Abstract
TGF-β/Smads signaling plays a significant role in the regulation of growth of normal and prostate cancer cells. Smad proteins function as important mediators of intracellular signal transduction of transforming growth factor-β (TGF-β). TGF-β signaling pathway is known to regulate cell proliferation, differentiation, apoptosis and play a major role in some human diseases and cancers. Following their phosphorylation by TGF-β receptor-I, Receptor-regulated Smads (including Smad2 and Smad3 proteins) form a heteromeric complex with co-Smad (Smad4) and then translocate into the nucleus where they bind and regulate the expression of target genes. ERG (Ets Related Gene) belongs to the ETS family of transcriptional factors. Chromosomal rearrangement of TMPRSS2 gene and ERG gene has been found in majority of prostate cancers. Over-expression of full length or truncated ERG proteins have been shown to associate with a higher rate of recurrent and unfavorable prognosis of prostate cancer. In order to understand how ERG oncoprotein regulates TGF-β/Smads signaling pathway, we have studied the effect of ERG on TGF-β/Smad3 signaling pathway. In this study, we demonstrate that ERG oncoprotein physically interacts with Smad3 protein and stabilizes phospho-Smad3 protein and thereby enhance TGF-β/Smad3 signaling pathway in prostate cells. Thus, ERG oncoprotein plays an important role in prostate tumorigenesis by using a novel mechanism to activate TGF-β/Smad3 signaling pathway.
Keywords: TGF-β, Smad Protein, ERG, Phosphorylation, Prostate Cancer
INTRODUCTION
ERG (ETS Related Gene), an ETS family of transcriptional factor was discovered by Drs. Reddy and Rao in 1987 (Rao, 1987, Reddy, 1987). ERG was shown to function as a specific DNA transcriptional activator (Reddy, 1991, Siddique, 1993). ERG (DNA binding protein) was shown to be fused with RNA binding proteins resulting in fusion proteins that function as transcriptional activators in some Ewing tumors and leukemias (Ohno, 1994, Prasad, 1994, Rao, 1988, Tsuzuki, 2011). The gene fusion between ERG and the androgen responsive gene TMPRSS2 (transmembrane protease, serine 2) is a common genetic event on chromosome 21 in prostate cancer (Tomlins, 2005). Approximately 50% of prostate cancer patients are “fusion-positive” (Furusato, 2008, Shah, 2009). After the gene rearrangement, ERG gene expression is up regulated significantly by the androgen-responsive promoter of TMPRS2. TMPRSS2-ERG fusions accelerate prostate cancer progression by promoting cell invasion, activating C-MYC oncogene and abrogating prostate epithelial differentiation (Yin, 2011). Recently, Dr. Reddy’s group has shown that antiepileptic drug targets ERG-positive prostate cancer cells through activation of tumor suppressors and nuclear receptors (Fortson, 2011). Similar results were also observed in the Ewing family of tumors (Kayarthodi et al. 2014).
Transforming growth factor-β1 (TGF- β1) signaling pathway is involved in key biological processes including cell proliferation, apoptosis, differentiation, human diseases, and tumors. TGF-β1 binds to the TGF-β receptor type II (TGF-β RII) and recruits TGF-β receptor I (TGF-βRI) and makes a heterodimeric receptor complex. TGF-βRII phosphorylates TGF-βRI which in turn phosphorylates Smad2 and Smad3 proteins. Phosphoryated Smd2 and Smad3 interact with Smad4 and shown translocate into the nucleus as a complex where they bind to transcriptional co-factors and regulate transcription of target genes (Feng, 2005, Massague, 2012, Sakaki-Yumoto, 2013). TGF-β signaling pathway has been accepted to have a dual role in tumor progression, which is a tumor suppressor for normal epithelial and early stages of cancer cells, and the other is a tumor promoter in last steps of the metastatic disease (Kocic, 2012, Miles, 2012).
TGF-β/Smad3 signaling pathway regulates many genes at the transcription level in various cell types. Although both ERG and Smad3 have been shown to exert differential biological effects on prostate cells, intracellular relationships of the two proteins in TGF-β/Smad3 signaling pathways remain unknown. In this study, we have studied the effect of ERG on TGF-β/Smad3 signaling pathway. We demonstrate that ERG induces the transcriptional activity of TGF-β/Smad3. In addition, we demonstrate that ERG binds to Smad3 protein and stabilizes phospho-Smad3 protein. These results suggest that ERG can induce either endogenous or exogenous TGF-β/Samd3-mediated transcriptional activity through interaction and stabilization of phosphorylated Smad3 protein. Thus, it appears that ERG may play an important role in regulating TGF-β/Smad3 pathway in ERG-positive prostate cancer cells.
MATERIAL AND METHODS
Plasmids and Antibodies
ERG2 expression plasmid (pSG5/erg2) and β-galactosidase expression plasmid pCMVβ were described previously. Luciferase reporter plasmid p3TP and pCMV5B/Flag/Smad3 was obtained from Addgene. Horseradish peroxidase (HRP)-conjugated antibodies to mouse or to rabbit IgG was obtained from GE healthcare. Erg 1/2/3 (c-20) rabbit polyclonal IgG antibody, Smad1/2/3 (H-2) mouse monoclonal antibody, and β-actin(C-4) mouse monoclonal IgG1), and p-Smad3 (Ser 208) rabbit polyclonal antibody was obtained from Santa Cruz. Anti-flag-M2-peroxidase (HRP) cloneM2 and anti-Flag-M2-affinity gel was obtained from Sigma. Anti-phospho-Smad3 (ser423/425) rabbit serum and anti-Smad3 (clone EP568Y) rabbit monoclonal antibody was obtained from Milipore.
Cell Culture and Treatments
PNT1A cells (Immortalized normal prostate epithelium cells) were obtained from Sigma-Aldrich and were cultured in RPMI 1640 (Cellgro) and supplemented with 10% fetal bovine serum (American Type Culture Collection). Cells were treated with TGF-β and SB431542 as described previously (Halder, 2005, Mordasky Markell, 2010). SB431542, TGF-β type I receptor inhibitor (from Tocris) was dissolved in DMSO and used at 10 μM. Recombinant Human TGF-β1 from R&D system, Inc, was dissolved in sterile 4 mM HCl containing 1 mg/ml human or bovine serum albumin and made stock solution at 2 μg/mL and used at 5 ng/ml concentration.
Reporter Gene Assays
Cells were plated into 24-well plates 24 hours prior to transfection and were co-transfected with p3TP reporter gene (0.02 μg), a pCMVβ (0.01 μg) and expression plasmids as indicated. The expression plasmid pSG5/erg2 (0.08 to 0.64 μg) and pCMV5B/Flag/Smad3 (0.08 to 0.32 μg) were co-transfected into cells using Lipofectamine TM 2000 (Invitrogen) according to the manufacturer’s protocol. The total amount of DNA in all transfection experiments was kept constant using appropriate parent empty vector expression plasmids. Cells were treated with or without TGF-β1 (5 ng/ml) for 20 hours before harvesting the cells. Reporter activity was measured at 48 hours after transfection. The reporter activity is expressed as light units. Luciferase activity was measured using the Dual luciferase reporter 100 assay system (Promega). These values were normalized against β-galactosidase activity. All reporter activity was expressed as mean ± S.E. from three duplicate samples. Experiments were done at least three times.
Western Blotting Analysis
Cells were transiently transfected with various expression plasmids or parental expression vectors using transfection reagent Lipofectamine 2000 (Invitrogen). Forty-eight hours after transfection, cell lysates were prepared using lysis buffer [(20 mM Hepes (pH 7.5), 100 mM KCl, 0.4 mM EDTA (pH 8.0), 0.2% Igepal CA630, 10 mM β-mercaptoethanol, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), and PI Cocktail (Roche Complete Mini EDTA-free)]. The cell lysates were homogenized by passing through a 27-gauge needle for several times and subjected to centrifugation at 13,000 rpm in a bench top Eppendorf centrifuge. Protein concentration was determined using the Bradford method (Bio-Rad). Proteins were separated on 4–12% SDS-PAGE gel and transferred to nitrocellulose membranes. Membrane was blocked with membrane blocking solution (Invitrogen) and incubated with appropriate primary antibodies. Proteins were revealed using the ECL detection kit (GH Healthcare). The density of the protein bands were compared using Multi gauge software.
Immunoprecipitation
Cells cultured in a 10 cm dish were harvested in 500 μl of lysis buffer (50 mM Tris, pH7.5, 150 mM NaCl, 0.1% Nonidet P-40, 1 mM EDTA, 0.25% sodium deoxycholate) containing 1 mM phenylmethylsufony fluoride (PMSF), protease inhibitor cocktail, and phosphatase inhibitor cocktail 2, then passed through 27-gauge needle for several times. Cell lysate were incubated for 30 min on ice and then centrifuged at 13,000 rpm in a bench top Eppendorf centrifuge. The supernatant was incubated with specific antibodies or control sera and protein A/G-agarose beads for overnight at 4 °C. Immunoprecipitates were washed four times in lysis buffer, solubilized with SDS sample buffer and separated on 4–12% SDS-polyacryamide gels followed by Western blotting.
Statistical Analysis
Transcriptional activity analysis results shown by column were from three different experiments. Means and standard deviation were calculated. Data were analyzed by student’s t-test. P values < 0.05 were considered as statistically significant.
RESULTS
The Transcriptional Activity of TGF-β/Smad3 was Enhanced by ERG
We have investigated that effect of ERG on TGF-β/Samd3 transcriptional activity using the reporter gene p3TP which contains three consecutive TPA response elements (TREs) and a portion of the plasminogen activator inhibitor 1 (PAI-1) promoter region. In PNT1A cell, the endogenous activity of TGF-β/Samd3 was increased by increasing amounts of ERG (Fig. 1(a)) and also by Smad3 (Fig. 1(c)). Further, the reporter activity induced by TGF-β/Samd3 was significantly enhanced by the expression of both ERG and Smad3 proteins (Fig. 1(b)). These results suggest that ERG can induce either endogenous or exogenous TGF-β/Samd3-mediated transcriptional activity.
Figure 1.
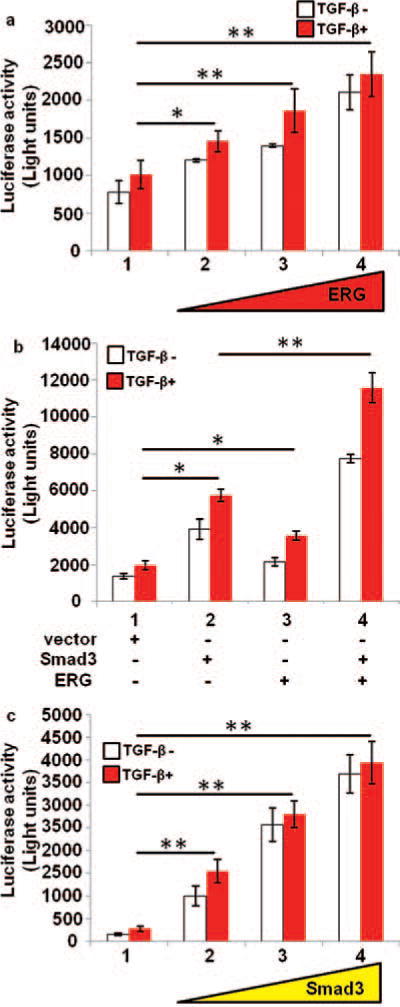
Effect of ERG on TGF-β/Smad3 transfection activity. PNT1A cells were transiently co-transfected with p3TP reporter gene, pCMVβ and expression plasmids or parental empty expression vector as indicated. Cells were treated with or without TGF-β1 for 20 hours before harvest. Reporter activity was measured at 48 hours after the transfection. All reporter activity was shown as mean ± S.E. from three samples. The reporter activity is expressed as light units. (a), Endogenous TGF-β/smad3 activity is increased by ERG. PNT1A cells were transiently transfected with increasing amount of expression plasmid ERG. (b), The activity of TGF-β/Smad3 is increased by ERG. PNT1A cells were transiently co-transfected with expression plasmids ERG and Smad3. (c), The TGF-β/Smad3 activity is increased by increasing amount smad3 transfection. PNT1A cells were transiently transfected with increasing amount of expression plasmid Smad3. *, p<0.05; **, p < 0 01
Phosphorylated Smad3 Protein was Increased by ERG
Smad3 proteins are known to be phosphorylated by TGF-β receptor and thereby regulate target gene expression. The level of phospho-Smad3 protein is increased in the presence TGF-β as expected (Fig. 2(a)). Interestingly, we observed that the expression level of phospho-Smad3 protein was increased significantly by ERG in presence of TGF-β (compare lane 4 to lane 2, the p-Smad3 protein was increased by 44% in Fig. 2(a)).
Figure 2.
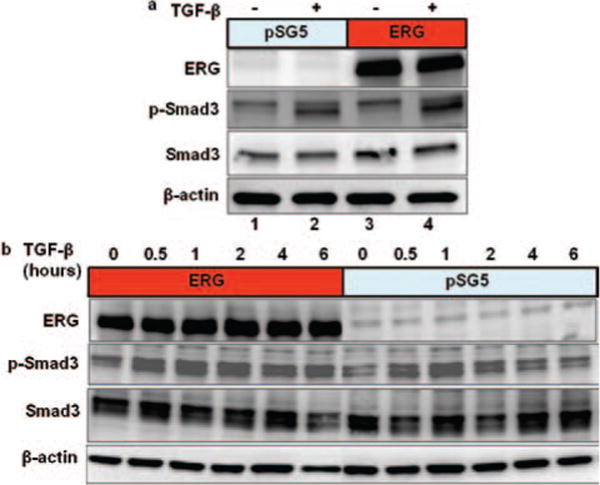
Endogenous phospho-Smad3 increased by ERG. (a) and (b), PNT1A cells were transfected with expression plasmid pSG5/ERG or parental empty expression vector. The cells were treated with or without TGF-β1 as shown for 2 hours (a) and for 30 minute, 1 hour, 2 hours, 4 hours 6 hours (b) before harvest. Total cell extracts were prepared and subjected to SDS-PAGE followed by western blot analysis using anti-ERG, anti-phospho-Smad3, anti-Smad3, and anti β-actin antibody.
Furthermore, we tested the levels of phospho-Smad3 protein at different time points after treatment with TGF-β in presence or absence of ERG (Fig. 2(b)). The level of phospho-Smad3 protein was significantly increased in the presence of ERG (Compared to control, ERG increased the p-Smad3 protein by 89.5% and 95.9% at 4 hours and 6 hours respectively) and these higher levels remained in the presence of ERG even after six hour treatment with TGF-β. These results indicate that ERG can increase and/or stabilize the phospho-Smad3 protein.
ERG Protein Binds to Both Smad3 and Phospho-Smad3 Protein
To investigate whether ERG and Smad3 proteins interact with each other, we performed co-immunoprecipitation assay after co-transfecting ERG and Smad3 expression plasmids into PNT1A cells (Fig. 3(a)). Anti-Flag-Smad3 brings down ERG suggesting that exogenous Smad3 interacts with ERG (Fig. 3(a), lane 4). Similarly, we observed that Anti-Smad3 brings down endogenous Smad3 or phospho-Smad3 protein along with ERG in ERG transfected PNT1A cells (Fig. 3(b), Compare lane 8 to lane 7, ERG proteins increased more than 200%). These results suggest that ERG interact with Smad3 and phospho-Smad3 protein in vivo.
Figure 3.
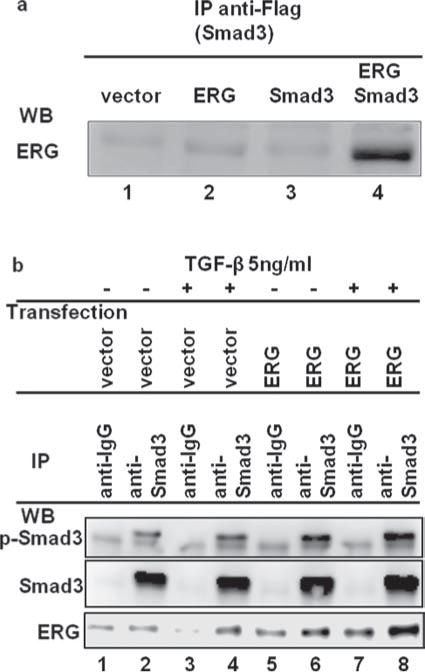
ERG protein binds to Smad3 and phospho-Smad3 protein in PNT1A cells. (a), ERG interacts with exogenous Smad3 proteins. PNT1A cells were transiently co-transfected with expression plasmids Smad3 and ERG or parental empty expression vector. Total cell extracts were prepared and subjected to immunoprecipitation (IP) using antibody against Flag (anti-Flag-M2-affinity gel). Immunoprecipitates were subjected to western blot analysis using anti-ERG antibody. (b), ERG protein binds to endogenous Smad3 and phospho-Smad3 protein. PNT1A cells were transiently transfected with expression plasmid ERG2 or parental empty expression vector. Cells were treated with TGF-β for 2hours before harvest. Total cell extracts were prepared and subjected to immunoprecipitation (IP) using normal mouse IgG and antibody against anti-Smad3. Immunoprecipitates were subjected to western blot analysis using anti-p-Smad3, anti-Smad3, and anti-ERG antibody.
ERG Inhibits the Effect of SB431542 on TGF-β/Smad3 Signaling Pathway
SB431542, a small molecular inhibitor of TGF-β type I receptor, is known to inhibit TGF-β/Smad3 signaling pathway. It is also known to inhibit phosphorylation of Smad3 and Smad2 (Mordasky Markell, 2010). Since above results suggest that ERG interacts with Smad3 and stabilizes the phosphorylation of Smad3, we tested the effect of ERG on the inhibitory properties of SB431542 on transcriptional activity of TGF-β/Smad3. We co-transfected increasing amounts ERG expression plasmid along with Smad3 expression plasmid into PNT1A cells and then treated the cells with SB431542. As expected, we found that the transcriptional activity of TGF-β/Smad3 was inhibited by SB431542. However, this inhibition of SB431542 on transcriptional activity of TGF-β/Smad3 was relieved by increasing expression of ERG (Fig. 4(a)). These results suggest that ERG counteracts the inhibitory properties of SB431542. It is possible that ERG may interact with TGF-β type I receptor and thereby interfere with the interaction of SB431542 and TGF-β type I receptor as shown in Figure 4(b). It is likely that ERG may also activate TGF-β type I receptor through its interaction.
Figure 4.
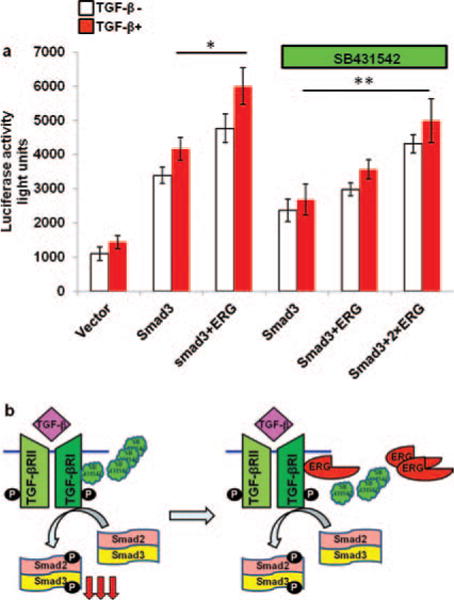
ERG relieves the effect of SB431542, small molecule inhibitor of TGF-β receptor I on TGF-β/Smad3 signaling pathway. (a) PNT1A cells were transiently co-transfected with p3TP reporter gene, pCMVβ and the expression plasmids for ERG and Smad3 or parental empty expression vector. The cells were treated with or without SB431542 at 10 μM for 24 hours as shown and with TGF-β1 for 6 hours before harvesting the cells. Reporter activity was measured at 48 hours after transfection. All reporter activity was shown as mean ± S. E. from 3 samples. *, p < 0 05; **, p < 0 01. (b) Possible mechanism of action by ERG on SB431542. Smad3 is phosphorylated by TGF-β binding to its receptor. SB431542 inhibits the activity of TGF-β receptor I and decrease the amount of phosphorylated Smad3. ERG activates TGF-β receptor I activity through direct or indirect interaction with TGF-β receptor I. (P: Phosphorylated group).
DISCUSSION
TGF-β/Smad3 signaling plays an important role in the regulation of normal cells and cancer cells, but more details are still needed to understand its role in prostate and other cancers (de Caestecker, 2000, Tian, 2011, Yue, 2001). In the present study, we have investigated the role of ERG oncoprotein on Smad3, which functions as a key intracellular mediator of the TGF-β signaling pathway. We tested the effect of ERG on TGF-β/Smad3 signaling pathway in normal prostate cells (PNT1A). We show that the transcriptional activity of both endogenous and exogenous TGF-β/Smad3 was enhanced by ERG. We also demonstrate that ERG oncoprotein interacts with both Smad3 and increased phosphorylated Smad3 proteins. It appears that ERG induces phosphorylation of Smad3 protein by activation of TGF-β receptor I kinase activity (Fig. 4(b)) and also stabilizes Smad3 by direct interaction with Smad3 and phospho-Smad3.
As expected SB431542, small molecule inhibitor of TGF-β receptor I decreased TGF-β/Smad3 transcriptional activity. Interestingly, ERG reverses the inhibitory function of SB431542 on TGF-β/Smad3 transcriptional activity. These results suggest that ERG activates TGF-β receptor I activity by interaction with the receptor and stabilizes phosphorylated Smad3 protein by binding to Smad3 and phospho-Smad3 and thereby enhances TGF-β/Smad3 signaling pathway. These results strongly support that ERG oncoprotein plays an important role in regulating TGF-β/Smad3 pathway in ERG-positive prostate cancer cells (Fig. 5).
Figure 5.
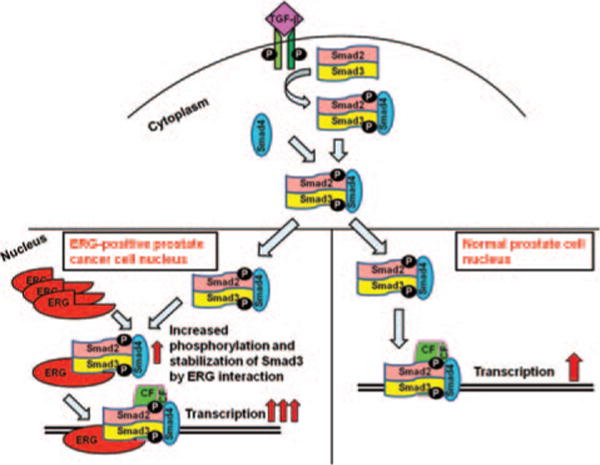
Phosphortlated Smad3 is stabilized by ERG in prostate cells. Smad3 is phosphorylated by TGF-β through binding to its receptor. Phosphorylated Smad3 forms a complex with phosphorylated Smad2 and the co-Smad (Smad4) and translocates to the nucleus. Phosphorylated Smad3 is stabilized by binding to ERG in nucleus. (CF: Co-factor; P: Phosphorylated group).
Variants of ETS gene fusions have been reported (Liu, 2013, Ohno, 1994, Sreenath, 2011). TMPRSS2-ERG is the most common fusion detected in prostate cancer (45–70%), with great diversity in the precise structure of the TMPRSS2-ERG hybrid transcript found in human prostates (Tomlins, 2005). The role of ETS gene fusions in prostate carcinogenesis is not fully understood. It is reported that there is a low or no expression of ERG in benign prostate cells, and high expression of ERG in prostate cancer cells because of TMPRSS2:ERG fusion (Huang, 2009). These studies suggest that compared with ERG-negative prostate cancer patients, the ERG-positive patients have a low rate of high Gleason grade, poor prostate cancer cell differentiation, and African American ethnicity (Hu, 2008). Some studies suggest causal roles of ERG protein in prostate cancers. In early prostate cancer development, TMPRSS2-ERG gene fusions may be an initial-trigger point and resulted in over expression of ERG (Klezovitch, 2008, Polson, 2013). Our results suggest that over expressed ERG proteins bind to phospho-Smad3 protein and make phospho-Smad3 more stabilized leading to increased amount of phospho-Smad3 in the nucleus, resulting in enhancing the activity of TGF-β/Smad3 signaling pathway. TGF-β/Smad3 signaling activity was completely abrogated by TGF-β receptor1 inhibitor SB431542 but increasing amount of ERG relieved the inhibitory activity of TGF-β/Smad3. These results indicated that ERG binds phospho-Smad3 and stabilizes it in the nucleus even though import amount of phospho-Smad3 from cytoplasm to nucleus is decreased because of TGF-β receptor inhibition (Fig. 5).
Ubiquitin-dependent protein degradation plays a key role in many biological processes. Smad3, a mediator for TGF-β, is degraded by the ubiquitin-proteasome pathway. In the nucleus, small amounts of Smad3 is dephosphorylated by phosphatase and directly exported from the nucleus to cytoplasm (Shi, 2003). Most of Smad3 protein in the nucleus interacts with a RING finger protein, ROC1, through its C-terminal MH2 domain. Smad3 bound to ROC1-SCFFbw1a (an E3 ubiquitin ligase complex) is then exported from the nucleus to the cytoplasm where it is subjected to proteasomal degradation (Fukuchi, 2001). This ubiquitin-proteasome-mediated degradation of Smads regulates the levels of Smads in cells (Derynck, 2003). Even though Smads are not enzymes, they still function as key regulators of TGF-β/Smads pathway and thereby show profound effect on cellular responses with little change in Smad protein levels (de Caestecker, 2000).
In this study, we find that ERG can enhance the activity of Smad3, in absence or presence of TGF-β. Furthermore, we showed that ERG directly interacts with Smad3 and phospho-Smad3 and also stabilized phospho-Smad3 protein levels in the nucleus. Our results demonstrate that ERG can also relieve the function of SB431542 on TGF-β/Smad3 signaling pathway. Possible mechanism are:
-
(1)
ERG binds to phospho-Smad3 and make latter not to bind to other proteins especially involved in ubiquination and reduce the amount of ubiquited Smad3;
-
(2)
ERG bound to phospho-Smad3 and thereby inhibits the dephosphorylation of Smad3 and consequently decrease the amount of Smad3 exported from nucleus to cytoplasm;
-
(3)
ERG activates TGF-β receptor I activity through direct or indirect interaction with TGF-β receptor I (Fig. 4(b)). The above-mentioned possibilities result in an increased amount of phosphorylated-Smad3 in the nucleus and enhanced the transcriptional activity of TGF-β/Smad3 (Fig. 5).
The TGF-β signaling pathway have a dual role in cancer progression, which is a tumor suppressor for normal epithelial and early stages of cancer cells, and also, it is a tumor promoter in final stages of the metastatic diseases (Kocic, 2012, Miles, 2012, Tarasewicz, 2012). Our results provide support that ERG plays a major role in prostate cancer progression through the regulation of TGF-β signaling pathway. Thus, these results have great implications in diagnosis and treatment of ERG-positive prostate cancers (Fang, 2014).
Acknowledgments
We thank all the members of Reddy and Rao laboratories. This study was funded by in part by the U.S. Army Medical Research and Materiel Command under W81XWH-08-1-0628, W81XWH-09-1-0236, W81XWH-10-1-0418 (E. Shyam P. Reddy) and the Georgia Cancer Coalition Distinguished Cancer Scholar award (E. Shyam P. Reddy and Veena N. Rao), NIH 2U54CA118948, 3U54CA118638-05S1, U54/56 Morehouse School of Medicine/University of Alabama at Birmingham/Tuskegee University Partnership Grant (U54 CA118638) and the Research Centers in Minority Institutions (G-12-RR003034).
References
- de Caestecker MP, Piek E, Roberts AB. Role of transforming growth factor-beta signaling in cancer. J Natl Cancer Inst. 2000;92:1388–402. doi: 10.1093/jnci/92.17.1388. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in TGF-beta signaling through smads. Annu Rev Cell Dev Biol. 2005;21:659–93. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Fortson WS, Kayarthodi S, Fujimura Y, Xu H, Matthews R, et al. Histone deacetylase inhibitors, valproic acid and trichostatin-a induce apoptosis and affect acetylation status of p53 in erg-positive prostate cancer cells. Int J Oncol. 2011;39:111–9. doi: 10.3892/ijo.2011.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi M, Imamura T, Chiba T, Ebisawa T, Kawabata M, et al. Ligand-dependent degradation of smad3 by a ubiquitin ligase complex of roc1 and associated proteins. Mol Biol Cell. 2001;12:1431–43. doi: 10.1091/mbc.12.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusato B, Gao CL, Ravindranath L, Chen Y, Cullen J, et al. Mapping of TMPRSS2-erg fusions in the context of multi-focal prostate cancer. Mod Pathol. 2008;21:67–75. doi: 10.1038/modpathol.3800981. [DOI] [PubMed] [Google Scholar]
- Halder SK, Beauchamp RD, Datta PK. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia. 2005;7:509–21. doi: 10.1593/neo.04640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Dobi A, Sreenath T, Cook C, Tadase AY, et al. Delineation of TMPRSS2-erg splice variants in prostate cancer. Clin Cancer Res. 2008;14:4719–25. doi: 10.1158/1078-0432.CCR-08-0531. [DOI] [PubMed] [Google Scholar]
- Huang W, Waknitz M. Ets gene fusions and prostate cancer. Am J Transl Res. 2009;1:341–51. [PMC free article] [PubMed] [Google Scholar]
- Fang J, Xu H, Yang C, Kayarthodi S, Matthews R, Rao VN, Reddy ESP. Molecular mechanism of activation of transforming growth factor beta/smads signaling pathway in ets related gene-positive prostate cancers. J Pharm Sci Pharmacol. 2014;1:82–85. doi: 10.1166/jpsp.2014.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klezovitch O, Risk M, Coleman I, Lucas JM, Null M, et al. A causal role for erg in neoplastic transformation of prostate epithelium. Proc Natl Acad Sci USA. 2008;105:2105–10. doi: 10.1073/pnas.0711711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocic J, Bugarski D, Santibanez JF. Smad3 is essential for transforming growth factor-beta1-induced urokinase type plasminogen activator expression and migration in transformed keratinocytes. Eur J Cancer. 2012;48:1550–7. doi: 10.1016/j.ejca.2011.06.043. [DOI] [PubMed] [Google Scholar]
- Liu F, Gao L, Jing Y, Xu YY, Ding Y, et al. Detection and clinical significance of gene rearrangements in chinese patients with adult acute lymphoblastic leukemia. Leuk Lymphoma. 2013 doi: 10.3109/10428194.2012.754888. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles FL, Tung NS, Aguiar AA, Kurtoglu S, Sikes RA. Increased TGF-beta1-mediated suppression of growth and motility in castrate-resistant prostate cancer cells is consistent with smad2/3 signaling. Prostate. 2012;72:1339–50. doi: 10.1002/pros.22482. [DOI] [PubMed] [Google Scholar]
- Mordasky Markell L, Perez-Lorenzo R, Masiuk KE, Kennett MJ, Glick AB. Use of a TGFbeta type I receptor inhibitor in mouse skin carcinogenesis reveals a dual role for tgfbeta signaling in tumor promotion and progression. Carcinogenesis. 2010;31:2127–35. doi: 10.1093/carcin/bgq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T, Ouchida M, Lee L, Gatalica Z, Rao VN, et al. The ews gene, involved in ewing family of tumors, malignant melanoma of soft parts and desmoplastic small round cell tumors, codes for an RNA binding protein with novel regulatory domains. Oncogene. 1994;9:3087–97. [PubMed] [Google Scholar]
- Polson ES, Lewis JL, Celik H, Mann VM, Stower MJ, et al. Monoallelic expression of TMPRSS2/ERG in prostate cancer stem cells. Nat Commun. 2013;4:1623. doi: 10.1038/ncomms2627. [DOI] [PubMed] [Google Scholar]
- Prasad DD, Ouchida M, Lee L, Rao VN, Reddy ES. Tls/fus fusion domain of tls/fus-erg chimeric protein resulting from the t(16;21) chromosomal translocation in human myeloid leukemia functions as a transcriptional activation domain. Oncogene. 1994;9:3717–29. [PubMed] [Google Scholar]
- Rao VN, Modi WS, Drabkin HD, Patterson D, O’Brien SJ, et al. The human erg gene maps to chromosome 21, band q22: Relationship to the 8; 21 translocation of acute myelogenous leukemia. Oncogene. 1988;3:497–500. [PubMed] [Google Scholar]
- Rao VN, Papas TS, Reddy ES. Erg, a human ets-related gene on chromosome 21: Alternative splicing, polyadenylation, and translation. Science. 1987;237:635–9. doi: 10.1126/science.3299708. [DOI] [PubMed] [Google Scholar]
- Reddy ES, Rao VN. Erg, an ets-related gene, codes for sequence-specific transcriptional activators. Oncogene. 1991;6:2285–9. [PubMed] [Google Scholar]
- Reddy ES, Rao VN, Papas TS. The erg gene: A human gene related to the ets oncogene. Proc Natl Acad Sci USA. 1987;84:6131–5. doi: 10.1073/pnas.84.17.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki-Yumoto M, Katsuno Y, Derynck R. TGF-beta family signaling in stem cells. Biochim Biophys Acta. 2013;1830:2280–96. doi: 10.1016/j.bbagen.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RB, Chinnaiyan AM. The discovery of common recurrent transmembrane protease serine 2 (TMPRSS2)-erythroblastosis virus E26 transforming sequence (ETS) gene fusions in prostate cancer: Significance and clinical implications. Adv Anat Pathol. 2009;16:145–53. doi: 10.1097/PAP.0b013e3181a12da7. [DOI] [PubMed] [Google Scholar]
- Kayarthodi Shubhalaxmi, Fujimura Yasuo, Fang Jinbo, Morsalin Sharif, Rao Veena N, Reddy E Shyam P. Anti-epileptic drug targets Ewing Sarcoma. J Pharm Sci Pharmacol. 2014;1:87–100. doi: 10.1166/jpsp.2014.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Siddique HR, Rao VN, Lee L, Reddy ES. Characterization of the DNA binding and transcriptional activation domains of the erg protein. Oncogene. 1993;8:1751–5. [PubMed] [Google Scholar]
- Sreenath TL, Dobi A, Petrovics G, Srivastava S. Oncogenic activation of erg: A predominant mechanism in prostate cancer. J Carcinog. 2011;10:37. doi: 10.4103/1477-3163.91122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasewicz E, Jeruss JS. Phospho-specific smad3 signaling: Impact on breast oncogenesis. Cell Cycle. 2012;11:2443–51. doi: 10.4161/cc.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Neil JR, Schiemann WP. Transforming growth factor-beta and the hallmarks of cancer. Cell Signal. 2011;23:951–62. doi: 10.1016/j.cellsig.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, et al. Recurrent fusion of TMPRSS2 and ets transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- Tsuzuki S, Taguchi O, Seto M. Promotion and maintenance of leukemia by erg. Blood. 2011;117:3858–68. doi: 10.1182/blood-2010-11-320515. [DOI] [PubMed] [Google Scholar]
- Yin L, Rao P, Elson P, Wang J, Ittmann M, et al. Role of TMPRSS2-erg gene fusion in negative regulation of psma expression. PLoS One. 2011;6:e21319. doi: 10.1371/journal.pone.0021319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J, Mulder KM. Transforming growth factor-beta signal transduction in epithelial cells. Pharmacol Ther. 2001;91:1–34. doi: 10.1016/s0163-7258(01)00143-7. [DOI] [PubMed] [Google Scholar]


