Abstract
Objective
To evaluate sensitization, myofascial trigger points, and quality of life in women with chronic pelvic pain with and without endometriosis.
Methods
A cross-sectional prospective study of women aged 18 to 50 with pain suggestive of endometriosis and healthy, pain-free volunteers without history of endometriosis. Patients underwent a physiatric neuro-musculoskeletal assessment of clinical signs of sensitization and myofascial trigger points in the abdominopelvic region. Pain symptoms, psychosocial, and quality-of-life measures were also assessed. All pain participants underwent laparoscopic excision of suspicious lesions to confirm endometriosis diagnosis by histologic evaluation.
Results
Patients included 18 with current, biopsy-proven endometriosis, 11 with pain only, and 20 healthy volunteers. The prevalence of sensitization as measured by regional allodynia and hyperalgesia was similar in both pain groups (83% and 82%) but much lower among healthy volunteers (15%, p<0.001). Nearly all women with pain had myofascial trigger points (94% and 91%). Adjusting for study group, those with high anxiety (OR=1.05, 95% CI:1.004–1.099; p=0.031) and depression (OR=1.06, 95% CI:1.005–1.113; p=0.032) scores were more likely to have sensitization. Pain patients with any history of endometriosis had the highest proportion of sensitization compared to the others (87% v 67% v 15%; p<0.001). Adjusting for any history of endometriosis, those with myofascial trigger points were most likely sensitized (OR=9.41, 95% CI:1.77–50.08, p=0.009).
Conclusions
Sensitization and myofascial trigger points were common in women with pain regardless of whether they had endometriosis at surgery. Those with any history of endometriosis were most likely to have sensitization. Traditional methods of classifying endometriosis-associated pain based on disease, duration, and anatomy are inadequate and should be replaced by a mechanism-based evaluation, as our study illustrates.
Introduction
Endometriosis affects reproductive-aged women and is associated with chronic pelvic pain (1). Pain is challenging because pain severity and location do not correlate with endometriosis extent or lesion location (2,3). The relationship of lesions and hormonal environment to pain initiation, amplification and maintenance in individuals with endometriosis is poorly understood. Chronic pain broadly impacts an individual’s quality of life (4–6), resulting in depression, anxiety, and fatigue (2,7–9).
Chronic pain states are characterized by sensitization (10). Clinical manifestations of sensitization include regional allodynia and regional hyperalgesia (11–13). Central and peripheral sensitization are reported in regional pain syndromes including endometriosis (14,15), migraine (16), fibromyalgia (17), painful bladder syndrome (18), irritable bowel syndrome (19), and overlapping pain conditions (20).
Myofascial pain is a common, non-articular musculoskeletal disorder characterized by myofascial trigger points - hard, palpable, discrete, localized nodules within taut bands of skeletal muscle that are painful upon compression (21). Abdominal wall myofascial trigger points are reported in those with endometriosis (22) and in a rat endometriosis model where pain symptoms consist of vaginal hyperalgesia and increased abdominal muscle activity (23,24).
We compared clinical signs of sensitization and myofascial dysfunction in women with chronic pelvic pain and healthy volunteers. After evaluation, the pain group underwent laparoscopy to diagnose and treat endometriosis enabling classification of pain participants as having biopsy-proven endometriosis or not. Pain-free healthy participants had no endometriosis history or symptoms suggestive of endometriosis. Psychosocial and quality-of-life measures were also assessed. We hypothesized that women with chronic pelvic pain, regardless of endometriosis, would be more likely to have clinical signs of sensitization and myofascial dysfunction and impaired quality of life.
Materials and Methods
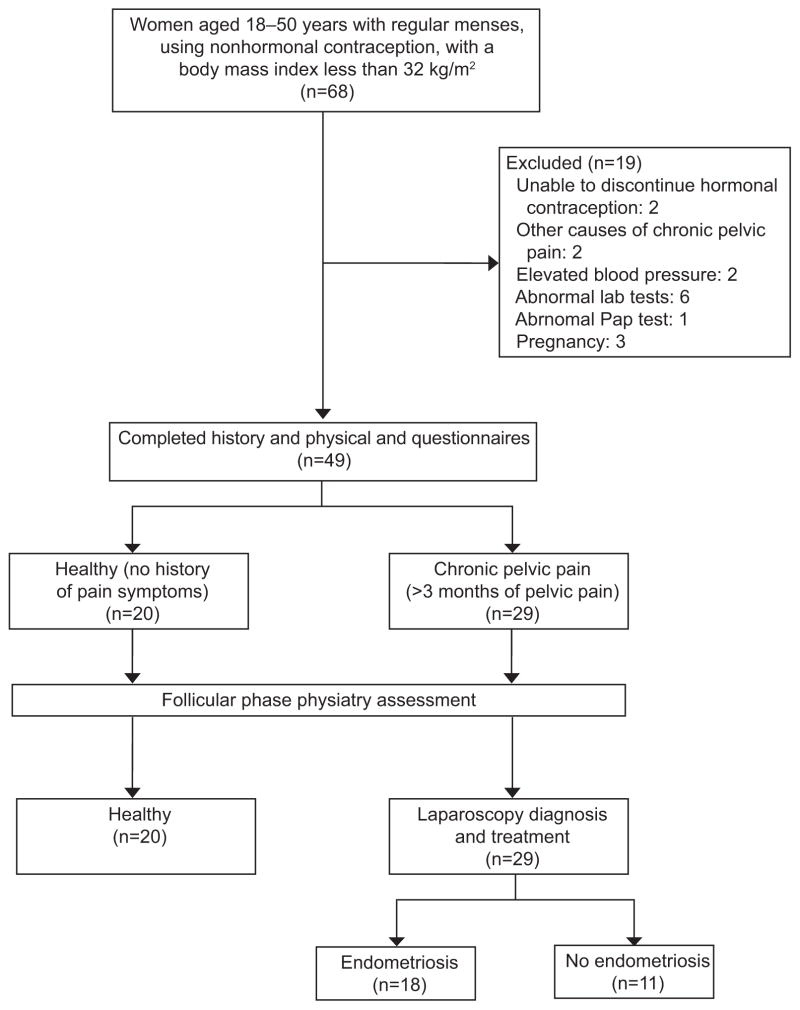
Women between ages 18 and 50 were recruited at the Clinical Center, National Institutes of Health, for a prospective study approved by the NICHD IRB (NCT00073801) from April 2004 to 2011. Women with chronic pelvic pain symptoms suggestive of endometriosis including dysmenorrhea, dyspareunia, and non-menstrual pain for at least 3 months were enrolled. Participants were then later categorized as having endometriosis or not based on histology findings at a study surgery. We also included women with no history of these pain symptoms or of endometriosis, as a healthy control group (Figure 1). All participants were not pregnant, had regular menstrual cycles, were not on any hormonal treatment, had not had recent surgical treatment and were otherwise healthy. We excluded those who had pain symptoms initiated by other causes, including infections, thyroid disease, autoimmune diseases, gastrointestinal disease, or fibromyalgia. In addition, we excluded participants with abnormal renal or liver function more than twice the normal range. Each participant was assessed by self-report and structured interview for headaches, depression, and abuse, sexually transmitted diseases, and gynecologic conditions and underwent a physical examination including a pelvic examination and laboratory assessments including gonorrhea and chlamydia by PCR (Figure 1). At pelvic examination, patients were assessed for levator muscle spasm. Each woman’s cycle phase was confirmed by menstrual calendar, twice weekly assessment of estradiol and progesterone levels, and use of a luteinizing hormone kit (Ovuquick, Conception Technologies, San Diego, CA). After one month in the study, those with chronic pelvic pain underwent laparoscopic surgery at which all lesions suspicious of endometriosis were excised and the diagnosis of endometriosis was confirmed by histologic evaluation as described in our previous studies (25) (Figure 1).
Figure 1.
Study design. Those with chronic pelvic pain underwent laparoscopic surgery to assess for current diagnosis of endometriosis (n = 18) or no endometriosis (n = 11) confirmed on biopsy of all suspicious lesions. Of those without histology-confirmed endometriosis at study laparoscopy, six had a history of endometriosis (three had adhesions, one had fibroids, and two were without surgical findings). Those without prior history of endometriosis included: two women with adhesions, two without findings at surgery (one of these reported a history of abuse; the other had a vagal response to intraoperative bowel manipulation), and one had an urachal cyst and possible interstitial cystitis.
Pain symptoms were prospectively assessed prior to surgery. The presence and severity of pain within the previous month was assessed using a visual analog scale (VAS) in which the level of pain was rated from 0–10 (no pain–worst pain). A VAS score of 4 or more was classified as having pain, based on the IMMPACT guidelines (26). All participants also underwent a comprehensive neuro-musculoskeletal assessment performed by a physiatrist blinded to study group. Since pain perception varies across the menstrual cycle (27), neuro-musculoskeletal system assessment was restricted to the follicular phase (Figure 1).
The neuro-musculoskeletal assessment (Appendix 1, available online at http://links.lww.com/xxx) included clinical signs of sensitization, measurement of local tenderness or pressure-pain-threshold over the supraspinous ligament, clinical signs of myofascial dysfunction, and measurement of pressure-pain-threshold of muscles. Both allodynia (pain due to a non-noxious stimulus) and hyperalgesia (an increased pain response to a noxious stimulus) were assessed bilaterally over the skin, approximately 2.5 cm lateral to the spinous process, for each spinal segment from thoracic-9 through sacral-2. Allodynia was assessed using a pinch and roll technique (28,29). Hyperalgesia was assessed by rolling a Wartenberg pinwheel vertically from cephalad to caudad along the skin adjacent to the spinous processes (30). During the evaluation, for each segmental level, the subject’s report of whether the stimulus evoked pain (allodynia) or increased pain (hyperalgesia) was recorded. Women with six or more affected segments on a side were classified as having regional allodynia or regional hyperalgesia, respectively, as this indicates that more than half of the assessed segments were affected. Sensitization was defined as the presence of either regional allodynia or regional hyperalgesia.
The threshold at which pressure elicits pain (pressure-pain-threshold) can be measured, with lower thresholds observed in affected areas (8,31,32). A pressure algometer (Pain Diagnostics and Treatment, Great Neck, NY) was used to measure the pressure-pain-threshold (29) over the supraspinous ligament, between each vertebra from thoracic-9 to sacral-2. A lowered pressure-pain-threshold was defined as less than 9 lbs/cm2 (31) in six or more segments per side, as this indicated that more than half of the assessed segments were affected.
The physiatrist who performed the neuro-musculoskeletal assessment also had expertise in assessing myofascial trigger points and examined seven paired muscles: the iliacus (deep to the anterior superior iliac spine), external oblique, rectus abdominis, adductor longus, adductor magnus, vastus medialis, and gluteus maximus. Some of these muscles share segmental innervation with pelvic floor muscles (lumbar-5 to sacral-5) while others are innervated by adjacent segments. Myofascial dysfunction was defined as having myofascial trigger points in four or more muscles per side, identified through palpation, as this indicated that more than half of the assessed muscles were affected. Pressure-pain-threshold of each paired muscle was also assessed. If a myofascial-trigger-point was palpated in the muscle, pressure-pain-threshold was measured at the trigger point; otherwise, pressure-pain-threshold was measured over the uninvolved muscle. A subject was classified as having a lowered pressure-pain-threshold if the measurement was less than 4 lbs/cm2 (8) in at least four muscle groups per side, as this indicated that more than half of the assessed muscles were affected.
All participants completed the Duke Health Profile and Endometriosis Health Profile-30 to assess the impact of pain on quality of life and other psychosocial variables (Figure 1) (4,33). The Duke Health Profile, a 17-item general health-quality of life questionnaire, assessed patients’ perceptions of themselves, their health, and their relationships and was used to create a score for six health (physical, mental, social, general, perceived health, and self-esteem) and four self-reported functional measures (anxiety, depression, pain, and disability). The Endometriosis Health Profile-30 questionnaire assessed health-related quality of life, specifically in women with endometriosis, to evaluate work life, sexual intercourse, treatment, infertility, and their relationship with their children and the medical profession.
Participants were grouped by whether they currently had pain and biopsy-proven endometriosis diagnosed at study laparoscopy as: 1) women with endometriosis and chronic pelvic pain; 2) women with chronic pelvic pain only; or 3) healthy volunteers. The clinical findings of persistent sensitization and myofascial dysfunction are suggestive of a chronic pain state and can manifest even long after a disease has been treated. Thus, further analyses included: 1) women with any prior history of surgically-diagnosed endometriosis along with those currently diagnosed with endometriosis at study laparoscopy; 2) women with pain only and no history of endometriosis at any surgery in a second group; and 3) healthy volunteers.
Data were described by frequency distributions and simple descriptive statistics, and are reported as percents or means ± standard deviation (SD), respectively. Unordered categorical variables were analyzed by Fisher’s exact tests; ordered categories were analyzed by the Kruskal-Wallis or Jonckheere-Terpstra test for trend, as appropriate. Continuous variables were analyzed between two groups by t-tests, among the three groups by analysis of variance (ANOVA), and repeated measures by mixed modeling when appropriate; post-hoc tests were corrected by the Bonferroni adjustment and, where applicable, only adjusted p-values are reported. Wilcoxon rank sum and Kruskal-Wallis tests compared non-parametric continuous variables with two and three groups, respectively. Trends based on continuous variables were assessed by the Abelson-Tukey linear contrast ANOVA. Logistic regression modeling adjusted for study group or history of endometriosis when assessing various central sensitization and myofascial dysfunction associations, and results are reported as Odds Ratios (OR) with 95% Confidence Intervals (CI). A p-value less than or equal to 0.05 was considered statistically significant. All data were analyzed using SAS v 9.2 (SAS Institute, Inc, Cary, NC). Post hoc analysis showed the study was adequately powered (beta<0.20, alpha=0.05) for its primary endpoints of sensitization and myofascial dysfunction comparing the three study groups.
Results
Eighteen patients with current, biopsy-proven endometriosis and chronic pelvic pain, 11 patients with chronic pelvic pain without biopsy-proven endometriosis (pain only), and 20 healthy volunteers were assessed. Of the 49 participants, 36 were non-Hispanic Caucasian, nine were non-Hispanic Black, three were Asian, and one was Hispanic (Table 1). Age, BMI, and race or ethnicity did not differ among groups, but history of abuse was significantly different, with chronic pelvic pain only reporting the most. Over two-thirds of women with chronic pelvic pain, regardless of the diagnosis of endometriosis, had a history of migraine headaches (Table 1). Overall, those with migraines were more likely to be sensitized, an association that did not persist after adjusting for group, as those with migraine headaches all had chronic pelvic pain. In considering those with endometriosis, sensitization did not occur more frequently among those with deep infiltrating lesions (compared to those with superficial lesions) or moderate-to-severe endometriosis (compared to those with minimal or mild endometriosis). Sensitization rates were similar among 10 women reporting a history of abuse, regardless of groups.
Table 1.
Demographics and clinical characteristics in women with chronic pelvic pain with or without current diagnosis of biopsy-proven endometriosis, and healthy volunteers
| Pain and current biopsy-proven endometriosis CPP-endo-now (N=18) n (%) |
Pain only CPP- no-endo-now (N=11) n (%) |
Healthy Volunteers (N=20) n (%) |
Total (N=49) n (%) |
P-value | |
|---|---|---|---|---|---|
| Age, years [mean (SD)] | 32.1 (8.0) | 35.9 (9.3) | 35.4 (8.8) | 34.3 (8.6) | 0.456 |
| BMI [mean (SD)] | 24.8 (4.3) | 26.3 (4.2) | 23.7 (4.3) | 24.7 (4.3) | 0.205 |
| Race | |||||
| White, Non-Hispanic | 14 (78%) | 8 (73%) | 14 (70%) | 36 (74%) | 0.898 |
| Black, Non-Hispanic | 3 (17%) | 3 (27%) | 3 (15%) | 9 (18%) | |
| Hispanic | 0 | 0 | 1 (5%) | 1 (2%) | |
| Asian | 1 (6%) | 0 | 2 (10%) | 3 (6%) | |
| History of self-reported abusea (N=40) | 1 (6%) | 5 (45%) | 4 (36%) | 10 (25%) | 0.024 |
| Migraine headache | 11 (61%) | 7 (64%) | 0 | 18 (38%) | <0.001 |
| Non-migraine headache | 1 (6%) | 1 (9%) | 3 (17%) | 5 (11%) | 1.0 |
| Duration of Chronic Pelvic Pain, years [mean (SD)] | 11.6 (6.1) | 11.6 (9.4) | --- | 11.6 (7.4) | 1.0 |
| Biopsy-confirmed Endometriosis | |||||
| Stage I | 7 (39%) | --- | --- | --- | --- |
| Stage II | 3 (17%) | --- | --- | --- | --- |
| Stage III | 5 (28%) | --- | --- | --- | --- |
| Stage IV | 3 (17%) | --- | --- | --- | --- |
| Deep lesions | 8 (44%) | --- | --- | --- | --- |
| Superficial lesions | 10 (56%) | --- | --- | --- | --- |
Data are n (%), unless otherwise indicated; SD = standard deviation; --- = not applicable
P-values are from comparisons among the three study groups.
Percents are based on a total denominator of 40, as 9 healthy volunteers were missing data on self-reported abuse.
The occurrence of pain (VAS over 4) on the day of evaluation and in the last month was similar in the pain groups; pain level over 4 in the last month was reported more commonly in the current biopsy-proven endometriosis group compared to the other groups (p<0.001). Levator spasm was significantly more common in women with current biopsy-proven endometriosis compared to those pain only versus healthy volunteers (61% versus 36 % versus 0%; p<0.001).
Regional allodynia was detected more commonly in women with pain than healthy volunteers (p<0.001); however, women with chronic pelvic pain had similar proportions, regardless of whether they currently had endometriosis (Table 2). Regional hyperalgesia was observed most commonly in women with current biopsy-proven endometriosis compared to those with pain only, and both were significantly more common than healthy volunteers (p<0.001). Allodynia and hyperalgesia for the left and right side of the body involved more segments for women with chronic pelvic pain, regardless of whether they currently had endometriosis, compared to very few segments for healthy volunteers (all p<0.001; see Appendix 2 online at http://links.lww.com/xxx). Women within either chronic pelvic pain group had a lower pressure-pain-threshold of the supraspinous ligaments at more segments compared to healthy volunteers (see Appendix 2 online at http://links.lww.com/xxx; p=0.003). The prevalence of clinical signs of sensitization was similar in both chronic pelvic pain groups (83% versus 82%), but much lower among healthy volunteers (15%, p<0.001; Table 2). Healthy volunteers without myofascial trigger points tended to have less evidence of sensitization (p=0.046).
Table 2.
Sensitization and myofascial trigger points in women with chronic pelvic pain with or without and current diagnosis of biopsy-proven endometriosis, and healthy volunteers
| Pain and current biopsy-proven endometriosis CPP-endo-now (N=18) n (%) |
Pain only CPP-no-endo-now (N=11) n (%) |
Healthy Volunteers (N=20) n (%) |
P-value | |
|---|---|---|---|---|
| Sensitization | 15 (83%) | 9 (82%) | 3 (15%) | <0.001 |
| Regional Allodynia | 14 (78%) | 9 (82%) | 3(15%) | <0.001 |
| Regional Hyperalgesia | 14 (78%) | 7 (64%) | 1(5%) | <0.001 |
| Lowered pressure-pain-threshold PPT of supraspinous ligament | 9 (50%) | 5 (45%) | 1(5%) | 0.003 |
| Myofascial-trigger-points TrPs | 17 (94%) | 10 (91%) | 3(15%) | <0.001 |
| Lowered pressure-pain-threshold PPT of muscles | 4(22%) | 1(9%) | 0 | 0.026 |
| Pain in last month (VAS> 4) | 16 (89%) | 7 (64%) | 0 | <0.001 |
P-values are from tests for trend comparing chronic pelvic pain and current biopsy-proven endometriosis CPP-endo-now vs versus chronic pelvic pain only (without current biopsy-proven endometriosis) CPP-no-endo-now versus vs healthy volunteers.
CPP-endo-now comprises women diagnosed with endometriosis at laparoscopy in this study.
PPT = pressure pain threshold
Myofascial TrPs = myofascial trigger points
VAS = Visual Analogue Scale
Nearly all women with chronic pelvic pain had myofascial trigger points, which occurred more commonly than in healthy volunteers (p<0.001). The average pressure-pain-threshold in myofascial trigger points was statistically significantly higher in either pain group compared to healthy volunteers (see Appendix 3 online at http://links.lww.com/xxx; p<0.001 for both). Adjusting for study group, those with clinical signs of myofascial dysfunction were more likely to have clinical signs of sensitization (OR=6.81, 95% CI: 1.04, 44.36, p=0.045). Eighty-seven percent of those with levator spasm had myofascial dysfunction whereas 45% of those with myofascial dysfunction had levator spasm.
As shown in Table 3, chronic pelvic pain patients with any history of endometriosis had the highest prevalence of regional allodynia (p<0.001) and regional hyperalgesia (p<0.001), resulting in higher proportions of clinical evidence of central sensitization (87%) compared to those with pain who never had endometriosis (67%) and healthy volunteers (15%, p<0.001). Adjusting for any history of endometriosis, those with myofascial dysfunction were most likely to have clinical signs of sensitization (OR=9.41, 95% CI: 1.77–50.08; p=0.009). Lowered pressure-pain-thresholds in muscles were only observed in the group with any history of endometriosis (p=0.018), but not the other two groups.
Table 3.
Sensitization and myofascial trigger points in women with any history of endometriosis and chronic pelvic pain, and healthy volunteers
| Pain and current or prior history of endometrisois CPP-any-endo-history (N=23) n (%) |
Pain and never history of endometriosis CPP-never-endo (N=6) n (%) |
Healthy Volunteers (N=20) n (%) |
P-value | |
|---|---|---|---|---|
| Sensitization | 20 (87%) | 4 (67%) | 3 (15%) | <0.001 |
| Regional Allodynia | 19 (83%) | 4 (67%) | 3 (15%) | <0.001 |
| Regional Hyperalgesia | 17 (74%) | 4 (67%) | 1 (5%) | <0.001 |
| Lowered pressure-pain-threshold PPT of supraspinous ligament | 11 (48%) | 3 (50%) | 1 (5%) | 0.003 |
| Myofascial-trigger-points TrPs | 21 (91%) | 6 (100%) | 3 (15%) | <0.001 |
| Lowered pressure-pain-threshold PPT of muscles | 5 (22%) | 0 | 0 | 0.018 |
P-values are from tests for trend comparing pain and current or prior history of endometriosis versus pain and never any history of endometriosis versus CPP-endo-now vs CPP-no-endo-now vs healthy volunteers.
Chronic pelvic pain with any endometriosis history CPP-any-endo-history comprises all women with any history of surgically-diagnosed endometriosis in the past or those diagnosed with endometriosis at the study laparoscopy
PPT = pressure pain threshold
Myofascial TrPs = myofascial trigger points
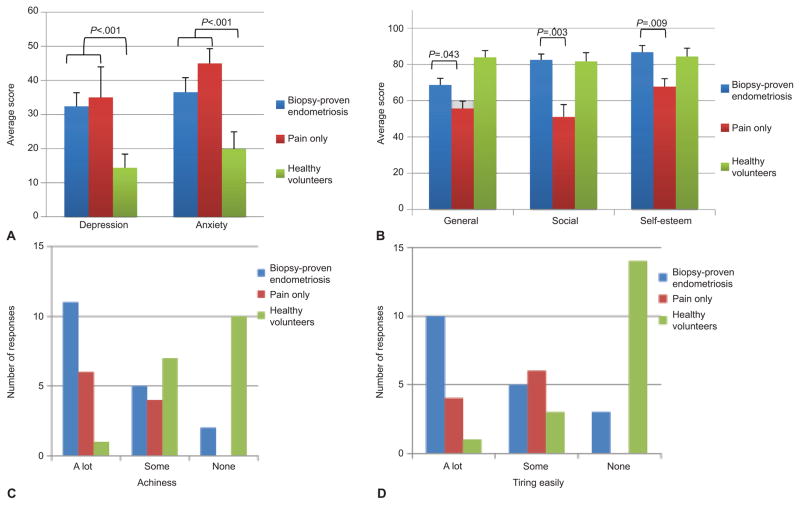
Table 4 and Figure 2 describe summary scores of the various sections of the Endometriosis Health Profile-30 and Duke Health Profile, with statistically significant differences among the study groups in nearly all areas. In assessing the pattern of depression and anxiety scores, statistically significant trends were observed with worst scores in current biopsy-proven endometriosis, followed by those with pain only, then healthy volunteers (p<0.001 and p<0.001, respectively; Figure 2A). Adjusting for study group, those with high anxiety and depression scores (anxiety: OR=1.05, 95% CI: 1.004–1.099; p= 0.031; depression: OR=1.06, 95% CI: 1.005–1.113; p=0.032) were more likely to have clinical signs of sensitization.
Table 4.
Quality of life scores from the Duke Health Profile and Endometriosis Health Profile-30 in women with chronic pelvic pain (with and without and current biopsy-proven diagnosis of endometriosis), and healthy volunteers
| Pain and current biopsy-proven endometriosis CPP-endo-now (N=18) Mean (SD) |
Pain without current biopsy-proven endometriosis only CPP-no-endo-now (N=11) Mean (SD) |
Healthy Volunteers (N=20) Mean (SD) |
P-value | |
|---|---|---|---|---|
| Duke Health Profile | ||||
| Physical health | 48.9 (29.7) | 42.0 (18.7) | 84.4 (17.6) | <0.001 |
| Mental health | 75.6 (15.8) | 69.0 (18.5) | 85.6 (17.2) | 0.001 |
| Social healtha | 81.7 (12.9) | 56.0 (21.2) | 81.7 (19.8) | 1.0 |
| General healtha | 68.7 (15.5) | 55.7 (13.0) | 83.9 (15.9) | <0.001 |
| Pain | 75.0 (35.4) | 80.0 (25.8) | 25.0 (30.9) | <0.001 |
| Disability | 16.7 (24.3) | 20.0 (25.8) | 0 | <0.001 |
| Self-esteema | 86.1 (15.4) | 71.0 (13.7) | 84.4 (19.2) | 0.6 |
| Anxiety | 36.6 (17.7) | 45.0 (13.7) | 19.9 (21.0) | <0.001 |
| Depression | 32.4 (16.9) | 35.0 (13.5) | 14.4 (17.1) | <0.001 |
| Perceived health*c | 80.6 (25.1) | 55.0 (28.4) | 91.7 (19.2) | <0.001 |
| Endometriosis Health Profile-30 | ||||
| Pain | 42.9 (24.3) | 40.3 (23.0) | 4.1 (9.2) | <0.001 |
| Sense of control | 62.5 (35.7) | 57.8 (36.1) | 4.6 (9.0) | <0.001 |
| Emotional well-being | 29.9 (20.6) | 34.9 (23.1) | 8.3 (9.7) | <0.001 |
| Social support | 33.7 (26.6) | 43.0 (26.8) | 2.8 (7.6) | <0.001 |
| Self image | 28.2 (22.9) | 30.2 (25.6) | 7.6 (12.0) | <0.001 |
| Work life | 42.5 (28.4) | 25.0 (21.4) | 3.1 (8.8) | <0.001 |
| Concern about children | 42.5 (39.1) | 0 | 0 | 0.002 |
| Sexual intercourse | 47.2 (32.9) | 54.6 (32.5) | 1.0 (2.2) | <0.001 |
| Feelings about medical profession | 17.9 (26.7) | 16.3 (23.2) | 0 | 0.002 |
| Feelings about treatment | 33.3 (26.7) | 31.2 (20.8) | 0 | <0.001 |
| Concern about infertility | 38.6 (13.9) | 56.3 (6.3) | 0 | 0.009 |
P-values are from tests for trend comparing pain and current biopsy-proven endometriosis CPP-endo-now versus pain without biopsy-proven endometriosis CPP-no-endo-now versus healthy volunteers.
p<0.05 for the comparison between chronic pelvic pain subsets
Figure 2.
Duke Health Profile scores by study group. A. A trend of worse scores was observed in current biopsy-proven endometriosis followed by pain only, then healthy volunteers for depression and anxiety (P<.001 for both). B. General health scores were statistically significantly different among all three groups (P<.001); however, social health and self-esteem scores were not (P=1.0 and P=0.6, respectively). Women with pain only had worse general (P=.043) and social (P =.003) health with lower self-esteem (P=.009) than women with current biopsy-proven endometriosis. C. Current biopsy-proven endometriosis patients were most likely to indicate achiness (P <.001). D. Current biopsy-proven endometriosis were most likely to indicate tiring easily (P <.001) though both pain groups together indicated tiring easily more often than healthy volunteers (P <.001).
In comparing the three groups, current biopsy-proven endometriosis patients were most likely to indicate achiness and tiring easily (p<0.001 and p<0.001, respectively; Figures 2C and 2D) on the Duke Health Profile. Participants suffering from pain also reported more fatigue; those with current biopsy-proven endometriosis were most likely to indicate being unable to sleep properly (p=0.002) and need to lie down or rest (p<0.001).
Discussion
Clinical signs of sensitization and myofascial dysfunction were documented in the abdomino-pelvic regions of women with endometriosis-associated chronic pelvic pain. Myofascial dysfunction, as extensive myofascial trigger points, was present in nearly all pain patients. All pain patients had clinical evidence of sensitization (i.e. regional allodynia and/or regional hyperalgesia). However, women with any history of endometriosis were most likely to have sensitization when compared to those without a history of endometriosis and healthy volunteers. Similarly, adjusting for any endometriosis history, those with myofascial trigger points were most likely to have sensitization. These findings suggest that long-term remodeling of the central nervous system (resulting in allodynia, hyperalgesia, and myofascial dysfunction) may persist after lesions are treated in women with history of endometriosis. Accordingly, any history of endometriosis (trait) may be more clinically relevant to initiating, amplifying and maintaining pain than the current finding of endometriotic lesions (state).
Prolonged noxious stimulation leads to central sensitization in which the central nervous system changes, distorts, or amplifies the perception of pain (7,12). This neuroplasticity is responsible for sustaining pain states (34), and may be measured by clinical signs of sensitization.
Painful endometriotic lesions send noxious signals to wide dynamic range spinal cord neurons. Anti-dromic signals are sent to somatic structures within the same levels resulting in segmentally-related allodynia, hyperalgesia, and myofascial trigger points in abdomino-pelvic skeletal muscles, as we observed. Jarrell demonstrated that abdominal wall myofascial trigger points correlated with endometriosis in patients with chronic pelvic pain and myofascial dysfunction (22). Visceral disease was diagnosed in 90% of women with myofascial trigger points but absent in 64% without palpable myofascial trigger points. Painful myofascial trigger points can sensitize segmentally-related visceral structures. Symptoms without apparent visceral disease create diagnostic confusion and possibly result in unnecessary (or harmful) procedures.
Although ectopic growths could be a nociceptive source in painful endometriosis, the relationship between lesions and pain is unclear. Women with minimal disease (at surgery) were most likely to complain of pelvic pain sooner, suggesting sensitization occurred before surgery (1). Our findings suggest that endometriosis may initiate sensitization and myofascial pain via neurogenic inflammation.
Chronic pain broadly impacts one’s social life, mental health, and physical ability resulting in depression, anxiety and fatigue (35). Those with depression and anxiety were more likely to have sensitization. Our findings suggest that reduced quality-of-life might contribute to the neurobiological underpinnings of chronic pain (36). Multidimensional treatment strategies addressing psychosocial factors in addition to pain warrant study.
This study has several strengths. First, no patients were using hormones or had recent surgery for endometriosis. Prior surgical diagnosis of endometriosis was ascertained. Additionally, women had regular menstrual cycles and lacked co-morbidities associated with pain. Importantly, women with pain underwent laparoscopy, in which all suspicious endometriosis lesions were excised and examined by pathology. The neuro-musculoskeletal assessment was restricted to the follicular phase to minimize hormonal variations. Lastly, the physiatrist was blinded to study cohort and had expertise in neuro-musculoskeletal assessments.
This study has limitations. The absence of endometriosis was confirmed in only one healthy woman who underwent tubal ligation and women with pain-free endometriosis undergoing surgery were not recruited. Group and subgroup sample sizes were small, limiting power for secondary analyses. Additionally, study participants with pain had symptoms suggesting endometriosis which limited the number with pain without an endometriosis history. We did not assess sensitization in locations remote from the pelvis nor did we identify a second physiatrist skilled in performing these neuro-musculoskeletal exams which hampered our ability to determine inter-rater reliability and may limit generalizability (37,38). The reproducibility of these techniques and findings warrant confirmation, given our small sample size. Depression, abuse and anxiety were defined using only self-reported data. Catastrophization was not assessed.
Nevertheless, our findings suggest a comprehensive neuro-musculoskeletal exam utilizing objective findings of allodynia, hyperalgesia, pressure-pain-threshold, and myofascial trigger points, better describes a pain experience and may reveal potential sources of persistent somatic or visceral nociception (39). This simple, office-based exam is useful for evaluating pain because it is poorly localized, of unpredictable intensity, and may manifest in structures unrelated to or remote from endometriosis lesions. Our findings are consistent with research on sensitization and the association of myofascial trigger points with other pain syndromes (10,11,40,41).
Traditional methods of classifying pain based on disease, duration, and anatomy are inadequate and being replaced by a mechanism-based evaluation. The development of successful treatment approaches depends upon targeting mechanisms and perpetuating factors of this common pain syndrome. Accordingly, clinicians and investigators should expand their focus beyond lesions to include pain assessment. Ultimately, this broader focus will enable a better understanding of how endometriosis affects the central nervous system, compared to mechanisms underlying other chronic pain conditions.
Supplementary Material
Acknowledgments
Funded by the Intramural Program of the NIH, Clinical Center, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and clinical trial NCT00073801.
The authors thank the OR nurses, clinic staff, research staff and the Reproductive Endocrinology staff and fellows for their help in conducting this study.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented in part at the Society for Gynecologic Investigation, Glasgow, Scotland, March 17–21, 2009, American Society for Reproductive Medicine, Denver, CO, October 18–21, 2010, and Society for Gynecologic Investigation, Miami Beach, FL, March 17–19, 2011.
Clinical Trial Registration: ClinicalTrials.gov, www.clinicaltrials.gov, NCT00073801.
References
- 1.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011 May-Jun;17(3):327–46. doi: 10.1093/humupd/dmq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007 Jan;22(1):266–71. doi: 10.1093/humrep/del339. [DOI] [PubMed] [Google Scholar]
- 3.Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011 Aug;118(2 Pt 1):223–30. doi: 10.1097/AOG.0b013e318223fed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones G, Kennedy S, Barnard A, Wong J, Jenkinson C. Development of an endometriosis quality-of-life instrument: The Endometriosis Health Profile-30. Obstet Gynecol. 2001 Aug;98(2):258–64. doi: 10.1016/s0029-7844(01)01433-8. [DOI] [PubMed] [Google Scholar]
- 5.Jones G, Jenkinson C, Kennedy S. The impact of endometriosis upon quality of life: a qualitative analysis. J Psychosom Obstet Gynaecol. 2004 Jun;25(2):123–33. doi: 10.1080/01674820400002279. [DOI] [PubMed] [Google Scholar]
- 6.Laursen BS, Bajaj P, Olesen AS, Delmar C, Arendt-Nielsen L. Health related quality of life and quantitative pain measurement in females with chronic non-malignant pain. Eur J Pain. 2005 Jun;9(3):267–75. doi: 10.1016/j.ejpain.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007 Aug 2;55(3):365–76. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Giesecke T, Williams DA, Harris RE, Cupps TR, Tian X, Tian TX, et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003 Oct;48(10):2916–22. doi: 10.1002/art.11272. [DOI] [PubMed] [Google Scholar]
- 9.Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005 Nov-Dec;11(6):595–606. doi: 10.1093/humupd/dmi029. [DOI] [PubMed] [Google Scholar]
- 10.Clauw DJ. Perspectives on fatigue from the study of chronic fatigue syndrome and related conditions. PMR. 2010;2(5):414–30. doi: 10.1016/j.pmrj.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011 Mar;152(3 Suppl):S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009 Sep;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007 Jun;36(6):339–56. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Bajaj P, Madsen H, Arendt-Nielsen L. Endometriosis is associated with central sensitization: a psychophysical controlled study. J Pain. 2003 Sep;4(7):372–80. doi: 10.1016/s1526-5900(03)00720-x. [DOI] [PubMed] [Google Scholar]
- 15.As-Sanie S, Harris RE, Harte SE, Tu FF, Neshewat G, Clauw DJ. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet Gynecol. 2013 Nov;122(5):1047–55. doi: 10.1097/AOG.0b013e3182a7e1f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giamberardino MA, Tafuri E, Savini A, Fabrizio A, Affaitati G, Lerza R, et al. Contribution of myofascial trigger points to migraine symptoms. J Pain. 2007 Nov;8(11):869–78. doi: 10.1016/j.jpain.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Labat JJ, Guerineau M, Delavierre D, Sibert L, Rigaud J. Symptomatic approach to musculoskeletal dysfunction and chronic pelvic and perineal pain. Prog Urol. 2010 Nov;20(12):982–9. doi: 10.1016/j.purol.2010.08.059. [DOI] [PubMed] [Google Scholar]
- 18.Dell JR, Mokrzycki ML, Jayne CJ. Differentiating interstitial cystitis from similar conditions commonly seen in gynecologic practice. Eur J Obstet Gynecol Reprod Biol. 2009 Jun;144(2):105–9. doi: 10.1016/j.ejogrb.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 19.Mease PJ, Clauw DJ, Arnold LM, Goldenberg DL, Witter J, Williams DA, et al. Fibromyalgia syndrome. J Rheumatol. 2005 Nov;32(11):2270–7. [PubMed] [Google Scholar]
- 20.Giamberardino MA, Costantini R, Affaitati G, Fabrizio A, Lapenna D, Tafuri E, et al. Viscero-visceral hyperalgesia: characterization in different clinical models. Pain 2010. 2010 Nov;151(2):307–22. doi: 10.1016/j.pain.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 21.Shah JP, Gilliams EA. Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: an application of muscle pain concepts to myofascial pain syndrome. J Body Mov Ther. 2008 Oct;12(4):371–84. doi: 10.1016/j.jbmt.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Jarrell J. Myofascial dysfunction in the pelvis. Curr Pain Headache Rep. 2004 Dec;8(6):452–6. doi: 10.1007/s11916-004-0066-0. [DOI] [PubMed] [Google Scholar]
- 23.Berkley KJ, Cason A, Jacobs H, Bradshaw H, Wood E. Vaginal hyperalgesia in a rat model of endometriosis. Neurosci Lett. 2001 Jun 29;306(3):185–8. doi: 10.1016/s0304-3940(01)01906-1. [DOI] [PubMed] [Google Scholar]
- 24.Berkley KJ, McAllister SL, Accius BE, Winnard KP. Endometriosis-induced vaginal hyperalgesia in the rat: effect of estropause, ovariectomy, and estradiol replacement. Pain. 2007 Nov;132(Suppl 1):S150–9. doi: 10.1016/j.pain.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stegmann BJ, Sinaii N, Liu S, Segars J, Merino M, Nieman LK, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008 Jun;89(6):1632–6. doi: 10.1016/j.fertnstert.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003 Dec;106(3):337–45. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Giamberardino MA, Berkley KJ, Iezzi S, de Bigontina P, Vichy L. Pain threshold variations in somatic wall tissues as a function of menstrual cycle, segmental site and tissue depth in non-dysmenorrheic women, dysmenorrheic women and men. Pain. 1997 Jun;71(2):187–97. doi: 10.1016/s0304-3959(97)03362-9. [DOI] [PubMed] [Google Scholar]
- 28.Fischer A. Functional Diagnosis of Musculoskeletal Pain and Evaluation of Treatment Results by Quantitative and Objective Techniques. In: ESR, editor. Myofascial pain and fibromyalgia: trigger point management. 2. St. Louis: Mosby; 2002. pp. 145–73. [Google Scholar]
- 29.Fischer AA. Algometry in diagnosis of musculoskeletal pain and evaluation of treatment outcome: an update. J Musculoskelet Pain. 1998;6(1):5–32. [Google Scholar]
- 30.Schafer RCaACA. Basic chiropractic procedural manual, emphasizing geriatric considerations. 4. Arlington, Virginia: Associated Chiropractic Academic Press for the American Chiropractic Association; 1984. [Google Scholar]
- 31.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990 Feb;33(2):160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 32.Gracely RH, Grant MA, Giesecke T. Evoked pain measures in fibromyalgia. Best Pract Res Clin Rheumatol. 2003 Aug;17(4):593–609. doi: 10.1016/s1521-6942(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 33.Parkerson GR, Jr, Broadhead WE, Tse CK. The Duke Health Profile. A 17-item measure of health and dysfunction. Med Care. 1990 Nov;28(11):1056–72. doi: 10.1097/00005650-199011000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Imamura M, Imamura ST, Kaziyama HH, Targino RA, Hsing WT, de Souza LP, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis Rheum. 2008 Oct 15;59(10):1424–31. doi: 10.1002/art.24120. [DOI] [PubMed] [Google Scholar]
- 35.Weisberg JN, Boatwright BA. Mood, anxiety and personality traits and states in chronic pain. Pain. 2007 Dec 15;133(1–3):1–2. doi: 10.1016/j.pain.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and naturopathic pain. Front Basic. 2009;14:5291–338. doi: 10.2741/3598. [DOI] [PubMed] [Google Scholar]
- 37.Erwin RD, Shannon S, Hong CZ, Hubbard D, Evarts R. Interpreter reliability in myofascial trigger point examination. Pain. 1997 Jan;69(1–2):65–73. doi: 10.1016/s0304-3959(96)03248-4. [DOI] [PubMed] [Google Scholar]
- 38.Barber M, Britoil P, Cession C, Macmillan F, Courts F, GATT R. Intra-rater reliability of an experienced physiotherapist in locating myofascial trigger points in upper trapeziums muscle. The Journal of manual & manipulative therapy. 2012 Nov;20(4):171–7. doi: 10.1179/2042618612Y.0000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howard FM. Endometriosis and mechanisms of pelvic pain. J Minim Invasive Gynecol. 2009 Sep-Oct;16(5):540–50. doi: 10.1016/j.jmig.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Affaitati G, Costantini R, Fabrizio A, Lapenna D, Tafuri E, Giamberardino MA. Effects of treatment of peripheral pain generators in fibromyalgia patients. Eur J Pain. 2011 Jan;15(1):61–9. doi: 10.1016/j.ejpain.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Giamberardino MA, Affaitati G, Fabrizio A, Costantini R. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol. 2011 Apr;25(2):185–98. doi: 10.1016/j.berh.2011.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.