Abstract
Background
The impact of replacing the NCEP/ ATPIII cholesterol guidelines with the new 2013 ACC/AHA guidelines for primary prevention of cardiovascular disease is unclear.
Methods
We used risk factor and 10-year clinical event rate data from the Multi-Ethnic Study of Atherosclerosis (MESA), combined with estimates of efficacy of moderate and high intensity statin therapy from meta-analyses of statin primary prevention trials to estimate 1.) the change in number of subjects eligible for drug therapy, and 2.) the anticipated reduction in atherosclerotic cardiovascular disease (ASCVD) events and increment in Type II diabetes (T2DM) associated with the change in cholesterol guidelines.
Results
Of the 6814 MESA participants, 5437 were not on statins at baseline and had complete data for analysis (mean age 61.4 ±10.3). Using the NCEP/ATP III guidelines 1334 (24.5%) would have been eligible for statin therapy compared with 3015 (55.5%) under the new ACC/AHA guidelines. Among the subset of newly eligible, 127/1742 (7.3%) had an ASCVD event during 10 years of follow-up. Assuming 10 years of moderate intensity statin therapy, the estimated absolute reduction in ASCVD events for the newly eligible group was 2.06% (NNT: 48.6) and the estimated absolute increase in T2DM was 0.90% (NNH: 110.7). Assuming 10 years of high intensity statin therapy, the corresponding estimates for reductions in ASCVD and increases in T2DM were: ASCVD; 2.70% (NNT: 37.5) and T2DM: 2.60% (NNH: 38.6). The estimated effects of moderate intensity statins on 10 year risk for ASCVD and T2DM in participants eligible for statins under the NCEP/ATP III were: 3.20% (NNT: 31.5) and 1.06% (NNH: 94.2) respectively.
Conclusion
Substituting the NCEP/ATP III cholesterol guidelines with the 2013 ACC/AHA cholesterol guidelines in MESA more than doubled the number of participants eligible for statin therapy. If the new ACC/AHA cholesterol guidelines are adopted and extend the primary prevention population eligible for treatment, the risk-benefit profile is much better for moderate intensity than high intensity statin treatment.
Keywords: Cholesterol guidelines, statins, type 2 diabetes mellitus, atherosclerotic cardiovascular event
Introduction
Over the past decade, statin therapy for primary coronary heart disease (CHD) prevention was based on the 2001 National Cholesterol Education Program (NCEP)/ Adult Treatment Program (ATP) III guidelines and an update in 2004, developed by collaboration between the National Heart, Lung and Blood Institute (NHLBI), the American College of Cardiology (ACC) foundation and the American Heart Association (AHA) 1,2. Recently, the ACC/AHA published new guidelines for statin therapy to reduce primary atherosclerotic cardiovascular disease (ASCVD)3.
Since the initial publication of the 2013 ACC/AHA cholesterol guidelines, there has been a great deal of attention to the increase in the number of Americans who will now qualify for statin therapy under this new guideline compared with previous recommendations 4-7. In addition, among the newly eligible statin users, the risk-benefit profile may be less favorable due to a higher number of people needed to treat for each prevented ASCVD event and a corresponding increase in adverse events such as type 2 diabetes mellitus (T2DM) and rhabdomyolysis – which are especially undesirable outcomes in the context of a purely primary prevention intervention. These considerations have contributed to uncertainty among practicing physicians and their patients about whether or not to fully embrace the new guidelines.
To provide some greater clarity about how many more individuals would be newly eligible; and the likely benefits and risks of statin therapy among newly eligible users, we applied relative risk estimates of CVD risk reduction and incident T2DM from primary prevention statin trials8-16 to actual adjudicated incident ASCVD and T2DM event rates in participants from the Multi Ethnic Study of Atherosclerosis (MESA) who would have been newly eligible for statin therapy according to the previous1,2 and newly published guidelines3 at the time of their baseline exam.
Methods
Study Population and Data Collection
The study design for the MESA study has been published elsewhere 17. A brief description of the MESA cohort, collection of data, event adjudication and how the ASCVD events were calibrated in this analysis is attached as an appendix (Appendix I).
Statistical Analysis
Only MESA participants’ aged 40-75years during the baseline examination were included in these analyses. Descriptive statistics of all MESA participants who were not on statins during the baseline exam and have complete data for assessing statin eligibility under the NCEP/ATP III and 2013 ACC/AHA cholesterol guidelines are presented as mean(sd) for continuous variables and percentages for categorical variables. 10 year ATP III risk was calculated for each participant. Under the NCEP/ATP III cholesterol guidelines(2001 and the 2004 update); all T2DM as well as individuals with a) 2+ risk factors with calculated 10 year CHD risk20 of >20% and LDLc > 100 mg/dl; b) 2+ risk factors with 10 year risk 10-20%18 and LDLc ≥ 130mg/dl; c) 2+ risk factors with 10 year risk <10%18 and LDLc ≥160mg/dl and d) LDLc ≥190mg/dl are eligible for statin therapy for primary prevention. The optional recommendation of 2+ risk factors with calculated CHD risk 10-20% and LDLc ≥ 100mg/dl was also included as a sensitivity analysis to estimate statin eligibility under the old guidelines2.
The new pooled ASCVD risk estimator19 was also used to calculate the estimated 10 year ASCVD risk for each MESA participant. The 10 year ASCVD risk for Hispanics and Chinese participants was estimated using the Pooled ASCVD risk equation for Caucasians. Under the 2013 AHA/ACC cholesterol guidelines; all T2DM (40-75 years), participants with LDLc ≥190mg/dl and all participants with calculated new pooled risk score≥ 7.5% are eligible for statin therapy for primary prevention. The optional recommendation of statin therapy for individuals with ASCVD risk 5-7.5% was also included for sensitivity analysis19. The difference between the number who qualified for statin therapy under the new guidelines and the number who qualified for statin therapy under the NCEP/ATP III(old guideline) was used as the additional number of participants who will qualify for statin therapy under the current guidelines(newly eligible)(N=1742). The rate at which ASCVD events accumulated in the newly eligible MESA sub cohort was analyzed with a Kaplan-Meier plot and a Poisson rate model. Robust standard errors were used for the Poisson regression.
Relative risk estimates based on the type and dose of statins administered in selected clinical trials were applied to the calibrated 10 year ASCVD and T2DM events which occurred in participants who qualified under a) NECP/ATP III, b) new ACC/AHA and c) newly eligible MESA cohorts to provide estimates of the reduction in ASCVD8-11 and increment in T2DM12-15 that would have occurred due to statin therapy in this MESA subcohort (Table 1).
Table 1.
Relative Risk Estimates based on the 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Disease (ASCVD) Risk in Adults by the intensity of statin therapy.
| Event | Statin Therapy | Source of Relative Risk Estimate | Relative Risk Estimate |
|---|---|---|---|
| ASCVD | Moderate Intensity | Cochrane | 0.74 (0.66, 0.85) |
| ASCVD | High Intensity | Cochrane combined with Mills et al. | 0.67 (0.59, 0.77) |
| Diabetes Mellitus | Moderate Intensity | Sattar et al*. | 1.08 (1.01, 1.15) |
| Diabetes Mellitus | High Intensity | JUPITER and CORONA Pooled | 1.24 (1.05, 1.46) |
The estimated re ative risk for moderate intensity [1·08 (1·01–1·15), /2=1·5%] was obtained by eliminating high intensity statins estimates from that provided in the paper by Sattar et al.
The accurate duration of therapy of all the MESA participants who were not on statins at the baseline exam but took statins during the follow up period could not be determined. We therefore undertook a sensitivity analysis in which we considered the observed events to have occurred while 1.) 100% of each subcohort took statins throughout the follow up period and 2) none of the participants in each of the subcohort took statins during the follow up period; to provide the extreme bounds of the estimates in our main analysis.
Classification of statin intensity and their corresponding relative risk estimates in published clinical trials were based on the 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults3. Estimates from the Cochrane Collaboration Meta-Analysis8 were used for the effects of moderate intensity therapy on ASCVD events. The relative risk estimate for high intensity statins was obtained by combining data from the Cochrane collaboration Meta analysis8 and Mills et al11. The effects of statin therapy on incident T2DM were obtained by eliminating high intensity statin studies from risk estimate provided in the meta-analysis by Sattar et al.12 for moderate intensity(OR: 1·08,(1·01–1·15), I2=1·5%), and by taking a weighted average of the JUPITER14 and CORONA15 studies for high intensity statin therapy. The expected change in the number of events was calculated by multiplying the number of calibrated events by (RR – 1). The 95% prediction intervals were calculated from the 95% confidence intervals in clinical trials.
Number needed to treat( NNT= # of participants that needs to be treated with statins over a 10 year period to prevent one ASCVD event) and Number needed to harm( NNH= # of participants that needs to be treated with statins over a 10 year period to cause one T2DM) were calculated. The statistical analysis was performed using STATA 12.0 and Excel 2010.
Results
5437 out of the total 6814 MESA participants were not taking statins during the baseline exam, had sufficient data to determine statin eligibility under both cholesterol guidelines (the 2013 ACC/AHA and the NCEP/ATP III guidelines) and were therefore included in this analysis. 1334(24.5%) participants were eligible under the 2001/2004 NCEP/ATP III guidelines (excluding the optional recommendation) while 1624(29.9%) were eligible when the optional criteria was considered for statin therapy. 3015(55.5%) were eligible under the 2013 ACC/AHA guidelines (excluding the optional recommendation) while 3610(66.4%) were eligible when the optional criteria was considered for statin therapy. Thus 1742(32.0%) were newly eligible without and 2012(37.0%) were newly eligible with the inclusion of both optional criteria for statin therapy during the MESA baseline examination. 61(4.6%) of participants who were eligible for statin therapy under the NCEP/ATP III cholesterol guidelines were no longer eligible for statin therapy under the 2013 ACC/AHA guidelines.
Table 2 and 3 shows the demographic characteristics of the total MESA cohort, participants who were eligible for statin therapy under NCEP/ATP III guidelines, participants who are eligible for statin under the new ACC/AHA guidelines and their corresponding net newly eligible participants.
Table 2.
Demographic Characteristics of the MESA cohort (Using Strict criteria and excluding optional recommendations)
| Variable | Total MESA Sample (N=5437) | Recommended for Statin Therapy Under the NCEP/ATPIII Guidelines (N=1334) | Recommended for Statin Therapy Under the New AHA/ACC 2013 Guidelines (N=3015) | Net MESA Participants Recommended Statin Therapy (N=1742) |
|---|---|---|---|---|
| Age (years) | 61.4 ±10.3 | 65.4 ±9.9 | 67.5 ±8.8 | 68.6 ±7.9 |
| Female (%) | 2845(52.3) | 470(35.2) | 1238(41.1) | 802(46.0) |
| Race/Ethnicity (%) | ||||
| Caucasian | 2055(37.8) | 409(30.7) | 1040(34.5) | 660(37.9) |
| Chinese | 672(12.4) | 140(10.5) | 340(11.3) | 201(11.5) |
| African American | 1482(27.3) | 412(30.9) | 966(32.0) | 566(32.5) |
| Hispanic | 1228(22.6) | 373(28.0) | 669(22.2) | 315(18.1) |
| BMI( Kg/m2) | 28.2 ±5.5 | 29.3 ±5.3 | 28.4 ±5.3 | 27.7 ±5.1 |
| Diabetes Mellitus (%) | 575(10.6) | 575(43.1) | 575(19.1) | 0(0.0) |
| Cholesterol(mg/dl) | ||||
| Total | 195.6 ±34.4 | 210.2 ±39.6 | 197.1 ±36.4 | 188.6 ±31.3 |
| LDL | 119.5 ±31.2 | 136.7 ±36.2 | 121.7 ±33.2 | 111.8 ±26.8 |
| HDL | 51.2 ±15.0 | 44.5 ±11.0 | 49.2 ± 14.4 | 52.7 ±15.5 |
| Triglycerides | 125.0 ±65.5 | 145.4 ±68.6 | 130.7 ±67.6 | 120.3 ±65.0 |
| Blood Pressure(mmHg) | ||||
| Systolic | 125.7 ±21.3 | 134.7 ±22.3 | 134.3 ±21.3 | 133.3 ±20.5 |
| Diastolic | 72.0 ±10.3 | 74.5 ±10.4 | 73.8 ±10.5 | 73.2 ±10.5 |
| Cigarette Smoking (%) | ||||
| Never | 2750(50.6) | 588(44.1) | 1385(45.9) | 822(47.2) |
| Former | 1966(36.2) | 518(38.8) | 1165(38.6) | 666(38.2) |
| Current | 721(13.3) | 228(17.1) | 465(15.4) | 254(14.6) |
| Antihypertensive Medication use (%) | 1765(32.5) | 665(49.9) | 1374(45.6) | 726(41.7) |
| Statin use (%) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) |
| NCEP/ATP III Risk Score (%) | 8.0 ±7.8 | 14.5 ±9.0 | 12.4 ±7.8 | 10.6 ±6.2 |
| Low Risk (<10%) | 3719(68.4) | 449(33.7) | 1311(43.5) | 914(52.5) |
| Intermediate Risk (10-20%) | 1227(22.6) | 498(37.3) | 1213(40.2) | 724(41.6) |
| High Risk (>20%) | 491(9.0) | 387(29.0) | 491(16.3) | 104(6.0) |
| ASCVD Risk Score (%) | 12.6 ±12.7 | 23.1 ±15.5 | 20.2 ±12.7 | 17.4 ±9.5 |
| Low Risk (<7.5%) | 2540(46.7) | 179(13.4) | 118(3.9) | 0(0.0) |
| High Risk (≥ 7.5%) | 2897(53.3) | 1155(86.6) | 2897(96.1) | 1742(100.0) |
| ASCVD Event (%) | 318(5.9) | 144(10.8) | 269(8.9) | 127(7.3) |
Footnote: BMI indicates body mass index; NCEP indicates National Cholesterol Education Program; ATP indicates Adult Treatment Program; ASCVD indicated Atherosclerotic cardiovascular disease.
Table 3.
Demographic Characteristics of the MESA cohort (Including the optional statin eligibility criteria for ATP III and New ACC/AHA 2013 guidelines)
| Variable | Total MESA Sample (N=5437) | Recommended for Statin Therapy Under the NCEP/ATPIII Guidelines (N=1624) | Recommended for Statin Therapy Under the New AHA/ACC 2013 Guidelines (N=3610) | Net MESA Participants Recommended Statin Therapy (N=2012) |
|---|---|---|---|---|
| Age (years) | 61.4 ±10.3 | 65.5 ±9.6 | 66.0 ±9.1 | 66.2 ±8.8 |
| Female (%) | 2845(52.3) | 507(31.2) | 1523(42.2) | 1034(51.4) |
| Race/Ethnicity (%) | ||||
| Caucasian | 2055(37.8) | 507(31.2) | 1265(35.0) | 771(38.3) |
| Chinese | 672(12.4) | 180(11.1) | 406(11.3) | 227(11.3) |
| African American | 1482(27.3) | 498(30.7) | 1148(31.8) | 655(32.6) |
| Hispanic | 1228(22.6) | 439(27.0) | 791(21.9) | 359(17.8) |
| BMI( Kg/m2) | 28.2 ±5.5 | 29.1 ±5.2 | 28.4 ±5.2 | 27.8 ±5.2 |
| Diabetes Mellitus (%) | 575(10.6) | 575(35.4) | 575(15.9) | 0(0.0) |
| Cholesterol(mg/dl) | ||||
| Total | 195.6 ±34.4 | 205.9 ±37.6 | 197.1 ±35.8 | 190.7 ±33.0 |
| LDL | 119.5 ±31.2 | 132.9 ±34.0 | 121.8 ±32.5 | 113.4 ±28.8 |
| HDL | 51.2 ±15.0 | 44.3 ±10.8 | 49.4 ±14.4 | 53.4 ±15.5 |
| Triglycerides | 125.0 ±65.5 | 143.9 ±68.1 | 130.2 ±67.5 | 119.2 ±64.9 |
| Blood Pressure(mmHg) | ||||
| Systolic | 125.7 ±21.3 | 134.2 ±22.0 | 132.2 ±21.1 | 130.3 ±20.2 |
| Diastolic | 72.0 ±10.3 | 74.8 ±10.3 | 73.6 ±10.3 | 72.6 ±10.3 |
| Cigarette Smoking (%) | ||||
| Never | 2750(50.6) | 685(42.2) | 1661(46.0) | 990(49.2) |
| Former | 1966(36.2) | 632(38.9) | 1382(38.3) | 759(37.7) |
| Current | 721(13.3) | 307(18.9) | 567(15.7) | 263(13.1) |
| Antihypertensive Medication use (%) | 1765(32.5) | 805(49.6) | 1514(41.9) | 713(35.4) |
| Statin use (%) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) |
| NCEP/ATP III Risk Score (%) | 8.0 ±7.8 | 14.6 ±8.2 | 11.2 ±7.7 | 8.5 ± 6.0 |
| Low Risk (<10%) | 3719(68.4) | 449(27.7) | 1892(52.4) | 1469(73.0) |
| Intermediate Risk (10-20%) | 1227(22.6) | 788(48.5) | 1227(34.0) | 439(21.8) |
| High Risk (>20%) | 491(9.0) | 387(23.8) | 491(13.6) | 104(5.2) |
| ASCVD Risk Score (%) | 12.6 ±12.7 | 22.1 ±14.5 | 17.9 ±12.8 | |
| Low Risk (<7.5%) | 2540(46.7) | 182(11.2) | 713(19.8) | 557(27.7) |
| High Risk (≥ 7.5%) | 2897(53.3) | 1442(88.8) | 2897(80.3) | 1455(72.3) |
| ASCVD Event (%) | 318(5.9) | 166(10.2) | 291(8.1) | 126(6.3) |
Footnote: BMI indicates body mass index; NCEP indicates National Cholesterol Education Program; ATP indicates Adult Treatment Program; ASCVD indicated Atherosclerotic cardiovascular disease.
Risk – Benefit for Participants Eligible under the NCEP/ATP III Guidelines (Table 4)
Table 4.
Predicted Change in Number of Event Counts with the ASCVD Guidelines by Intensity of statin therapy in the MESA cohort accounting for actual statin use during the follow up. Assuming similar adherence rate as reported in clinical trials over 10 years.
| ASCVD(95% PI)* | NNT | Diabetes Mellitus(95% PI)* | NNH | |
|---|---|---|---|---|
| NCEP/ATP III | N=1334, #Event=144 | N=736, # Event=101 | ||
| Moderate Intensity | −42.2(−57.3, −23.1) | 31.7 | 7.8(1.0,14.2) | 94.2 |
| High Intensity | −55.4(−71.7,−36.8) | 24.1 | 22.0(4.9,38.8) | 33.5 |
| −13.2 | 14.2 | |||
| New ACC/AHA | N=3015, # Event =269 | N=2353, # Event =285 | ||
| Moderate Intensity | −77.2(−104.2,−42.7) | 39.1 | 22.2(2.8, 40.6) | 106.2 |
| High Intensity | −100.7(−129.4,−67.5) | 29.9 | 63.0(14.0, 112.5) | 37.4 |
| −23.6 | 40.6 | |||
| Newly Eligible | N=1742, # Event=127 | N= 1678, #Event =194 | ||
| Moderate Intensity | −35.8(−48.1, −20.0) | 48.7 | 15.2(1.9, 27.9) | 110.7 |
| High Intensity | −46.5(−59.4, −31.4) | 37.45 | 43.4(9.6, 78.4) | 38.6 |
| Difference | −10.7 | 28.3 |
Note: 575 of the subcohort eligible under NCEP/ATP III and new ACC/AHA had diabetes mellitus; 23, 87 and 64 participants of the NCEP/ATP III, new ACC/AHA and newly eligible subcohorts had missing data on incident diabetes mellitus.
ASCVD: Indicates atherosclerotic cardiovascular disease, PI: Predicted interval, NNT: Number needed to treat, NNH: Number needed to harm
To obtain percent reduction in ASCVD events or T2DM caused = [(absolute number divided by #Event) × 100%]
Out of 1334 participants, 144(10.7%) had an adjudicated ASCVD and 101(7.7%) had T2DM during the follow up. Assuming 10 years of moderate intensity statin use and accounting for observed use of statins in this subcohort, 42.2(29.3%) (Predicted interval: 23.1 to 57.3) ASCVD events would have been prevented with a NNT of 31.7. 10 years of moderate intensity statin use would have caused 7.8(7.7%)(predicted interval: 1.0 to 14.2) T2DM with a NNH of 94.2.The corresponding NNT and NNH for high intensity statins were 24.1 and 33.5 respectively as shown in Table 2. Supplemental Table 1 and 2 shows the extreme bounds of the NNT and the NNH assuming all or none of these participants used statins during the 10 year follow up period.
Risk – Benefit for Participants Eligible under the new ACC/AHA Guidelines (Table 4)
Out of 3012 participants, 269(8.9 %) had an adjudicated ASCVD and 285(9.7%) had T2DM during the follow up. Assuming 10 years of moderate intensity statin use and accounting for observed use of statins in this subcohort, 77.2(28.7%)(predicted interval: 42.7 to 104.2) ASCVD events would have been prevented with a NNT of 39.1. 10 years of moderate intensity statin use would have caused 22.2(7.8%)(predicted interval: 2.8 to 40.6) T2DM with a NNH of 106.2. The corresponding NNT and NNH for high intensity statin use were 29.9 and 37.4 respectively as shown in Table 4. Supplemental Table 1 and 2 shows the extreme bounds of the NNT and the NNH assuming all and none of these participants used statins during the 10 year follow up period.
Risk- Benefit for participants in the Newly Recommended MESA Subcohort (Table 4)
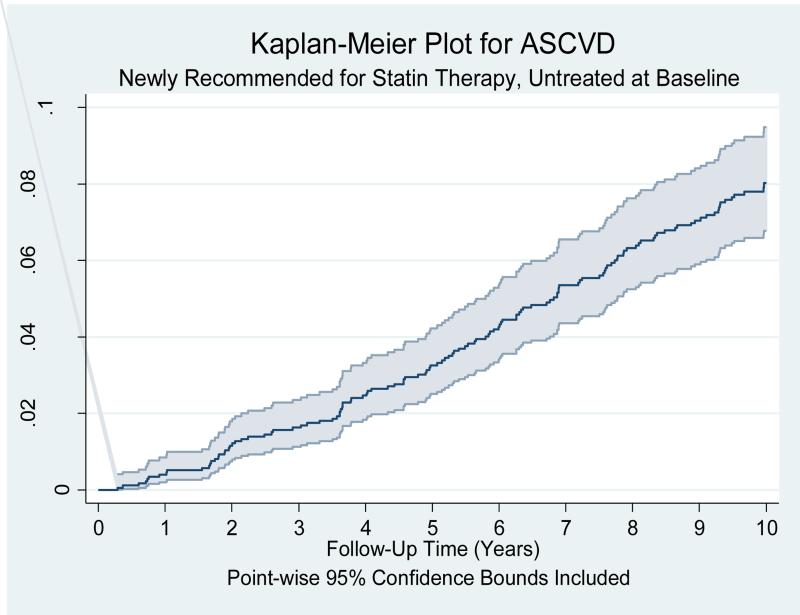
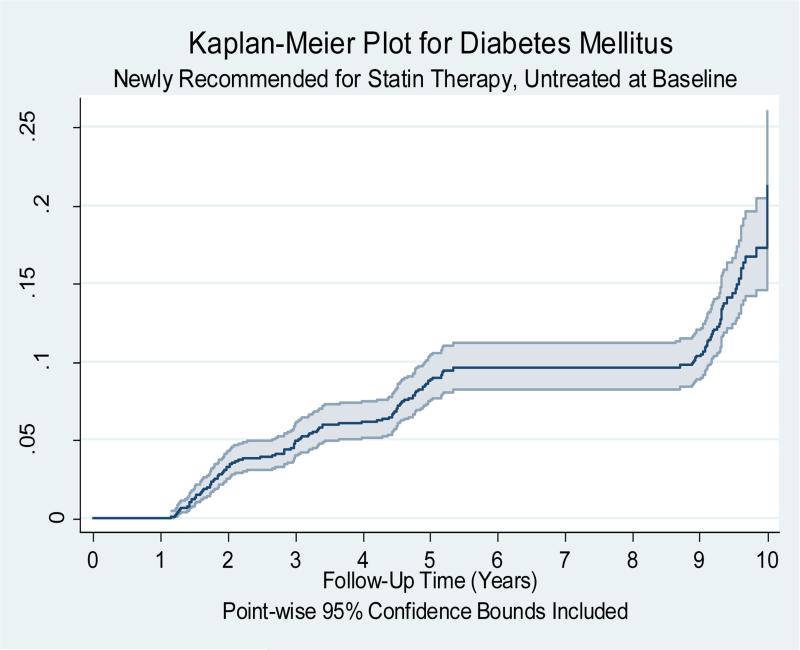
Out of 1742 participants, 127(7.3%) of the newly recommended MESA participants had an adjudicated ASCVD event and 194(11.1%) had T2DM during 10 years of follow up. Figure 1 is the Kaplan Meier cumulative probability plot of ASCVD events over 10 years of follow-up time for the newly recommended MESA participants [estimated annual ASCVD event rate of 0.82(0.69-0.97)/100 person years]. 194(11.6%) of the newly recommended MESA subcohort developed diabetes mellitus after 10 years of follow up. Figure 2 is the cumulative probability plot of diabetes mellitus events over 10 years of follow-up time for the newly recommended MESA participants. Assuming 10 years of moderate intensity statin use and accounting for observed statin use during the follow up period, 35.8(28.2%)(predicted interval: 20.0 to 48.1) ASCVD events would have been prevented with a NNT of 48.7. 10 years of moderate intensity statin use would have caused 15.2(7.8%)(predicted interval: 1.9 to 27.9) T2DM with a NNH of 110.7. 10 years of high intensity statin use would have prevented 46.5(36.6%)(predicted interval: 31.4 to 59.4) ASCVD events with a NNT of 37.5 and caused 43.4(22.4%)(predicted interval: 9.6 to 78.4) T2DM with a NNH of 38.6. Supplemental Table 1 and 2 shows the extreme bounds of the NNT and the NNH assuming all and none of these participants used statins during the10 year follow up period. Table 5 and 6 shows the change in ASCVD prevented and T2DM caused with change in adherence rate for moderate intensity statin therapy in the newly recommended MESA participants.
Figure 1.
Kaplan Meier cumulative probability plot of Atherosclerotic cardiovascular disease (ASCVD) event over 10 years of follow-up time for the 1,742 MESA subjects who are recommended statin therapy under the new AHA/ACC guidelines but not the NCEP/ATP III guidelines (Newly recommended) and were not taking statin during the baseline MESA
Figure 2.
Kaplan Meier cumulative probability plot of incident type 2 diabetes mellitus event over 10 years of follow-up time for the 1,679 MESA subjects who are recommended statin therapy under the new AHA/ACC guidelines but not the NCEP/ATP III guidelines (Newly recommended) and were not taking statin during the baseline MESA
Table 5.
Sensitivity analysis showing the predicted Change in Number of atherosclerotic cardiovascular disease (ASCVD) Events and corresponding number needed to treat (NNT) for different moderate intensity statin adherence rates in the MESA newly recommended subcohort.
| Event | Adherence Rate (Assumed) | Estimated Change in # of Event Counts | Number Needed to Treat (NNT) | 95% Prediction Interval | |
|---|---|---|---|---|---|
| ASCVD | 100% | −35.8 | 48.7 | −48.1 | −20.0 |
| ASCVD | 75% | −26.9 | 64.9 | −36.1 | −15.0 |
| ASCVD | 50% | −17.9 | 97.3 | −24.0 | −10.0 |
| ASCVD | 25% | −9.0 | 194.6 | −12.0 | −5.0 |
Table 6.
Sensitivity analysis showing the predicted Change in Number of incident type2 diabetes mellitus events and corresponding number needed to treat (NNT) for different moderate intensity statin adherence rates in the MESA newly recommended subcohort.
| Event | Adherence Rate (Assumed) | Estimated Change in # of Event Counts | Number Needed to Harm (NNH) | 95% Prediction Interval | |
|---|---|---|---|---|---|
| Diabetes Mellitus | 100% | 15.2 | 110.7 | 1.9 | 27.9 |
| Diabetes Mellitus | 75% | 11.4 | 147.6 | 1.5 | 20.9 |
| Diabetes Mellitus | 50% | 7.6 | 221.4 | 1.0 | 13.9 |
| Diabetes Mellitus | 25% | 3.8 | 442.7 | 0.5 | 7.0 |
Based on the estimates provided by Floyd et al16 on the incidence of statin-related rhabdomyolysis [5.2 events per 100,000 person years], the 16262.6 person -years exposure time in the current MESA subcohort (N=1742) yielded less than one case over the 10 year period of follow up.
Discussion
Replacing the NCEP/ATP III cholesterol guidelines with the new ACC/AHA cholesterol guidelines in this cohort would result in more than doubling of statin eligibility for primary prevention; and moderate intensity statin administration in the newly eligible subcohort was associated with reduce ASCVD events [NNT(bounds): 48.7(39.0-52.8)], increase T2DM [NNH(bounds): 110.7(108.1-116.8)] and negligible cases of rhabdomyolysis assuming similar adherence rate as reported in the clinical trials over 10 years in this multi ethnic cohort.
While the NCEP/ATP III adopts a modified version of the Framingham risk calculator18, the new ACC/AHA cholesterol guidelines recommend the use of a newly developed pooled ASCVD risk calculator19 for the estimation of 10 year risk. It is important to note that the 2 risk calculators predict different outcomes18,19. The new ACC/AHA ASCVD risk calculator predicts a composite endpoint consisting of non-fatal myocardial infarction, CHD death, non-fatal and fatal stroke while the outcome for the NCEP/ATP III is limited to hard CHD events1-3. Unlike the NCEP/ATP III cholesterol guidelines, the 2013 ACC/AHA cholesterol guidelines only apply to 10 year risk for individual's age 40-75years. However, compared with the observed event rates in several cohorts, the predicted rate using the new pooled ASCVD risk calculator has been shown to overestimate 10 year risk 5-7. In addition, the calculated cut off for recommending shared decision making for statin therapy by the new ACC/AHA cholesterol guideline is lower(≥7.5%) compared with the NCEP/ATP III cholesterol guidelines which recommended statin for a cut off without any caveat of ≥20% 10 year risk1-3. These two factors among others guarantee a significant increase in statin eligibility. However, the degree of increase in statin eligibility that would occur as a result of the change in cholesterol guidelines remains unclear. The authors of the new ACC/AHA ASCVD risk assessment tool compared the 10 year risk for hard CHD(using the ATP III risk calculator) and the 10 year risk for hard ASCVD events(using the new pooled ASCVD calculator) using the 2007-2010 NHANES data and reported a 31.9% vs. 32.9% eligibility for statin therapy respectively21. However, neither the 2001/2004 NCEP/ATP III cholesterol guidelines nor the new ACC/AHA cholesterol guidelines recommends statins solely based on these risk calculators. Pencina et al20 recently used a selected participants from NHANES 2005-2010 data and extrapolation to show that the replacement of the ATP III by the new ACC/AHA cholesterol guidelines will lead to an estimated 12.8 million more statin users in the USA. It is important to note that a significant proportion of the participants in that study20 had prevalent cardiovascular diseases and were also taking statins. In addition, the inherent limitations of the NHANES data coupled with the limitations of the analysis noted by the authors20 suggest that more data are needed in order to make a precise estimation especially for primary prevention. The present study used an asymptomatic community dwelling participants who were not taking statins and had no clinical cardiovascular disease at baseline to show that replacing the ATP III with the new ACC/AHA cholesterol guidelines resulted in more than doubling the statin eligibility.
Statins are considered very effective drugs with relative safe adverse effect profile9. Statins are also readily available and currently very affordable. Thus statin possesses characteristics of drugs or substances that could be deployed or implemented on a wide scale. However like all medications, statin use has been associated with side effects such as diabetes mellitus, cognitive impairment among others21. In the present study, application of the new ACC/AHA cholesterol guidelines to the newly eligible MESA subcohort would be expected to increase the number of incident T2DM cases over 10 years. Although the benefits of statins may outweigh the risk, the possible increment in disease prevalence/incidence that may be associated with statin therapy such as T2DM cannot be discounted. More studies on the potential impact of the adverse effects of statin and how they can be reduced are needed.
The present study predicts an overall reduction in ASCVD events over 10 years by the replacement of the NCEP/ATP III guidelines with the new ACC/AHA cholesterol guidelines in the MESA cohort. However, this analysis assumed a relatively high and an unlikely statin adherence over the 10 year period by physicians and patients. Subsequent analysis in Table 3 revealed that the number of ASCVD events that would have been prevented is greatly dependent on the overall statin adherence rate. Current literature suggests an average 2 year statin adherence rate of 48-65%22-24. Thus the actual percent reduction in ASCVD events and percent increase in diabetes mellitus may be much smaller given the relatively low percent of statin adherence in the general population and the unlikely scenario that all physicians will follow the new ACC/AHA cholesterol guidelines.
Although serious adverse effects and life threatening conditions such rhabdomyolysis are rare with statin therapy, conditions such as muscle aches (myalgia) and elevated liver enzymes appears to be the main reason for discontinuation22,24 of statins. This is despite the establishment of the liver enzyme cut offs for discontinuation and even recommendation against routine monitoring of liver enzymes of patients on statins3. For the implementation of the new ACC/AHA cholesterol guidelines to achieve its aim of reducing ASCVD events , improvement of statin adherence is key. Research into how we can prevent myalgia and wide scale education of primary care providers on liver enzymes and statin therapy will certainly improve statin adherence and reduce ASCVD events.
The strengths of this study include the large sample size, the use of observed 10 year adjudicated events and the multi-ethnicity of our cohort. In fact, of all the current ongoing population based studies, the race/ethnic makeup of the MESA cohort most resembles that of the USA population. We used best available data on relative risk estimates due to statin therapy in clinical trials. Despite this, the compositions of these trials were quite different from the MESA subcohort and the USA population. Our estimates were also based mainly on relatively high adherence rate for shorter duration of statin therapy in these clinical trials. We therefore provided estimates for other adherence rates since the actual 10 year statin adherence rate in the USA population is unknown. MESA is an observational study and thus residual confounding may have influenced our results. The authors of this paper also acknowledge the fact that event ascertainment is intensity dependent25 and therefore the exclusion of sources such as MEDICARE claims data in the MESA event adjudication process likely resulted in missed events. However in MESA the number of potentially missed events (unadjudicated), especially for outcomes under consideration in this paper has been determined to be very small and unlikely to significantly change the NNT and the NNH reported. MESA does not include other ethnic groups such as American Indians and other Asian groups except Chinese. Data on rhabdomyolysis and reason for statin noncompliance was not collected in MESA. The risk of incident diabetes may be significantly influenced by patient's baseline characteristics, which were not accounted for in the current study. Lastly, the proportion of each ethnic group in MESA does not accurately reflect that of the US population.
Conclusion
Substituting the NCEP/ATP III guidelines with the new ACC/AHA cholesterol guidelines in a multi-ethnic population based asymptomatic cohort resulted in a more than doubling of statin eligibility. The risk of T2DM vs. the benefit of preventing an ASCVD appears to be more favorable under the NCEP/ATP III compared with the new ACC/AHA cholesterol guidelines in this cohort. For the newly eligible MESA participants, moderate intensity statin therapy had a favorable risk of T2DM vs. prevention of ASCVD event ratio. In the newly eligible MESA subcohort, the NNT (ASCVD) and the NNH (T2DM) for high intensity statin therapy is approximately equal.
Supplementary Material
Highlights.
Replacing NCEP/ATPIII with new ACC/AHA cholesterol guidelines more than double statin eligibility for primary prevention.
Risk-benefit ratio profile for moderate is better than high intensity statin therapy for primary prevention of ASCVD event.
Risk-benefit ratio depends on statin compliance emphasizing the need for Physician and patient adherence to current guidelines.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding Sources
This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-RR-025005 from NCRR. Diversity Supplement to R01HL098445 (PI: J. Jeffrey Carr).
Footnotes
Conflict of Interest Disclosure: None of the authors have any conflict.
The authors are solely responsible for all the analyses, the drafting and editing of the paper and its final contents.
Reference
- 1.National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone JJ. National Heart, Lung and Blood Institute; American College of Cardiology Foundation; American Heart Association. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 3.Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW. ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 20132013:S0735–1097(13)06028-2. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Lenzer J. Majority of panelists on controversial new cholesterol guideline have current or recent ties to drug manufacturers. BMJ. 347(2013):f6989. doi: 10.1136/bmj.f6989. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762– 1765. doi: 10.1016/S0140-6736(13)62388-0. [DOI] [PubMed] [Google Scholar]
- 6.Muntner P, Safford MM, Cushman M, Howard G. Comment on the Reports of Over-estimation of ASCVD Risk Using the 2013 ACC/AHA Risk Equation. Circulation 2014. 2014;129:266–267. doi: 10.1161/CIRCULATIONAHA.113.007648. [DOI] [PubMed] [Google Scholar]
- 7.Ioannidis JPA. More than a billion people taking statins? Potential implications of the new cardiovascular guidelines. JAMA. 2014;311:463–464. doi: 10.1001/jama.2013.284657. [DOI] [PubMed] [Google Scholar]
- 8.Taylor F, Huffman MD, Macedo AF, Moore THM, Burke M, Davey Smith G, Ward K, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews. 2013;(1) doi: 10.1002/14651858.CD004816.pub5. Art. No.: CD004816. DOI:10.1002/14651858.CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor Fiona C, PhD, Huffman Mark, MD, MPH, Ebrahim Shah DM. Statin Therapy for Primary Prevention of Cardiovascular Disease. JAMA Clinical Evidence Synposis. JAMA. 310:2451–2452. doi: 10.1001/jama.2013.281348. [DOI] [PubMed] [Google Scholar]
- 10.Server Peter S, Dahlof Bijorn, Poulter Neil R, Wedel Hans, Beevers Gareth, Caulfield Mark, Collins Rory, Kjeldsen Sverre E, Kristinsson Ami, McInnes Gordon T, Mehlsen Jesper, Nieminen Markku, O'Brien Eoin, Ostergren Jan. Prevention of Coronary and Stroke Events with Atorvastatin in Hypertensive Patients who have Average or Lower-than-Average Cholesterol Concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial –Lipid Lowering Arm (ASCOT-LLA): a Multicentre Randomized Controlled Trial. The Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 11.Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol. 2008;52:1769–81. doi: 10.1016/j.jacc.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 12.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 13.Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–64. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Danielson E, Fonsca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willergon JT, Glynn RJ. JUPITER Study group. Rosuvastatin to prevent vascular events in men and women with elevated C - reactive protein. N Engl J Med. 2008;358:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 15.Raipathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes. A Meta analysis. Diabetes Care. 2009;32:1924–9. doi: 10.2337/dc09-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Floyd James S., Heckbert Susan R., Weiss Noel S., Carrell David S., Psaty Bruce M. Use of Administrative Data to Estimate the Incidence of Statin-Related Rhabdomyolysis. JAMA. 2012;307:1580–1582. doi: 10.1001/jama.2012.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. CHD risk prediction group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 19.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson J, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013:S0735–1097(13)06031-2. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pencina MJ, Navar-Boggan AM, D'Agostino RB, Williams K, Neely B, Sniderman AD, Peterson ED. Application of new cholesterol guidelines to a population- based sample. N. Engl J Med. 2014;370:1422–31. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 21.Bang CN, Okin PM. Statin treatment, new onset diabetes and other adverse effects, a systematic review. Curr cardiol rep. 2014;16:461–4. doi: 10.1007/s11886-013-0461-4. [DOI] [PubMed] [Google Scholar]
- 22.Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal statin adherence and discontinuation in primary and secondary prevention. J Gen Intern Med. 2004;19:638–45. doi: 10.1111/j.1525-1497.2004.30516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–7. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 24.Wei MY, Ito MK, Cohen JD, Brinton EA, Jacobson TA. Predictors of statin adherence, switching and discontinuation in the USAGE survey: understanding the use of statins in America and gaps in patient education. J Clin lipidol. 2013;7:472–83. doi: 10.1016/j.jacl.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Muntner P, Colantonio LD, Cushman M, Goff DC, Jr, Howard G, Howard VJ, Kissela B, Levitan EB, Lloyd-Jones DM, Safford MM. Validation of the Atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA. 2014;311:1406–15. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.