, despite a significant increase in the brain swelling, the increase in the ICP was rather modest suggests that the swelling had not peaked and the compensatory mechanisms were still able to keep the ICP from rising steeply. Further, though lesion sizes and brain swelling were different at this time point, we do not know how this translates into functional outcome and long-term recovery. A large animal survival model of combined TBI and multisystem trauma has been developed in our lab, and additional long-term survival studies are underway to address this important issue. Second, several modeling choices may affect clinical translatability. Most notably, we opted to use combined controlled and free hemorrhage in order to ensure reproducibility between animals, but this decision naturally comes at the price of translatability. Also, we acknowledge the fact that most modern resuscitation regimens call for combined resuscitation with FFP, PRBCs and platelets (PLTs) whereas large-volume NS resuscitation is infrequently used. The aim of this study was, however, to evaluate the effects of FFP resuscitation, and we thus opted to use this fluid without additional PRBCs and PLTs because this would, in essence, have evaluated the synergistic effects of these combined resuscitation strategies, rather than the effect of FFP alone. Further, studies have indicated that freeze-dried FFP may be equally efficacious,26,27 which may indicate a potential role for FFP-based products in early prehospital resuscitation. Our choice of NS as a control fluid can certainly be debated, but was based on the need for a well-studied, relatively inert control treatment. Further, the choice of a 3:1 ratio of saline resuscitation could be argued to result in under-resuscitation, which could, again, impact on our findings. The choice of ratio was based on the most commonly accepted strategy as advocated by currently accepted guidelines, such as the Advanced Trauma Life Support (ATLS).
The differences in the oncotic pressures and the volumes of distribution of the test fluids certainly contributed to the differential degree of brain swelling observed 6 h later. As is the case for any study comparing a novel treatment strategy to an accepted one, the interpretation of the results is only meaningful in the scope of those particular treatments. Interpretation of the presented results should thus be limited to the effect of FFP resuscitation, compared with the previously accepted crystalloid 3:1 resuscitation strategy, and care should be exercised when extrapolating these finding to other fluids or ratios.
It should also be recognized that the small sample size and large number of comparisons done in this study increase the risk of chance findings, and results should be interpreted with this in mind.
In conclusion, our results indicate that early administration of FFP decreases brain lesion size and swelling, compared to NS, in a large animal model of combined TBI and multi-system trauma.
Acknowledgments
The study was funded by a grant from the U.S. Army Medical Research and Materiel Command (GRANTT00521959; to H.B.A.).
Author Disclosue Statement
No competing financial interests exist.
References
- 1.Faul M.X.L., Wald M.M., and Coronado V.G. (2010). Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 2.Eastridge B.J., Mabry R.L., Seguin P., Cantrell J., Tops T., Uribe P., Mallett O., Zubko T., Oetjen-Gerdes L., Rasmussen T.E., Butler F.K., Kotwal R.S., Holcomb J.B., Wade C., Champion H., Lawnick M., Moores L., and Blackbourne L.H. (2012). Death on the battlefield (2001–2011): implications for the future of combat casualty care. J. Trauma Acute Care Surg. 73, S431–S437 [DOI] [PubMed] [Google Scholar]
- 3.Badjatia N., Carney N., Crocco T.J., Fallat M.E., Hennes H.M., Jagoda A.S., Jernigan S., Letarte P.B., Lerner E.B., Moriarty T.M., Pons P.T., Sasser S., Scalea T., Schleien C.L., and Wright D.W. (2008). Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehosp. Emerg. Care 12, Suppl. 1, S1–S52 [DOI] [PubMed] [Google Scholar]
- 4.Chesnut R.M., Marshall S.B., Piek J., Blunt B.A., Klauber M.R., and Marshall L.F. (1993). Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir. Suppl. (Wien) 59, 121–125 [DOI] [PubMed] [Google Scholar]
- 5.Bickell W.H., Wall M.J., Jr., Pepe P.E., Martin R.R., Ginger V.F., Allen M.K., and Mattox K.L. (1994). Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N. Engl. J. Med. 331, 1105–1109 [DOI] [PubMed] [Google Scholar]
- 6.Leijdesdorff H.A., van Dijck J.T., Krijnen P., Vleggeert-Lankamp C.L., and Schipper I.B. (2014). Injury pattern, hospital triage and mortality of 1,250 patients with severe traumatic brain injury caused by road traffic accidents. J. Neurotrauma 31, 459–65 [DOI] [PubMed] [Google Scholar]
- 7.Tan P.G., Cincotta M., Clavisi O., Bragge P., Wasiak J., Pattuwage L., and Gruen R.L. (2011). Review article: prehospital fluid management in traumatic brain injury. Emerg. Med. Australas 23, 665–676 [DOI] [PubMed] [Google Scholar]
- 8.Finfer S., Bellomo R., Boyce N., French J., Myburgh J., and Norton R. (2004). A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N. Engl. J. Med. 350, 2247–2256 [DOI] [PubMed] [Google Scholar]
- 9.Brown J.B., Cohen M.J., Minei J.P., Maier R.V., West M.A., Billiar T.R., Peitzman A.B., Moore E.E., Cushieri J., and Sperry J.L. (2012). Debunking the survival bias myth: characterization of mortality during the initial 24 hours for patients requiring massive transfusion. J. Trauma Acute Care Surg. 73, 358–364 [DOI] [PubMed] [Google Scholar]
- 10.Brown L.M., Aro S.O., and Cohen M.J; Trauma Outcomes Group, Holcomb J.B., Wade C.E., Brasel K.J., Vercruysse G., MacLeod J., Dutton R.P., Hess J.R., Duchesne J.C., McSwain N.E., Muskat P., Johannigamn J., Cryer H.M., Tillou A., Pittet J.F., Knudson P., De Moya M.A., Schreiber M.A., Tieu B., Brundage S., Napolitano L.M., Brunsvold M., Sihler K.C., Beilman G., Peitzman A.B., Zenait M.S., Sperry J., Alarcon L., Croce M.A., Minei J.P., Kozar R., Gonzalez E.A., Stewart R.M., Cohn S.M., Mickalek J.E., Bulger E.M., Cotton B.A., Nunez T.C., Ivatury R., Meredith J.W., Miller P., Pomper G.J., and Marin B. (2011). A high fresh frozen plasma: packed red blood cell transfusion ratio decreases mortality in all massively transfused trauma patients regardless of admission international normalized ratio. J. Trauma 71, S358–S363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasotakis G., Sideris A., Yang Y, de Moya M., Alam H., King D.R., Tompkins R., and Velhamos G.; Inflammation and Host Response to Injury Investigators. (2013). Aggressive early crystalloid resuscitation adversely affects outcomes in adult blunt trauma patients: an analysis of the Glue Grant database. J. Trauma Acute Care Surg. 74, 1215–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozar R.A., Peng Z., Zhang R., Holcomb J.B., Pati S., Park P., Ko T.C., and Paredes A. (2011). Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth. Analg. 112, 1289–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin G., DeMoya M.A., Duggan M., Knightly T., Mejaddam A.Y., Hwabejire J., Lu J., Smith W.M., Kasotakis G., Velmahos G.C., Socrate S., and Alam H.B. (2012). Traumatic brain injury and hemorrhagic shock: evaluation of different resuscitation strategies in a large animal model of combined insults. Shock 38, 49–56 [DOI] [PubMed] [Google Scholar]
- 14.Sillesen M., Johansson P.I., Rasmussen L.S., Jin G., Jepsen C.H., Imam A.M., Hwabejire J., Lu J., Duggan M., Velmahos G., deMoya M., and Alam H.B. (2013). Platelet activation and dysfunction in a large-animal model of traumatic brain injury and hemorrhage. J. Trauma Acute Care Surg. 74, 1252–1259 [DOI] [PubMed] [Google Scholar]
- 15.Prevost T.P., Jin G., de Moya M.A., Alam H.B., Suresh S., and Socrate S. (2011). Dynamic mechanical response of brain tissue in indentation in vivo, in situ and in vitro. Acta Biomater. 7, 4090–4101 [DOI] [PubMed] [Google Scholar]
- 16.Sillesen M., Jin G., Oklu R., Albadawi H., Imam A.M., Jepsen C.H., Hwabejire J.O., Ostrowski S.R., Johansson P.I., Rasmussen L.S., and Alam H.B. (2013). Fresh-frozen plasma resuscitation after traumatic brain injury and shock attenuates extracellular nucleosome levels and deoxyribonuclease 1 depletion. Surgery 154, 197–205 [DOI] [PubMed] [Google Scholar]
- 17.Jin G., Duggan M., Imam A., deMoya M.A., Sillesen M., Hwabejire J., Jepsen C.H., Liu B., Mejaddam A.Y., Lu J., Smith W.M., Velhamos G.C., Socrate S., and Alam H.B. (2012). Pharmacologic resuscitation for hemorrhagic shock combined with traumatic brain injury. J. Trauma Acute Care Surg. 73, 1461–1470 [DOI] [PubMed] [Google Scholar]
- 18.Lundblad C., Grande P.O., and Bentzer P. (2009). Hemodynamic and histological effects of traumatic brain injury in eNOS-deficient mice. J. Neurotrauma 26, 1953–1962 [DOI] [PubMed] [Google Scholar]
- 19.Kramer D.R., Winer J.L., Pease B.A., Amar A.P., and Mack W.J. (2013). Cerebral vasospasm in traumatic brain injury. Neurol. Res. Int. 2013, 415813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maier B., Lehnert M., Laurer H.L., and Marzi I. (2007). Biphasic elevation in cerebrospinal fluid and plasma concentrations of endothelin 1 after traumatic brain injury in human patients. Shock 27, 610–614 [DOI] [PubMed] [Google Scholar]
- 21.Salonia R., Empey P.E., Poloyac S.M., Wisniewski S.R., Klamerus M., Ozawa H., Wagner A.K., Ruppel R., Bell M.J., Feldman K., Adelson P.D., Clark R.S., and Kochanek P.M. (2010). Endothelin-1 is increased in cerebrospinal fluid and associated with unfavorable outcomes in children after severe traumatic brain injury. J. Neurotrauma 27, 1819–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hlatky R., Goodman J.C., Valadka A.B., and Robertson C.S. (2003). Role of nitric oxide in cerebral blood flow abnormalities after traumatic brain injury. J. Cereb. Blood Flow Metab. 23, 582–588 [DOI] [PubMed] [Google Scholar]
- 23.Liu P., Li Y.S., Quartermain D., Boutajangout A., and Ji Y. (2013). Inhaled nitric oxide improves short term memory and reduces the inflammatory reaction in a mouse model of mild traumatic brain injury. Brain Res. 1522, 67–75 [DOI] [PubMed] [Google Scholar]
- 24.Terpolilli N.A., Kim S.W., Thal S.C., Kuebler W.M., and Plesnila N. (2013). Inhaled nitric oxide reduces secondary brain damage after traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 33, 311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tisdall M.M., Rejdak K., Kitchen N.D., Smith M., and Petzold A. (2013). The prognostic value of brain extracellular fluid nitric oxide metabolites after traumatic brain injury. Neurocrit. Care 19, 65–68 [DOI] [PubMed] [Google Scholar]
- 26.Lee T.H., Van P.Y., Spoerke N.J., Hamilton G.J., Cho S.D., Watson K., Differding J., and Schreiber M.A. (2013). The use of lyophilized plasma in a severe multi-injury pig model. Transfusion 53, Suppl. 1, 72S–79S [DOI] [PubMed] [Google Scholar]
- 27.Spoerke N., Zink K., Cho S.D., Differding J., Muller P., Karahan A., Sondeen J., Holcomb J.B., and Schreiber M. (2009). Lyophilized plasma for resuscitation in a swine model of severe injury. Arch. Surg. 144, 829–834 [DOI] [PubMed] [Google Scholar]