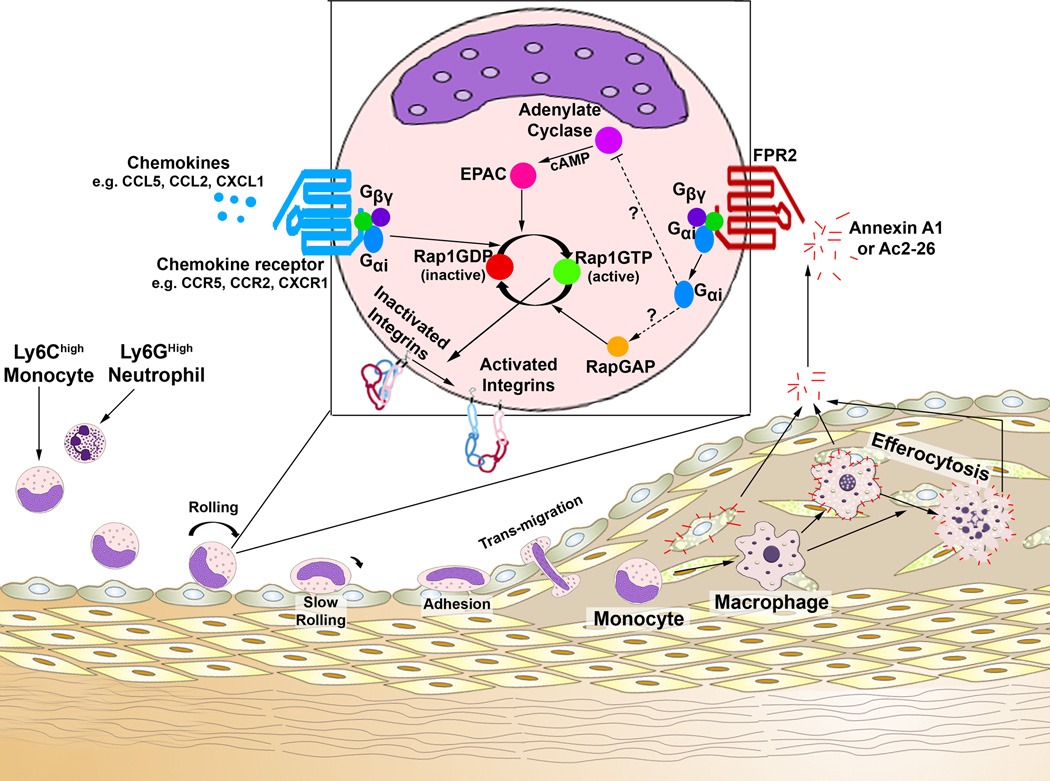
Within the past 30 years, the recruitment and accumulation of pro-inflammatory monocytes and macrophages within atherosclerotic lesions has been a major area of research, however the precise processes through which myeloid cells migrate are still being worked out. The formation of atherosclerotic lesions occurs in several well-characterized stages and the recruitment of pro-inflammatory monocytes and neutrophils plays key roles in the initiation and progression of the disease.1 The extravasation of myeloid cells occurs in several steps (Figure 1), ranging from capture, to rolling, activation, binding and spreading, intravascular creeping, to transendothelial migration; and these steps are regulated in part by leukocyte (e.g. β1, β2 integrins, and L-Selectin) and endothelial cell adhesion molecules (e.g. VCAM-1, ICAM-1, and E-Selectin), arrest chemokines (e.g. CCL5 in atherosclerosis), and chemoattractant chemokines (e.g. CCL2, CX3CL1, CXCL1, CXCL2, etc.).2 Based primarily on knockout studies in atherosclerotic mice, Ly6Chigh classical monocytes are known to be recruited in a CCL5-CCR5, CCL2-CCR2, and CX3CL1-CX3CR1-dependent fashion3–6 and neutrophils may utilize CCL5-CCR5, CCL3-CCR1/CCR5,7, 8 and CXCL2-CXCR2.8 While pro-inflammatory cytokines, chemokines, and an activated endothelium positively regulate myeloid cell recruitment in atherogenesis, several novel players such as pro-resolving eicosanoids and annexins may support the resolution of inflammation. In this issue of Circulation Research, Drechsler and colleagues9 examined the role of the promiscuous G protein coupled chemokine receptor Formyl peptide receptor 2 (FPR2) and one of its known anti-inflammatory ligands, Annexin A1, on myeloid cell recruitment in atherosclerosis. While the role of Annexin A1 as an athero-protective factor has been previously suggested, Dreschler and colleagues9 described a mechanism by which Annexin A1 may regulate integrin-dependent neutrophil and monocyte recruitment during atherogenesis. This study provides compelling evidence that Annexin A1/FPR2 signaling plays an important role in negative regulation of the Rap-1-dependent integrin activation and thus monocyte and neutrophil recruitment in atherosclerosis. These novel findings have significant implications for understanding the mechanisms of atherogenesis and importantly are also supported by evidence from symptomatic vs asymptomatic patient populations, which demonstrate a negative correlation between Annexin A1 expression and the severity of atherosclerotic lesions.10, 11
Figure 1. Potential mechanisms of action for how Annexin A1/FPR2 might antagonize integrin activation and avidity.
The recruitment of myeloid cells to atherosclerotic plaques represents a critical process in the initiation and progression of atherosclerosis. In this issue of Circulation Research, Dreshler and colleagues9 investigated the actions of Annexin A1, the Annexin A1 N-terminal peptide AC2-26, and its receptor, N-Formyl peptide receptor 2 (FPR2), on atherosclerosis in Apoe−/− mice. Ly6Chigh monocytes and Ly6Ghigh neutrophils (left) use several chemokine receptors and leukocyte adhesion molecules, in the steps of the adhesion cascade (rolling to transmigration), to migrate to atherosclerotic plaques. Importantly, in order for monocytes or neutrophils to roll, adhere, and transmigrate, membrane integrins need to activate and properly cluster, through outside-in activation, inside-out activation, or changes in avidity. This study demonstrates that Annexin A1, which is primarily present within lesional Mac2+ macrophages and foam cells, may be released from the plaque and act on circulating FPR2+ monocytes and neutrophils to antagonize integrin activation, clustering, and therefore migration. How does Annexin A1/FPR2 signaling work? While the full pathway was not examined, the pathway at least involves activation of the small GTPase Rap1 and integrin activation. So how might Annexin A1/FPR2 signaling impact Rap1-activation? FPR2 is a known G-protein coupled receptor and has been reported to associate with Gαi2, Gβ, and Gγ. Thus while the exact signaling mechanisms involved are unclear, several pathways could be involved. Annexin A1-FPR2-Gαi2 signaling might serve to antagonize Adenylate Cyclase, thereby lowering cyclic AMP levels, and antagonizing EPAC proteins, which are known guanine exchange factor for Rap1. These actions would result in a decrease in GTP-Rap1 dependent integrin activation. Alternatively, Annexin A1-FPR2-Gαi2 activation might promote the recruitment and activation of a RapGAP, which would promote hydrolysis of GTP and thereby deactivate Rap1; achieving the same end. All together, while additional work on the mechanisms of action is necessary, the results presented by Drescher and colleagues9 indicate that Annexin A1-might serve as an endogenous negative regulator of myeloid cell recruitment in early atherogenesis.
Annexin A1 is a member of the Annexin superfamily of calcium- and phospholipid binding proteins and it is primarily expressed within the subcellular granules of neutrophils, eosinophils, and monocytes.12 A protective role for Annexin A1 and peptide derivatives (e.g. the N terminal peptide Ac2-26) has been implicated in several biological processes, ranging from acute and chronic inflammation, to ischemia/reperfusion injury, growth and apoptosis, and leukocyte migration.12,13 Importantly, glucocorticoids may regulate the synthesis and function of Annexin A1 on the different cell types and thus regulate inflammatory processes.13 Given the actions of Annexin A1 on other systems, one would think that Annexin A1 might similarly affect atherogenesis.
Formyl peptide receptor 2 (FPR2) is a G protein coupled receptor that is expressed mainly by mononuclear phagocytes and serves to induce responses to various ligands including pro-inflammatory cathelicidin and serum amyloid A and anti-inflammatory lipoxin A4, and Annexin A1.13 FPR2 likely has different ligand-induced conformational states that may promote very different signaling and cellular responses.14,15 Indeed, recent mouse studies involving Fpr2−/−Ldlr−/− bone marrow chimeras demonstrated a pro-inflammatory role for FPR2, via elevated macrophage accumulation, activation, and the formation of stable atherosclerotic plaques.16 Additionally, FPR2 has been reported to support foam cell formation and increased CCL2 production by macrophages.17,18 However, given the promiscuity of FPR2, it is quite possible that FPR2 might also participate in the suppression of chronic inflammation in atherosclerosis via concomitant interactions with annexins, and resolvins.
In the present study9, Drescher and colleagues generated Fpr2−/−Apoe−/− and Anxa1−/−Apoe−/− mice to investigate the effects of Annexin A1 and potentially other FPR2 ligands on early atherosclerosis in four week high fat diet-fed mice. Interestingly, both Fpr2−/− Apoe−/− and Anxa1−/−Apoe−/− mice displayed a slight increase in early atherosclerotic lesion size, which corresponded with an overall increase in lesional macrophages and neutrophils without any change in total endothelial ICAM-1 or VCAM-1 expression, in comparison with Apoe−/− controls. In the context of leukocyte migration, Annexin A1 has been shown to induce L-Selectin shedding on neutrophils and the detachment of adhering leukocytes from the endothelium, likely by reducing α4β1 integrin clustering and activation.12 As administration of Annexin A1 inhibits neutrophil rolling and capture and the N terminal peptide Ac2-26 has been shown to antagonize neutrophil adhesion and chemotaxis,19 Drescher and colleagues sought to study monocyte and neutrophil extravasation to atherosclerotic carotid arteries by intravital microscopy. In agreement with migratory data from other studies and their own lesional data, Fpr2−/−Apoe−/− and Anxa1−/−Apoe−/− monocytes and neutrophils rolled more frequently and accumulated to a greater extent. Interestingly, as Ac2-26 and Annexin A1 regulated neutrophil and monocyte accumulation within the aortas without changes in tethering, thus, Ac2-26 and Annexin A1 may not impact L-selectin shedding in these conditions. Additionally, these results were extended with several well-controlled in vitro VCAM1/ICAM1 binding, LFA1 clustering, iCa2+ flux, and Rap1 activation assays; which demonstrate together that Ac2-26 at least partially antagonizes inside-out chemokine-induced integrin activation and clustering in a Rap1-GTPase dependent fashion. Importantly these effects are likely independent of Ca2+ dependent signaling cascades as Ac2-26 did not induce further intracellular Ca2+ flux.
These results are novel and important for a few reasons. The authors demonstrate that part of the mechanism of action for the antagonistic effects of Annexin A1 on myeloid cell recruitment involves antagonism of the GTPase Rap1, which is required for inside-out integrin activation and clustering via activation of downstream RIAM, PKD1, and Talin.20 Rap1 plays an essential role in integrin-dependent adhesion for the β2 integrin LFA-1 as well as the β1 integrin VLA-4.21 Chemokine receptor signaling is an important mechanism that helps to promote integrin clustering and activation, thereby controlling leukocyte rolling and transendothelial migration20. In this report, signaling via FPR2 inhibits both VLA-4 and LFA-1 integrin activation. Thus, the FPR2/Annexin signaling may be a “universal regulator” of Rap-1-dependent integrin activation for neutrophils and monocytes.
The FPRs are primarily coupled to GIα2 and GIα3 G proteins and signal through phopholipase C and intracellular Ca2+-dependent pathways (Figure 1). While experiments presented by Drescher and colleagues9 did not fully work out the signaling pathway(s) between Annexin A1, FPR2, and Rap1-GTP, several potential intermediates might be involved and could be investigated in future studies. As FPR2 is known to associate with Gαi proteins, Annexin A1-ligation might promote Gαi-dependent activation of RapGAP, which would serve to promote the hydrolysis of Rap1-GTP to GDP, inactivating Rap1. Alternatively, Annexin A1-FPR2-Gαi signaling might antagonize Adenylate cyclase, resulting in diminished cAMP production thereby antagonizing Epac, or perhaps GDF15 activity. Both of which are known guanine exchange factors for Rap122,23 (Figure 1). Interestingly, in later intravital microscopy experiments Annexin A1 is shown to antagonize chemokine-Rap-1-induced integrin activation and monocyte adhesion in vivo, for several pro-atherogenic chemokines, including CCL2, CCL5 and CXCL2. Together, these results demonstrate that super-physiologic doses of Ac2-26 might be useful in antagonizing myeloid cell recruitment during atherogenesis. Or, alternatively, that harnessing intraplaque Annexin A1 might be helpful as an endogenous negative regulator of myeloid cell recruitment.
While the authors focused their attention on the effects of Annexin A1 and Ac2-26 on leukocyte recruitment, a strong line of evidence also implicates Annexin-A1 in the regulation of efferocytosis at the sites of inflammation24 and the promotion of a favorable M2a macrophage phenotype.25 It is possible that in addition to the regulation of leukocyte recruitment, Annexin A1 might also accelerate efferocytosis within atherosclerotic lesions, thus helping to resolve arterial inflammation. Studying advanced atherosclerosis in Fpr2−/−Apoe−/− and Anxa1−/−Apoe−/− mice might help to address this question and dissect potential roles for FPR2 and Annexin A1 in plaque stability and regression. While the biology of Annexin A1 in the regulation of innate and adaptive immunity is complex and mainly depends on the pathology12, the notion that Annexin A1 can limit inflammation might also involve the adaptive immune system. Low levels of Annexin A1 are constitutively expressed by T cells, and are elevated upon T cell activation. Some evidence indicates that Annexin A1 can enhance Th1 differentiation and activation. As Th1 cells are pro-atherogeneic, it would be important to test effects of Annexin A1 and Ac2-26 on the generation of Th1 cells under atherosclerosis-prone conditions, to carefully rule out the potential effects of Ac2-26 on this population.
Much of the interest in Annexin A1 as a potential therapeutic has stemmed from its use as an exogenous anti-inflammatory agent in in vivo models of inflammation. So could Annexin A1 peptides be therapeutically useful in antagonizing atherogenesis? In the last set of experiments, Dreschler and colleagues9 sought to determine whether repeated super-physiological doses of Ac2-26 might attenuate early atherogenesis (3× ip injections of 50µg/mouse/week during a 4 week high fat diet). Repeated administration of Ac2-26 resulted in reduction of lesion plaques and lesional macrophages, but effects were relatively small, suggesting potential compensatory mechanisms for atherogenesis in this model. While Annexin A1 did seem to affect myeloid cell recruitment, the effects of intra-plaque Annexin A1 on efferocytosis, macrophage activation and the Th1 response in these recipients were not examined. In followed-up studies these questions could be addressed. Other question is still remained uncovered: Would using Ac2-26 to treat atherosclerosis make the recipient vulnerable to acute or chronic infections? The use of Ac2-26 may prevent the necessary recruitment of leukocytes to sites of infection, resulting in an inadequate host response to pathogens. Although the use of Ac2-26 to some extent provides the first proof of efficacy in blockading myeloid cell recruitment during atherogenesis, further studies will need to be conducted to target the homing of pro-inflammatory leukocytes specifically into the atherosclerotic aortic wall. Despite these setbacks, data presented by Drescher and colleagues are novel and interesting and future studies should further clarify the functions of Annexin A1/FPR2 and potential therapeutic approaches to target myeloid cell recruitment in atherosclerosis.
Acknowledgements
This work was supported by the NHLBI RO1HL107522 (to E. Galkina).
Footnotes
Disclosures
None.
Reference List
- 1.Libby P, Hansson GK. Inflammation and Immunity in Diseases of the Arterial Tree: Players and Layers. Circ Res. 2015;116:307–311. doi: 10.1161/CIRCRESAHA.116.301313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galkina E, Ley K. Leukocyte influx in atherosclerosis. Curr Drug Targets. 2007;8:1239–1248. doi: 10.2174/138945007783220650. [DOI] [PubMed] [Google Scholar]
- 3.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van RN, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 6.Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008;117:1642–1648. doi: 10.1161/CIRCULATIONAHA.107.743872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jager SC, Bot I, Kraaijeveld AO, Korporaal SJ, Bot M, van Santbrink PJ, van Berkel TJ, Kuiper J, Biessen EA. Leukocyte-specific CCL3 deficiency inhibits atherosclerotic lesion development by affecting neutrophil accumulation. Arterioscler Thromb Vasc Biol. 2013;33:e75–e83. doi: 10.1161/ATVBAHA.112.300857. [DOI] [PubMed] [Google Scholar]
- 8.Drechsler M, Megens RT, van ZM, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 9.Drechsler M, de Jong RJ, Rossaint J, Viola J, Leoni G, Wang JM, Grommes J, Hinkel R, Kupatt C, Weber C, Doering Y, Zarbock A, Soehnlein O. Annexin A1 Counteracts Chemokine-Induced Arterial Myeloid Cell Recruitment. Circ Res. 2015;116 doi: 10.1161/CIRCRESAHA.116.305825. xxx-xxx [in this issue] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheuk BL, Cheng SW. Annexin A1 expression in atherosclerotic carotid plaques and its relationship with plaque characteristics. Eur J Vasc Endovasc Surg. 2011;41:364–371. doi: 10.1016/j.ejvs.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Viiri LE, Full LE, Navin TJ, Begum S, Didangelos A, Astola N, Berge RK, Seppala I, Shalhoub J, Franklin IJ, Perretti M, Lehtimaki T, Davies AH, Wait R, Monaco C. Smooth muscle cells in human atherosclerosis: proteomic profiling reveals differences in expression of Annexin A1 and mitochondrial proteins in carotid disease. J Mol Cell Cardiol. 2013;54:65–72. doi: 10.1016/j.yjmcc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Gavins FN, Hickey MJ. Annexin A1 and the regulation of innate and adaptive immunity. Front Immunol. 2012;3:354. doi: 10.3389/fimmu.2012.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 14.Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 15.Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, Flower RJ, Perretti M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A. 2013;110:18232–18237. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petri MH, Laguna-Fernandez A, Gonzalez-Diez M, Paulsson-Berne G, Hansson GK, Back M. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc Res. 2015;105:65–74. doi: 10.1093/cvr/cvu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HY, Oh E, Kim SD, Seo JK, Bae YS. Oxidized low-density lipoprotein-induced foam cell formation is mediated by formyl peptide receptor 2. Biochem Biophys Res Commun. 2014;443:1003–1007. doi: 10.1016/j.bbrc.2013.12.082. [DOI] [PubMed] [Google Scholar]
- 18.Lee HY, Kim SD, Shim JW, Lee SY, Lee H, Cho KH, Yun J, Bae YS. Serum amyloid A induces CCL2 production via formyl peptide receptor-like 1-mediated signaling in human monocytes. J Immunol. 2008;181:4332–4339. doi: 10.4049/jimmunol.181.6.4332. [DOI] [PubMed] [Google Scholar]
- 19.Hayhoe RP, Kamal AM, Solito E, Flower RJ, Cooper D, Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood. 2006;107:2123–2130. doi: 10.1182/blood-2005-08-3099. [DOI] [PubMed] [Google Scholar]
- 20.Montresor A, Toffali L, Constantin G, Laudanna C. Chemokines and the signaling modules regulating integrin affinity. Front Immunol. 2012;3:127. doi: 10.3389/fimmu.2012.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duchniewicz M, Zemojtel T, Kolanczyk M, Grossmann S, Scheele JS, Zwartkruis FJ. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol Cell Biol. 2006;26:643–653. doi: 10.1128/MCB.26.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de RJ, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 23.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini-Vittori M, Korf-Klingebiel M, Napp LC, Hansen B, Kanwischer A, Bavendiek U, Beutel G, Hapke M, Sauer MG, Laudanna C, Hogg N, Vestweber D, Wollert KC. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17:581–588. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 24.Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4:587–598. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Cai L, Wang H, Wu P, Gu W, Chen Y, Hao H, Tang K, Yi P, Liu M, Miao S, Ye D. Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor-2. Oncogene. 2011;30:3887–3899. doi: 10.1038/onc.2011.112. [DOI] [PubMed] [Google Scholar]