Summary
The role of the weather as a trigger of sickle cell acute painful episodes has long been debated. To more accurately describe the role of the weather as a trigger of painful events, we conducted a case-crossover study of the association between local weather conditions and the occurrence of painful episodes. From the Cooperative Study of Sickle Cell Disease, we identified 813 patients with sickle cell anaemia who had 3570 acute painful episodes. We found an association between wind speed and the onset of pain, specifically wind speed during the 24-h period preceding the onset of pain. Analysing wind speed as a categorical trait, showed a 13% increase (95% confidence interval: 3%, 24%) in odds of pain, when comparing the high wind speed to lower wind speed (P = 0.007). In addition, the association between wind speed and painful episodes was found to be stronger among men, particularly those in the warmer climate regions of the United States. These results are in agreement with another study that found an association between wind speed and hospital visits for pain in the United Kingdom, and lends support to physiological and clinical studies that have suggested that skin cooling is associated with sickle vasoocclusion and perhaps pain.
Keywords: epidemiology, sickle cell anaemia, pain, weather
Sickle cell disease is the result of a point mutation in the β-globin chain of adult haemoglobin (HbA) that produces an abnormal haemoglobin, haemoglobin S (HbS) (Ingram, 1956). HbS polymerizes to form stiff rod-like structures when deoxygenated. When there are sufficient quantities of this polymer, red blood cells (RBC) distort and bend into the hallmark sickle shape (Herrick, 1910). Vasoocclusive complications of sickle cell disease result from blood flow interruption due to the tangling of these sickle cells in blood vessels. These complications include recurrent acute painful episodes, ischemic stroke, a pneumonia-like complication called acute chest syndrome (ACS), priapism, and avascular necrosis of the hip/shoulder (AVN) (Steinberg et al, 2001).
Both environmental and genetic factors are thought to contribute to the variability of severity and frequency of painful episodes among subjects with sickle cell disease (Jones et al, 2005). The role of the weather as a trigger of sickle cell pain crises has been studied for more than 30 years. Early studies were based on anecdotal evidence; such as case reports of sickle cell patients reporting pain during the colder parts of the day, or when swimming in the cold ocean on a particularly hot day (Redwood et al, 1976). Physiological and clinical studies have supported these suppositions and shown that skin cooling is related to vasoocclusion (Mohan et al, 1998) and perhaps pain (Resar & Oski, 1991). Further supporting cold weather as a trigger for painful episodes, several studies have associated pain with cold and rainy seasons (Amjad et al, 1974; Redwood et al, 1976; Ibrahim, 1980) and with windy weather and low humidity (Jones et al, 2005). Other studies, however, have shown no association of pain with the weather (Seeler, 1973; Slovis et al, 1986; Kehinde et al, 1987; Smith et al, 2003). The conclusions of all of these studies, with the exception of Jones et al (2005), are based on aggregated data, seasonal trend data, mean monthly temperatures, hospital-wide visit rates, but not data at the individual subject level.
Given that the induction period of the weather on sickle cell pain episodes is likely to be short, and its effect temporary due to inherent variation, it is a methodological challenge to assess the effect of the weather on pain episodes using traditional study designs. To more accurately describe the role of the weather as a trigger of pain crises, we studied the association between the weather and the occurrence of sickle cell painful episodes using a case-crossover design. The case-crossover study was specifically designed to study exposures, like the weather, that have a short induction time and transient effect. In short, the association was estimated by comparing the weather conditions preceding the occurrence of a painful episode with the weather conditions during another time when the case subject was not experiencing acute pain.
Methods
Study population
Study subjects were selected from the Cooperative Study of Sickle Cell Disease (CSSCD). During the three Phases of the CSSCD, data were gathered on all acute and chronic complications related to sickle cell disease. More detailed information was collected during Phase I, so all analyses were restricted to events occurring during that phase. During Phase 1, a ‘Painful Episode Form’ was completed each time a study patient presented at a participating clinic, emergency room, or hospital with a painful crisis (see definition below), not associated with bone pain lasting >7 d, avascular necrosis by culture, or joint swelling. All subjects/events for which a painful event form was completed were included in this study. Data collected included, but was not limited to: the time pain began, location and characterization of the pain, whether the patient was hospitalized and associated laboratory values. A detailed description of the CSSCD and patient demographics has been reported elsewhere (Gaston et al, 1987).
Data collection
The weather conditions for all of the participating centres were purchased from http://weather-source.com/; a commercial provider of local real time and historical weather data. Using the dates and times of each episode and the zip code of the emergency department/clinic attended, 48-h of weather data from the closest weather monitoring station were merged with clinical and demographic data for both a hazard period (preceding the onset of pain) and two control periods (that did not precede the onset of pain).
Outcome
During Phase I of the CSSCD, 813 patients had 3570 painful events over follow-up. Though Phase I was conducted over 10 years, from March 1979 to September 1988, most subjects were not followed for the entire duration of the study. All of the events described here occurred between March 1979 and December 1984.
A painful event was defined as a visit to a clinic/emergency department (ED) with pain in the extremities, back, abdomen, chest, or head, for which no other explanation could be found and which was not classified as one of the other special events (i.e. acute chest syndrome, avascular necrosis, stroke etc.). Pain should have lasted for at least 2 h, irritability in young children accompanied by pain on palpation was considered appropriate evidence, and finally, if patient was old enough, he/she must have stated that the pain was of the nature usually associated with a crisis (Platt et al, 1991). Painful events that occurred within 2 weeks after another painful event were considered a continuation of the preceding event and were eliminated from this study.
Exposures
Local weather data included temperature, humidity, precipitation, wind speed, cloud cover, and dew point. In addition to studying raw weather values, additional variables were derived from the raw values. The variables studied included minimum, maximum and means of all continuous measures and absolute change over the hazard period. Wind speed was also dichotomized using the Beaufort wind force scale (http://www.hpc.ncep.noaa.gov/html/beaufort.shtml) and its association with the occurrence of pain crises examined.
The hazard period consisted of the 48 h before the onset of pain up to the time of onset of pain. Control periods were two periods of 48 h, the first 2 weeks before and the second 2 weeks after the painful event. Preliminary analysis showed null associations between all weather conditions and the onset pain crises during the 48-h hazard period. Suggestive associations were, however, apparent when the hazard/control periods were limited to 24 h. Therefore, this study focused on the 24 h preceding the onset of pain.
Statistical analyses
Descriptive statistics were generated for all demographic and clinical variables (Table I). Crude associations of painful episodes and each of the described exposures were analysed using conditional logistic regression. Following crude analyses, potential confounders and effect modifiers were examined. Because of the nature of case-crossover design, characteristics that did not change between hazard and control periods, such as age, sex, geographic region etc., could not confound the association between the weather and pain. These may, however, act as modifiers.
Table I.
Subject characteristics.
| Males | 434 (53) |
| SS α | 254 (31) |
| Age, years | 25.1 ± 11.0 |
| Median (range) | 24 (7 to 68) |
| Number of painful episodes per study subject | 4.4 ± 5.8 |
| Median (range) | 2 (1 to 36) |
| Years follow-up per study subject | 3.1 ± 0.6 |
| Median (range) | 3.2 (1.6 to 4.1) |
| Regions | |
| (1) Cold without a dry season and a warm summer | 1910 (54) |
| (2) Temperate without a dry season and a hot summer | 1005 (28) |
| (3) Tropical | 483 (14) |
| (4) Temperate with a dry season and a warm summer | 172 (5) |
Values are presented as n (%), mean ± standard deviation or median (range).
CSSCD Sites in region: (1) Massachusetts (MA), Connecticut (CT), New York (NW), Pennsylvania (PA), Illinois (IL) and Missouri (MO); (2) Washington (WA), District of Columbia (DC), Tennessee (TN), North Carolina (NC), Georgia (GA) and Mississippi (MS); (3) Florida (FL) and (4) California (CA).
MA, NY, IL, DC, TN, NC and CA had more than one participating centre.
To study modification by geographic region, each centre was classified according to its Köppen-Geiger climate type (Peel et al, 2007). The 23 participating centres all reside in one of four climate types: (1) cold without a dry season, and a warm summer (2) temperate without a dry season, and a hot summer, (3) tropical, and (4) temperate with a dry season, and a warm summer. Stratified analyses were performed to determine if climate type was an important modifier of the association of the weather and the occurrence of painful episodes.
It is important to note that a limitation of this study is a speculative induction time and the potential for misclassification of the truly etiologically relevant exposure. To determine the relevant induction time, the hypothesized hazard/control period (24 h) was broken into 4 h intervals, analysed separately. Since misclassification of the exposure would bias the measure of association toward the null, the interval that maximizes the measure of association should reflect the appropriate induction time (Rothman, 1981).
Results
During Phase I of the CSSCD, 813 subjects had 3570 painful episodes. On average, study subjects were followed 3.1 years and experienced 4.4 painful episodes each (Table I). Subjects ranged in age from 7 to 68 years (mean 25.1 years), 53% were male and 31% had coincident α thalassaemia (Table I).
We found no substantial associations between temperature, cloud cover, relative humidity or precipitation and the occurrence of painful episodes. We did, however, find an association between wind speed and the onset of pain, specifically wind speed during the 24-h period preceding the onset of pain. Continuous measures of wind speed, mean and median wind speed during the 24 first hours of the hazard/control windows, showed significant associations with the occurrence of pain (P = 0.03 and P = 0.009, respectively). There was a 24% (95% confidence interval [CI] 1%, 52%) and 28% (95% CI: 6%, 56%) increase in the odds of pain with every 10 kph increase in mean and median wind speeds respectively, when comparing the hazard and control periods (Table II).
Table II.
Results of crude and sex-stratified analyses of the association between wind speed and the occurrence of sickle cell-related painful episodes.
| Crude | Male | Female | |
|---|---|---|---|
| Average wind speed | |||
| OR10 kph (95% CI) | 1.24 (1.01, 1.52) |
1.40 (1.05, 1.89) |
1.11 (0.84, 1.49) |
| P value | 0.03 | 0.02 | 0.46 |
| High versus low | |||
| OR10 kph (95% CI) | 1.13 (1.03, 1.24) |
1.27 (1.11, 1.45) |
1.02 (0.90, 1.16) |
| P value | 0.007 | 0.0004 | 0.73 |
OR, odds ratio; 95% CI, 95% confidence interval.
Wind speed was also analysed as a categorical trait by dichotomizing the 24-h average wind speed at Beaufort wind scale value of 2 (7 kph). This cut-off point was chosen since a Beaufort number of 2 is defined as ‘wind felt on exposed skin and leaves rustle’ and it was hypothesized that it is the skin cooling resulting from the wind which may trigger the acute painful event. This analysis showed a 13% increase (95% CI: 3%, 24%) in odds of pain when comparing the high wind speed with lower wind speed (P = 0.007; Table II).
Possible confounding by temperature was also studied. The adjusted measures of association however did not differ from the crude, so only the crude results are described here.
Potential effect modification of the association between wind speed and the occurrence of pain crises by climate zone, sex, and coincident α thalassemia was also studied by stratified analyses. There was no modification of the odds ratio by coincident α thalassaemia, however there was considerable effect modification by both climate zone and sex. When the analyses were stratified by sex, there was a stronger association of wind speed and pain among males when compared with females (Table III).
Table III.
Results of analyses of the association between wind speed and the occurrence of sickle cell-related painful episodes stratified by region.
| Region 1 | Region 2 | Region 3 | Region 4 | |
|---|---|---|---|---|
| Average wind speed | ||||
| OR10 kph (95% CI) |
1.17 (0.9, 1.52) |
1.52 (1.01, 1.51) |
1.28 (0.73, 2.22) |
0.65 (0.19, 2.22) |
| P value | 0.23 | 0.05 | 0.39 | 0.49 |
| High versus low | ||||
| OR10 kph (95% CI) |
1.07 (0.95, 1.21) |
1.26 (1.05, 1.51) |
1.23 (0.97, 1.55) |
0.94 (0.60, 1.45) |
| P value | 0.26 | 0.01 | 0.08 | 0.7 |
OR, odds ratio; 95% CI, 95% confidence interval.
After stratification by climate zone it was found that the temperate climate with hot summers (Region 2) had the strongest association with the occurrence of painful crises when compared with the remaining three climate zones. Stratification by both region and sex yielded similar results as the analyses stratified by region only, however we see a stronger association (OR 1.59; 95%CI: 1.15–2.21) between wind speed and the occurrence of painful episodes in Region 3 (tropical) than seen previously (Table IV). Three of the regions showed an increase in the odds of painful episodes as wind speed increased. In Region 4 (cold without a dry season), however, wind speed appeared to confer a protective effect, i.e. higher winds corresponded to fewer painful episodes. These results failed to reach significance, and subjects in this region made up a very small portion of the total number of painful events (53 of 813 subjects and 172 of 3570 events; 6% and 5% respectively).
Table IV.
Results of sex and region-stratified analyses of the association between wind speed and the occurrence of sickle cell-related painful episodes.
| Region 1
|
Region 2
|
Region 3
|
Region 4
|
|||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Average wind speed | ||||||||
| OR10 kph (95% CI) | 1.30 (0.88, 1.93) |
1.08 (0.76, 1.53) |
1.59 (0.89, 2.81) |
1.49 (0.79, 2.82) |
1.84 (0.84, 4.03) |
0.88 (0.40, 1.95) |
0.26 (0.024, 2.63) |
0.92 (0.22, 3.86) |
| P value | 0.18 | 0.67 | 0.12 | 0.2 | 0.13 | 0.76 | 0.28 | 0.91 |
| High versus low | ||||||||
| OR10 kph (95% CI) | 1.23 (1.02, 1.48) |
0.96 (0.82, 1.14) |
1.25 (0.98, 1.60) |
1.26 (0.97, 1.65) |
1.59 (1.15, 2.21) |
0.92 (0.66, 1.29) |
0.69 (0.29, 1.65) |
1.05 (0.63, 1.74) |
| P value | 0.03 | 0.18 | 0.08 | 0.09 | 0.005 | 0.65 | 0.40 | 0.86 |
OR, odds ratio; 95% CI, 95% confidence interval.
To address the potential limitation of an unknown induction time, the 24-h hazard/control periods were divided into 4 h intervals and their crude association with the occurrence of pain was analysed. It was found that the interval 13 to 16 h before the start of the painful was most strongly associated with the onset of pain and therefore was the most likely induction time (Fig 1).
Fig 1.
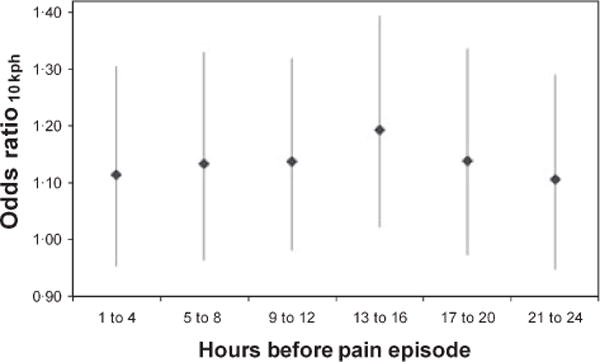
Determination of the etiologically relevant induction time of wind speed on the occurrence of sickle cell-related painful episodes. Figure 1 shows the results of an analysis to determine the most etiologically relevant induction time. Since misclassification of exposure should bias the measure of association (odds ratio) toward the null, the interval that maximizes the odds ratio should reflect the appropriate induction time. The interval 13 to 16 h before the start of the painful was most strongly associated with the onset of pain and therefore was the most likely induction time.
Discussion
The present study reports evidence of an association between wind speed and painful episodes in patients with sickle cell anaemia, particularly among males living in temperate climates with dry summers or humid sub-tropical climates. These results are in partial agreement with a study that found an association between wind speed and hospital visits for pain in the United Kingdom. The UK, however, is in a temperate climate zone with no dry season and a warm summer (Jones et al, 2005); a climate type not represented in the CSSCD.
Many studies have found an association with cold temperatures (Amjad et al, 1974; Redwood et al, 1976; Ibrahim, 1980) and analysis of our own data showed a clear seasonal trend (Fig 2). There was not, however, an association between temperature and painful episodes when data were analysed as a case-crossover study. This result may be due to the lack of variability in temperature between the hazard and control periods because they are separated by only 2 weeks. The case-crossover study is ideal for studying triggers or short-lived exposures, therefore, another possibility is that, although temperature may be a risk factor for painful episodes, it might not trigger painful episodes. Our results lend support to physiological and clinical studies that have suggested that skin cooling, and perhaps more importantly, the speed at which the skin is cooled, is associated with sickle vasoocclusion (Mohan et al, 1998) and pain (Resar & Oski, 1991).
Fig 2.
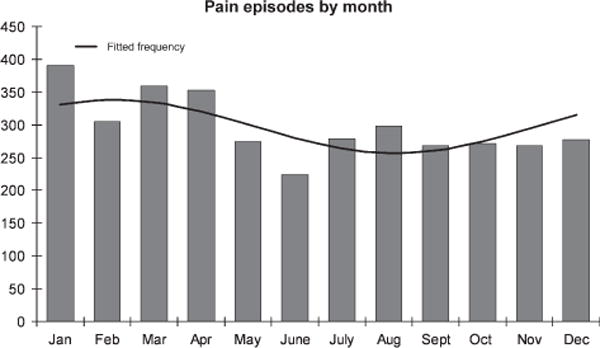
Seasonal trend analysis of the occurrence of sickle cell-related painful episodes. Figure 2 shows the total number of painful events by month over the follow up. The fitted frequency was determined using EpiSheet which uses the method described by Edwards (1961).
It is unsurprising that climate type is a modifier of the association between pain and wind speed as the overall weather conditions probably dictate clothing choices and time spent outdoors, and therefore exposure to the direct cooling effects of the wind. Another possible explanation for the strong associations seen in the warmer climates is rapid heat loss through the evaporation of sweat. The effect modification of the association by sex, however, is less obvious. We hypothesize that the varying magnitudes and directions of the odds ratios in each stratum may be due to body size, and therefore body surface area exposed to the wind, or perhaps because men perspire more than women, there is greater potential for evaporative cooling of the skin among men.
Since ‘time pain began’ was among the data collected during Phase 1 of the CSSCD, we were able to address the potential limitation of a speculative induction time and the potential for misclassification of the truly etiologically relevant exposure. The hypothesized hazard/control period (24 h) was broken into 4 h intervals, analysed separately. We acknowledge that there may be some misclassification of time of onset because we are relying on self-report, however this misclassification is likely to be non-differential and, therefore should bias the measure of association toward the null. We found the strongest association between the weather and pain occurred during the interval from 13 to 16 h preceding the onset of pain. As this significant association cannot be explained by the bias due to misclassification of the time of onset, this interval probably contains the most etiologically relevant induction time. This finding supports the length of our hypothesized hazard/control period (24 h) and further supports the choice of study design since case-crossover studies are ideal for exposures with short induction times.
Since data were available for multiple painful episodes for each subject, the issue of within subject correlation needed to be addressed. The simple solution was to only analyse one event, the first event or one randomly selected event, per subject. Though evidence of an association between wind speed and painful episodes was discovered, we lacked the power to detect a meaningful difference between the hazard and control periods (data not shown). A recent paper Luo and Sorock (2007) found that analysing multiple events with standard conditional logistic regression produced an unbiased effect estimate, however the estimate was less precise (wider confidence interval) than more sophisticated methods). As the effect estimates reported in the present study reached significance even with this loss of precision, we are confident in our finding of an association between wind speed and the occurrence of pain crises.
In conclusion, though pain is the most common complication of sickle cell disease, and likely to have many potential triggers, physicians may wish to advise patients to take precautions on windy days by limiting skin exposure.
Acknowledgments
Supported by NHLBI grant HL R01 68970 (MHS). VGN was supported by T32 HL 007501.
References
- Amjad H, Bannerman RM, Judisch JM. Letter: sickling pain and season. British Medical Journal. 1974;2:54. doi: 10.1136/bmj.2.5909.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JH. The recognition and estimation of cyclic trends. Annals of Human Genetics. 1961;25:83–87. doi: 10.1111/j.1469-1809.1961.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Gaston M, Smith J, Gallagher D, Flournoy-Gill Z, West S, Bellevue R, Farber M, Grover R, Koshy M, Ritchey AK. Recruitment in the Cooperative Study of Sickle Cell Disease (CSSCD) Controlled Clinical Trials. 1987;8:131S–140S. doi: 10.1016/0197-2456(87)90016-x. [DOI] [PubMed] [Google Scholar]
- Herrick J. Peculiar elongated sickle shaped red blood corpuscles in a case of severe anemia. Archives of Internal Medicine. 1910;6:517–520. [Google Scholar]
- Ibrahim AS. Relationship between meteorological changes and occurrence of painful sickle cell crises in Kuwait. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1980;74:159–161. doi: 10.1016/0035-9203(80)90236-9. [DOI] [PubMed] [Google Scholar]
- Ingram VM. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature. 1956;178:792–794. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- Jones S, Duncan ER, Thomas N, Walters J, Dick MC, Height SE, Stephens AD, Thein SL, Rees DC. Windy weather and low humidity are associated with an increased number of hospital admissions for acute pain and sickle cell disease in an urban environment with a maritime temperate climate. British Journal of Haematology. 2005;131:530–533. doi: 10.1111/j.1365-2141.2005.05799.x. [DOI] [PubMed] [Google Scholar]
- Kehinde MO, Marsh JC, Marsh GW. Sickle cell disease in North London. British Journal of Haematology. 1987;66:543–547. doi: 10.1111/j.1365-2141.1987.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Luo X, Sorock GS. Analysis of recurrent event data under the case-crossover design with applications to elderly falls. Statistics in Medicine. 2007;27:2890–2901. doi: 10.1002/sim.3171. [DOI] [PubMed] [Google Scholar]
- Mohan J, Marshall JM, Reid HL, Thomas PW, Hambleton I, Serjeant GR. Peripheral vascular response to mild indirect cooling in patients with homozygous sickle cell (SS) disease and the frequency of painful crisis. Clinical Science (London) 1998;94:111–120. doi: 10.1042/cs0940111. [DOI] [PubMed] [Google Scholar]
- Peel MC, Finlayson BL, McMahon TA. Updated world map of the Koppen-Geiger climate classification. Hydrology and Earth System Sciences. 2007;11:1633–1644. [Google Scholar]
- Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease. Rates and risk factors. New England Journal of Medicine. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- Redwood AM, Williams EM, Desal P, Serjeant GR. Climate and painful crisis of sickle-cell disease in Jamaica. British Medical Journal. 1976;1:66–68. doi: 10.1136/bmj.1.6001.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resar LM, Oski FA. Cold water exposure and vaso-occlusive crises in sickle cell anemia. Journal of Pediatrics. 1991;118:407–409. doi: 10.1016/s0022-3476(05)82156-0. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. Induction and latent periods. American Journal of Epidemiology. 1981;114:253–259. doi: 10.1093/oxfordjournals.aje.a113189. [DOI] [PubMed] [Google Scholar]
- Seeler RA. Non-seasonality of sickle-cell crisis. Lancet. 1973;2:743. doi: 10.1016/s0140-6736(73)92584-1. [DOI] [PubMed] [Google Scholar]
- Slovis CM, Talley JD, Pitts RB. Non relationship of climatologic factors and painful sickle cell anemia crisis. Journal of Chronic Diseases. 1986;39:121–126. doi: 10.1016/0021-9681(86)90068-8. [DOI] [PubMed] [Google Scholar]
- Smith WR, Coyne P, Smith VS, Mercier B. Temperature changes, temperature extremes, and their relationship to emergency department visits and hospitalizations for sickle cell crisis. Pain Management Nursing. 2003;4:106–111. doi: 10.1016/s1524-9042(02)54211-9. [DOI] [PubMed] [Google Scholar]
- Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Sickle Cell Disease. Cambridge University Press; Cambridge, UK: 2001. [Google Scholar]


