Abstract
Brain trauma is accompanied by regional alterations of brain metabolism, reduction in metabolic rates and possible energy crisis. We hypothesize that microdialysis markers of energy crisis are present during the critical period of intensive care despite the absence of brain ischemia. In all, 19 brain injury patients (mean GCS 6) underwent combined positron emission tomography (PET) for metabolism of glucose (CMRglu) and oxygen (CMRO2) and cerebral microdialysis (MD) at a mean time of 36 h after injury. Microdialysis values were compared with the regional mean PET values adjacent to the probe. Longitudinal MD data revealed a 25% incidence rate of metabolic crisis (elevated lactate/pyruvate ratio (LPR)>40) but only a 2.4% incidence rate of ischemia. Positron emission tomography imaging revealed a 1% incidence of ischemia across all voxels as measured by oxygen extraction fraction (OEF) and cerebral venous oxygen content (CvO2). In the region of the MD probe, PET imaging revealed ischemia in a single patient despite increased LPR in other patients. Lactate/pyruvate ratio correlated negatively with CMRO2 (P<0.001), but not with OEF or CvO2. Traumatic brain injury leads to a state of persistent metabolic crisis as reflected by abnormal cerebral microdialysis LPR that is not related to ischemia.
Keywords: brain injury, ischemia, lactate, lactate/pyruvate ratio, microdialysis, positron emission tomography, pyruvate
Introduction
Traumatic brain injury (TBI) results in primary cellular death in a limited region of the brain directly involved in the insult, while creating a more widespread state of metabolic dysfunction in remote areas of the brain (Feeney and Baron, 1986; Vink et al, 1988). This metabolic dysfunction is best characterized as a reduction in oxidative metabolism (CMRO2) (Vink et al, 1988; Hovda et al, 1991) with alteration in glucose metabolism (Yoshino et al, 1991, 1992; Kawamata et al, 2000) and has been shown both in experimental (Hayes et al, 1988; Yoshino et al, 1992; Hovda et al, 1991; Andersen and Maramarou, 1989) and in human brain injury (Bergsneider et al, 1997, 2000, 2001). Specifically, CMRO2 is reduced by at least 50% after TBI in the acute period. This reduction is thought to be due to a predominantly calcium-mediated impairment of mitochondrial respiratory function as well as early cell death. In the immediate postinjury period, there is a compensatory increase in glucose utilization to supply energy to membrane ionic pumps to facilitate restoration of ionic gradients. This increase in glucose utilization has been coined hyperglycolysis and is short-lived and followed rapidly by a reduction in glucose utilization. These changes in brain metabolism occur acutely during periods of increased energy demand and proceed through phasic changes initially with increased glucose metabolism and then evolve to a subacute period of apparent metabolic depression. The metabolic depression is distinct from that of ischemia, since oxygen extraction appears to be low or normal, and lasts for days to weeks in humans. During this acute period, secondary events such as seizures or elevated intracranial pressure can place demands on the tissue to supply additional energy. In such a setting of increased energy demand, the metabolic reserve of the tissue might be impaired.
Numerous clinical studies have documented ischemia early after TBI. The evidence of brain ischemia occurring after TBI comes from pathological autopsy series of fatal cases of brain injury and from hyperacute studies of cerebral blood flow and jugular venous oximetry (Bouma et al, 1991; De Deyne et al, 1996; Vigue et al, 1999). Early pathological studies of fatal brain injuries such as those by Graham and Adams (1971), Graham et al (1989) documented ischemic damage in 91% of cases. This ischemic injury was severe in 27% and moderate in 43%. Most of this ischemic damage was noted to occur in the hippocampus, basal ganglia, and cerebellum. The stability of these findings has been shown over the last two decades (Jennett et al, 2001; Kotapka et al, 1991). However, the overall prevalence of ischemia after the initial 24 h has been difficult to document despite persistent markers of disturbed brain metabolism seen in microdialysis monitoring (Vespa et al, 2003).
It is in this context that the present study attempts to determine if there is a metabolic crisis due to ischemia or due to mechanisms other than ischemia by examining a representative region of brain tissue remote from the primary injury site through the use of combined positron emission tomography and brain microdialysis. We define metabolic crisis as the elevation of the lactate/pyruvate ratio (LPR)>40 and we define ischemia by the PET criteria of OEF > 0.75 or by the combined microdialysis criteria of LPR> 40 and microdialysis glucose < 0.2 mmol/L. In this region of interest (ROI), we prospectively measure the metabolic state of the tissue and attempt to validate the usefulness of microdialysis as an indicator of ischemia in TBI.
Materials and methods
The University of California at Los Angeles institutional review board for human research approved of this study. This study was conducted as an integral part of the UCLA Brain Injury Program on patients with severe TBI with either GCS≤8 or evidence of traumatic mass lesion on computerized tomographic scan and GCS≤12. Subjects were identified in the emergency room, consented by proxy and enrolled into the study as soon as possible. The management of patients has been previously described (Vespa et al, 1999, 2003). Acute-phase studies were performed during the initial 10 days after hospital admission, subject to constraints including catheter removal, the patient’s graduation from intensive care, or death. Determination of the injury severity score (ISS) was performed using the conventional assessment tool (Baker et al, 1974).
Cerebral microdialysis was performed using the CMA70 probe (10 cm flexible shaft, 10 mm membrane length, 20 kDa cutoff, CMA, Stockholm, Sweden) inserted via a twist drill burr hole adjacent to an existing ventriculostomy. The microdialysis catheter was inserted into a depth of 1.5 to 2 cm below the dura at an angle 30° lateral to the trajectory of the ventriculostomy, to place the catheter tip into the white matter (which was confirmed by computerized tomography of the hyperdense tip located in white matter). The location was in the nondominant frontal lobe. The probe was tunneled 3 cm under the skin and secured to the scalp with a flat profile, and then attached to the CMA103 perfusion pump. Normal saline was perfused through the catheter at 2 µL/min, and fluid was collected in 60-min samples and then placed into dry ice or directly into the CMA600 instrument. The initial 60-min sample was not used for analysis because this was the time allowed for stabilization of the probe. Microdialysis was not interrupted for transport or bedside testing. In five most recent patients, subcutaneous probes were placed in the skin overlying the right lower abdominal quadrant to control for hourly systemic changes in glucose, lactate and urea. In one patient, two microdialysis probes were placed into the brain, with the second probe located in a pericontusional white matter location.
Positron emission tomography was performed using a quantitative method previously described (Bergsneider et al, 2001; Wu et al, 2004). Patients were placed into the scanner and physiological monitoring of ICP, end-tidal CO2, arterial blood gases, arterial blood pressure, continuous electroencephalography, core temperature, and heart rate wes monitored using a portable intensive care unit (ICU) monitoring system. These physiological parameters were kept similar to those that were present in the ICU. ICP was kept under 20 mm Hg, CO2 was kept at 30 to 34 mm Hg, and temperature was kept 37 to 37.6°C. Patients underwent serial O-15 PET scans using dynamic blood sampling to determine regional cerebral blood flow (CBF, mL/(100 g/min−1)), oxygen extraction fraction (OEF, %), and the cerebral metabolic rate of oxygen (CMRO2, mg/(100g/min−1)). Images of oxygen extraction coefficient (OEF) were generated using the initial 5 min of the 15O2 study and a method that is based on a compartmental model for oxygen that accounts for recirculated (Mintun et al, 1984; Ohta et al, 1992). The 15O2 raw images (voxel size: 1.471 mm × 1.471 mm × 2.45 mm) were smoothed with a 3D filter (in plane fwhm = 2.942 mm, axial fwhm = 2.45 mm) before OEF images were generated on a voxel basis. The extracranial tissue and CSF were excluded in addition to contusions and hemorrhage as described by Coles et al (2004). This was followed by an FDG-PET scan obtained using a quantitative technique for the calculation of the regional cerebral metabolic rate of glucose (CMRglc, mg/(100 g/min−1)). Using CMRO2 and CMRglc values derived from ROI analysis, the oxygen-glucose ratio (OGR, mg O2/mg glucose) was calculated. For the purpose of the present study, regional glucose metabolic rates were determined in a 2 cm3 region of the cerebral microdialysis probe. Microdialysis probe location was confirmed by computerized tomography (CT) or magnetic resonance image (MRI), and this location was then coregistered to the PET. With the MD membrane at the center, a 1.5-cm diameter ROI was selected for analysis. To evaluate the influence of heterogeneity of PET measures within the area of brain containing the microdialysis probe, we mapped the distribution of the metabolic parameter in the 2 cm3 ROI along with concentric ROIs from 1 to 5 cm3 that used the microdialysis probe as the epicenter. Both these procedures showed little variation of PET-derived mean and standard deviation values in the vicinity of the probe and <5% difference in the PET signal in progressively larger ROIs. Thus, 2 cm3 was chosen as the operational area for comparison.
A detailed patient event log was kept by the bedside nurse and research team to identify important events, and to record times of vial sampling. In addition, automated computerized capture of all physiological monitoring data was achieved. Sampling of the physiology occurs every 2 min and a 1-h mean is generated. An experienced nurse then confirms the hourly mean values. The following data on an hourly basis were recorded: intracranial pressure (ICP), mean arterial blood pressure (MAP), cerebral perfusion pressure (CPP), heart rate, arterial oxygen saturation, core temperature (jugular), jugular venous oxygen saturation (SjvO2), regional brain oxygen partial pressure (PTiO2), electroencephalography, and Glasgow coma score. In addition, serial measurements of dose of sedatives, mannitol, and other neurologically active medication were recorded for each hour.
Daily testing was performed using the radioactive Xenon-133 Kety–Schmidt technique for global measurement of CBF, and glucose and oxygen metabolic rates were calculated using methods previously defined (Lee et al, 2001). Matched samples of arterial and jugular bulb venous blood samples were taken on a daily basis and matched to the corresponding hourly microdialysis glucose sample. Blood glucose levels were determined using the glucose oxidase method. Intravenous injection of radioactive Xenon-133 was performed and the global CBF-15 was determined. Thereafter, global rates of glucose and oxidative metabolism were obtained. Determination of global glucose metabolism and CBF was performed for each patient and compared with a matched cerebral microdialysis sample taken during the hour of study.
Frozen samples were thawed, then briefly centrifuged, and then analyzed on the CMA600 in batch analysis with standard CMA600 reagents. These samples were analyzed for glucose, lactate, pyruvate, glutamate, glycerol, and urea. These hourly samples were run twice each for each analyte and the mean final value was used. Quality control measurements using normal saline and water blank samples, as well as standardized solutions across a range of concentrations (0.025 to 3.0 mmol/L) mimicking those of the human samples, were run weekly, with an additional internal control sample for each subject. Acceptable values of coefficient of variation (3% to 5%) and accuracy were obtained to validate very low sample concentrations of selected analytes. Samples with extremely low values (<0.05 mmol/L) underwent repeat testing for confirmation.
The incidence of ischemia in various brain regions on PET was determined by looking at all voxels contained in the individual slices across the entire brain and applying one of two sets of criteria for ischemia. For this analysis, contusions were excluded but pericontusional tissue was included. In the first instance, we used the criterion of OEF>0.75 and in the second we used the criteria of critical oligemia proposed by Coles et al (2004). Critical oligemia uses the concept that the lowest cerebral venous oxygen content (CvO2) in an infarction is below the threshold of 3.5 mL/100mL (Yundt and Diringer, 1997; Powers et al, 1985. Hence the formula for critical oligemia used was OEFCrit = (CaO2–3.5)/CaO2. Using this technique, we examined on average approximately 98,300 voxels per patient. With regard to the molar ratio of oxygen to glucose metabolism, OGR was considered to be high if it was greater than the expected stoichiometric ratio of 5.8 and decreased if ≤4.9.
Subjects were closely followed for recovery of function over the next 6 months by home and outpatient office visits. At 6 months, patients underwent in-person follow-up testing consisting of the extended Glasgow Outcome score (GOSe) (Wilson et al, 2000). The GOSe was deemed to be stable at 6 months after injury and was used to assess the profile of microdialysis based in each major category of GOSe.
General Management Protocol
All patients underwent continuous EEG (cEEG) monitoring in the neurosurgical ICU as part of the standardized care protocol. Patients requiring an initial emergency operation for a mass lesion were taken to operating room within 1 h of admission. Intracranial pressure (ICP) monitoring, starting within 2 h of injury, was performed using a ventriculostomy. ICP was kept below 20 mm Hg using a stepwise management strategy (i.e. cerebrospinal fluid drainage, hyperventilation to PCO2 of 30 to 34, and hypertonic saline). Ventriculostomy drainage was used for persistent elevation of the ICP > 20 mm Hg for >5 min. CSF was drained for 10 min and then the ventriculostomy was set to monitor. Cerebral perfusion pressure (CPP) was kept equal to or greater than 60 mm Hg using norepinephrine when required. Jugular venous oximetry (SJO2) was performed to monitor for jugular venous desaturation and for hyperemia. Blood pressure was adjusted to keep the SJO2 between 60% to 70%. Core temperature from the jugular vein was used and kept between 37°C to 37.6°C though the use of medications (acetominophen) and surface-cooling devices. Systemic glucose was controlled using subcutaneous insulin to achieve a serum glucose between 110 and 200 mg/dL. All patients received a loading dose of phenytoin (18 mg/kg) in the emergency room and were continued on phenytoin 300 mg/day for seven days.
Data analyses included Pearson product–moment correlations, analyses of proportions, analyses of variance, computation of odds ratios with 95% confidence intervals, and linear regression. Data acquisition was handled in Access 97 (Microsoft Corp., Redmond WA, USA), while statistical procedures were conducted within Statistica 5.5 (StatSoft, Inc., Tulsa, OK, USA).
Results
In all, 19 subjects underwent combined microdialysis and PET imaging after TBI. The main diagnostic and epidemiological indicators for each subject are outlined in Table 1. The patients had severe TBI with prolonged ICU stays. A total of 2614 samples of hourly microdialysis samples were obtained. The start time of cerebral microdialysis averaged 12 h after injury. During the period of monitoring microdialysis, longitudinal continuous monitoring for brain ischemia in the ICU was performed using jugular venous oximetry and daily global xenon-133 cerebral blood flow measures. The incidence of any single marker of global brain ischemia was low, namely <2% (SJVO2 <50% or CBF <20 cm3/(100 g/min−1)) (Glenn et al, 2003). In the present cohort, ischemia occurred in one of two situations: transient events lasting less than 60 min (6 patients) and terminal events occurring during the final few hours of monitoring (1 patient). Table 1 summarizes the per patient incidence of increased ICP, reduced CPP, and reduced SJVO2 in this cohort of subjects. The PaCO2 ranged from 28 to 41 mm Hg, with most patients in the range from 30 to 34 mm Hg. There was poor correlation between PaCO2 and CBF, OEF, CMRO2, and OGR (pearson correlation coefficients ranging from −0.18 to 0.07).
Table 1.
Demographic data
| Subject | Age (years) |
iGCS | Lesion type |
Surgery | PET time |
SJO2 at PET |
PaCO2 at PET |
% time ICP> 20 |
% time CPP< 60 |
% time SJO2<50 |
6GOSe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | 12 | Multi Ctx | N | 36 | 56 | 34 | 28 | 1 | 0 | 7 |
| 2 | 27 | 5 | tSAH | n | 96 | 56 | 28 | 0 | 1 | 0 | 3 |
| 3 | 15 | 14 | SDH, Evac | y | 28 | 78 | 34 | 0 | 40 | 15 | 4 |
| 4 | 38 | 8 | EDH, Multi Ctx | n | 60 | 80 | 41 | 0 | 6 | 0 | 8 |
| 5 | 24 | 3 | Multi Ctx | n | 98 | 65 | 32 | 14 | 14 | 0 | 1 |
| 6 | 47 | 7 | Multi Ctx | n | 96 | 75 | 34 | 0 | 0 | 0 | 3 |
| 7 | 29 | 3 | Multi Ctx | n | 70 | 69 | 29 | 8 | 17 | 1 | 1 |
| 8 | 53 | 14 | Multi Ctx | y | 20 | 88 | 33 | 11 | 1 | 1 | 5 |
| 9 | 44 | 7 | EDH, Multi Ctx | y | 30 | 68 | 36 | 0 | 3 | 0 | 6 |
| 10 | 35 | 14 | t SAH | n | 24 | 84 | 34 | 0 | 13 | 0 | 6 |
| 11 | 20 | 12 | Multi Ctx | y | 72 | 73 | 33 | 1 | 1 | 0 | 7 |
| 12 | 31 | 8 | SDH, Evac | y | 48 | 59 | 34 | 3 | 58 | 1 | 5 |
| 13 | 57 | 4 | Multi Ctx | y | 88 | 81 | 30 | 1 | 19 | 1 | 3 |
| 14 | 41 | 7 | Multi Ctx | n | 41 | 66 | 31 | 0 | 0 | 0 | 8 |
| 15 | 38 | 3 | EDH, SDH | y | 39 | 59 | 39 | 0 | 0 | 0 | 3 |
| 16 | 18 | 3 | t SAH | n | 115 | 66 | 31 | 0 | 0 | 0 | 6 |
| 17 | 16 | 3 | Multi Ctx | n | 12 | 92 | 31 | 2 | 4 | 0 | 7 |
| 18 | 25 | 8 | Multi Ctx | y | 98 | 54 | 30 | 11 | 21 | 1 | 3 |
| 19 | 31 | 3 | Multi Ctx | y | 77 | 77 | 33 | 15 | 5 | 1 | 5 |
ICP > 20—the percent time within subject of elevated intracranial pressure over 20 mm Hg, CPP <60—the percent time within subject of decreased cerebral perfusion pressure, SLJO <50%—the percent time within subject of decreased jugular venous oxygen below the global ischemic level, GOSe—the 6-month extended Glasgow outcome score for each patient. PET time—the postinjury hour during which PET was completed.
Probe Location
MRI was performed to locate the MD probe and to coregister the probe with the PET scan. The terminus of the MD probes was confirmed to be in the white matter u fibers directly adjacent to the cortical ribbon.
Longitudinal Microdialysis Study
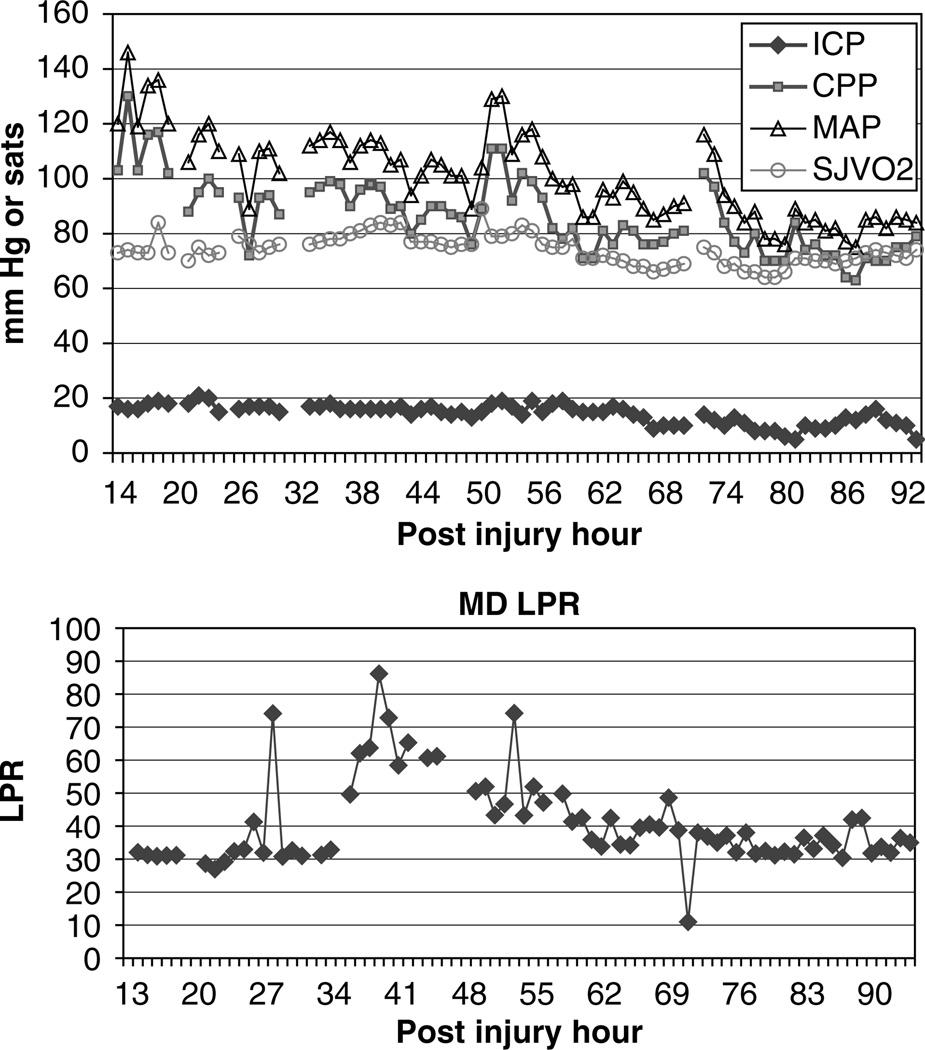
In the longitudinal study of microdialysis markers of ischemia, the mean LPR day by day is shown in Figure 1. Overall, 25% of all LPR values exceeded the predefined critical value of 40. Elevation of LPR>40 occurred in 12/18 patients, with a mean duration of each episode of 2.4±0.3 h. The highest incidence of LPR> 40 occurred on postinjury day zero (31% of all values). An example patient in whom the LPR was persistently elevated despite having ICP, SJVO2, and CPP actively treated to maintain values within the normal treatment range is illustrated in Figure 2. Based on the available literature of microdialysis markers of ischemia (Landolt et al, 1994; Langemann et al, 2001; Hlatky et al, 2004) and the single patient who showed ischemia during PET in Figure 3, we utilized a more stringent pattern of microdialysis to determine the incidence of ischemia. The criteria for ischemia were simultaneous glucose <0.2 mmol and LPR>40. When this combination of microdialysis markers was applied to this cohort of patients, the incidence of ischemia in the longitudinal data set is 2.4%, with a mean duration of 0.5 ± 0.4h.
Figure 1.
Mean daily LPRs over the first 8 days after injury. Mean LPR values day by day are shown in solid bars. The percentage of LPR >40 on each postinjury day are shown in hatched bars.
Figure 2.
An example patient in whom the LPR was elevated above 40 for several hours. During this time, the ICP was being actively treated to maintain the ICP <20 mm Hg. The jugular venous oximetry (sjvo2) % saturation, arterial carbon dioxide pressure in mm Hg (PaCO2), the CPP, and MAP were all within normal limits and show no evidence of brain ischemia.
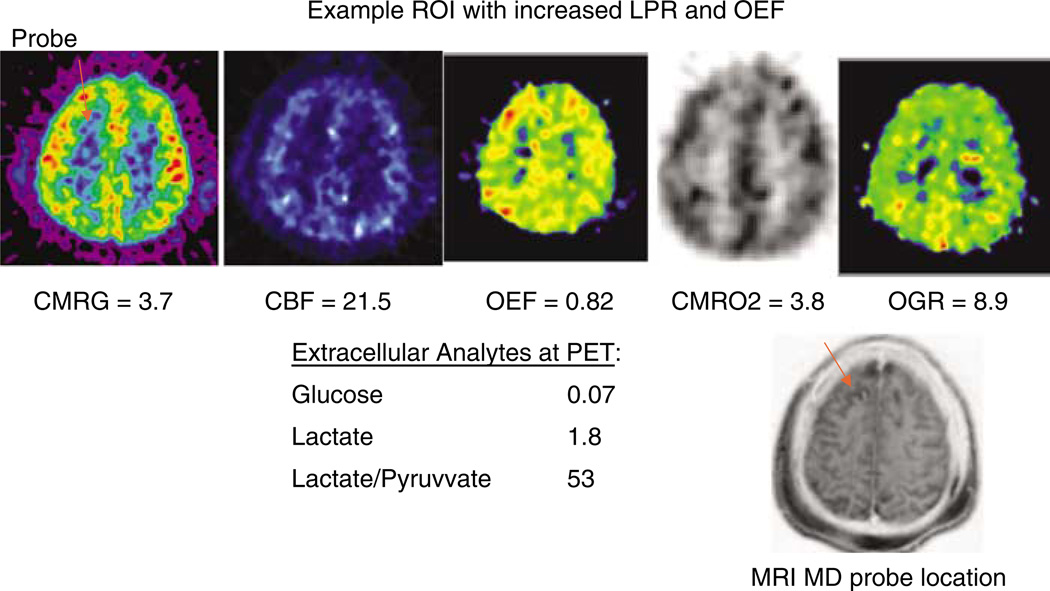
Figure 3.
An example patient who showed ischemic values on OEF on PET scan. There was a corresponding increase in LPR and reduction in glucose. This patient was used to define the ischemic pattern of microdialysis in the entire cohort. Values within the region of interest are shown below each PET image. Abbreviations: CMRgluc—metabolic rate of glucose, CMRO2—metabolic rate of oxygen, CBF—cerebral blood flow, OGR—oxygen to glucose ratio, OEF—oxygen extraction fraction.
Evidence of Regional Ischemia Based on Positron Emission Tomography
The incidence of ischemia in various brain regions on PET was determined by looking at all voxels contained in the individual slices across the entire brain and applying one of two sets of criteria for ischemia. The latency from injury to PET was 60 ± 30 h after injury. For this analysis, CT and MRI coregistration was performed to determine the location and size of hemorrhagic contusions, and areas of hemorrhage, including parenchymal and CSF within the core of these lesions, were excluded. These structural images were obtained on the day of the PET. However, pericontusional tissue, defined as hypodense edematous tissue, was included. In the first instance we used the criterion of OEF>0.75, and in the second we used the criteria of critical oligemia proposed by Coles et al (2004). Critical oligemia uses the concept that the lowest cerebral venous oxygen content (CvO2) in an infarction is below the threshold of 3.5 mL/100 mL (Yundt and Diringer, 1997; Powers et al, 1985). Hence, the formula for critical oligemia used was OEFcrit = (CaO2–3.5)/CaO2. Using this technique, we examined on average approximately 98,300 voxels per patient. Using the OEF >0.75 threshold, we found the mean incidence rate of ischemic voxels to be 0.11 ± 0.18%, with a maximum of 1% of voxels in a single patient. Using the critical oligemia threshold as defined by Coles et al (2004), the incidence of ischemic voxels averaged 0.14 ± 0.28% of voxels. This translated into a calculated mean ischemic brain volume of 1.5 ± 3.1 cm3 (range 0.00 to 11.0 cm3). Separate analysis was performed that incorporated contusions and these data did not differ significantly from the above analysis (data not shown). Only a single ROI containing the microdialysis probe showed ischemia based on the above definitions (Table 2). In this patient, the microdialysis markers of low glucose (glucose = 0.07) and LPR>40 (LPR=57) were found to be present.
Table 2.
Microdialysis and PET values by region of interest
| ROI | Glucose | Glutamate | Lactate | Glycerol | LPR | LGR | CMRG | CRF | CMRO2 | OEF | OGR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.27 | 30.42 | 1.61 | 191.1 | 34.5 | 1.3 | 3.5 | 38.0 | 1.6 | 0.5 | 6.9 |
| 2 | 0.75 | 2.23 | 7.61 | 488.0 | 100.0 | 10.2 | 2.1 | 29.0 | 1.2 | 0.4 | 4.6 |
| 3 | 0.32 | 1.40 | 0.56 | 18.0 | 12.4 | 1.8 | 2.4 | 46.0 | 1.6 | 0.1 | 2.7 |
| 4 | 0.10 | 1.90 | 0.49 | 7.4 | 28.7 | 4.9 | 3.8 | 31.0 | 1.3 | 0.4 | 2.8 |
| 5 | 0.07 | 1.60 | 0.90 | 7.8 | 53.0 | 12.0 | 3.7 | 21.5 | 3.8 | 0.8 | 8.9 |
| 6 | 1.72 | 2.00 | 0.58 | 28.7 | 10.4 | 0.3 | 4.3 | 41.0 | 2.0 | 0.3 | 3.5 |
| 7 | 0.23 | 1.50 | 0.51 | 27.6 | 24.0 | 2.2 | 3.3 | 30.9 | 1.8 | 0.3 | 3.9 |
| 8 | 0.34 | 1.14 | 0.36 | 23.4 | 30.0 | 1.1 | 3.5 | 28.0 | 2.2 | 0.6 | 5.3 |
| 9 | 2.19 | 0.58 | 1.41 | 13.4 | 11.2 | 0.6 | 4.1 | 36.8 | 2.6 | 0.5 | 5.0 |
| 10 | 1.20 | 1.90 | 0.85 | 14.8 | 46.0 | 0.7 | 3.8 | 86.0 | 1.4 | 0.2 | 4.9 |
| 11 | 0.36 | 4.00 | 0.30 | 2.4 | 22.0 | 0.8 | 2.7 | 24.0 | 1.4 | 0.5 | 4.0 |
| 12 | 0.25 | 5.00 | 0.96 | 15.0 | 38.1 | 3.9 | 2.9 | 31.0 | 1.7 | 0.4 | 4.8 |
| 13 | 0.05 | 59.00 | 4.85 | 40.0 | 68.4 | 107.9 | 3.3 | 33.0 | 0.8 | 0.5 | 5.4 |
| 14 | 0.41 | 1.62 | 2.12 | 95.7 | 64.2 | 5.2 | 2.0 | 34.0 | 0.8 | 0.1 | 2.4 |
| 15 | 0.40 | 0.29 | 0.42 | 37.5 | 40.0 | 1.0 | 3.7 | 84.0 | 1.4 | 0.1 | 7.0 |
| 16 | 0.32 | 1.02 | 0.55 | 18.1 | 80.6 | 1.7 | 3.1 | 31.0 | 0.7 | 0.4 | 4.2 |
| 17 | 1.62 | 1.35 | 0.78 | 8.9 | 12.2 | 0.5 | 2.9 | 51.0 | 2.8 | 0.4 | 7.9 |
| 18 | 0.60 | 1.97 | 0.30 | 8.5 | 20.6 | 0.5 | 4.7 | 48.0 | 2.5 | 0.4 | 5.4 |
| 19 | 1.13 | 1.08 | 0.80 | 12.1 | 18.2 | 0.7 | 3.2 | 23.0 | 2.0 | 0.4 | 3.4 |
| 20 | 0.13 | 2.50 | 1.20 | 29.3 | 26.3 | 9.2 | 3.0 | 29.0 | 2.5 | 0.4 | 5.4 |
Microdialysis metabolites of glucose, glutamate, lactate, glycerol, LPR are shown along with the PET metabolic rates of glucose (CMRgluc) and oxygen (CMRO2), CBF, OEF and OGR.
Regional Metabolism
Microdialysis metabolites including LPR were compared with each of the PET-derived measures of metabolism including CBF, CMRO2, CMRglucose, and OGR. In all, 20 ROIs were examined in 19 subjects, with one subject having two MD probes. These results are presented in Table 2, which shows a wide range of microdialysis LPR values (12 to 100) and corresponding range of CMRO2 values (0.7 to 3.8). Overall only one patient showed PET evidence of ischemia (Figure 3). This patient shows increased OEF without a decrease in CMRO2, suggesting viable yet ischemic tissue. Commensurate with this increase in OEF is an increase in OGR. Correlation analysis was made between each metabolite and each PET parameter. LPR was negatively correlated with CMRO2 (r=−0.44, P<0.001) (Figure 4), but not correlated with other PET metabolic parameters. Of note, there were strong correlations between several PET measures and microdialysis metabolite levels: lactate vesus OGR (r=0.48), lactate versus OEF (0.54) (Table 3).
Figure 4.
Region of interest cerebral metabolic rate of oxygen (CMRO2) and the microdialysis LPR. Increasing LPR correlated with reducing CMRO2.
Table 3.
Correlation matrix of PET parameters and microdialysis metabolites
| CMRgluc | CBF | CMRO2 | OEF | OGR | |
|---|---|---|---|---|---|
| Glucose | 0.3 | 0.25 | 0.23 | −0.05 | 0.09 |
| P | 0.19 | 0.27 | 0.32 | 0.83 | 0.69 |
| Glutamate | 0.01 | −0.09 | −0.31 | 0.22 | 0.17 |
| P | 0.96 | 0.19 | 0.19 | 0.37 | 0.46 |
| Lactate | −0.42 | −0.18 | −0.32 | 0.06 | −0.01 |
| P | 0.06 | 0.44 | 0.17 | 0.79 | 0.99 |
| Glycerol | −0.43 | −0.12 | −0.27 | −0.01 | 0 |
| P | 0.06 | 0.65 | 0.26 | 0.96 | 0 |
| LPR | −0.41 | −0.09 | −0.49 | 0.02 | 0.04 |
| P | 0.07 | 0.7 | 0.03 | 0.92 | 0.86 |
| LGR | −0.04 | −0.14 | −0.27 | 0.19 | 0.1 |
| P | 0.83 | 0.57 | 0.27 | 0.43 | 0.69 |
The bold characters highlight the only statistically significant findings.
In examining the PET measures, the oxygen-to-glucose ratio (OGR) was found to be abnormal in 15 of the 19 patients evaluated. OGR was considered to be high if it was greater than the expected ratio of 5.8 and decreased if ≤4.9. OGR was increased in four patients and decreased in 11 patients. Given that low OGR may indicate a disproportionate increase in anaerobic glucose metabolism, we endeavored to evaluate the microdialysis metabolites as a function of the OGR. The microdialysis levels of glucose, lactate, glutamate, glycerol, and LPR are similar in these regions, despite statistically significant differences in OGR. Hence, microdialysis levels did not differentiate between reductions or increases in OGR. Four ROIs showed normal OGR with no distinguishing features seen in the micro-dialysate. In four subjects, the OGR is increased without a significant difference in the levels of cerebral microdialysis levels compared with those subjects with normal or low OGR. Similar comparisons were made by segregating the microdialysis values according to normal, low or high CMRO2, OEF and CMRgluc; however, no significant relationships were forthcoming.
Significance of LPR
LPR>40 was found to be a sensitive but nonspecific indicator of ischemia in the present cohort. Seven individuals showed LPR>40, but only a single patient showed regional ischemia (Figure 3). In this patient, the OEF in the region of the microdialysis was increased and CBF reduced, suggesting an ischemic response with compensatory increase in oxidative metabolism. Examination of other patients with increased LPR showes a trend towards lower glucose and higher glutamate and glycerol levels, however, given the small sample size and standard deviation, these differences were not statistically significant. Cluster analysis using a composite of abnormal microdialysis parameters including the combination of glucose <0.1mmol and LPR>25 was performed to determine if this combination more accurately reflected compromised metabolism. Only three subjects showed this pattern. The corresponding mean PET parameters of these three patients were CMRO2 2.1 ± 1.8, OEF 0.55 ± 0.24, 5.7 ± 3.1, and CBF 28.5 ± 6.1. Sequential analysis of various combinations of abnormal microdialysis parameters was performed, but no other specific cluster was better able to reflect the abnormalities seen on the PET images. Hence, LPR >40 alone was not a specific indicator of ischemia in this cohort.
Discussion
Traumatic brain injury results in a reduction in oxidative metabolism and metabolic crisis that is long lasting. The current study used PET combined with cerebral microdialysis to study the regional and temporal course of this disturbance. The principal findings of our study were that (1) the incidence of posttraumatic regional and global ischemia was low, accounting for a minority of the metabolic disturbance, (2) there was ongoing nonischemic metabolic crisis indicated by elevated microdialysis lactate/pyruvate ratio (LPR) that cannot be accounted for by brain ischemia; (3) LPR correlated negatively with CMRO2; (4) that LPR was a nonspecific indicator of posttraumatic brain ischemia. Our findings are restricted temporally to the period of observation in the ICU, generally from postinjury hour 18 to postinjury day 10, and cannot address the incidence of ultra-early brain ischemia or the usefulness of LPR during the ultra-early period.
The Incidence of Ischemia in the ICU after TBI
Several lines of evidence from clinical brain injury research suggest that ischemia occurs frequently in TBI (Graham and Adams, 1971; Bouma et al, 1991). These data are most robust for the initial 12 h after injury in which brain-imaging studies as well as whole brain oxygenation monitoring has shown that ischemia occurs in 30% of the population. Moreover, autopsy series have shown that necrotic cellular changes are frequent in fatal TBI and these changes are thought to be due to ischemia, rather than other mechanisms of cell death. In contradistinction to these studies, recent PET studies performed in TBI patients both early and late after the injury have failed to show an elevated incidence of brain ischemia. In similarly conducted PET studies of oxidative metabolism conducted by Diringer and colleagues, PET studies were performed at a mean of 12 h after injury. In the Diringer study, the OEF, CBF, CMRO2 values are similar to those in the current study at a baseline arterial carbon dioxide (PaCO2) concentration of 40 mm Hg. With induced hyperventilation, to PaCO2 of 30 or 25 mm Hg, Diringer found global and regional increases in OEF without reduction in CMRO2. In ROIs with initial CBF <10 mL/(100 g/min−1), OEF increased from 0.3 to 0.71, without a reduction in CMRO2. These results indicate that, under basal conditions, ischemia was not present even in areas of low CBF, but those areas could become ischemic without energy failure during hyperventilation. Similarly, Coles and colleagues used oxidative PET studies in TBI patients and found that at baseline CO2 of 30 to 34 mm Hg, the mean ischemic brain volume was 67 cm3. This translates into ischemia being present in approximately 6% of the total brain volume. However, the volume of potentially ischemic tissue ranged up to 224 cm3 and in six of 15 patients the ischemic volume averaged 50 cm3 T.
In the current study, we found a lower mean ischemic brain volume, 1.5 cm3, which translates into 1.5% of total brain volume. In addition, using an ROI of 1.5 cm3 that theoretically covers the region that is sampled by the microdialysis probe, we found only a single instance of ischemia in the region of the probe. Hence, of the three contemporary studies, using different volumes of tissue sampling by PET, the incidence of ischemia across all voxels is low and constitutes between 1.5% and 6% of the total brain. In contrast to previous studies, we performed PET at later time points after injury, and during the delivery of intensive care. Our study is limited in part because the timing of PET was later than that in the Diringer study (mean 60 h after injury) and ischemia might have been present during at earlier times. However, among the four patients studied within 28 h within our study, we did not see a difference in the volume of tissue in the ischemic range. We did not perform within-subjects baseline and hyperventilation studies, however, we did not find increased volume of potentially ischemic brain in those subjects who had PaCo2 in the 28 to 31mm Hg range compared with those who had higher PaCo2 values. The cohorts may differ in ways that are not known. Thus, several factors could explain the differences, yet the main result is the same, namely that ischemia is not the common physiological state in the brain after brain injury. However, early PET imaging performed by Diringer and colleagues (2002) also showd that the presence of ischemia was low, as indicated on oxygen extraction fraction values in multiple ROIs. Thus, the incidence of ischemia after TBI on PET appears to be uncommon.
LPR as a Marker of Metabolic Crisis
There is a robust and growing literature on cerebral microdialysis in patients with TBI and other neurocritical care illnesses such as subarachnoid hemorrhage (Vespa et al, 2004). Microdialysis is a safe and effective monitoring technique that enables sampling of brain neurochemistry and inferential assessment of brain metabolism. Despite this growing body of knowledge (see also Hutchinson et al, 2002), the exact relationship between microdialysis markers of metabolism and independent measures of metabolism has yet to be clearly defined in various disease states. Hence, the validation of microdialysis markers of cellular metabolism in human subjects is of paramount priority to clinicians and researchers alike. The current paper contributes to this validation process.
The ratio of lactate to pyruvate has been one of the commonly reported features of microdialysis monitoring since proposed by Hillered (Persson and Hillered, 1992; Valtysson et al, 1998). LPR ratio normally ranges <20 under conditions of uncomplicated metabolism. The LPR is considered to be a marker of the relative redox state of the tissue, with increases in LPR indicating ischemia and a consequent shift in the NAD/NADH ratio. Thus, LPR has been proposed to be a reliable marker of ischemia. Indeed, the combination of reduction in extracellular glucose and elevation in LPR has been robustly shown to reflect energy crisis (Landolt et al, 1994; Langemann et al, 2001) and these have been shown to be preterminal events when glucose is reduced to undetectable levels. These preterminal events occur in TBI patients under conditions of cerebral circulatory arrest and loss of all brain function. Under experimental conditions, LPR has been documented to indicate severely impaired oxidative respiration (Enblad et al, 2001) and the impaired redox state that occurs with ATP depletion. Under conditions of permanent ischemia, the LPR increases to above 40 and often plateaus in the range of 80 to 120. Under conditions of impending brain death, LPR has been documented in the range of 500 to 1000. With reversible ischemia, the LPR does normalize within 60 to 90 min of restoration of the CBF. The increase in the ratio is comprised primarily of a reduction in the pyruvate concentration by 10- to 100-fold compared with increases in lactate by 2- to 5-fold (Vespa et al, 2003). Reversible increases of the LPR followed by restoration to normal values have been seen with specific reversible mitochondrial poisoning (Clausen et al, 2001. Thus, the LPR appears to be a sensitive marker of reversible mitochondrial dysfunction.
In the present study, we found that increased LPR most tightly corresponds to nonischemic reduction in the CMRO2. Since CMRO2 is a measure of mitochondrial oxidative function, our findings correspond to the previous experimental observations. The PET-derived measures of CMRO2 potentially correspond to reversible mitochondrial dysfunction. In our current PET cohort, areas adjacent to contusions or within the center of contusions shown very low CMRO2 values which were consistent with irreversible cell loss. However, these areas were not included in the ROIs used for the comparison with microdialysis. In contrast, the regions containing the microdialysis probes did not show anatomic injury on CT and MRI, and hence the tissue is not considered irreversibly injured. Therefore, the relationship between LPR and CMRO2, which we found in our cohort, is most likely related to reversible metabolic dysfunction rather than cell loss. As such, it appears that LPR is able to reflect this metabolic dysfunction even in the absence of tissue damage or brain ischemia.
The Nature of Posttraumatic Metabolic Crisis
In our data, we found that ischemia is uncommon in PET and microdialysis measurements taken during the period of early intensive care. Yet, the LPR and other microdialysis measurements were abnormal, suggesting that even in the absence of brain ischemia, the brain is in metabolic crisis. Indeed, in a recent microdialysis paper (Vespa et al, 2003), persistent and long-lasting evidence of metabolic crisis lasting several days despite the absence of brain ischemia was documented. The exact nature of this metabolic crisis remains to be determined. However, we did find that LPR elevations were correlated with reduced oxidative metabolism, suggesting that LPR is an indicator of mitochondrial dysfunction.
Alterations in other PET parameters such as OGR support the concept of nonischemic metabolic crisis, but also point to the heterogeneity of metabolism after TBI. Both reductions and increases in the OGR have been shown to occur after TBI in the current study. This alteration of OGR was previously characterized by Wu et al (2004). OGR is the stochiometric relationship between oxidative to glucose metabolism, which is normally in a ratio of approximately 6. Reduction in OGR indicates a disproportionate reduction in oxidative metabolism, thought to be due to impaired mitochondrial function. Reduction in OGR has been seen primarily in white matter structures reflecting the widespread metabolic dysfunction after TBI. In some circumstances, reduction in OGR is related to an observed absolute increase in glucose metabolism. However, this is not frequently seen; instead, the absolute rates of glucose metabolism are decreased as well. Hence, there is a combined reduction in both oxidative and glucose metabolism, but the degree of reduction in each is not equivalent on a molar basis. This implies a relative increase in glucose metabolism, compared with that of oxidative metabolism.
However, increases in OGR have been seen in some circumstances. Increased OGR indicates an increase in oxidative metabolism that is not dependent on an increase in glucose utilization. The increase in OGR would indirectly indicate utilization of a fuel other than glucose to generate the oxidative rates seen. The nature of this fuel is not known at the present time, but experimental evidence suggests that the brain is capable of utilizing ketones, lactate, and pyruvate as alternative fuels under selected conditions. Thus, the nature of the metabolic crisis might be predicated on impaired oxidative metabolism. Hence, cerebral microdialysis monitoring of brain metabolism should be interpreted in the context that changes in metabolites may reflect important nonischemic changes in metabolism.
Defining Ischemia after Traumatic Brain Injury
The definition of ischemia and determining the incidence of ischemia after TBI remain elusive. In this paper, we have used classic thresholds of PET OEF >0.75 as well as microdialysis metabolite values to define ischemia. The PET OEF >0.75 threshold is based in part on the work of Senda et al (1989) and Marchal et al (1999). In the former study (Senda et al, 1989), mean OEF in acute ischemic territories was 0.78 within 2 days of the ischemic stroke. In the latter study, Marchal et al report that the upper limit of OEF beyond which acutely ischemic tissue was irreversibly injured was >0.73. Experimentally, a similar OEF >0.75 threshold has been reported (Sakoh et al, 2000). Arguably, defining ischemia at a different threshold, such as OEF >0.5, potentially would result in an alternative interpretation. For example, if we use an OEF >0.50, then 2 of 19 patients would have regional ischemia. Indeed, a single valid OEF threshold for ischemia has not been agreed on in the literature. Alternatively, a reduction in CMRO2 <1.4mL/(100 mL/min−1) has been shown to be a threshold below which tissue viability is lost under conditions of ischemia (Powers et al, 1985; Ackerman et al, 1989). If we use this CMRO2 definition of irreversibly injured tissue, then in the three subjects who displayed CMRO2 ≤1.3, 2/3 had an LPR >40 and might be considered ischemic, while one did not. Given that CMRO2 is reversibly reduced in TBI, the CMRO2 <1.3 definition may not be valid. However, it is intriguing that LPR negatively correlates with CMRO2, and hence LPR may be a marker of cellular viability rather than of ischemia per se. Presently, we are exploring the long-term tissue viability as a function of LPR to determine if in fact elevated LPR predicts long-term tissue atrophy.
To be independent of a single OEF threshold, we used an alternative definition of ischemia, namely CvO2 <3/5 mL/100 mL, proposed by Powers et al (1985), Sutton et al (1990), and recently by Coles et al (2004). The CvO2-based analysis reveals that only 1% of all regions imaged were in the ischemic range. Hence, the present study shows that ischemia is rare at either PET threshold for ischemia. Similarly, if we use an alternative microdialysis threshold for ischemia, LPR alone, then we would determine that the incidence of ischemia is between 30% and 40% of all hourly values. This incidence would be inconsistent with other continuous measures of brain oxygenation in our study, and in all others (Gopinath et al, 1994, 1999; Coles et al, 2004). The microdialysis definition of ischemia that we used, namely a reduction in glucose <0.2 mmol/L and LPR >40, was initially proposed by Landolt et al (1994) and Langemann et al (2001) in the setting of terminal herniation leading to ischemia and cell death after TBI. Indeed, Hlatky et al (2004) recently confirm this definition of ischemia, in which microdialysis glucose values decrease to to <0.2 mmol/L and LPR >40. In the same study (Hlatky et al, 2004), elevated LPR >40 alone did not correlate with ischemia, as measured by a brain tissue oxygen probe. Our study differs from that of Hlatky, since we make use of a direct comparison of OEF in the region of the probe rather than a comparison with the partial pressure of oxygen. In addition, there are limitations to these microdialysis criteria for ischemia. However, the finding that LPR alone is not a sensitive measure of brain ischemia in the TBI patient is similar to that of the Hlatky study. Hence, we consider our PET and microdialysis definitions of ischemia to be robust and, even with the use of alternative, more conservative definitions of ischemia, the incidence of ischemia is low despite the persistent abnormal LPR.
Summary
The primary findings of the current study were that the injured brain has persistent impairments in metabolism that can be reflected by cerebral microdialysis. Specifically, the LPR best reflects impaired oxidative metabolism, but is not specific for brain ischemia. Moreover, the metabolic crisis was not primarily a result on persistent brain ischemia, and hence elevations in LPR are not specific for brain ischemia. Given that most clinical monitors such as the brain parenchymal oxygen monitor and the jugular venous oximeter are designed to look for ischemia, the finding of a low incidence of brain ischemia is important. Moreover, these findings suggest that the use of microdialysis monitoring of various metabolites to determine the overall state of energy balance between supply and demand might be the most appropriate monitoring tool in TBI.
Acknowledgements
This research was supported by NINDS 03039, NS02089, and the State of California Neurotrauma Initiative grant.
References
- Ackerman RH, Lev MH, Mackay BC, Katz PM, Babikian VL, Alpert NM. PET studies in acute stroke findings and relevance to therapy (abstract) J Cereb Blood Flow Metab. 1989;9(S1):S359. [Google Scholar]
- Andersen BJ, Maramarou A. Isolated stimulation of glycolysis following traumatic brain injury. In: Hoff JT, Betz AL, editors. Intracranial pressure VII. Berlin: Springer; 1989. pp. 575–580. [Google Scholar]
- Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- Bergsneider MA, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, Phelps ME, McArthur DL, Caron MJ, Kraus JF, Becker DP. Cerebral hyperglycolysis following severe human traumatic brain injury: a positron emission tomography study. J Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- Bergsneider M, Hovda DA, Lee SM, Kelly DF, McArthur DL, Vespa PM, Lee JH, Huang SC, Martin NA, Phelps ME, Becker DP. Dissociation of cerebral glucose metabolism and level of consciousness during the period of metabolic depression following human traumatic brain injury. J Neurotrauma. 2000;17:389–401. doi: 10.1089/neu.2000.17.389. [DOI] [PubMed] [Google Scholar]
- Bergsneider M, Hovda DA, McArthur DL, Etchepare M, Huang SC, Sehati N, Satz P, Phelps ME, Becker DP. Metabolic recovery following human traumatic brain injury based on FDG-PET: time course and relationship to neurological disability. J Head Trauma Rehabil. 2001;16:135–148. doi: 10.1097/00001199-200104000-00004. [DOI] [PubMed] [Google Scholar]
- Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF. Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg. 1991;75:685–693. doi: 10.3171/jns.1991.75.5.0685. [DOI] [PubMed] [Google Scholar]
- Clausen T, Zauner A, Levasseur JE, Rice AC, Bullock R. Induced mitochondrial failure in the feline brain: implications for understanding acute posttraumatic metabolic events. Brain Res. 2001;20:358. doi: 10.1016/s0006-8993(01)02566-5. [DOI] [PubMed] [Google Scholar]
- Coles JP, Fryer TD, Smielewski P, Rice K, Clark JC, Pickard JD, Menon DK. Defining ischemic burden after traumatic brain injury using 150 PET imaging of cerebral physiology. J Cereb Blood Flow Metab. 2004;24:191–201. doi: 10.1097/01.WCB.0000100045.07481.DE. [DOI] [PubMed] [Google Scholar]
- de Deyne C, Vandekerckhove T, Decruyenaere J, Colardyn F. Analysis of abnormal jugular bulb oxygen saturation data in patients with severe head injury. Acta Neurochir (Wien) 1996;138:1409–1415. doi: 10.1007/BF01411119. [DOI] [PubMed] [Google Scholar]
- Diringer MN, Videen TO, Yundt K, Zazulia AR, Aiyagari V, Dacey RG, Jr, Grubb RL, Powers WJ. Regional cerebrovascular and metabolic effects of hyperventilation after severe traumatic brain injury. J Neurosurg. 2002;96:103–108. doi: 10.3171/jns.2002.96.1.0103. [DOI] [PubMed] [Google Scholar]
- Enblad P, Frykholm P, Valtysson J, Silander HC, Anders-son J, Fasth KJ, Watanabe Y, Langstrom B, Hillered L, Persson L. Middle cerebral artery occlusion and reperfusion in primates monitored by microdialysis and sequential positron emission tomography. Stroke. 2001;32:1574–1580. doi: 10.1161/01.str.32.7.1574. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Baron JC. Diaschisis. Stroke. 1986;17:817–830. doi: 10.1161/01.str.17.5.817. [DOI] [PubMed] [Google Scholar]
- Glenn TC, Kelly DF, Boscardin WJ, McArthur DL, Vespa PM, Oertel M, Hovda DA, Bergsneider M, Hillered L, Martin NA. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose and lactate metabolism. J Cereb Blood Flow Metab. 2003;23:1239–1250. doi: 10.1097/01.WCB.0000089833.23606.7F. [DOI] [PubMed] [Google Scholar]
- Gopinath SP, Robertson CS, Contant CF, Hayes C, Feldman Z, Narayan RK, Grossman RG. Jugular venous desaturation and outcome after head injury. J Neurol Neurosurg Psychiatry. 1994;57:717–723. doi: 10.1136/jnnp.57.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath SP, Valadka AB, Uzura M, Robertson CS. Comparison of jugular venous oxygen saturation and brain tissue Po2 as monitors of cerebral ischemia after head injury. Crit Care Med. 1999;27:2337–2345. doi: 10.1097/00003246-199911000-00003. [DOI] [PubMed] [Google Scholar]
- Graham DI, Adams JH. Ischaemic brain damage in fatal head injuries. Lancet. 1971;6:265–266. doi: 10.1016/s0140-6736(71)91003-8. [DOI] [PubMed] [Google Scholar]
- Graham DI, Ford I, Adams JH, Doyle D, Teasdale GM, Lawrence AE, McLellan DR. Ischaemic brain damage is still common in fatal non-missile head injury. J Neurol Neurosurg Psychiatry. 1989;52:346–350. doi: 10.1136/jnnp.52.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes RL, Katayama Y, Jenkins LW, Lyeth BG, Clifton GL, Gunter J, Povlishock JT, Young HF. Regional rates of glucose utilization in the cat following concussive head injury. J Neurotrauma. 1988;5:121–137. doi: 10.1089/neu.1988.5.121. [DOI] [PubMed] [Google Scholar]
- Hlatky R, Valadka AB, Goodman JC, Contant CF, Robertson CS. Patterns of energy substrates during ischemia measured in the brain by microdialysis. J Neurotrauma. 2004;21:894–906. doi: 10.1089/0897715041526195. [DOI] [PubMed] [Google Scholar]
- Hovda DA, Yoshino A, Kawamata T, Katayama Y, Becker DP. Diffuse prolonged depression of cerebral oxidative metabolism following concussive brain injury in the rat: a cytochrome oxidase histochemistry study. Brain Res. 1991;3:1–10. doi: 10.1016/0006-8993(91)91429-5. [DOI] [PubMed] [Google Scholar]
- Hillered L, Persson L. Neurochemical monitoring of the acutely injured human brain. Scand J Clin Lab Invest Suppl. 1999;229:9–18. doi: 10.1080/00365519950185904. [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, Gupta AK, Fryer TF, Al-Rawi PG, Chatfield DA, Coles JP, O’Connell MT, Kett-White R, Minhas PS, Aigbirhio FI, Clark JC, Kirkpatrick PJ, Menon DK, Pickard JD. Correlation between cerebral blood flow, substrate delivery, and metabolism in head injury: a combined microdialysis and triple oxygen positron emission tomography study. J Cereb Blood Flow Metab. 2002;22:735–745. doi: 10.1097/00004647-200206000-00012. [DOI] [PubMed] [Google Scholar]
- Jennett B, Adams JH, Murray LS, Graham DI. Neuropathology in vegetative and severely disabled patients after head injury. Neurology. 2001;56:486–490. doi: 10.1212/wnl.56.4.486. [DOI] [PubMed] [Google Scholar]
- Kawamata T, Katayama Y, Aoyama N, Mori T. Heterogeneous mechanisms of early edema formation in cerebral contusion: diffusion MRI and ADC mapping study. Acta Neurochir Suppl. 2000;76:9–12. doi: 10.1007/978-3-7091-6346-7_2. [DOI] [PubMed] [Google Scholar]
- Kotapka MJ, Gennarelli TA, Graham DI, Adams JH, Thibault LE, Ross DT, Ford I. Selective vulnerability of hippocampal neurons in acceleration-induced experimental head injury. J Neurotrauma. 1991;8:247–258. doi: 10.1089/neu.1991.8.247. [DOI] [PubMed] [Google Scholar]
- Landolt H, Langemann H, Mendelowitsch A, Gratzl O. Neurochemical monitoring and on-line pH measruements using brain microdialysis in patients in intensive care. Acta Neurochir. 1994;60:47578. doi: 10.1007/978-3-7091-9334-1_130. [DOI] [PubMed] [Google Scholar]
- Langemann H, Alessandri B, Mendelowitsch A, Feuer-stein T, Landolt H, Gratzl O. Extracellular levels of glucose and lactate measured by quantitative microdialysis in the human brain. Neurol Res. 2001;23:531–536. doi: 10.1179/016164101101198785. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kelly DF, Oertel M, McArthur DL, Glenn TC, Vespa P, Boscardin WJ, Martin NA. Carbon dioxide reactivity, pressure autoregulation, and metabolic suppression reactivity after head injury: a transcranial Doppler study. J Neurosurg. 2001;95:222–232. doi: 10.3171/jns.2001.95.2.0222. [DOI] [PubMed] [Google Scholar]
- Marchal G, Benali K, Iglesias S, Viader F, Derlon JM, Baron JC. Voxel-based mapping of irreversible ischaemic damage with PET in acute stroke. Brain. 1999;122:2387–2400. doi: 10.1093/brain/122.12.2387. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Martin WR, Herscovitch P. Brain oxygen utilization measured with 0–15 radiotracers and positron emission tomography. J Nucl Med. 1984;25:177–187. [PubMed] [Google Scholar]
- Ohta S, Meyer E, Thompson CJ, Gjedde A. Oxygen consumption of the living human brain measured after a single inhalation of positron emitting oxygen. J Cereb Blood Flow Metab. 1992;12:179–192. doi: 10.1038/jcbfm.1992.28. [DOI] [PubMed] [Google Scholar]
- Persson L, Hillered L. Chemical monitoring of neurosurgical intensive care patients using intracerebral microdialysis. J Neurosurg. 1992;76:72–80. doi: 10.3171/jns.1992.76.1.0072. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Grubb RL, Jr, Darriet D, Raichle ME. Cerebral blood flow and cerebral metabolic rate of oxygen requirements for cerebral function and viability in humans. J Cereb Blood Flow Metab. 1985;5:600–608. doi: 10.1038/jcbfm.1985.89. [DOI] [PubMed] [Google Scholar]
- Sakoh M, Ostergaard L, Rohl L, Smith DF, Simonsen CZ, Sorensen JC, Poulsen PV, Gyldensted C, Sakaki S, Gjedde A. Relationship between residual cerebral blood flow and oxygen metabolism as predictive of ischemic tissue viability: sequential multitracer positron emission tomography scanning of middle cerebral artery occlusion during the critical first 6 hours after stroke in pigs. J Neurosurg. 2000;93:647–657. doi: 10.3171/jns.2000.93.4.0647. [DOI] [PubMed] [Google Scholar]
- Senda M, Alpert NM, Mackay BC, Buxton RB, Correia JA, Weise SB, Ackerman RH, Dorer D, Buonanno FS. Evaluation of the 11C02 positron emission tomographic method for measuring brain pH. II. Quantitative pH mapping in patients with ischemic cerebrovascular diseases. J Cereb Blood Flow Metab. 1989;9:859–873. doi: 10.1038/jcbfm.1989.120. [DOI] [PubMed] [Google Scholar]
- Sutton LN, McLaughlin AC, Dante S, Kotapka M, Sinwell T, Mills E. Cerebral venous oxygen content as a measure of brain energy metabolism with increased intracranial pressure and hyperventilation. J Neurosurg. 1990;73:927–932. doi: 10.3171/jns.1990.73.6.0927. [DOI] [PubMed] [Google Scholar]
- Valtysson J, Persson L, Hillered L. Extracellular ischaemia markers in repeated global ischaemia and secondary hypoxaemia monitored by microdialysis in rat brain. Acta Neurochir (Wien) 1998;140:387–395. doi: 10.1007/s007010050113. [DOI] [PubMed] [Google Scholar]
- Vespa PM, Nuwer MR, Nenov V, Ronne-Engstrom E, Hovda DA, Bergsneider M, Kelly DF, Martin NA, Becker DP. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroen-cephalographic monitoring. J Neurosurg. 1999;91:750–760. doi: 10.3171/jns.1999.91.5.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa P, McArthur D, Glenn T, O’Phelan K, Etchepare M, Kelly D, Bergsneider M, Martin NA, Hovda DA, Neurotrauma J. Persistently reduced levels of extracellular glucose early after traumatic brain injury correlate with poor outcome at six months: A microdialysis study. J Cereb Blood Flow Metab. 2003;23:865–877. doi: 10.1097/01.WCB.0000076701.45782.EF. [DOI] [PubMed] [Google Scholar]
- Vespa P, McArthur D, Alger J, O’Phelan K, Glenn T, Bergsneider B, Martin NA, Hovda DA. Regional heterogeneity of brain metabolism using cerebral microdialysis: concordance with magnetic resonance spectroscopy and positron emission tomography. Brain Pathol. 2004;14:210–214. doi: 10.1111/j.1750-3639.2004.tb00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigue B, Ract C, Benayed M, Zlotine N, Leblanc PE, Samii K, Bissonnette B. Early SjvO2 monitoring in patients with severe brain trauma. Intens Care Med. 1999;25:445–451. doi: 10.1007/s001340050878. [DOI] [PubMed] [Google Scholar]
- Vink R, Faden AI, Mcintosh TK. Changes in cellular bioenergetic state following graded traumatic brain injury in rats: determination by phosphorus 31 magnetic resonance spectroscopy. J Neurotrauma. 1988;5:315–330. doi: 10.1089/neu.1988.5.315. [DOI] [PubMed] [Google Scholar]
- Wilson JT, Pettigrew LE, Teasdale GM. Emotional and cognitive consequences of head injury in relation to the glasgow outcome scale. J Neurol Neurosurg Psychiatry. 2000;69:204–209. doi: 10.1136/jnnp.69.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HM, Huang SC, Hattori N, Glenn TC, Vespa PM, Yu CL, Hovda DA, Phelps ME, Bergsneider M. Selective metabolic reduction in gray matter acutely following human traumatic brain injury. J Neurotrauma. 2004;21:149–161. doi: 10.1089/089771504322778613. [DOI] [PubMed] [Google Scholar]
- Yoshino A, Hovda DA, Kawamata T, Katayama Y, Becker DP. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;4:106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- Yoshino A, Hovda DA, Katayama Y, Kawamata T, Becker DP. Hippocampal CA3 lesion prevents postcon-cussive metabolic dysfunction in CAl. J Cereb Blood Flow Metab. 1992;12:996–1006. doi: 10.1038/jcbfm.1992.137. [DOI] [PubMed] [Google Scholar]
- Yundt KD, Diringer MN. The use of hyperventilation and its impact on cerebral ischemia in the treatment of traumatic brain injury. Crit Care Clin. 1997;13:163–184. doi: 10.1016/s0749-0704(05)70300-6. [DOI] [PubMed] [Google Scholar]