Abstract
A comparative view of the brain, comparing related functions across species and sensory systems, offers a number of advantages. In particular, it allows separating the formal purpose of a model structure from its implementation in specific brains. Models of auditory cortical processing can be conceived by analogy to the visual cortex, incorporating neural mechanisms that are found in both the visual and auditory systems. Examples of such canonical features on the columnar level are direction selectivity, size/bandwidth selectivity, as well as receptive fields with segregated versus overlapping on- and off-sub-regions. On a larger scale, parallel processing pathways have been envisioned that represent the two main facets of sensory perception: 1) identification of objects and 2) processing of space. Expanding this model in terms of sensorimotor integration and control offers an overarching view of cortical function independent of sensory modality.
Keywords: Hierarchical processing, simple and complex cells, ventral and dorsal streams, sensorimotor integration, internal models
Comparative Approaches
A comparative view of the brain, comparing similar functions across species and sensory systems, offers a number of advantages (Rauschecker & Marler, 1987). In particular, it allows separating the formal purpose of a model structure from its implementation in specific brains. Different animal species have to solve the same problems in the interest of behavior and survival. Similarly, different sensory systems have to identify and recognize objects in their environment and localize them in space. In higher mammals, cerebral cortex in different sensory systems shows the same basic columnar structure with the same number of layers, cell types and input-output organization (Scheich et al., 2007). The notion that a cortical module in, say, visual and auditory cortex performs the same operation on different input signals with the same canonical micro-circuit (Douglas & Martin, 2004) is, therefore, not far-fetched. Furthermore, advancing from early to late cortical regions, specificity of neuronal responses increases, while receptive field size and ability to generalize and to form invariances increase as well (Riesenhuber & Poggio, 1999). Finally, the specificity of cortical areas for one function or another is organized along processing streams that are manifested neuroanatomically as well as physiologically (Rauschecker & Scott, 2009; Romanski & Averbeck, 2009). Although I largely restrict my analysis to two primate species, rhesus monkeys and humans, and two sensory systems, visual and auditory, I believe that the conclusions may be generalizable to other species and cortical systems as well (Lomber & Malhotra, 2008; Read et al., 2002).
Hierarchical Organization
Work on the visual cortex of higher mammals has led to two major results: The anatomical organization of the visual cortical system follows a largely hierarchical principle, i.e. lower-order regions project, both serially and in parallel, to higher-order regions, where increasingly complex content is processed (Felleman & Van Essen, 1991). This process starts already in primary visual cortex, which contains “simple” and “complex” cells (Hubel & Wiesel, 1962, 1977), although their laminar distribution may differ between species, such as cats or monkeys. The output of primary visual cortex then projects to secondary and tertiary areas forming two processing pathways or “streams” (Kravitz et al., 2013): a ventral stream that projects into temporal lobe and ultimately to ventrolateral prefrontal cortex; and a dorsal stream that projects into parietal cortex, premotor cortex and onto dorsolateral prefrontal cortex. If one is interested in general brain organization, the question immediately follows: Are cortical representations of other sensory systems organized similarly or differently? Can the auditory system, for instance, be understood also as a hierarchical system that is organized in series and in parallel, feedback connections notwithstanding? Can we find analogues of simple and complex cells early on in the auditory cortex? And are the auditory cortical pathways divided into a ventral and a dorsal stream that subserve similar functions as in the visual system? These questions are meant to lead to answers about how sensory systems in the brain are organized in general.
Canonical Circuits in Early Cortical Areas
Much has been said about differences in neural architecture of the visual and auditory systems at the level of thalamus and below (King & Nelken, 2009; Masterton, 1992), sometimes claiming a larger number of synaptic steps from sensory receptors to primary cortical neurons and more complicated wiring in the auditory brainstem. What is often overlooked, however, is that the retina evolutionarily is a part of the brain and contains a comparable number of processing steps as the auditory brainstem. Therefore, and given the fact that the basic organization of neocortex into six layers is the same across areas, the argument that primary auditory cortex is at a higher level of processing than primary visual cortex and/or performs more complex operations is not easy to defend. In this brief review chapter, I will instead focus on similarities in the organization of the visual and auditory systems at the level of cortex and above.
Core and belt organization
First of all, sensory cortices seem to be set up by a general ‘bauplan’ (Sanides, 1969): Primary sensory areas are characterized by a prominent granular layer (hence the term koniocortex) receiving input from the principal thalamic relay nucleus. A number of “belt” areas surround the core. While the terms “core” and “belt” have become standard terminology for auditory cortex, they are used less commonly for visual cortex, where the terms parastriate and peristriate cortex refer to the same form of organization. The core/belt terminology of Sanides and his associates is similar but distinct from that used later by Jones (2007), who talks about core and “matrix”, where matrix refers to the diffuse thalamic projections from nonprimary thalamic nuclei characterized by distinct histochemical markers (see also Hackett, 2011).
Receptive field properties
Primary cortical areas (or “core” areas; Pandya & Sanides, 1973; Kaas & Hackett, 2000) are central representations of the peripheral receptor surface. As such, visual and somatosensory cortices reflect the two-dimensional nature of their respective sense organs: retina and skin. Auditory cortex, on the other hand, represents frequency, as it is coded along the one-dimensional basilar membrane of the cochlea. Tonotopic (or cochleotopic) organization, as found in primary core areas of auditory cortex as well as secondary (“belt”) areas surrounding them, is the equivalent of retinotopic organization. Sweeps of frequency-modulated tones (“FM sweeps”) are equivalent to moving bars of light (Mendelson & Cynader, 1985), and bandwidth selectivity, as described in lateral auditory belt of rhesus monkeys (Rauschecker et al., 1995) may be analogous to size selectivity in visual cortical area V4 (Desimone & Schein, 1987; Rauschecker, 2012a).
Simple and complex cells
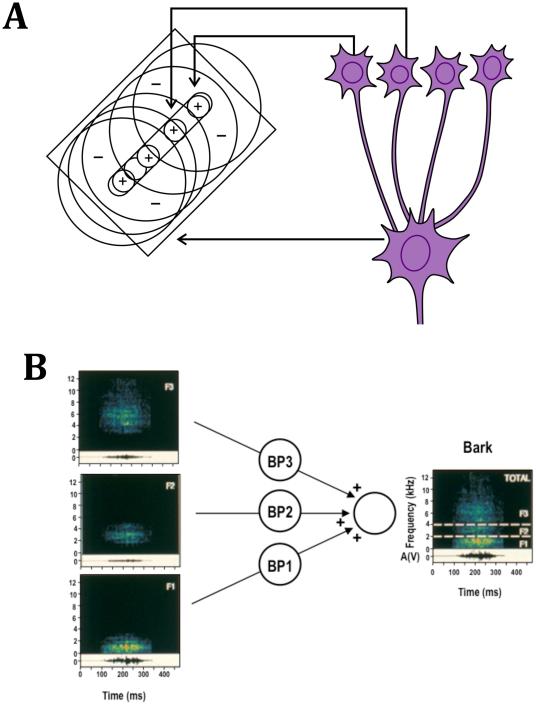
When it gets to the temporal organization of receptive fields (RFs), sensory stimuli usually begin with an onset and finish with an offset. In one of their fundamental discoveries, Hubel and Wiesel (1962) described two RF types in primary visual cortex of the cat that showed different responses to the on- and offset of a light bar flashed on the RF: “Simple-cell” RFs had segregated subregions that responded to either the on- or offset of the bar and showed spatial summation within each of these regions. In this respect they resemble cells in the thalamic lateral geniculate nucleus (LGN), which provide input to cortical simple cells and also have segregated On- and Off-subregions. By contrast, “complex” cells, as described by Hubel and Wiesel (1962), had On- and Off-regions that were coextensive in space. These two RF types were considered the beginning of a hierarchical pathway from visual thalamus to cortex that continued into secondary (and higher) areas of visual cortex. The first step was a convergence of thalamic input onto cortical simple cells, which led to the input alignment of thalamic RFs with On- and Off-responses onto the discrete subregions of simple-cell RFs (Figure 1A). Although it was later shown that, in the monkey, concentric RFs still exist in the input layers of visual cortex, the basic model has remained the same (Hubel & Wiesel, 1977). In particular, a preponderance of simple cells in or near the input layers of visual cortex and a more common occurrence of complex cells above and below layer IV has been confirmed in both species.
Figure 1. Hierarchical convergence of inputs in visual and auditory cortex.
A: Input alignment model of Hubel and Wiesel (1962). Edge detectors are created by convergence of input from lateral geniculate neurons. B: “Bark” detector postulated for rhesus monkey auditory cortex (Rauschecker, 1998). Neurons selective to specific monkey vocalizations are created by convergence from lower-order neurons selective to specific bandwidths.
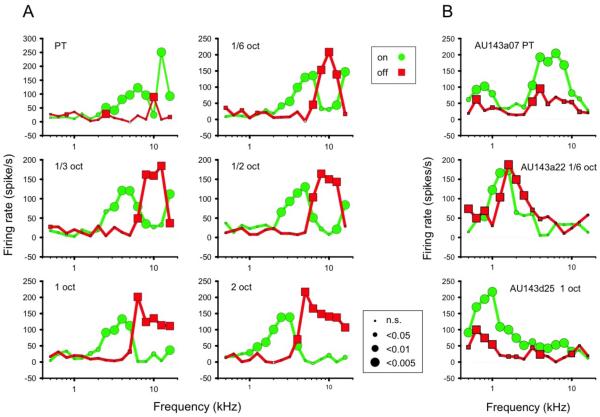
Recently, RF types were discovered in primary auditory cortex of the macaque that were in some ways analogous to simple and complex cells (Tian et al., 2013): On- and Off-responses to band-passed noise (BPN) bursts showed different frequency tuning in S-type neurons, whereas frequency tuning was the same or overlapping for C-type cells (Figure 2). This similarity of RF organization in visual and auditory cortex suggests that 1) discrete On- and Off-channels exist in the afferent auditory pathway as in the visual pathway, and 2) the organization of a thalamo-cortical processing module or column may be similar, suggesting indeed a canonical model of cortical processing.
Figure 2. Excitatory response profiles of A1 neurons determined from peak firing rates in response to the ON- and OFF-set of a BPN burst with varying frequency (from Tian et al., 2013).
Peak firing rates were extracted from peri-stimulus time histograms. Green circles connected by green lines show ON-responses. Red squares and lines show OFF-responses. Size of the symbols indicates significance level of the response in Wilcoxon Signed Rank test. A: Response profiles of a typical type-S (“simple-like”) cell. In addition to center frequency, bandwidth of the BPN stimuli was also varied, as indicated within each panel. ON- and OFF-response profiles appear largely separated into discrete frequency ranges. The effect was consistent for different bandwidths, although OFF-responses were often markedly diminished for pure-tone stimuli (upper left panel). B: Three examples of type-C (“complex-like”) cells. In all cases, the response profiles of ON- and OFF-responses are similar or show significant overlap, though OFF-responses are considerably weaker in two of the examples (top and bottom).
To prove or disprove the latter assumption, it will be useful to demonstrate a preponderance of S-type cells in layer III/IV of primary auditory cortex and a majority of C-type cells in supra- and infragranular layers. Furthermore, one would want to test S- and C-cells with the equivalent of drifting gratings, e.g. dynamic ripple sounds, to test the linearity of responses in S- and C-cells as in their visual analogues (Tian et al., 2013).
As to the perceptual purpose of such cell types, it is generally assumed that visual edge detectors with discrete adjacent On- and Off-regions improve the spatio-temporal contrast in vision and contribute to the perception of line segments (Hubel & Wiesel, 1977). In audition, S-cells could serve the purpose of enhancing spectro-temporal contrast between successive sounds with different spectra, thus detecting the boundaries between events or facilitating the segmentation of auditory scenes (Bregman, 1990) and in stream segregation (Micheyl et al., 2005). The existence of such cell types may have also enabled the emergence of mechanisms for the segmentation of speech in humans. It is reasonable to assume that such a process of boundary detection and event segmentation would occur relatively early in the cortical hierarchy. By contrast, C-cells (like their equivalent in the visual system) could contribute to the formation of invariance, in this case invariance against changes of frequency (“transposition invariance”).
Convergence of inputs in the lateral belt
Characteristic for the response specificity of neurons in the lateral belt of auditory cortex in nonhuman primates is their ability to integrate over a specific set of frequencies, which leads to RFs with bandwidth selectivity and better responses to BPN bursts compared to pure tones (Rauschecker et al., 1995). An increasing number of neurons in the anterior LB also respond to species-specific communication calls and/or harmonic complex tones (Kikuchi et al., 2014). The mechanism for generating this response specificity is nonlinear spectral and temporal summation, which is commonly referred to as “combination sensitivity” (Margoliash & Fortune, 1992; Suga et al., 1978) (Figure 1B). Such nonlinear summation may be realized with an AND-gate mechanism similar to the input alignment model of Hubel and Wiesel (Figure 1A).
Parallel Processing Streams and Internal Models
Ventral and dorsal streams
Neuroanatomically, two major cortico-cortical pathways exist in each of the sensory systems: a ventral stream projecting from primary regions to ventrolateral prefrontal cortex (VLPFC; Brodmann areas 12 and 45) and a dorsal stream projecting to dorsolateral prefrontal cortex (DLPFC; areas 46 and 8a) (Goldman-Rakic, 1988; Hackett, 2011; Romanski et al., 1999). The ventral stream is almost universally agreed upon as an object recognition or “what”-stream in both the visual and auditory system, whereas the functional definition of the visual dorsal stream has varied (“where”: Ungerleider & Mishkin, 1982; “how”: Goodale & Milner, 1992).
Magno- and parvo-cellular pathways
A similarly influential finding in the visual system was the discovery of sustained and transient channels that were later substantiated as different types of retinal ganglion cells with distinct physiological and anatomical properties. Owing to the different size of their somata and dendritic arbors, the cell types were named parvo- and magnocellular (or P and M), and the respective pathways formed by them carry the same name. P and M pathways project to distinct layers of the lateral geniculate nucleus (LGN), which in turn give rise to projections targeting different layers within primary and secondary visual cortex (V1 and V2; Horton & Hubel, 1981; Livingstone & Hubel, 1988). P and M cells are also characterized by the sustained versus transient nature and the linearity versus non-linearity of their visual responses, respectively. P cells are sensitive to fine detail and color, and M-cells are selective for motion, so some have argued that P and M cells may give rise to the ventral and dorsal visual cortical pathways, respectively. While it seems to be true that P cells provide the dominant input to the ventral stream and M cells to the dorsal stream, there is no one-to-one correspondence.
Little is known about the equivalency of the parvo- and magnocellular pathways in the auditory system. Although various researchers have looked for corresponding cell types beginning with the cochlear nuclei, especially given the spatial tuning of neurons in the dorsal cochlear nucleus (DCN) (Yu & Young, 2000), no clear equivalency has been established. It is tempting to think that the ventral cochlear nucleus (VCN), via the central nucleus of the inferior colliculus (ICC) and the ventral nucleus of the medial geniculate (MGv), provides dominant input into the auditory ventral pathway, while the DCN, the external nucleus of the inferior colliculus (ICx) and the dorsal and/or magnocellular nucleus of the medial geniculate (MGd or MGm) form the beginning of the dorsal stream, but this is mostly hypothetical. Lesion and tract-tracing studies do show a significant input, however, from the MGd to the caudomedial belt area CM in the rhesus monkey (Rauschecker et al., 1997), which is one of the early areas of the auditory dorsal stream.
Auditory dorsal stream, sensorimotor integration, and internal models
The auditory dorsal stream was originally defined by analogy to the visual system as a “where”-stream for the processing of space and motion (Rauschecker & Tian, 2000). Synthesizing data from humans and nonhuman primates in the auditory system, however, the definition of the dorsal stream was recently expanded to include sensorimotor integration and control (Rauschecker, 2011; Rauschecker & Scott, 2009), thus unifying elements of the “where” and how” streams in vision.
For instance, the production, storage and anticipation of sound sequences resulting from action sequences, such as musical melodies or spoken speech in humans, may be mediated by the dorsal stream through its close relationship to sensorimotor structures in parietal and premotor cortex and in the basal ganglia (Leaver et al., 2009). Expressed in the language of control theory, dorsal-stream function may entail the encoding of actions as “forward models” informing sensory structures of actions that are about to occur, i.e. as internal models that produce a predicted sensation. Conversely, dorsal-stream function also relates to the programming of motor structures by sensory information as “inverse models”. In this case, the premotor system (“controller”) receives the desired sensation as input and must find actions that cause actual sensations to be as close as possible to desired sensations (Grush, 2004; Jordan & Rumelhart, 1992)(Figure 3).
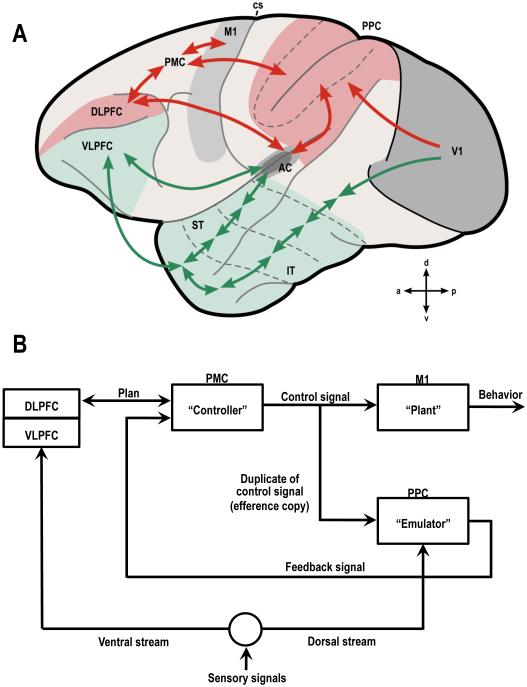
Figure 3. Generalized scheme for the organization of sensory systems in the cerebral cortex.
A: Auditory and visual processing pathways are shown superimposed on the left surface of a macaque brain (ventral streams in green; dorsal streams in red) (adapted from (Rauschecker & Scott, 2009)). B: Block diagram of information flow for sensorimotor integration and control (after Grush, 2004). Different parts of the internal model are hypothetically assigned to different parts of the cerebral cortex.
Similar models of audio-motor function have been proposed for the vocal system of songbirds (Doupe, 1993) and have obvious relevance for the understanding of communication and its evolution (Rauschecker, 2012b). Language and music in humans are two cognitive functions that are highly evolved but are likely based on the same mechanisms (Rauschecker, 2014; Bornkessel-Schlesewsky et al., 2015).
The existence of internal models has long been postulated for the visual system as well (Grush, 2004). A redefinition of the visual dorsal stream in these terms appears adequate and necessary and comprises both ‘where’ and ‘how’-models of the visual dorsal stream (Ungerleider & Mishkin, 1982; Goodale & Milner, 1992). The need for feedback connections (for the transmission of efference copies; Kauramäki et al., 2010) in the service of visuomotor integration is obvious, if only to compensate for the effect of eye movements. However, the need for internal models goes farther than that: As the eyes move almost constantly between temporary fixations, a stitching together of snapshots taken during these fixation periods is necessary. Thus, the function of the visual dorsal stream may be analogous to that in audition, that is, to serve with the production, storage and anticipation of visuomotor sequences. To be sure, this expanded definition of the visual dorsal stream includes the processing of space as a framework for its visuomotor functions.
Conclusions
A generalized split of sensory cortical systems into ventral and dorsal streams projecting from primary sensory areas into segregated PFC regions appears meaningful and has been widely accepted. The consensus is that ventral streams in both vision and audition project to the temporal lobe and on to VLPFC for purposes of object identification, whereas the dorsal streams project into parietal and premotor cortices and DLPFC for sensorimotor integration in the direct service of sensory-controlled behavior. While the definition of function in the ventral stream clearly has profited from work in the visual system that is being generalized to the auditory system, a redefinition of the dorsal stream is now beginning to take shape by generalizing knowledge gained from the study of the auditory system. This new body of knowledge emerging from audition may now prove beneficial for understanding vision across a number of species by greater emphasis on features of “active perception” in the service of behavior (e.g. Lewicki et al., 2014).
Acknowledgements
This chapter is dedicated to David Hubel and Peter Marler, two giants and heroes in the field of neurophysiology and neuroethology, who passed away within less of a year of each other on September 22, 2013 and July 5, 2014, respectively.
The research summarized here was supported by grants from the National Science Foundation (BCS-0519127 and OISE-0730255 to JPR) and the National Institutes of Health (R01DC03489 and R01NS052494 to JPR). The manuscript was prepared with partial support from the Technische Universität München – Institute for Advanced Study, funded by the German Excellence Initiative and the European Union Seventh Framework Programme under grant agreement n° 291763 (JPR).
Abbreviations
- AC
early auditory cortex (core and belt)
- V1
primary visual cortex
- ST
superior temporal
- IT
inferior temporal
- PFC
prefrontal cortex (DLPFC, VLPFC: dorsolateral, ventrolateral prefrontal cortex)
- PPC
posterior parietal cortex
- PMC
premotor cortex
- M1
primary motor cortex
- cs
central sulcus
- a
anterior
- p
posterior
- d
dorsal
- v
ventral. Numbers denote Brodmann Areas
References
- Bornkessel-Schlesewsky I, Schlesewsky M, Small SL, Rauschecker JP. The neurobiological roots of language in primate audition: common computational properties. Trends in Cognitive Science. 2014 doi: 10.1016/j.tics.2014.12.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman AS. Auditory Scene Analysis: The Perceptual Organization of Sound. MIT Press; Cambridge, MA: 1990. [Google Scholar]
- Chevillet MA, Riesenhuber M, Rauschecker JP. Functional localization of the ventral auditory “what” stream hierarchy. J. Neurosci. 2011;(25):9345–9352. doi: 10.1523/JNEUROSCI.1448-11.2011. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevillet MA, Jiang X, Rauschecker JP, Riesenhuber M. Automatic phoneme category selectivity in the dorsal auditory stream. J. Neurosci. 2013;33(12):5208–5215. doi: 10.1523/JNEUROSCI.1870-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Schein SJ. Visual properties of neurons in area V4 of the macaque: sensitivity to stimulus form. J. Neurophysiol. 1987;57:835–68. doi: 10.1152/jn.1987.57.3.835. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu. Rev. Neurosci. 2004;27:419–51. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Doupe AJ. A neural circuit specialized for vocal learning. Curr. Opin. Neurobiol. 1993;3:104–11. doi: 10.1016/0959-4388(93)90043-x. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu. Rev. Neurosci. 1988;11:137–56. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–5. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grush R. The emulation theory of representation: motor control, imagery, and perception. Behav. Brain Sci. 2004;27:377–96. doi: 10.1017/s0140525x04000093. discussion 396-442. [DOI] [PubMed] [Google Scholar]
- Hackett TA. Information flow in the auditory cortical network. Hear. Res. 2011;271:133–46. doi: 10.1016/j.heares.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, Hubel DH. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature. 1981;292:762–764. doi: 10.1038/292762a0. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. 1962;160:106–54. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc. R. Soc. Lond. B Biol. Sci. 1977;198(1130):1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. 2nd Cambridge University Press; Cambridge: 2007. [Google Scholar]
- Jordan MI, Rumelhart DE. Forward models: supervised learning with a distal teacher. Cog. Sci. 1992;16:307–354. [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. PNAS. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauramäki J, Jääskeläinen IP, Hari R, Möttönen R, Rauschecker JP, Sams M. Transient adaptation of auditory cortex organization by lipreading and own speech production. J. Neurosci. 2010;30(4):1314–1321. doi: 10.1523/JNEUROSCI.1950-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Horwitz B, Mishkin M, Rauschecker JP. Processing of harmonics in the lateral belt of macaque auditory cortex (in revision) Front. Neurosci. 2014 doi: 10.3389/fnins.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Nelken I. Unraveling the principles of auditory cortical processing: can we learn from the visual system? Nat. Neurosci. 2009;12:698–701. doi: 10.1038/nn.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, Mishkin M. The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn. Sci. 2013;17:26–49. doi: 10.1016/j.tics.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver A, Van Lare JE, Zielinski BA, Halpern A, Rauschecker JP. Brain activation during anticipation of sound sequences. J Neurosci. 2009;29(8):2477–2485. doi: 10.1523/JNEUROSCI.4921-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewicki MS, Olshausen BA, Surlykke A, Moss CF. Scene analysis in the natural environment. Front. Psychol. 2014;5:199. doi: 10.3389/fpsyg.2014.00199. doi: 10.3389/fpsyg.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Malhotra S. Double dissociation of 'what' and 'where' processing in auditory cortex. Nat. Neurosci. 2008;11:609–16. doi: 10.1038/nn.2108. [DOI] [PubMed] [Google Scholar]
- Margoliash D, Fortune ES. Temporal and harmonic combination-sensitive neurons in the zebra finch's HVc. J. Neurosci. 1992;12:4309–26. doi: 10.1523/JNEUROSCI.12-11-04309.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterton RB. Role of the central auditory system in hearing: the new direction. Trends Neurosci. 1992;15:280–285. doi: 10.1016/0166-2236(92)90077-l. [DOI] [PubMed] [Google Scholar]
- Mendelson JR, Cynader MS. Sensitivity of cat primary auditory cortex (AI) neurons to the direction and rate of frequency modulation. Brain Res. 1985;327(1-2):331–335. doi: 10.1016/0006-8993(85)91530-6. [DOI] [PubMed] [Google Scholar]
- Micheyl C, Tian B, Carlyon RP, Rauschecker JP. Perceptual organization of sound sequences in the auditory cortex of awake macaques. Neuron. 2005;48:139–48. doi: 10.1016/j.neuron.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Sanides F. Architectonic parcellation of the temporal operculum in rhesus monkey and its projection pattern. Z. Anat. Entwicklungsgesch. 1973;139(2):127–161. doi: 10.1007/BF00523634. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Pons T, Mishkin M. Serial and parallel processing in rhesus monkey auditory cortex. J. Comp. Neurol. 1997;382:89–103. [PubMed] [Google Scholar]
- Rauschecker JP. Parallel processing in the auditory cortex of primates. Audiol. Neurootol. 1998;3:86–103. doi: 10.1159/000013784. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. An expanded role for the dorsal auditory pathway in sensorimotor integration and control. Hear. Res. 2011;271:16–25. doi: 10.1016/j.heares.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP. Processing streams in the auditory cortex. In: Cohen Y, Fay RR, Popper AN, editors. Neural Correlates of Auditory Cognition. Springer Handbook of Auditory Research. Springer; New York: 2012a. [Google Scholar]
- Rauschecker JP. Ventral and dorsal streams in the evolution of speech and language. Front Evol. Neurosci. 2012b;4:7. doi: 10.3389/fnevo.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP. Is there a tape recorder in your head? How the brain stores and retrieves musical melodies. Front Syst. Neurosci. 2014;8:149. doi: 10.3389/fnsys.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Marler P. Cortical plasticity and imprinting: Behavioral and physiological contrasts and parallels. In: Rauschecker JP, Marler P, editors. Imprinting and Cortical Plasticity. Comparative Aspects of Sensitive Periods. John Wiley & Sons; New York: 1987. [Google Scholar]
- Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat. Neurosci. 2009;12:718–24. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of "what" and "where" in auditory cortex. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11800–6. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Hauser M. Processing of complex sounds in the macaque nonprimary auditory cortex. Science. 1995;268:111–4. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- Read HL, Winer JA, Schreiner CE. Functional architecture of auditory cortex. Curr. Opin. Neurobiol. 2002;12:433–40. doi: 10.1016/s0959-4388(02)00342-2. [DOI] [PubMed] [Google Scholar]
- Riesenhuber M, Poggio T. Hierarchical models of object recognition in cortex. Nat. Neurosci. 1999;2:1019–25. doi: 10.1038/14819. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Averbeck BB. The primate cortical auditory system and neural representation of conspecific vocalizations. Annu. Rev. Neurosci. 2009;32:315–46. doi: 10.1146/annurev.neuro.051508.135431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat. Neurosci. 1999;2:1131–6. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanides F. Comparative architectonics of the neocortex of mammals and their evolutionary interpretation. Ann. N. Y. Acad. Sci. 1969;167:404–423. [Google Scholar]
- Scheich H, Brechmann A, Brosch M, Budinger E, Ohl FW. The cognitive auditory cortex: task-specificity of stimulus representations. Hear. Res. 2007;229:213–24. doi: 10.1016/j.heares.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Suga N, O'Neill WE, Manabe T. Cortical neurons sensitive to combinations of information-bearing elements of biosonar signals in the mustache bat. Science. 1978;200:778–781. doi: 10.1126/science.644320. [DOI] [PubMed] [Google Scholar]
- Tian B, Kusmierek P, Rauschecker JP. Analogues of simple and complex cells in rhesus monkey auditory cortex. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7892–7. doi: 10.1073/pnas.1221062110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two visual systems. In: Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. MIT Press; Cambridge: 1982. [Google Scholar]
- Wessinger CM, Van Meter J, Tian B, Van Lare J, Pekar J, Rauschecker JP. Hierarchical organization of human auditory cortex revealed by functional magnetic resonance imaging. Journal of Cognitive Neuroscience. 2001;13(1):1–7. doi: 10.1162/089892901564108. [DOI] [PubMed] [Google Scholar]
- Yu JJ, Young ED. Linear and nonlinear pathways of spectral information transmission in the cochlear nucleus. Proc. Natl. Acad. Sci. U S A. 2000;97(22):11780–11786. doi: 10.1073/pnas.97.22.11780. [DOI] [PMC free article] [PubMed] [Google Scholar]