Abstract
Magnetization transfer (MT) imaging has been explored in prior studies of HIV patients and showed the potential capacity to assess brain injury after HIV infection. In the present study, adult pig-tailed macaques were infected with a highly neuropathogenic virus SIVsmmFGb. MT imaging was exploited to examine the monkey brains before simian immunodeficiency virus (SIV) inoculation and 2, 4, 8, 12, 16, and 20 weeks post-SIV inoculation. Blood samples were collected from each animal for monitoring CD4+ and CD8+ T cells before each MRI scan. The MT ratios (MTR) in several brain regions of interest were evaluated longitudinally. Significant reductions of MTR were observed in whole brain and selected regions of interest (genu, splenium, thalamus, caudate, centrum semiovale, frontal white matter, frontal gray matter, and putamen) in the SIV-infected monkeys, consistent with those reported previously in HIV patients. In particular, the longitudinal results indicate that abnormal MTR reduction can be detected as early as in 2 weeks and MTR may be more sensitive to the brain injury in cortical regions than in subcortical regions during acute SIV infection. In addition, MTR reduction in genu, centrum semiovale, and thalamus significantly correlated with the CD4+ T cell percentage decrease. Also, the MTR reduction in thalamus correlated with the CD8+ T cell percentage elevation. Taken together, this study reported the longitudinal evolution of MTR in different brain regions during SIV infection and further validates previous findings in HIV patients. The preliminary results suggest that MT imaging could be a robust and sensitive approach to characterize the neurodegeneration after SIV or HIV infection.
Introduction
Magnetization transfer (MT) imaging, or magnetization transfer contrast (MTC) magnetic resonance imaging, is a magnetic resonance imaging (MRI) technique based upon the exchange between proton magnetization in free water and bonded water in the brain tissue to characterize brain tissue property quantitatively.1,2 The magnetization transfer ratio (MTR), derived from MT imaging, has been explored and used to evaluate brain injury in various brain diseases (such as multiple sclerosis, Alzheimer's disease, stroke, and epilepsy).3–8 Also, a few studies in HIV patients have demonstrated that MT imaging might be a robust technique to assess neurodegeneration after HIV infection.9–13
However, prior MT imaging studies in HIV patients did not provide the dynamic MTR changes during the course of infection and those results could be biased with unknown conditions such as time of infection, patient age, and treatment history. Therefore, a longitudinal study of the disease under controlled condition will offer valuable information about the clinical relevance of global and regional temporal changes of MTR and about the evolution of the disease. Due to the difficulty of studying HIV in humans, such a study can be performed with an animal model of NeuroAIDS.
The simian immunodeficiency virus (SIV)-infected macaque models exhibit neuropathological symptoms similar to those seen in HIV-infected humans, and have been widely used for studying the cognitive and neuropathological sequelae of AIDS14–17 or vaccine development.18,19 SIVsmmFGb is a novel simian lentivirus strain inducing neuropathology in over 90% of infected pig-tailed macaques.15,17 The SIVsmm FGb-infected pig-tailed macaques are regarded as an excellent model for studying the mechanism of HIV-induced neurological disease and therapeutic development.15,20,21 In the present study, MT imaging was employed to evaluate brain injury longitudinally in this novel macaque model of NeuroAIDS. The purpose of this study was to characterize the temporal MTR changes during SIV infection under controlled conditions and compare the findings with previous studies of HIV patients. In addition, the relationship between MTR measures in the brain regions of interest and the immune system response was evaluated.
Materials and Methods
Three adult male pig-tailed macaques (Macaca nemestrina, 4 years old) were infected with the SIVsmmFGb, a highly neuropathogenic virus in pig-tailed macaques.15,20
MRI scans were performed on a Siemens 3T Trio scanner with the CP extremity coil (Siemens Medical Solutions, Malvern, PA) before SIV inoculation and in 2, 4, 8, 12, 16, and 20 weeks post-inoculation (WPI). Blood samples were collected from each animal for monitoring CD4+ and CD8+ T cells before each MRI scan. During MRI scanning, animals were anesthetized and immobilized with a custom-built monkey head holder. Anesthesia was maintained with 1–1.5% isoflurane. Et-CO2, inhaled CO2, O2 saturation, blood pressure, heart rate, respiration rate, and body temperature were monitored continuously and maintained in the normal ranges.22 The MT images (Fig. 1, middle row) were acquired using a custom-designed EPI sequence with the following parameters: TR/TE=3,810 ms/18 ms, FOV=96×96 mm, data matrix=64×64, slice thickness=2.2 mm, 60 measurements (30-pair images with/without presaturation RF irradiation for average purpose), and 16 slices were acquired. The MT pulse was achieved with an RF pulse train that consisted of 20 repeated RF blocks with a width of 100 ms for each pulse and a 1,000 μs gap in between. Corresponding T2 weighted images (Fig. 1, top row) were acquired with the same slice positions using fast spin-echo sequences with TR/TE=4870 ms/121 ms, FOV=96 mm×96 mm, matrix=128×128, slice thickness=2.2 mm, 16 slices, and 2 averages.
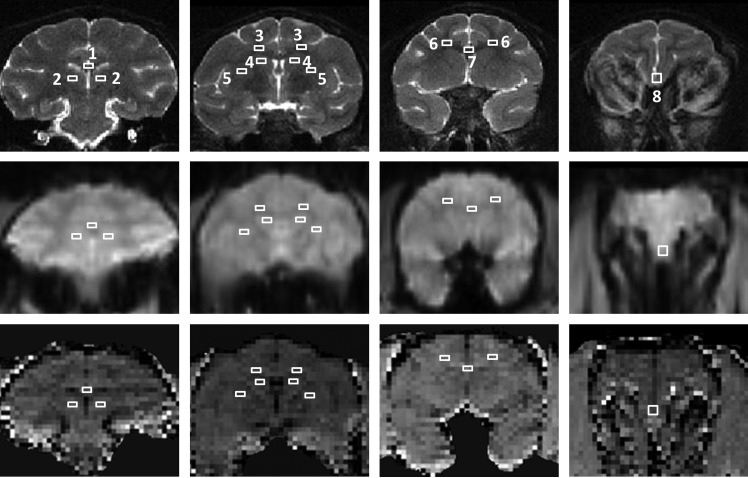
FIG. 1.
The regions of interests (ROIs) (rectangular markers) from (1) splenium, (2) thalamus, (3) CSEM, (4) caudate, (5) putamen, (6) FWM, (7) genu, and (8) FGM are illustrated in the coronal T2 weighted images (top), EPI images (middle), and magnetization transfer ratio maps (bottom) of an adult macaque brain. CSEM, centrum semiovale; FWM, frontal white matter; FGM, frontal gray matter; EPI, echoplanar imaging.
The MTR maps (Fig. 1, bottom row) were generated with MATLAB scripts constructed in-house with the following formula: MTR=(M0 – MS)/M0×100%, where MS and M0 are the signal intensities in a given voxel obtained with or without the MT pulse. The regions of interest (ROIs) were selected based upon previous studies of HIV patients and defined with the monkey brain atlas.23 The entire cortical, subcortical, and global regions, and specific brain structures including the genu, splenium, thalamus, caudate, centrum semiovale (CSEM), putamen, frontal gray matter (FGM), and frontal white matter (FWM), were selected for ROI analysis (Fig. 1). The MTR values in each ROI were calculated with Stimulate software.24 To conduct the MTR comparison of pre- vs. post-SIV infection, the mean MTR value of each ROI was obtained by averaging the MTR values across all the time points either before or post-SIV infection. A histogram of the whole parenchyma MTR was generated with the home-built Matlab script. The peak height of each histogram was normalized using the histogram frequency value (i.e., the total number of voxels with a given MTR value) divided by the total number of voxels in the ROI.12
Analysis of variance (ANOVA) for repeated measures was performed to check the differences across different time points; a paired Student's t-test was applied to compare averaged MTR values between pre- and post-SIV inoculation. Relationships between the T cells (or CD4/CD8 ratio) and MTR changes were examined using linear mixed regression analysis.25 All the p-values were adjusted for multiple comparisons by means of the false discovery rate (FDR) procedure.26 SPSS 20.0 was used for statistical analyses. Corrected p-values if less than 0.05 were considered statistically significant.
Results
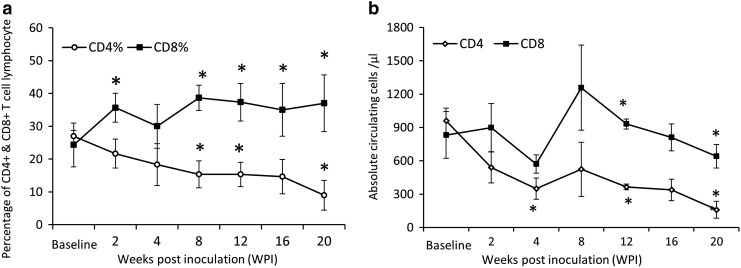
The temporal changes of the CD4+ and CD8+ T cell percentages and numbers are illustrated in Fig. 2. The CD4+ T cell percentage decreased progressively after SIV infection, differing significantly at 8, 12, and 20 WPI. The CD8+ T cell percentages were significantly elevated at 2, 8, 12, 16, and 20 WPI, though the CD8+ T cell numbers were significantly decreased in comparison to baseline level (Fig. 2b).
FIG. 2.
Percentage (a) and absolute circulating cells (b) of CD4+ and CD8+ T cell lymphocytes in macaques after simian immunodeficiency virus (SIV) inoculation. Error bars represent standard deviation. *p<0.05 compared with baseline (before SIV inoculation).
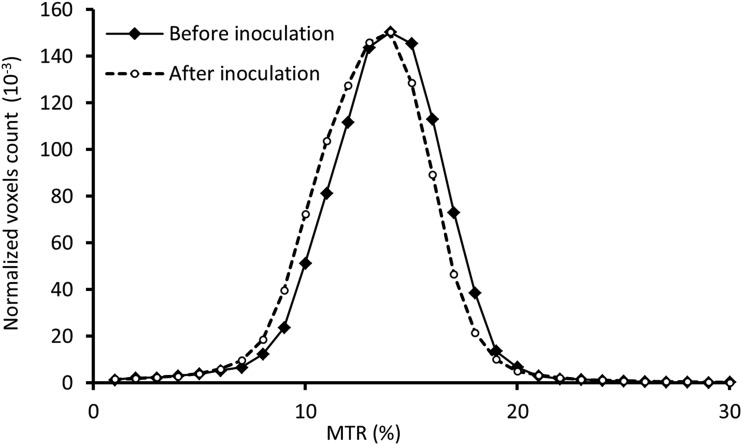
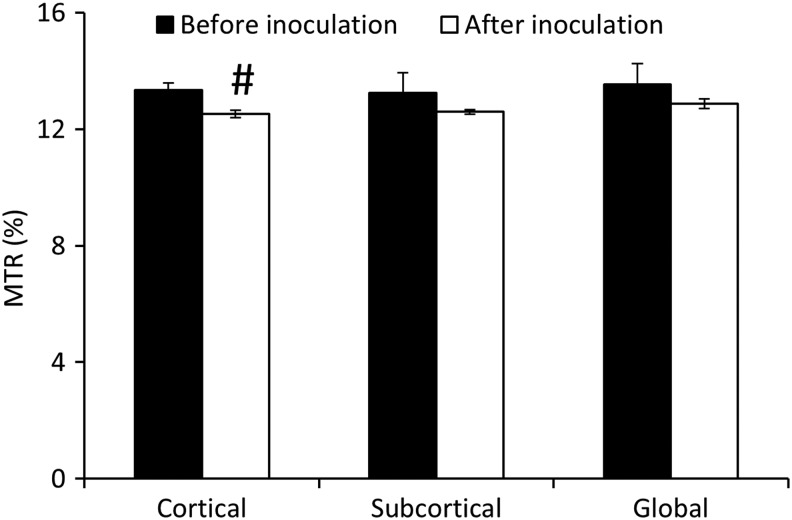
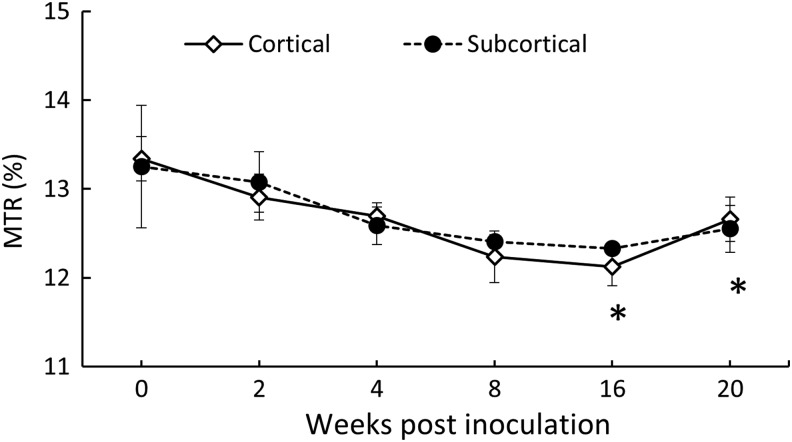
The histograms of whole brain MTR pre- and post-SIV inoculation are shown in Fig. 3. The MTR histogram after SIV infection was shifted to the lower MTR almost evenly and the whole brain mean MTR after SIV infection was reduced but did not reach a significant difference probably due to the small sample size. In addition, the mean MTR in the cortical, subcortical, and whole brain declined after SIV infection, but a nearly significant reduction was observed only in the cortical region (Fig. 4). The temporal evolution of MTR in the cortical and subcortical regions is exhibited in Fig. 5. A significant MTR reduction was observed in the cortical region 16 and 20 WPI. MTR in the subcortex exhibited a decreasing tendency but did not reach statistical significance at any time point compared to the baseline.
FIG. 3.
Comparison of whole brain magnetization transfer ratio (MTR) histograms before and after SIV inoculation.
FIG. 4.
Magnetization transfer ratio (MTR) changes in the cortical, subcortical, and whole brain between pre-SIV and post-SIV inoculation. Error bars represent the standard deviation error. #p=0.09.
FIG. 5.
Longitudinal magnetization transfer ratio (MTR) changes in the cortical and subcortical regions of macaque brains after SIV inoculation. Error bars represent the standard deviation error. *p<0.05 compared with baseline (before SIV inoculation).
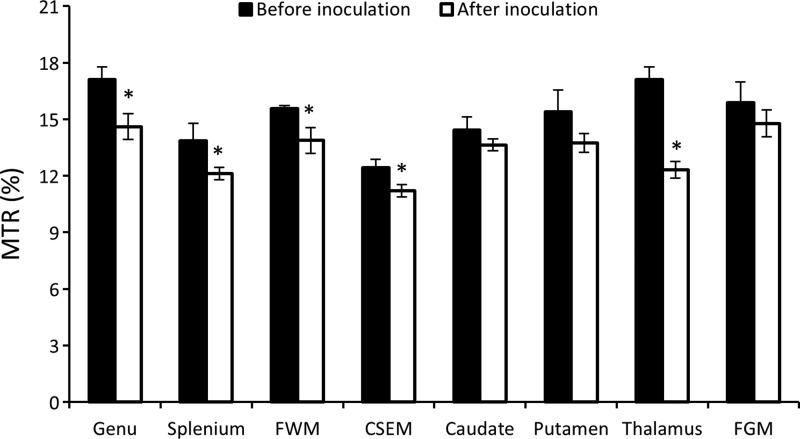
The MTR values in white matter structures (including the centrum semiovale, genu, splenium, and frontal white matter) and gray matter structures (such as the basal ganglia [caudate and putamen] thalamus, and frontal gray matter) were evaluated as determined by the paired Student's t-test for detecting the difference between pre- and post-SIV inoculation. The results showed that MTR in all the ROIs in white matter significantly decreased after SIV inoculation compared to those of the pre-SIV inoculation (Fig. 6, four pairs of bars in the left). All the MTR values in gray matter showed a decreasing tendency, but the significant MTR reduction was seen only in the thalamus (Fig. 6).
FIG. 6.
Magnetization transfer ratio (MTR) changes of selected regions of interest in white matter (the genu, splenium, FWM, and CSEM) and gray matter (caudate, putamen, FGM, and thalamus) between pre-SIV and post-SIV inoculation. CSEM, centrum semiovale; FWM, frontal white matter; FGM, frontal gray matter. Error bars represent the standard deviation. *p<0.05.
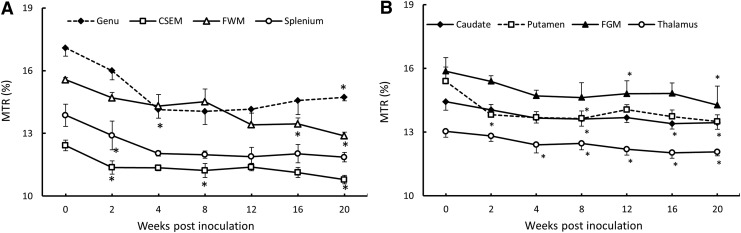
The longitudinal evolution of MTR in different brain regions is illustrated in Fig. 7. Repeated measures ANOVA analysis indicated that MTR in the centrum semiovale, splenium, and putamen significantly decreased as early as 2 WPI. In contrast, the earliest MTR reduction in genu and thalamus was seen 4 WPI. Meanwhile, the MTR in frontal white matter, caudate and frontal grey matter, declined progressively during the SIV infection, and significant MTR decrease was observed at 8, 16 and 20 WPI in caudate, 12 and 20 WPI in FGM, and 16 and 20 WPI in FWM.
FIG. 7.
Longitudinal magnetization transfer ratio (MTR) changes in white matter (A) and gray matter (B) of macaque brains after SIV inoculation. Error bars represent the standard deviation error. *p<0.05 compared with baseline (before SIV inoculation).
The relationships between the temporal MTR changes and immune response parameters (CD4+, CD8+ T cell percentage and CD4/CD8 ratio) were evaluated with mixed regression analysis (Table 1). The MTR reduction in genu, centrum semiovale, and thalamus correlated significantly with the CD4+ T cell percentage. Such a correlation was also observed between the MTR in thalamus and CD8+ T cell percentage. No correlations between the MTR changes in the whole brain, cortical and subcortical regions, and the CD4+, CD8+ T cell percentage and CD4/CD8 ratio were observed.
Table 1.
Standardized β Value Between CD4+, CD8+ T Cell Percentages and CD4/CD8 with Magnetization Transfer Ratio Changes
| % | Genu | Splenium | CSEM | FWM | Thalamus | Caudate | Putamen | FGM |
|---|---|---|---|---|---|---|---|---|
| CD4 | 0.31* | 0.19 | 0.55* | −0.66 | 0.64* | 0.30 | −0.36 | −0.09 |
| CD8 | −0.61 | 0.25 | 0.35 | −0.43 | −0.75* | 0.21 | 0.14 | −0.14 |
| CD4/CD8 | −0.47 | 0.29 | 0.57 | 0.44 | 0.47 | 0.51 | −0.44 | 0.25 |
p<0.05 after FDR correction.
Changes in genu, splenium, FWM, CSEM, caudate, putamen, FGM, and thalamus.
CSEM, centrum semiovale; FWM, frontal white matter; FGM, frontal gray matter.
Discussion and Conclusions
In the present study, MT imaging was utilized to examine brain injury in adult macaques infected with SIVsmmFGb. It is found the SIV infection results in general MTR reduction in most brain regions of interest. Significant MTR reduction was observed in genu, splenium, centrum semiovale, and putamen and thalamus during acute SIV infection. Also, the temporal MTR changes in genu, centrum semiovale, and thalamus were correlated significantly with immune responses (CD4+ and CD8+ percentages), suggesting that temporal evolution of MTR is region-dependent. The MTR alterations after SIV infection are in good agreement with those previously reported in HIV patients.
Neurodegeneration is generally observed in HIV-associated neurocognitive disorder (HAND) patients. Abnormal brain areas including corpus callosum, frontal white matter, basal ganglia, and thalamus were exhibited previously using MT imaging in HIV patients with dementia.27,28 The prior studies revealed that MTR reduction was significantly correlated with severity of cognitive impairment in HIV patients,12,13,29 demonstrating that MT imaging might be a robust means to assess brain injury after HIV infection. However, due to the difficulty of studying HIV in humans, there are very limited reports about temporal changes of brain structures during the course of HIV infection using MT imaging (or other MRI modalities). Therefore, macaque models can serve as an excellent platform to explore novel imaging techniques in HIV/AIDS studies. To characterize MTR changes during HIV infection and validate the prior findings in HIV patients, those ROIs used in patients were selected and examined in the SIV-infected monkeys.
Previous MT imaging studies have demonstrated that MTR is not sensitive to age in humans more than 2 years old.30–32 As a result, in the current data analysis, the baseline MTR (measured before SIV infection) was assumed not changing during the entire study period (<6 months). Therefore, it was used as a reference for comparison as the adult monkeys in the present study were 4 years old. MTR reduction was seen in most ROIs during acute SIV infection and the results (pre- vs. post-SIV infection) are in good agreement with prior findings, although the reduction in MTR in a few ROIs did not reach statistical significance probably due to the small study cohort.
The mean MTR changes in both cortex and subcortex demonstrate a declining tendency after SIV infection. However, a significant reduction in MTR was seen in the cortical but not in the subcortical regions, indicating that MTR may be a more sensitive measure of damage in the cortex, perhaps because there is more white matter in the cortex and the MT contrast is mediated through macromolecules and structural material, and thus is highly sensitive to myelin.33,34
MTR alterations in different brain structures including the corpus callosum (genu, splenium), frontal white matter, centrum semiovale, caudate, putamen, and thalamus have been seen in HIV patients.11,13 These areas were used in the present macaque study for comparison purposes. We also added frontal gray matter because it has been reported to be a vulnerable region in prior in vivo magnetic spectroscopy studies.35–37 Interestingly, MTR decreased significantly after SIV inoculation in all white matter ROIs (genu, splenium, centrum semiovale, and frontal white matter). In contrast, decreases of MTR in gray matter were less pronounced by showing a declining tendency in frontal gray matter, caudate, and putamen but a significant reduction only in thalamus.
This finding of MTR reduction is largely in agreement with previous reports in HIV patients.13 Previous postmortem studies of HIV patients indicate that all the selected ROIs are vulnerable in HIV infection.27,28 Our results further suggest that MTR is more sensitive in white matter than in gray matter. During HIV infection, reactive astrocytosis contributed substantially to HIV-associated brain damage.38 In addition, characteristic multinucleated giant cells (particularly in the central white matter of the cerebral hemispheres and the basal ganglia28) might be responsible for the vulnerability of these regions in HIV patients.
Previous MRS and MRI studies in SIV-infected macaques demonstrated regional differences in brain injury during the course of SIV infection.35,39–42 The region-dependent patterns in evolution were seen in the present study as well. Significant reduction of MTR in the centrum semiovale, splenium, and putamen was observed as early as 2 weeks after SIV infection while such alterations lagged behind in genu, thalamus, FWM, caudate, and FGM, revealing regional differences in MTR changes in the cerebral microstructural. Meanwhile, the time point at 2 weeks postinfection is associated with the beginnings of viral-infected cells in brain.43 Such regional differences during SIV infection were also observed in a previous report on the same group of animals examined with diffusion tensor imaging (DTI) and perfusion MRI.40
Thus, the results from various in vivo imaging modalities exhibit different sensitivity to immunodeficiency virus-induced brain damage but offer complementary information as well. As each imaging modality can be related to one or more tissue or physiological properties, the multimodality results can be integrated to investigate the neural substrates of SIV- or HIV-associated brain damage. Further study with a larger cohort of animals should provide more conclusive findings.
As seen in Table 1, the significant correlation between MTR reduction and CD4+ T and CD8+ T cell percentage changes indicates that reduction in MTR is associated with progression of the disease. CD4+ depletion and CD8 elevation are routinely used to monitor the progression of HIV infection.44–47 It was reported that the MTR of corpus callosum might be informative in studies of HIV-associated cognitive impairment and the MTR of thalamus and putamen was significantly correlated with the severity of cognitive impairment in HIV patients.13 Our findings suggest that the reduction in MTR in genu, centrum semiovale, and thalamus might be directly associated with the temporal progression of SIV or HIV infection and play an important role in patients with HAND.
In conclusion, this study reported the longitudinal evolution of MTR in a macaque model of NeuroAIDS. The spatial-temporal MTR changes are closely associated with the progression of SIV infection. The MT imaging findings in SIV monkeys validate the results reported previously in HIV patients and reveal the regional differences in temporal MTR changes during SIV infection. The preliminary results suggest that MT imaging could be a robust approach to examine early HIV infection.
Acknowledgments
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University. The Yerkes National Primate Research Center is fully accredited by AAALAC. The authors thank the Amelia Komery and vet team of Yerkes Imaging Center and the Animal Resources of Yerkes National Primate Research Center for their assistance in this study. The project was supported by the National Center for Research Resources P51RR000165 and is currently supported by the Office of Research Infrastructure Programs/OD P51OD011132 and NIH Grant MH067769 (to F.J.N.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hoffman SF. and Hoffman RA: Study of moderately rapid chemical exchange reactions by means of nuclear magnetic double resonance. J Chem Phys 1963;39(2892):2892–2901 [Google Scholar]
- 2.Wolff SD. and Balaban RS: Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med 1989;10(1):135–144 [DOI] [PubMed] [Google Scholar]
- 3.Jiang Q, Ewing JR, et al. : Magnetization transfer MRI: Application to treatment of middle cerebral artery occlusion in rat. J Magn Reson Imaging 2001;13(2):178–184 [DOI] [PubMed] [Google Scholar]
- 4.Knight RA, Nagesh V, et al. : Acute blood-brain barrier opening in experimentally induced focal cerebral ischemia is preferentially identified by quantitative magnetization transfer imaging. Magn Reson Med 2005;54(4):822–832 [DOI] [PubMed] [Google Scholar]
- 5.Tourdias T, Dousset V, et al. : Magnetization transfer imaging shows tissue abnormalities in the reversible penumbra. Stroke 2007;38(12):3165–3171 [DOI] [PubMed] [Google Scholar]
- 6.Mangia S, Carpenter AF, et al. : Magnetization transfer and adiabatic T1rho MRI reveal abnormalities in normal-appearing white matter of subjects with multiple sclerosis. Mult Scler 2014;20(8):1066–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bigot C, Vanhoutte G, et al. : Magnetization transfer contrast imaging reveals amyloid pathology in Alzheimer's disease transgenic mice. Neuroimage 2014;87:111–119 [DOI] [PubMed] [Google Scholar]
- 8.Seiler S, Ropele S, et al. : Magnetization transfer imaging for in vivo detection of microstructural tissue changes in aging and dementia: A short literature review. J Alzheimers Dis 2014;42:5229–5237 [DOI] [PubMed] [Google Scholar]
- 9.Grossman RI, Gomori JM, et al. : Magnetization transfer: Theory and clinical applications in neuroradiology. Radiographics 1994;14(2):279–290 [DOI] [PubMed] [Google Scholar]
- 10.Mehta RC, Pike GB, et al. : Magnetization transfer magnetic resonance imaging: A clinical review. Top Magn Reson Imaging 1996;8(4):214–230 [PubMed] [Google Scholar]
- 11.Dousset V, Armand JP, et al. : Magnetization transfer study of HIV encephalitis and progressive multifocal leukoencephalopathy. Groupe d'Epidemiologie Clinique du SIDA en Aquitaine. AJNR Am J Neuroradiol 1997;18(5):895–901 [PMC free article] [PubMed] [Google Scholar]
- 12.Ge Y, Kolson DL, et al. : Whole brain imaging of HIV-infected patients: Quantitative analysis of magnetization transfer ratio histogram and fractional brain volume. AJNR Am J Neuroradiol 2003;24(1):82–87 [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Storey P, et al. : Whole brain and localized magnetization transfer measurements are associated with cognitive impairment in patients infected with human immunodeficiency virus. AJNR Am J Neuroradiol 2008;29(1):140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClure HM. and Novembre FJ: Simian immunodeficiency virus variants: Threat of new lentiviruses. Am J Med Sci 1996;311(1):30–33 [DOI] [PubMed] [Google Scholar]
- 15.Novembre FJ, De Rosayr J, et al. : Isolation and characterization of a neuropathogenic simian immunodeficiency virus derived from a sooty mangabey. J Virol 1998;72(11):8841–8851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams R, Bokhari S, et al. : Nonhuman primate models of NeuroAIDS. J Neurovirol 2008;14(4):292–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeve AB, Pearce NC, et al. : Neuropathogenic SIVsmmFGb genetic diversity and selection-induced tissue-specific compartmentalization during chronic infection and temporal evolution of viral genes in lymphoid tissues and regions of the central nervous system. AIDS Res Hum Retroviruses 2010;26(6):663–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan C, Marthas M, et al. : The use of nonhuman primate models in HIV vaccine development. PLoS Med 2008;5(8):e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apetrei C, Pandrea I, et al. : Nonhuman primate models for HIV cure research. PLoS Pathog 2012;8(8):e1002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Neil SP, Suwyn C, et al. : Correlation of acute humoral response with brain virus burden and survival time in pig-tailed macaques infected with the neurovirulent simian immunodeficiency virus SIVsmmFGb. Am J Pathol 2004;164(4):1157–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeve AB, Patel K, et al. : Reduced genetic diversity in lymphoid and central nervous system tissues and selection-induced tissue-specific compartmentalization of neuropathogenic SIVsmmFGb during acute infection. AIDS Res Hum Retroviruses 2009;25(6):583–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li CX, Patel S, et al. : Dose-dependent effect of isoflurane on regional cerebral blood flow in anesthetized macaque monkeys. Neurosci Lett 2013;541:58–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Logothetis KSS, et al. : A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates, 1st ed. Academic Press, San Diego, CA, 2007, p. 326 [Google Scholar]
- 24.Strupp JP: Stimulate: A GUI based fMRI analysis software package. Neuroimage 1996;3:S607 [Google Scholar]
- 25.Blackwell E, de Leon CF, et al. : Applying mixed regression models to the analysis of repeated-measures data in psychosomatic medicine. Psychosom Med 2006;68(6):870–878 [DOI] [PubMed] [Google Scholar]
- 26.Benjamini YH, et al. : Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995;57(1):289–300 [Google Scholar]
- 27.Everall I, Barnes H, et al. : Assessment of neuronal density in the putamen in human immunodeficiency virus (HIV) infection. Application of stereology and spatial analysis of quadrats. J Neurovirol 1995;1(1):126–129 [DOI] [PubMed] [Google Scholar]
- 28.Bell JE: An update on the neuropathology of HIV in the HAART era. Histopathology 2004;45(6):549–559 [DOI] [PubMed] [Google Scholar]
- 29.Ragin AB, Storey P, et al. : Disease burden in HIV-associated cognitive impairment: A study of whole-brain imaging measures. Neurology 2004;63(12):2293–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engelbrecht V, Rassek M, et al. : Age-dependent changes in magnetization transfer contrast of white matter in the pediatric brain. AJNR Am J Neuroradiol 1998;19(10):1923–1929 [PMC free article] [PubMed] [Google Scholar]
- 31.van Buchem MA, Steens SC, et al. : Global estimation of myelination in the developing brain on the basis of magnetization transfer imaging: A preliminary study. AJNR Am J Neuroradiol 2001;22(4):762–766 [PMC free article] [PubMed] [Google Scholar]
- 32.Rovaris M, Iannucci G, et al. : Age-related changes in conventional, magnetization transfer, and diffusion-tensor MR imaging findings: Study with whole-brain tissue histogram analysis. Radiology 2003;227(3):731–738 [DOI] [PubMed] [Google Scholar]
- 33.Gringel T, Schulz-Schaeffer W, et al. : Optimized high-resolution mapping of magnetization transfer (MT) at 3 Tesla for direct visualization of substructures of the human thalamus in clinically feasible measurement time. J Magn Reson Imaging 2009;29(6):1285–1292 [DOI] [PubMed] [Google Scholar]
- 34.Corben LA, Kashuk SR, et al. : Myelin paucity of the superior cerebellar peduncle in individuals with Friedreich ataxia: An MRI magnetization transfer imaging study. J Neurol Sci 2014;343(1–2):138–143 [DOI] [PubMed] [Google Scholar]
- 35.Greco JB, Westmoreland SV, et al. : In vivo 1H MRS of brain injury and repair during acute SIV infection in the macaque model of neuroAIDS. Magn Reson Med 2004;51(6):1108–1114 [DOI] [PubMed] [Google Scholar]
- 36.Lentz MR, Kim JP, et al. : Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology 2005;235(2):461–468 [DOI] [PubMed] [Google Scholar]
- 37.Lentz MR, Kim WK, et al. : Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology 2009;72(17):1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Budka H: Neuropathology of human immunodeficiency virus infection. Brain Pathol 1991;1(3):163–175 [DOI] [PubMed] [Google Scholar]
- 39.Ratai EM, Pilkenton SJ, et al. : In vivo proton magnetic resonance spectroscopy reveals region specific metabolic responses to SIV infection in the macaque brain. BMC Neurosci 2009;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C, Zhang X, et al. : Longitudinal diffusion tensor imaging and perfusion MRI investigation in a macaque model of neuro-AIDS: A preliminary study. Neuroimage 2011;58(1):286–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X. and Li C: Quantitative MRI measures in SIV-infected macaque brains. J Clin Cell Immunol 2013;Suppl 7:S7–005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li C, Zhang X, et al. : Longitudinal cerebral metabolic changes in pig-tailed macaques infected with the neurovirulent virus SIVsmmFGb. J Neurovirol 2014; [Ebpub ahead of print]; DOI: 10.1007/s13365-014-0286-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams KC, Corey S, et al. : Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: Implications for the neuropathogenesis of AIDS. J Exp Med 2001;193(8):905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregersen JP, Mehdi S, et al. : A CD 4 immunoglobulin fusion protein with antiviral effects against HIV. Arch Virol 1990;111(1–2):29–43 [DOI] [PubMed] [Google Scholar]
- 45.Aranda-Anzaldo A: A role for CD8+ T lymphocytes in the pathogenesis of AIDS. Res Immunol 1991;142(7):541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coutinho SG, Da-Cruz AM, et al. : CD4+ and CD8+ T cell immune responses of immunocompetent and immunocompromised (AIDS) patients with American tegumentary leishmaniasis. Mem Inst Oswaldo Cruz 1996;91(3):381–384 [DOI] [PubMed] [Google Scholar]
- 47.Brenchley JM, Schacker TW, et al. : CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004;200(6):749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]