Abstract
Object
The early pathophysiological features of traumatic brain injury observed in the intensive care unit (ICU) have been described in terms of altered cerebral blood flow, altered brain metabolism, and neurochemical excitotoxicity. Seizures occur in animal models of brain injury and in human brain injury. Previous studies of posttraumatic seizures in humans have been based principally on clinical observations without a systematic approach to electroencephalographic (EEG) recording of seizures. The purpose of this study was to determine prospectively the incidence of convulsive and nonconvulsive seizures by using continuous EEG monitoring in patients in the ICU during the initial 14 days post-injury.
Methods
Ninety-four patients with moderate-to-severe brain injuries underwent continuous EEG monitoring beginning at admission to the ICU (mean delay 9.6 ± 5.4 hours) and extending up to 14 days postinjury. Convulsive and non-convulsive seizures occurred in 21 (22%) of the 94 patients, with six of them displaying status epilepticus. In more than half of the patients (52%) the seizures were nonconvulsive and were diagnosed on the basis of EEG studies alone. All six patients with status epilepticus died, compared with a mortality rate of 24% (18 of 73) in the nonseizure group (p < 0.001). The patients with status epilepticus had a shorter mean length of stay (9.14 ± 5.9 days compared with 14 ± 9 days [t-test, p < 0.03]). Seizures occurred despite initiation of prophylactic phenytoin on admission to the emergency room, with maintenance at mean levels of 16.6 ± 2.8 mg/dl. No differences in key prognostic factors (such as the Glasgow Coma Scale score, early hypoxemia, early hypotension, or 1-month Glasgow Outcome Scale score) were found between the patients with seizures and those without.
Conclusions
Seizures occur in more than one in five patients during the 1st week after moderate-to-severe brain injury and may play a role in the pathobiological conditions associated with brain injury.
Keywords: brain trauma, seizure, neurointensive care, head injury, nonconvulsive seizure, status epilepticus, continuous electroencephalography
MOST patients with severe traumatic brain injury (TBI) have a prolonged stay in the intensive care unit (ICU), the outcome being a long-term disability or death, with a minority of patients (20–30%) achieving a functionally independent outcome.1,42,43 Recognized prognostic factors that influence outcome, which are present early after injury, are early hypoxemia, early hypotension, severity of primary insult (assessed on computerized tomography [CT] scans), and admission Glasgow Coma Scale50 (GCS) score. Adverse secondary events including sustained intracranial hypertension,1,14,29,43,52 reduced or hyperemic cerebral blood flow (CBF),25,46 and frank ischemia45 influence outcome. These prognostic factors indicate that the brain exists in a vulnerable state during the first few days after trauma when secondary insults may worsen the injury and the resultant outcome.
Paramount to this state of vulnerability are altered glucose and oxidative metabolism,5 altered CBF,13,31 and ongoing neurochemical changes in extracellular excitatory amino acids, potassium, and lactate.9,16,34,53 The origin of these changes is thought to be ionic perturbations initiated by the trauma. However, seizures and other epileptiform activity are known to stimulate glycolysis and similar neurochemical changes.10,11,15,20,54 Therefore, seizures that occur during the early posttraumatic period may augment the neurochemical perturbation and worsen injury.
It is well known that clinically evident seizure activity occurs after brain injury, with investigators in clinical series reporting an incidence of 4 to 10%.3,23,28,49,54 The peak incidence of early posttraumatic clinically observed seizure activity is during the initial 48 hours, with the high-risk period continuing up to 1 week postinjury. Focal as well as generalized clinically recognized seizures occur with greater frequency (8–10%) in more severely injured patients.3 Although electroencephalographic (EEG) studies have been used to assist in determining the prognosis after TBI, the detection of nonconvulsive and convulsive seizures in the ICU by prospective continuous EEG monitoring during this period of enhanced vulnerability has not been previously addressed. The aim of this study is to discover the incidence of electroencephalographically defined nonconvulsive and convulsive seizures after TBI by using continuous EEG monitoring and to assess the impact of seizures on outcome.
Clinical Material and Methods
Patient Selection
Ninety-four consecutive patients with moderate-to-severe TBI (GCS score ≤ 12) were enrolled prospectively in this nonblinded, nonrandomized observational study. This study was approved by our institutional human subjects protection committee. All patients admitted consecutively during the study period (between December 1992 and May 1997) were recruited during the initial 24 hours after TBI (or on admission in one case) and subsequently enrolled. Patients with an initial GCS score of 13 or higher in whom a decline in mental status occurred in the first 36 hours postinjury also routinely underwent EEG monitoring but were not included in this analysis. Patients who were included in this analysis represent a subset of individuals previously reported on from our center.31,46
General Management Protocol
All patients underwent continuous EEG monitoring in the neurosurgical ICU as part of the standardized care protocol.25 In patients who required an initial emergency operation, EEG monitoring was started after the operation, on admission to the ICU. Intracranial pressure (ICP) monitoring was performed using ventriculostomy and/or pressure transducer systems, and ICP was kept below 20 mm Hg by using a stepwise management strategy (cerebrospinal fluid drainage, hyperventilation to PCO2 levels of 30–35 mm Hg, and mannitol administration). The ventriculostomy drainage procedure included drainage of cerebrospinal fluid for ICP higher than 20 mm Hg for more than 5 minutes, with brief periods of drainage only. Cerebral perfusion pressure (CPP) was kept at 70 mm Hg or higher by using phenylephrine when required. All patients received a loading dose of phenytoin (18 mg/kg) in the emergency room and continued to receive the drug (300 mg/day) for at least 7 days. Daily trough levels of total serum phenytoin were determined, and additional boluses were given to maintain levels between 10 and 20 mg/dl. If seizures were detected, additional phenytoin was given to raise levels to a range of 18 to 25 mg/dl. Patients suffering from status epilepticus were treated using a stepwise protocol that included midazolam and lorazepam injections and culminated with induction of burst suppression with pentobarbital or propofol. Burst suppression was maintained for 24 hours, then tapered while the EEG readings were observed on each successive day, and treatment was repeated until the status was controlled. Additional anti-epileptic agents, principally phenobarbital, were added as a daily agent if seizures recurred on withdrawal of pentobarbital or propofol.
Continuous EEG Monitoring Protocol
A 14-channel electroencephalography unit provided continuous monitoring at the patient’s bedside beginning at the earliest opportunity after admission to the ICU. The EEG tracings were continuously displayed at the bedside, 24 hours per day, for moment-to-moment on-line observation by physicians and nurses. A physician trained in interpretation of EEG readings reviewed the ongoing EEG activity at the bedside at least three times each day and additionally when informed by the bedside nurse of suspicious EEG activity. A 14-channel, 12-electrode montage was used, including the following electrode derivations: F4-CZ, T4-CZ, P4-CZ, O2-CZ, F3-CZ, T3-CZ, P3-CZ, O1-CZ, F4-T4, T4-P4, P4-O2, F3-T3, T3-P3, P3-O1 (according to the 10-20 international system, International Federation of Societies for Electroencephalography and Clinical Neurophysiology). After scalp measurement and skin preparation, needle electrodes were implanted subcutaneously and then covered with mesh soaked in collodion and air dried using a portable pump. Needle electrodes were disposable and were not reused. Placement of electrodes was selectively altered to avoid wound sites and skin incisions. When this occurred, a proportional adjustment was made in the placement of the homolateral sensor. Electrodes that had been removed for bedside surgical procedures were replaced with new ones. Head wraps were routinely removed and replaced after placement of electrodes, which were inspected daily for evidence of infection, dislodgment, or wire breaks.
Filter settings were as follows: 1-Hz low linear–frequency filter, 70-Hz high linear–frequency filter, and a 60-Hz notch filter. Frequency analysis was performed on every 2-second epoch of EEG readings by using the fast Fourier transform. In turn, these readings were averaged across 2 minutes and displayed as a continuous histogram of either mean amplitude (referred to as total power) or relative alpha (% alpha). This resulted in 8- to 12-hour trends of total power displayed as continuous histograms (Fig. 1). Trends of total power were reviewed twice daily, and selected segments of raw EEG data were screened for seizure activity (for example, sudden increases or bursts in total power). In addition a minimum of five random checks of the raw EEG data were made at times of non-specific monotonous or at otherwise unremarkable total power activity.
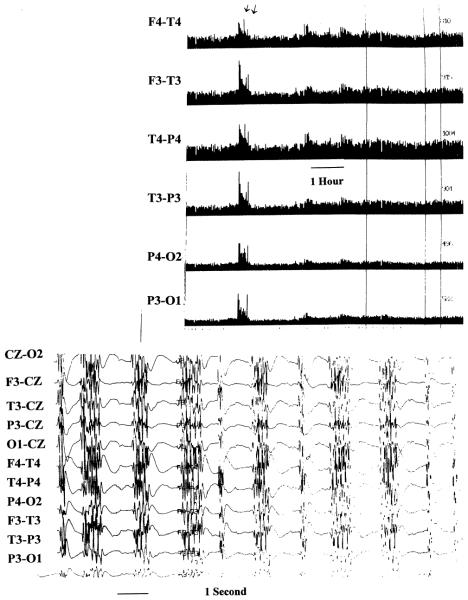
Fig. 1.
Electroencephalographic tracings showing total power trend of 15 hours duration (upper) with the electrode derivations shown at the left. The arrows show an increase in total power that corresponds to generalized seizure activity (lower) that was accompanied by subtle facial twitching. The remainder of the trend demonstrates reduced total power that results from induction of pentobarbital burst-suppression coma.
Each total power increase was further investigated by recall and review of the raw EEG data during a total power surge, and seizures were identified according to standard criteria (Fig. 1). The EEG tracing from 10 minutes before the burst until 10 minutes after the burst was reviewed. This method of seizure detection has been described previously.40 Thus, seizures were detected in one of three ways: by on-line identification of seizures by the neurological ICU nurse or neurointensivist; by total power trend seizure detection; or by detection during random EEG segment review. Nurses received classroom instruction on seizure identification supplemented by bedside tutoring with on-line EEG samples. The date and time of the seizure and the clinical behavior noted by the bedside neurological ICU nurse or neurointensivist were recorded. Each seizure was confirmed by an independent electroencephalographer blinded to the patient’s clinical condition (M.R.N.). The seizure type was characterized as focal, hemispheric, or generalized according to the EEG reading at time of onset. The duration of individual seizures was recorded along with the total (aggregate) duration of seizures that occurred during the patient’s stay in the ICU. The interval delay in starting EEG monitoring was noted.
Prehospital/ICU Data Analysis
Ambulance and initial emergency room resuscitation documents and arterial blood gas level records were screened to determine the presence of hypoxemia during the initial postinjury period. The initial postinjury period was considered to be the time spent in the field up to the end of the 1st hour in the emergency room. Hypoxemia was determined to be present if one of the following criteria were met: 1) desaturation below 90% in the field or in the emergency room; 2) complicated intubation; 3) absence of spontaneous breathing before intubation; or 4) arterial oxygen level lower than 70 mm Hg on initial available blood gas level reading. Hypotension was defined as a systolic blood pressure reading of 90 or less in the field or within the 1st hour in the emergency room. The admission Injury Severity Scale score was calculated for each patient.
The CT scans were assessed to determine the location of the initial traumatic lesion. Brain injuries were categorized according to the dominant one, or if multiple lesions were present, they were recorded as such. Lesions considered dominant were subdural hematoma (SDH), epidural hematoma (EDH), brain contusion, subarachnoid hemorrhage (SAH) and/or intraventricular hemorrhage, or diffuse brain edema with petechial hemorrhage.
Retrospective analysis was made of other concomitant secondary insults, namely elevated ICP, decreased CPP, and severe metabolic abnormalities, such as fever or hyponatremia. Elevated ICP was defined as ICP higher than 20 mm Hg for more than 5 minutes duration that required specific therapy. Using hourly ICP and CPP values, maximum daily ICP and minimum CPP were noted. Maximum ICP and minimum CPP on the day of seizure were recorded.
Outcome was assessed using the 1-month Glasgow Outcome Scale22 (GOS) score as determined by the institutional trauma case manager. In patients who died before discharge, deaths were clinically determined to be from brain death or other causes (that is, multiple organ failure).
Statistical Analysis
Analysis of prehospital data, physiological parameters measured in the ICU, and quantitative EEG data was performed using Student’s t-test for analysis of continuous parametric variables, the Wilcoxon rank-sum test for non-parametric data, and chi-square tests for dichotomous data. The ICP and CPP data were found to have a normal distribution. Subgroup analysis was made of patients with seizures and those without by using daily mean ICP and CPP levels, with comparison of mean values between groups for the entire ICU stay. The influence of seizures on outcome was determined by comparing dichotomized GOS scores (1–3 compared with 4–5) between the subgroups with and without seizures or with and without status epilepticus by using the chi-square test. Similarly, chi-square analysis was used to determine if the mean EEG background rhythm or the duration of monitoring affected the GOS score. A commercially available software package (StatSoft, Tulsa, OK) was used for analysis.
Results
Patient Characteristics
A total of 94 patients with moderate-to-severe brain injuries were studied over a 3-year period. There were 78 men and 16 women in the study (age range 18–78 years). The mean initial GCS score was 7.1 ± 4.1, with a median initial score of 6. The mean Injury Severity Scale score was 30.4 ± 9. The mechanism of injury and number of patients affected were as follows: motor vehicle accident (27), pedestrian hit by automobile (22), fall (20), motorcycle accident (10), bicycle accident (two), blunt injury (nine), and gunshot wound (four). The lesions seen on admission CT scans and the number of patients with each type were as follows: multiple injuries including multiple contusions, SDHs, and SAH (33), traumatic SAH and edema (19), focal contusion (16), SDH (15), EDH (nine), and gunshot wound with bullet retained (two). In the sub-group of 21 patients with seizures the individuals had similar admission CT profiles: multiple injuries in six (29%), contusion in six (29%), traumatic SAH in three (14%), EDH in three (14%), and SDH in three (14%).
Admission to our institution occurred within the first 24 hours of injury in 92 of the 94 patients, with one patient admitted on Day 6 postinjury and one on postinjury Day 2. The mean length of stay (LOS) was 14 ± 9 days. Thirty-seven patients underwent evacuation of mass lesions on admission or within the initial 48 hours postinjury; the lesions principally consisted of contusions or SDHs. Two patients each underwent two operations to control mass lesions.
The EEG monitoring began at a mean of 9.6 ± 5.4 hours postinjury (range 3–24 hours), except in one patient in whom monitoring was begun 6 days postinjury immediately on transfer to our institution. Patients underwent continuous EEG monitoring for a mean of 7.5 ± 4 days (median 7 days). The duration of monitoring was not related to the global severity of EEG abnormality (that is, mean frequency; r = 0.06), indicating no temporal bias to monitor the more severely injured patients.
Risk Factors for Seizures
One patient each in the seizure and the nonseizure groups had a history of seizures. None of the patients had ethanol or sedative-withdrawal seizures, and none had been exposed to drugs that provoke seizures (for example imipenem). The mean phenytoin level in the seizure group on the day of seizure was 16.6 ± 2.98 mg/dl. There was no significant change in levels of phenytoin in the 48 hours preceding the seizure in the 16 patients who suffered seizures during the ICU stay. Phenytoin levels in the seizure group were not statistically different from those in the nonseizure group. The mean ionized calcium and sodium levels were not statistically different from those in the nonseizure group. There were no statistical differences in the mean initial GCS score in the seizure group (7 ± 3.96, median 6) and that in the nonseizure group (7.1 ± 4.1, median 6). In addition, a similar proportion of patients with GCS scores of 8 or lower had seizures (18 of 68) when compared with those (six of 26) in whom GCS scores of 9 to 13 were recorded (chi-square test, p < 0.765). The occurrence of preadmission hypotension, hypoxemia, or seizures did not differ between the seizure and nonseizure subgroups. These data are summarized in Table 1.
TABLE 1.
Summary of possible risk factors for occurrence of early posttraumatic seizures*
| Risk Factor | Nonseizure Group (73 patients) |
Seizure Group (21 patients) |
Relative Risk |
|---|---|---|---|
| hypotension | 19 | 5 | 0.91 |
| hypoxemia | 18 | 4 | 0.77 |
| ethanol withdrawal | 15 | 2 | 0.46 |
| contusional injury | 39 | 12 | 1.1 |
| depressed skull fractures | 1 | 0 | 0 |
| linear skull fractures | 10 | 4 | 1.4 |
No significant differences between subgroups according to Student’s t-test (p < 0 05)
The impact of early evacuation of mass lesions on the incidence of seizures was addressed. We found an inverse relationship between early operation and the occurrence of seizures. Five (23%) of 21 patients with seizures underwent early surgery to evacuate a hematoma compared with 32 (45%) of 71 in the nonseizure group (p < 0.05, two-tailed t-test). The type of acute lesion identified on CT scans in the 21 patients with seizures did not differ from that in the group as a whole, and there was no change in operative indications over the study period.
Seizure Characteristics
Overall, 21 patients suffered electroencephalographically or clinically identified seizures. These electroencephalographically defined seizures had typical epileptic morphology, with sudden onset of repetitive spike-and-wave discharges that increased in amplitude and evolved over time. An example of a nonconvulsive focal seizure is presented in Fig. 2. This demonstrates a right frontal epileptic focus seen in the patient in Case 1. The corresponding total power trend is also shown, with clear-cut bursts in total power during the seizures. Examples of generalized seizures are presented in Figs. 1 and 3. Figure 1 demonstrates ongoing polyspike and wave nonconvulsive status epilepticus, which correlated clinically with subtle facial and eyelid twitching. Figure 3 demonstrates the evolution of generalized spike-wave status epilepticus that was unaccompanied by clinical behavior.
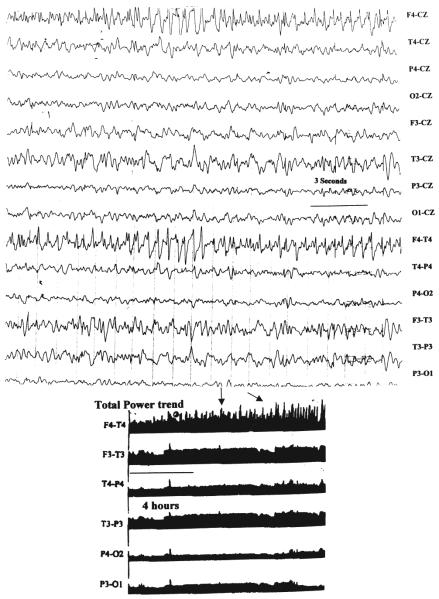
Fig. 2.
Upper: Segment of an EEG tracing demonstrating a focal nonconvulsive seizure emanating from the right frontal area, predominant over the F4 electrode. Lower: The corresponding total power trend demonstrating repeated bursts of total power (arrows) over a 12-hour epoch.
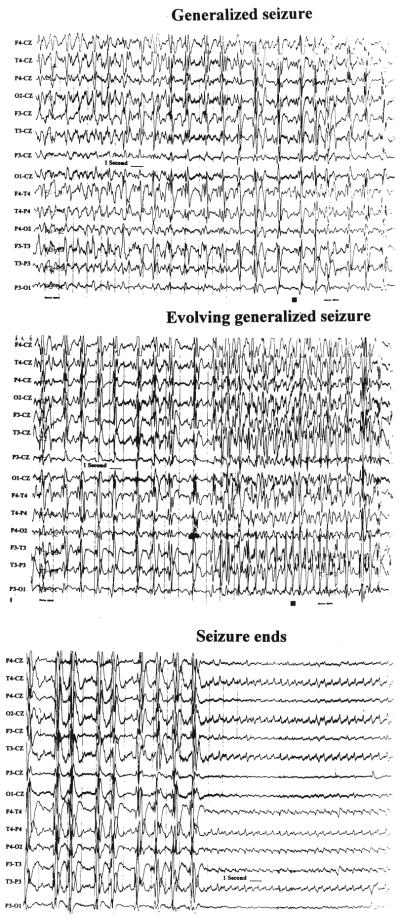
Fig. 3.
Tracings showing an example of a generalized electroencephalographically detected seizure that evolves over time. The EEG study demonstrates polyspike and wave discharges, repetitive spike discharges, and an eventual decrease in amplitude with rhythmic low-amplitude activity that gradually abates. No clinically observed activity accompanied the electroencephalographically defined seizure.
The demographic and clinical profile of the seizure sub-group reveals a total of 13 men and eight women with a mean age of 42.1 ± 17.9 years. Table 2 shows diagnostic information for the 21 patients with seizures (CT category, GCS score, postinjury day of seizure, duration of seizures, and ICP level). The mean GCS score in the seizure group was 6.4 ± 3.6, and the median score was 5. There were five patients who suffered ultra-early seizures witnessed by emergency room staff or ambulance staff. All of these were generalized tonic–clonic seizures. Sixteen patients had seizures while in the ICU and one patient had seizures in the emergency room and the ICU. Clinical seizure behavior in the ICU was characterized as the following: no clinical behavior (12 patients), stereotypical generalized tonic–clonic activity (one), rhythmic myoclonic activity (one), rhythmic facial twitching (one), and altered attention and lethargy (one).
TABLE 2.
Clinical data for 21 patients in the posttraumatic seizure group*
| ICP (mm Hg) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Case No. |
Age (yrs) |
GCS Score |
CT Lesion |
Day of Seizure† |
Ictal EEG |
Ictal Behavior‡ |
Max/ Seizure§ |
Max |
| 1 | 80 | 5 | con | 2 | focal spw | none | 20 | 20 |
| 2 | 31 | 4 | EDH | 7 | focal spw | none | 22 | 34 |
| 3 | 65 | 3 | con/SAH | 3 | gen spw | none | 46 | 46 |
| 4 | 56 | 3 | EDH/con | 7 | gen spw | eyelid twitch |
7 | 10 |
| 5 | 21 | 8 | con/SAH | 2 | gen spw | none | 15 | 15 |
| 6 | 22 | 6 | SDH/con | 0 | gen spw | convulsion | 18 | 28 |
| 7 | 33 | 3 | EDH/con | 2 | gen spw | none | 35 | 36 |
| 8 | 46 | 3 | edema/ SAH |
1 | gen spw | facial twitch |
NM | NM |
| 9 | 27 | 12 | SDH | 1 | focal spw | facial twitch |
NM | NM |
| 10 | 65 | 7 | EDH | 1 | focal spw | none | 18 | 23 |
| 11 | 48 | 3 | SDH | 0 | gen spw | none | 45 | 45 |
| 12 | 20 | 5 | con | 0 | focal spw | nystagmus | 20 | 22 |
| 13 | 18 | 4 | SDH | 2 | focal spw | none | 30 | 31 |
| 14 | 68 | 12 | con | 1 | focal spw | none | 16 | 26 |
| 15 | 56 | 12 | SDH | 0 | focal/gen spw |
none | 12 | 18 |
| 16 | 30 | 11 | con | 0 | pre-EEG | GTC | NM | 10 |
| 17 | 47 | 5 | con | 0 | pre-EEG | GTC | NM | 17 |
| 18 | 75 | 12 | SDH | 0 | pre-EEG | GTC | NM | 22 |
| 19 | 19 | 3 | con | 0 | pre-EEG | GTC | NM | 15 |
| 20 | 54 | 9 | con | 0 | pre-EEG | GTC | NM | 25 |
| 21 | 22 | 4 | edema | 1 | focal spw | none | 31 | 37 |
Con = contusion; EEG = electroencephalography; focal/gen = focal spike and wave discharge with secondary generalization; focal spw = focal spike and wave seizure; gen spw = generalized spike and wave seizure; GTC = generalized tonic-clonic motor activity; max = maximum ICP during ICU stay; max/seizure = maximum ICP on the day of seizure; NM = not measured at time of seizure.
Postinjury Day 0 indicates the day of injury.
The majority of patients had subtle clinical seizure behavior or no behavior during electroencephalographically defined seizures.
Elevated ICP was present in eight of 14 patients who had simultaneous EEG and ICP monitoring; however, detailed analysis comparing ICP before the seizure to that on the day of the seizure did not indicate a significant change (p < 0.65).
Six patients were in status epilepticus, a state consisting of frequent, repeated seizures occurring at a rate of more than three per hour. The group of patients with status epilepticus had a mean GCS score of 4.8 ± 3.6 (median GCS score 3). Status epilepticus occurred on postinjury Days 1 through 11 (median postinjury Day 3, see Table 2). The following EEG status epilepticus patterns were seen: secondarily generalized polyspike and wave (four patients), secondarily generalized repetitive spike discharges (one), and focal status epilepticus with waxing/waning spread into the contralateral frontal lobe and adjacent temporal lobe (one). The mean total duration of status epilepticus before seizures were brought under control was 8.6 ± 7.7 hours. Seizure control was defined as induction of burst suppression or resolution of repetitive spike discharges to fewer than 10 per minute. Individual seizures that comprised status epilepticus could be distinguished in five patients, who had seizures lasting a mean of 80 ± 14 seconds; the other had continuous seizures lasting several minutes before being treated. The time taken to control status epilepticus averaged 26 ± 15 minutes. Clinical behavior during the EEG monitoring of status epilepticus consisted of rhythmic facial twitching (two patients), eyelid fluttering (one), irregular myoclonus (one), and no clinical motor behavior (one).
In the remaining 11 patients who suffered seizures while in the ICU, each had isolated incidents with a median of two seizures per patient. The mean duration of each of these seizures was 212 ± 105.5 seconds. Six patients had generalized onset, whereas five had focal-onset seizures. Individual seizures occurred on postinjury Days 1 to 9 (median postinjury Day 2). The clinical correlate to the electroencephalographically defined seizures was as follows: eight patients had no clear motor signs of seizures, two had generalized tonic–clonic motor activity, and one had rhythmic eye clonic activity. Five patients had additional interictal epileptiform activity: this was characterized as focal isolated spikes (three patients), focal sharp waves (one), and pseudoperiodic lateralized epileptiform discharges ([PLEDs] one). In Case 15, the PLEDs occurred on Days 1 to 6 with electrographically defined seizures occurring on postinjury Days 0 and 5. The PLEDs were suppressed by induction of burst suppression with continuous propofol infusion at doses of 100 to 120 μg/minute. When propofol was tapered off, the PLEDs returned, followed by a seizure 30 seconds in duration.
Electroencephalographic Studies in Nonseizure Group
In the subgroup with no clinically or electrographically detected seizures, the EEG studies displayed nonepileptiform abnormalities in 90% of patients, with 10% having both epileptiform and nonepileptiform abnormalities. The most common nonepileptiform abnormality was symmetrical disorganized slowing in the 5 to 7–Hz range (83% of patients) with peak mean frequency of 6.8 ± 2.7 Hz. Asymmetrical disorganized delta waves slowing with a persistent focus occurred in 17%. Frontal intermittent rhythmic delta wave activity was present in 26% of patients. The absence of sleep potentials, namely vertex sharp waves and sleep spindles, occurred in 78% of patients. Progressive loss of EEG amplitude into burst suppression and complete EEG silence occurred in nine of 94 patients, and they all suffered brain death.
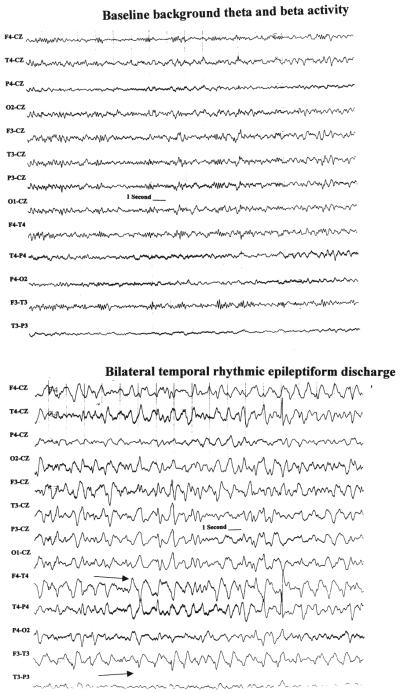
The epileptiform activity occurred in the form of isolated spikes or sharp waves in 80% of the nonseizure patients and as repetitive sharp waves in the remainder. Pseudoperiodic lateralized epileptiform activity occurred in two patients who did not have classic electroencephalographically defined seizures. An example of sudden-onset rhythmic bilateral temporal epileptiform discharges that occurred in a patient without classic electroencephalographically defined seizures is demonstrated in Fig. 4. These discharges were not considered to be seizures because they failed to meet all of the pattern recognition criteria.
Fig. 4.
Tracings showing paroxysmal discharges that occurred in patients in whom seizure criteria were not fulfilled. This demonstrates an example of a patient who at baseline has low-amplitude beta and theta frequencies (upper) and suddenly develops rhythmic bilateral temporal epileptiform discharges (lower). The discharges have a few features of sharp waves (arrows) but do not evolve over time and gradually fade back into the previous background activity. These were not considered to be seizures in this study.
The EEG changes that were common to both the seizure and nonseizure groups consisted of reaction to sedative hypnotic drugs and progressive deterioration of EEG amplitudes in patients who later suffered brain death. All patients who were given sedative hypnotic drugs experienced the expected changes (increased beta wave activity, suppression of amplitude, or burst suppression). Among these drugs the effects of propofol were unique: an initial increase in beta activity with moderate-to-high voltage (30–45 μV) occurred at infusion rates of 20 to 60 mg/kg/minute, followed by higher amplitude 2 to 3–Hz rhythmic delta wave slowing, followed by incomplete burst suppression with interburst intervals of 3 to 6 seconds. Propofol-induced burst suppression was transient, requiring hourly dosage adjustments to sustain the burst suppression–like pattern. On withdrawal of sustained propofol infusions lasting 24 hours or more, both high-amplitude beta wave activity (60–90 μV) and epileptiform spike discharges occurred in 40% of patients. None of these “propofol withdrawal” abnormalities were considered seizures.
Impact of Seizures on ICP and CPP Values
The impact of seizures on ICP and CPP was analyzed by comparing the group mean ICP and CPP values from the seizure and the nonseizure groups. In all, 13,659 hours of ICP and CPP data obtained during concurrent EEG monitoring were analyzed. The incidence of increased ICP (> 20 mm Hg) was similar in the seizure and non-seizure groups (42% and 38%, respectively). The mean overall ICP was higher in the nonseizure group (15.6 ± 9.9 mm Hg) than in the seizure group (11.8 ± 7.3 mm Hg, p < 0.001; two-tailed t-test). Similarly, CPP was slightly lower in the nonseizure group (83.6 ± 14.5 mm Hg) compared with the seizure group (85.1 ± 14.5 mm Hg; t-test, p < 0.03; Fig. 5). To determine seizure-related changes in ICP and CPP, a separate analysis of 1510 ICP and CPP values was performed, comparing the mean ICP and CPP values on the day of the seizure with the values on non-seizure days. There was no difference in ICP on the day of the seizure (13.9 ± 14 mm Hg) when compared with the nonseizure days (13.5 ± 17 mm Hg; t-test, p < 0.65). However, the mean CPP (86.6 ± 16.7 mm Hg) was statistically higher on the day of seizure when compared with nonseizure days (84.3 ± 19.1 mm Hg; t-test, p < 0.04). Serial trends for ICP did not demonstrate a clear seizure-related effect on ICP or CPP in the hours directly before, during, or after a seizure.
Fig. 5.
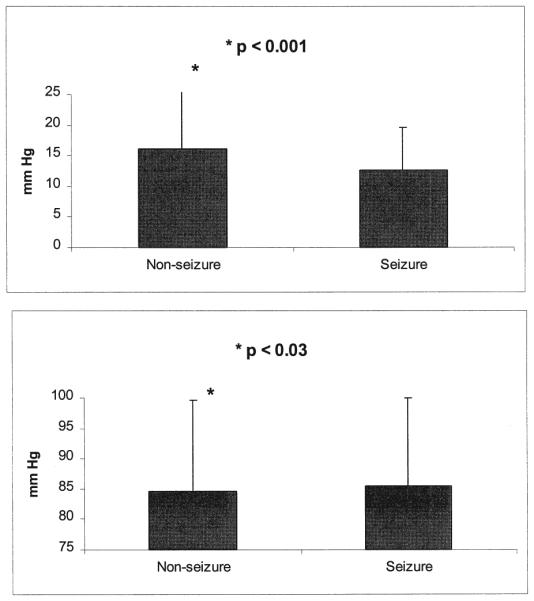
Bar graphs showing comparison of the overall means of ICP (upper) and CPP (lower) in the seizure and the nonseizure group. Data from a total of 13,659 hours of continuous recording of ICP and CPP were analyzed by comparing overall means of the nonseizure and seizure subgroups across all days of monitoring. As indicated, significant differences in ICP (p < 0.001) and CPP (p < 0.03) were found between the two groups, with the seizure group demonstrating overall lower mean ICP and higher CPP than the nonseizure group. The magnitude of the differences may not be clinically significant.
Outcome Correlated With LOS
The mean LOS was 14 ± 9 days. There was no difference between the nonseizure (14 ± 9 days) and seizure groups (15 ± 9.5 days). However, the subgroup with status epilepticus had a shorter mean LOS (9.14 ± 5.9 days, p < 0.03) in comparison with the nonseizure group. This shorter LOS was due to early death in five of six patients, with the one late death occurring in a skilled nursing facility. Two of the five early deaths were due to sepsis after control of status epilepticus; the remaining three patients suffered progressive neurological deterioration culminating in brain death despite intervention. The one patient who died late suffered respiratory arrest.
Of the 94 patients studied, 25 died during the acute-phase hospitalization, and no statistical difference was found between the seizure and nonseizure groups (seven of 21 and 18 of 73, respectively; p < 0.11). Conversely, there was no difference in good outcome at the time of discharge (four of 21 in the seizure group and 17 of 73 in the nonseizure group; t-test, p < 0.64). When considered more globally there was no difference in poor outcomes (GOS Scores 1–3) in the seizure compared with the non-seizure group (t-test, p < 0.15).
Discussion
This is a systematic study of continuous EEG monitoring during the acute phase of intensive care in patients with moderate-to-severe brain injuries. The main results are summarized as follows. Seizures occur at a higher rate than previously reported after brain injury, with a frequency of one in five patients. These seizures are often nonconvulsive and may escape clinical detection if patients are not monitored with electroencephalography. The presence of status epilepticus is associated with an excessive mortality rate. Seizures occur despite routine therapeutic administration of phenytoin. Early surgical evacuation of mass lesions was associated with a decreased incidence of seizures. There is a small statistical relationship between seizures and ICP and CPP values, but the magnitude of these differences may not be clinically significant. Finally, it is feasible to perform continuous EEG monitoring in the ICU with the proper fundamental skills and personnel.
Limitations and Self Critique
The start of continuous EEG monitoring was uniformly delayed until admission to the neurosurgical ICU, and the study thereby was insensitive to the ultra-early seizures. This systematic deficiency is partly caused by the logistical difficulties in capturing EEG recordings in this population. Given that five of 21 patients had early clinically detected seizures, it is possible that other patients had early seizures that were not witnessed electroencephalographically, and early clinically witnessed seizures may have represented nonseizure events.
Our method of screening the EEG tracings to detect seizures was based on detection of increases in total power and confirmation by subsequent review of the desired raw EEG epoch. This technique is sensitive to high-amplitude, rhythmic events that last for at least 30 seconds but is non-specific, affected by artifacts, and insensitive to low-amplitude temporal lobe seizures. Thus a knowledgeable clinician must manually screen the total power events, and short-duration seizures may have been missed. Alternative seizure detection methods, such as the Gotman method,18 are very sensitive to ictal activity but are also sensitive to artifact, and they were not available to us at the inception of the study.
It was difficult retrospectively to correlate changes in ICP and CPP with the presence of seizures. Short-duration changes in ICP and CPP lasting for 1 to 2 minutes during the actual seizure were not reliably reported. Therefore, the momentary dynamic relationships of EEG and ICP and CPP values remain to be studied in humans by using an on-line simultaneous data collection technique. The reported results of subtle differences in mean ICP and CPP therefore address trends and not momentary, dynamic relationships.
The evaluation of outcome in this study is limited to patient survival and general outcome as determined using the GOS score. This analysis focuses on the early clinical course and is limited by lack of long-term follow up. However, the impact of early short-lived events, such as seizures, may be better addressed by focusing on the immediate impact on the patient and not on later outcome that may be affected by other comorbid factors. Follow-up studies of long-term outcome are underway, with patient follow up ongoing at 36 months, but data accrual is still proceeding.
Incidence of Posttraumatic Seizures
We found that approximately 20% of patients with moderate-to-severe brain injuries suffered clinically and electroencephalographically detected seizures during the acute intensive care period. This value is higher than in previous studies, in which the incidence of early posttraumatic seizures ranged between 2.8% and 10%,3,21,23,24,51 with two studies demonstrating higher rates (15%) in severe brain injury.6,26 The previous studies used clinically witnessed seizure activity as the primary endpoint and did not report the use of electroencephalographically defined seizures as an endpoint, nor did they use routine frequent EEG recordings or continuous EEG monitoring. In their study, Dawson, et al.,12 obtained serial short-duration electroencephalograms in 45 brain-injured patients every few days during the initial 2 weeks of care and found a 25% incidence of electroencephalographically defined seizure activity. The latter study used “snapshot” electroencephalograms independent of clinical activity and obtained results similar to our own.
The incidence of isolated seizures or status epilepticus was not associated with specific causes or risk factors, such as a specific lesion type or location. As opposed to previous studies in which contusions and skull fractures were associated with seizures, our study population consisted entirely of more severely injured patients, rather than a spectrum of mild-to-severe injury. Thus, classic risk factors were seen commonly in both subgroups in our study. We did find an increased incidence of hypoxia in the patients with status epilepticus. Thus, anoxic injury together with brain trauma may have precipitated seizures in the six patients with status epilepticus and may be a unique risk factor, but interpretation of these data are limited because of the small sample size. In addition, neither high ICP nor low CPP predisposed patients to seizures. These data are difficult to interpret because of tight regulation of the ICP and CPP in the ICU and the small differences between groups. Moreover, patients in the ICU with secondary ischemic injury, as evidenced by low CPP, do not suffer seizures as a direct result. Thus, early hypoxia may contribute more to seizures than later ischemic events. Obviously, more data are needed to investigate the proximate causes and risk factors of seizures and status epilepticus in this population.
Electrophysiological Findings in Comatose Patients
Our study differs from previous reports in several ways. Previous electrophysiological studies of comatose patients (traumatic and nontraumatic) have focused on the longer subacute period, weeks to months postinjury. In addition they have used compressed spectral array and other quantitative EEG methods.8,35,44,47 Occasional cases of posttraumatic nonconvulsive status epilepticus have been reported.4 In these studies truly continuous monitoring was not maintained, and the raw EEG data were not routinely examined. We report on the power of combining EEG trend quantitation with ongoing analysis of raw EEG studies that are obtained in the early, critical phase of intensive care. Our results demonstrate that this method of continuous EEG monitoring allows detection of a higher incidence of seizures and status epilepticus than suspected clinically.
Others have studied the use of continuous EEG monitoring in comatose patients without head injuries. Young and colleagues58 found that 43 (34%) of 127 critically ill patients with neurological conditions who underwent continuous EEG monitoring in the ICU had seizures, with 17 of 43 in nonconvulsive status epilepticus. This population of patients with neurological conditions differed from ours (that is, half had remote structural causes of seizures and there was a higher percentage of primary medical conditions causing seizures). An important finding by Young, et al., was that the duration of status epilepticus and the delay in its diagnosis were associated with worsened outcome. In fact, a delay in diagnosis of more than 24 hours resulted in a mortality rate of 39%. In our study, diagnosis of early seizures and status epilepticus was delayed by at least 9.5 hours, and all of those patients died. We caution that our study was not designed specifically to answer the delay question, and therefore comparison with the study of Young, et al., is difficult. Nonetheless, our findings are in keeping with theirs and further indicate that an EEG study should be obtained early after admission to aid in diagnosis of nonconvulsive seizures.
Epileptiform activity other than classic electroencephalographically defined seizures occurred in the form of isolated spikes, sharp waves, or PLEDs. We found no physiological alteration associated with isolated spikes or sharp waves. The significance of spikes and sharp waves in the setting of acute brain injury has not been entirely defined but should alert the physician that the patient is predisposed to seizures. Pseudoperiodic lateralized epileptiform discharges are seen with a variety of acute brain insults and often are short lived, resolving within a few weeks of injury. However, PLEDs are considered by some to be a form of seizure with similar pathophysiological and pathological consequences. Indeed, Treiman52 has demonstrated that prolonged status epilepticus can evolve into PLEDs when left untreated, with resultant characteristic pathological findings at autopsy. The PLEDs may resolve after treatment with anticonvulsant agents. Despite these data, many epileptologists do not consider PLEDs to be a form of seizure, and therefore we used the more conservative electrographic criteria for documenting these phenomena. Parenthetically, if PLEDs were considered to be a form of seizure, the incidence of seizures in our study would be even higher. Nonetheless, PLEDs may have pathophysiological consequences similar to seizures after brain injury20,52 and may need to be electroencephalographically recognized. Although it is beyond the scope of this paper to recommend treatment for PLEDs after brain injury, we chose aggressive treatment consisting of continuous infusions of propofol for PLEDs that were temporally associated with seizures. It is apparent that further study is required on the impact of posttraumatic PLEDs and the effect of anticonvulsant therapy directed toward their rapid suppression.
Pathobiological Significance of Posttraumatic Seizures
Early after brain injury there are marked metabolic and neurochemical alterations that have been shown to affect outcome. These changes have been shown to occur in conjunction with abnormalities in the EEG studies.32,33,36,37,41 In this study we confirm that seizures occur during a critical period of neurochemical disarray. It is plausible that the neurochemical changes and the seizures are somehow related, and one must consider the contribution of seizures to these neurochemical and metabolic changes. Some of these changes, such as increased amounts of extracellular glutamate and lactate and decreased glucose, may occur as a result of seizures. In addition, the hyperglycolysis5 found after brain trauma may result from seizure activity. However, the majority of occurrences of hyperglycolysis were not accompanied by seizures.55 Thus, seizures may be responsible for some posttraumatic neurochemical and metabolic abnormalities54 but do not represent the sole cause of these abnormalities.
Alternatively, seizures may occur due to the increased ability of neurons to depolarize in the setting of disturbed ionic flux after trauma. In animal models of brain injury significant disturbances in neuronal membrane potential with spontaneous depolarizations resembling spreading depression have been documented. Seizures occur during the initial moments after injury in certain animal models.37 A vulnerability to seizures by the loss of inhibitory neurons has been anatomically confirmed.27 Thus, seizures represent something of a paradox in the pathophysiology of brain injury, representing a product of the injury as well as a stimulus for secondary neurochemical and metabolic insults.
Seizures and Mortality Rates
The prominent finding of death in all six patients with posttraumatic status epilepticus indicates that ongoing status epilepticus after brain injury plays a key role in determining mortality. Furthermore, the nonconvulsive nature of the status epilepticus in three of the five patients with individual seizures indicates that this important comorbidity may go unnoticed. Clearly, nonconvulsive seizures cannot be anticipated by the initial CT appearance of the lesions or GCS score. Thus, the diagnosis of seizures relies on the EEG findings. Our findings are in concert with previous reports of excessive mortality due to a symptomatic lesion57 and after a long duration of status epilepticus.2,49 One can only speculate about the negative impact of nonconvulsive status epilepticus on outcome after brain injury and the role of this phenomenon in explaining the variety of outcomes in patients with a similar severity of injury. Although we could not demonstrate an overall adverse effect of seizures on outcome, status epilepticus was correlated with an increased mortality rate.
Early hypoxia may be a risk factor for posttraumatic status epilepticus. Four of the six patients with status epilepticus had additional early hypoxic injury and may have had superimposed hypoxic ischemic insult resulting in poor outcome.39 Consequently, hypotension and hypoxemia during the initial resuscitation may be causally related to status epilepticus. A more conservative explanation is that status epilepticus merely signaled a severe injury and portended death. Nonetheless, early posttraumatic hypoxia should be an indication for continuous EEG monitoring to detect status epilepticus.
Conclusions
The results of this study indicate that a potentially important pathological process (that is, a seizure) occurs frequently after brain injury and is often undiagnosed based on clinical assessment alone. We therefore suggest that brain-injured patients should be monitored for seizures. Caution should be exercised in assigning importance to a single new monitoring modality (for example, continuous electroencephalography). Traditional monitoring and therapeutic management, including early operation, monitoring and management of ICP and CPP, and evaluation of CBF and metabolism, remain of paramount importance.7,17,19,30,38,45,48,56 However, implementing continuous EEG monitoring to detect seizure activity after brain injury appears to be logical given our results. The effect of seizures on primary brain injury and outcome needs to be studied further, and the efficacy of continuous EEG monitoring on outcome needs to be studied in a prospective trial.
Acknowledgments
The authors extend their sincerest gratitude to the EEG technicians and neurosurgical intensive care nurses who made this study possible.
This study was supported by National Institute of Neurological Disorders and Stroke Grant NS30308 and the Lind Lawrence Foundation.
References
- 1.Aldrich EF, Eisenberg HM, Saydjari C, et al. Predictors of mortality in severely head-injured patients with civilian gunshot wounds: a report from the NIH Traumatic Coma Data Bank. Surg Neurol. 1992;38:418–423. doi: 10.1016/0090-3019(92)90109-z. [DOI] [PubMed] [Google Scholar]
- 2.Aminoff MJ, Simon RP. Status epilepticus. Causes, clinical features and consequences in 98 patients. Am J Med. 1980;69:657–666. doi: 10.1016/0002-9343(80)90415-5. [DOI] [PubMed] [Google Scholar]
- 3.Annegers JF, Grabow JD, Groover RV, et al. Seizures after head trauma: a population study. Neurology. 1980;30:683–689. doi: 10.1212/wnl.30.7.683. [DOI] [PubMed] [Google Scholar]
- 4.Beni L, Constantini S, Matoth I, et al. Subclinical status epilepticus in a child after closed head injury. J Trauma. 1996;40:449–451. doi: 10.1097/00005373-199603000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Bergsneider MA, Hovda DA, Shalmon E, et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- 6.Black P, Shepard RH, Walker AE. Outcome of head trauma: age and post-traumatic seizures. Ciba Found Symp. 1975;34:215–226. doi: 10.1002/9780470720165.ch12. [DOI] [PubMed] [Google Scholar]
- 7.Bouma GJ, Muizelaar JP, Choi SC, et al. Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg. 1991;75:685–693. doi: 10.3171/jns.1991.75.5.0685. [DOI] [PubMed] [Google Scholar]
- 8.Bricolo A, Turazzi S, Faccioli F, et al. Clinical application of compressed spectral array in long-term EEG monitoring of comatose patients. Electroencephalogr Clin Neurophysiol. 1978;45:211–225. doi: 10.1016/0013-4694(78)90005-6. [DOI] [PubMed] [Google Scholar]
- 9.Bullock R, Zauner A, Myseros JS, et al. Evidence for prolonged release of excitatory amino acids in severe human head trauma. Relationship to clinical events. Ann NY Acad Sci. 1995;765:290–298. doi: 10.1111/j.1749-6632.1995.tb16586.x. [DOI] [PubMed] [Google Scholar]
- 10.Carlson H, Ronne-Engström E, Ungerstedt U, et al. Seizure related elevations of extracellular amino acids in human focal epilepsy. Neurosci Lett. 1992;140:30–32. doi: 10.1016/0304-3940(92)90674-v. [DOI] [PubMed] [Google Scholar]
- 11.Collins RC, Tearse RG, Lothman EW. Functional anatomy of limbic seizures: focal discharges from medial entorhinal cortex in rat. Brain Res. 1983;280:25–40. doi: 10.1016/0006-8993(83)91170-8. [DOI] [PubMed] [Google Scholar]
- 12.Dawson RE, Webster JE, Gurdjian ES. Serial electroencephalography in acute head injuries. J Neurosurg. 1951;8:613–630. doi: 10.3171/jns.1951.8.6.0613. [DOI] [PubMed] [Google Scholar]
- 13.DeWitt DS, Prough DS, Taylor CL, et al. Reduced cerebral blood flow, oxygen delivery, and electroencephalographic activity after traumatic brain injury and mild hemorrhage in cats. J Neurosurg. 1992;76:812–821. doi: 10.3171/jns.1992.76.5.0812. [DOI] [PubMed] [Google Scholar]
- 14.Eisenberg HM, Gary HE, Jr, Aldrich EF, et al. Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1990;73:688–698. doi: 10.3171/jns.1990.73.5.0688. [DOI] [PubMed] [Google Scholar]
- 15.Engel J, Jr, Kuhl DE, Phelps ME, et al. Local cerebral metabolism during partial seizures. Neurology. 1983;33:400–413. doi: 10.1212/wnl.33.4.400. [DOI] [PubMed] [Google Scholar]
- 16.Goodman JC, Valadka AB, Gopinath SP, et al. Lactate and excitatory amino acids measured by microdialysis are decreased by pentobarbital coma in head-injured patients. J Neurotrauma. 1996;10:549–556. doi: 10.1089/neu.1996.13.549. [DOI] [PubMed] [Google Scholar]
- 17.Gopinath SP, Robertson CS, Contant CF, et al. Jugular venous desaturation and outcome after head injury. J Neurol Neurosurg Psychiatry. 1994;57:717–723. doi: 10.1136/jnnp.57.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotman J. Automatic recognition of epileptic seizures in the EEG. Electroencephalogr Clin Neurophysiol. 1982;54:530–540. doi: 10.1016/0013-4694(82)90038-4. [DOI] [PubMed] [Google Scholar]
- 19.Gower DJ, Lee KS, McWhorter JM. Role of subtemporal decompression in severe closed head injury. Neurosurgery. 1988;23:417–422. doi: 10.1227/00006123-198810000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Handforth A, Cheng JT, Mandelkern MA, et al. Markedly increased mesiotemporal lobe metabolism in a case with PLEDs: further evidence that PLEDs are a manifestation of partial status epilepticus. Epilepsia. 1994;35:876–881. doi: 10.1111/j.1528-1157.1994.tb02526.x. [DOI] [PubMed] [Google Scholar]
- 21.Hauser WA, Tabaddor K, Factor PR, et al. Seizures and head injury in an urban community. Neurology. 1984;33:746–751. doi: 10.1212/wnl.34.6.746. [DOI] [PubMed] [Google Scholar]
- 22.Jennett B, Bond M. Assessment of outcome after severe brain damage. A practical scale. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 23.Jennett WB. Epilepsy after Nonmissile Head Injuries. 2 Heinemann; London: 1975. [Google Scholar]
- 24.Jennett WB, Lewin W. Traumatic epilepsy after closed head injuries. J Neurol Neurosurg Psychiatry. 1980;23:295–301. doi: 10.1136/jnnp.23.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly DF, Martin NA, Kordestani R, et al. Cerebral blood flow as a predictor of outcome following traumatic brain injury. J Neurosurg. 1997;86:633–641. doi: 10.3171/jns.1997.86.4.0633. [DOI] [PubMed] [Google Scholar]
- 26.Kollevold T. Immediate and early cerebral seizures after head injuries. Part I. Int J Oslo City Hosp. 1976;26:99–114. [PubMed] [Google Scholar]
- 27.Lee SM, Smith ML, Hovda D, et al. Concussive brain injury results in chronic vulnerability to post-traumatic seizures. Soc Neurosci Abstr. 1995;21:762. [Google Scholar]
- 28.Lee ST, Lui TN, Wong CW, et al. Early seizures after moderate closed head injury. Acta Neurochir. 1995;137:151–154. doi: 10.1007/BF02187187. [DOI] [PubMed] [Google Scholar]
- 29.Marmarou A. Increased intracranial pressure in head injury and influence of blood volume. J Neurotrauma. 1992;19(Suppl 1):S327–S332. [PubMed] [Google Scholar]
- 30.Marshall LF, Becker DP, Bowers SA, et al. The National Traumatic Coma Data Bank. Part 1: Design, purpose, goals, and results. J Neurosurg. 1983;59:276–284. doi: 10.3171/jns.1983.59.2.0276. [DOI] [PubMed] [Google Scholar]
- 31.Martin NA, Patwardhan R, Alexander MJ, et al. Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia, and vasospasm. J Neurosurg. 1997;87:9–19. doi: 10.3171/jns.1997.87.1.0009. [DOI] [PubMed] [Google Scholar]
- 32.Martins da Silva A, Nunes B, Vaz AR, et al. Posttraumatic epilepsy in civilians: clinical and electroencephalographic studies. Acta Neurochir Suppl. 1992;55:56–63. doi: 10.1007/978-3-7091-9233-7_16. [DOI] [PubMed] [Google Scholar]
- 33.Mayevsky A, Doron A, Manor T, et al. Cortical spreading depression recorded from the human brain using a multiparametric monitoring system. Brain Res. 1996;740:268–274. doi: 10.1016/s0006-8993(96)00874-8. [DOI] [PubMed] [Google Scholar]
- 34.McIntosh TK, Noble L, Andrews B, et al. Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent Nerv Syst Trauma. 1987;4:119–134. doi: 10.1089/cns.1987.4.119. [DOI] [PubMed] [Google Scholar]
- 35.Moulton RJ, Marmarou A, Ronen J, et al. Spectral analysis of the EEG in craniocerebral trauma. Can J Neurol Sci. 1988;15:82–86. doi: 10.1017/s0317167100027244. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson P, Hillered L, Olsson Y, et al. Regional changes in interstitial K+ and Ca2+ levels following cortical compression contusion trauma in rats. J Cereb Blood Flow Metab. 1993;13:183–192. doi: 10.1038/jcbfm.1993.22. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson P, Ronne-Engström E, Flink R, et al. Epileptic seizure activity in the acute phase following cortical impact trauma in rat. Brain Res. 1994;637:227–232. doi: 10.1016/0006-8993(94)91237-8. [DOI] [PubMed] [Google Scholar]
- 38.Nordström CH, Messeter K, Sundbärg G, et al. Severe traumatic brain lesions in Sweden. Part I: Aspects of management in non-neurosurgical clinics. Brain Inj. 1989;3:247–265. doi: 10.3109/02699058909029639. [DOI] [PubMed] [Google Scholar]
- 39.Nordström CH, Sundbärg G, Messeter K, et al. Severe traumatic brain lesions in Sweden. Part 2: Impact of aggressive neurosurgical intensive care. Brain Inj. 1989;3:267–281. doi: 10.3109/02699058909029640. [DOI] [PubMed] [Google Scholar]
- 40.Nuwer MR. Electroencephalograms and evoked potentials. Monitoring cererbal function in the neurosurgical intensive care unit. Neurosurg Clin North Am. 1994;5:647–659. [PubMed] [Google Scholar]
- 41.Ozawa Y, Nakamura T, Sunami K, et al. Study of regional cerebral blood flow in experimental head injury: changes following cerebral contusion and during spreading depression. Neurol Med Chir. 1991;31:685–690. doi: 10.2176/nmc.31.685. [DOI] [PubMed] [Google Scholar]
- 42.Pal J, Brown R, Fleiszer D. The value of the Glasgow Coma Scale and Injury Severity Score: predicting outcome in multiple trauma patients with head injury. J Trauma. 1989;29:746–748. doi: 10.1097/00005373-198906000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Piek J, Chesnut RM, Marshall LF, et al. Extracranial complications of severe head injury. J Neurosurg. 1992;77:901–907. doi: 10.3171/jns.1992.77.6.0901. [DOI] [PubMed] [Google Scholar]
- 44.Rae-Grant AD, Eckert N, Barbour PJ, et al. Outcome of severe brain injury: a multimodality neurophysiologic study. J Trauma. 1996;40:401–407. [PubMed] [Google Scholar]
- 45.Robertson C. Desaturation episodes after severe head injury: influence on outcome. Acta Neurochir Suppl. 1993;59:98–101. doi: 10.1007/978-3-7091-9302-0_17. [DOI] [PubMed] [Google Scholar]
- 46.Robertson CS, Contant CF, Gokaslan ZL, et al. Cerebral blood flow, arteriovenous oxygen difference, and outcome in head injured patients. J Neurol Neurosurg Psychiatry. 1992;55:594–603. doi: 10.1136/jnnp.55.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogatsky G, Mayevsky A, Zarchin N, et al. Continuous multiparametric monitoring of brain activities following fluid-percussion injury in rats: preliminary results. J Basic Clin Physiol Pharmacol. 1996;7:23–43. doi: 10.1515/jbcpp.1996.7.1.23. [DOI] [PubMed] [Google Scholar]
- 48.Rosner MJ, Rosner SD, Johnson AH. Cerebral perfusion pressure: management protocol and clinical results. J Neurosurg. 1995;83:949–962. doi: 10.3171/jns.1995.83.6.0949. [DOI] [PubMed] [Google Scholar]
- 49.Scholtes FB, Renier WO, Meinardi H. Generalized convulsive status epilepticus: causes, therapy, and outcome in 346 patients. Epilepsia. 1994;35:1104–1112. doi: 10.1111/j.1528-1157.1994.tb02562.x. [DOI] [PubMed] [Google Scholar]
- 50.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 51.Temkin NR, Dikmen SS, Wilensky AJ, et al. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- 52.Treiman DM. Electroclinical features of status epilepticus. J Clin Neurophysiol. 1995;12:343–362. [PubMed] [Google Scholar]
- 53.Uzzell BP, Dolinskas CA, Wiser RF. Relation between intracranial pressure, computed tomographic lesion, and neuropsychological outcome. Adv Neurology. 1990;52:269–274. [PubMed] [Google Scholar]
- 54.Vespa PM, Prins M, Ronne-Engstrom E, et al. Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: a microdialysis study. J Neurosurg. 1998;89:971–982. doi: 10.3171/jns.1998.89.6.0971. [DOI] [PubMed] [Google Scholar]
- 55.Vespa PM, Ronne-Engstrom E, Nuwer MR, et al. Altered global cerebral metabolism in traumatic brain injury: etiology of hyperglycolysis. J Cereb Blood Flow Metab. 1997;17(Suppl 1):S31. [Google Scholar]
- 56.Wilberger JE, Jr, Harris M, Diamond D. Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg. 1991;74:212–218. doi: 10.3171/jns.1991.74.2.0212. [DOI] [PubMed] [Google Scholar]
- 57.Yaffe K, Lowenstein DH. Prognostic factors of pentobarbital therapy for refractory generalized status epilepticus. Neurology. 1993;43:895–900. doi: 10.1212/wnl.43.5.895. [DOI] [PubMed] [Google Scholar]
- 58.Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47:83–89. doi: 10.1212/wnl.47.1.83. [DOI] [PubMed] [Google Scholar]