Introduction
This monograph describes a cohort of 4685 HIV-positive persons, most of whom were recently infected, who volunteered to dedicate several years as participants in a research study that addresses the question of when antiretroviral therapy (ART) should be initiated. Should it be started early after HIV infection occurs, or should it be deferred until the infection has started to impair immune function but before the risk of AIDS increases?
There has been consensus based on robust data for many years that ART should be initiated following the development of AIDS. Also, within the past six to seven years data from randomised trials (1-3) and observational studies (4-7) emerged that supported the initiation of ART when the CD4 cell count declined to <350 cells/μL among asymptomatic individuals. It has been hotly debated whether the clinical benefits of initiating ART at a CD4 cell count >500 cells/μL, compared to deferring ART until CD4 cell count decreases to 350 cells/μL, as is being tested in the Strategic Timing of AntiRetroviral Treatment (START) study, outweigh the risks (8-10). The debate is lively because the data available are not optimal. The fact that 4685 persons from 215 clinics in 35 countries volunteered to be randomised in START reflects the considerable uncertainty that many individuals with HIV and investigators around the world have about the answer to the question being addressed by START.
How START got started
In early 2006, the SMART study (11) was prematurely stopped because the interim data established that use of ART intermittently rather than continuously led to an excess risk of not only AIDS events, but also what is now known collectively as serious non-AIDS events (major cardiovascular, renal or liver disease or non-AIDS cancers) (12,13). The mortality rate, which was primarily attributed to end-organ disease and cancer and not AIDS, was also greater among participants randomised to receive ART intermittently compared to those assigned to continuous ART(11). This was a surprising and unexpected finding. Before data from SMART were available, many experts were of the opinion that intermittent ART was safe (14). Opinions were based on data from observational studies, uncontrolled studies of ART cessation, and small, short-term randomised trials. The definitive evidence provided by SMART trumped these opinions, and treatment guidelines were quickly modified.
The key unanswered question in the aftermath of SMART was whether initiation of ART early in the course of HIV infection – when risk of AIDS is low to negligible and morbidity and mortality is almost entirely driven by serious non-AIDs events – would provide clinical benefits that outweighed the risks. For this to be the case, early ART would likely have to result in a reduced risk of serious non-AIDS events as well as AIDS (13).
By the summer of 2006, a few months following the early termination of SMART, the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) study group prioritised “when to start ART” as the next key question for which they would mount an international trial. At that time, there was still a paucity of information as to whether the initiation of ART at CD4 cell count >250 cells/μL had a favourable risk/benefit profile compared to deferral to a CD4 cell count <200 cells/μL. Thus, considerable time was spent discussing the target population and deferral strategies of the trial for proposal to the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH). There was even consideration of the possibility of doing two trials because of the uncertainty around the benefit of the deferral strategy of 350 cells compared to a lower count as the control group. By September 2007, the current design of START had been approved, and NIAID agreed to fund a pilot study of 900 participants. If the pilot study demonstrated enrolment was feasible, the definitive trial of 4000 participants would be funded.
It was also realised that a trial design where half the study participants were randomly allocated to remain untreated for several years would present a unique opportunity to assess the impact of early ART on various end-organ disorders. Thus, following the decision to support START by NIAID, the INSIGHT group sought funding from other governments and from other NIH institutes for the substudies that are described in this monograph. INSIGHT also began working with NIAID on clinical trial agreements with pharmaceutical companies for the donation of antiretroviral treatment.
Shortly before START was to begin, an administrative challenge arose. INSIGHT was informed by NIAID in July 2008 that they had been advised by their general council that they could not sponsor a trial including subjects from European Union member states because of the requirements of the sponsor to indemnify or provide insurance for research-related injury (15). After several months of negotiation, the University of Minnesota agreed to be the sponsor in November 2008. Version 1.0 of the protocol was released in December 2008, and plans for implementing the trial in 2009 were initiated. Enrolment began in April 2009, the pilot study was judged to be successful in 2010, and as described in the paper on enrolment in this monograph, the last participant enrolled entered START on 23 December 2013.
There have been a number of other challenges during the enrolment phase of START. About the time enrolment in START was finally able to begin, the first (4) of several observational studies (5-7) aimed at determining at what CD4 cell count ART should be initiated was published. The authors argued that initiating ART at counts >500 cells/μL resulted in a survival benefit (4). Later, other observational studies were published that came to different conclusions (5, 7). It remains unclear which of these diverging findings are correct and whether indeed the claim made that observational studies can provide reliable answers (10) to a question like the one posed by START is correct. START will assist in clarifying this controversy.
Two randomised trials addressing the question of when to start ART were also completed during the enrolment phase of START: the Haiti trial (2) and HPTN 052 (16). Both trials were designed and executed with a belief that HIV infection could be left untreated as long as the CD4 cell count remained above 200 cells/μL; a belief that also guided the design of SMART. The results of these trials affirmed that the deferral strategy used in START as opposed to a lower one that was considered in the design stage was a better control group. Indeed, global consensus was already reached in 2007, as reflected in the change of all guidelines, that ART should commence once CD4 cell count decreased to around 350 cells/μL.
A concerted effort was initiated in 2007-2008 to determine a plausible biological explanation for why the results of SMART turned out the way they did. Interruption of ART was found to lead to activation of inflammation and coagulation pathways (17). Subsequent extensive research, workshops, and reviews have debated whether the activation of the immune system that results from untreated HIV (like any infection) has long-term clinical consequences. The status on this debate remains a resounding “perhaps.” Inflammatory biomarkers, like interleukin-6 (18-22), and coagulation biomarkers, like D-dimer (19,21,23), are strong prognostic indicators of these clinical outcomes. However, none of the studies to date have been able to address the key question in relation to this, namely whether use of an intervention (e.g., ART) that dampens activated inflammatory and coagulation processes in the body, results in a reduced risk of clinical disease. Concerted efforts are underway in the cardiovascular field to address directly the continued uncertainty for whether a causal link exists between inflammation and cardiovascular disease (24). The START study will contribute to clarifying this question in the field of HIV.
The science in START
The science to be derived from the findings in START, once the follow-up data are unblinded, will address two principle questions. The narrow and focused question is whether initiation of ART at CD4 cell counts >500 cells/μL (immediate ART) is superior to deferral of ART until CD4 declines to 350 cells/μL in terms of reducing the morbidity and mortality that is expected to be largely attributed to serious non-AIDS events. The broader question that the results of START will contribute to, relates to the public health strategic role ART may serve. ART unquestionably lowers the infectiousness of the person taking the treatment (16). If START demonstrates clinical benefit from early use of ART, the benefits to the individual HIV-positive person and to public health are aligned. This will not be the case if START fails to demonstrate early benefit.
The design of START
The design of START (Figure 1) is simple (25). Participants with a CD4 cell count >500 cells/μL are randomised to one of two equal-sized groups, where one group initiates ART immediately and the other initiates ART once CD4 has decreased to <350 cells/μL. The primary endpoint is any AIDS event (excluding oesophageal candidiasis and chronic Herpes simplex), serious non-AIDS event or death from any cause. Originally, a total of 4000 participants were to be enrolled and followed for three years after the last participant was randomised. This follow-up period was estimated to be sufficient to accrue 370 primary endpoints. This event target provided 90% power to detect a 27% difference in risk between the two arms of the study (25). In 2012, these assumptions were modified based on a planned sample size re-estimation. The sample size re-estimation was performed while participants were still being enrolled so that if sample size had to increase the enrolment period could be extended. The re-estimation resulted in the required number of primary endpoints being lowered to 213 to maintain the 90% power, while the sample size was increased to 4600 and remaining enrolment was restricted to persons older than 35 years. These changes were made because: 1) baseline CD4 cell count was much greater than originally assumed (median about 650 cells/μL compared to 566 cells/μL - as originally assumed) and this led to a greater predicted risk difference between the two treatment groups than originally hypothesised; and 2) the pooled (both treatment groups combined) primary event rate was lower than assumed.
Figure 1. The design of the START trial.
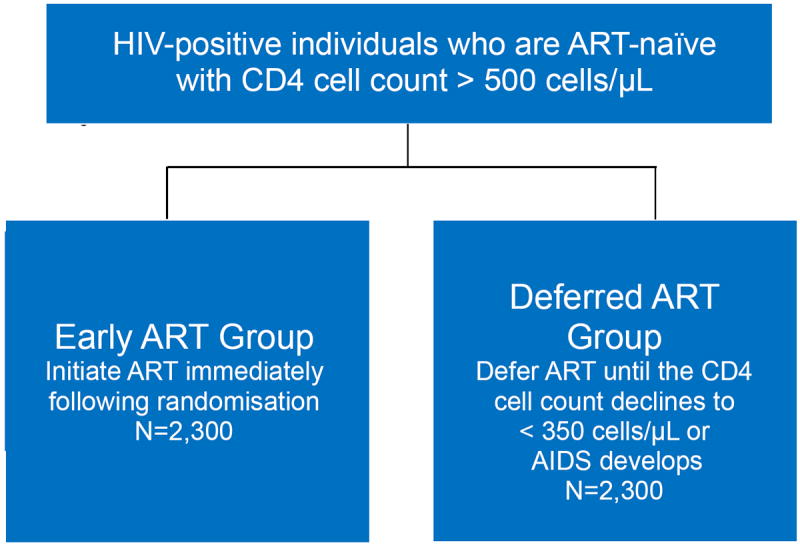
Legend: ART-naïve (i.e, persons not previously using antiretroviral therapy [ART]) HIV-positive persons with normal immune function (i.e., a CD4 cell count above 500 cells/μL) are randomly allocated to start ART immediately or until the CD4 count has further decreased to levels below which the person starts to become at risk of contracting opportunistic diseases (i.e., AIDS) if left untreated; if AIDS develops when the CD4 count is still above 350 cells/μL then ART should also be initiated.
The number of primary events in the two treatment groups remains blinded except to an independent Data and Safety Monitoring Board (DSMB). These data will remain blinded until either: 1) the DSMB determines that an answer to the question posed by START has been clearly shown or that it is unlikely to obtain a reliable answer; or 2) a total of 213 primary events have accumulated. At the time of writing this monograph, it remains the projection that the latter scenario will occur at the end of 2016, three years after enrolment of the last trial participant as stipulated in the protocol. The findings disclosed at the time will then guide decisions on possible future research direction, including the relevance of continued follow-up and use of ART in the deferred arm of the trial.
Serious non-AIDS events
Most primary endpoints are projected to be serious non-AIDS events as opposed to AIDS-defining events. In addition to the data from the main trial, data collected in the substudies will examine these serious non-AIDS outcomes and others in more detail. These outcomes include kidney impairment as determined by the decline in estimated glomerular filtration rate (eGFR) and the development of proteinuria, neurological dysfunction, chronic obstructive lung disease, arterial elasticity, major cardiovascular risk factors, including ischaemic ECG abnormalities, bone mineral density, and liver fibrosis. The monograph describes and discusses the details of the design and methodology of each of these planned investigations. Together, with the major clinical findings of START, these other outcomes will be instrumental in better defining the benefits and risks of early use of ART.
START Central Repository of Specimens
For consenting participants, specimens are collected at baseline and at each follow-up visit for future research. A central repository of biological samples of blood, plasma, urine, and host DNA as well as a separate repository of tissue samples from participants that develop cancer during the study are being maintained. Specimens in the sample repository from the SMART study and other INSIGHT studies have been used to generate a large number of research investigations, and we project that this will also be true for START specimens. INSIGHT invites the larger research community to exploit the wealth of research possibilities once the results of START are available.
Overview of the Baseline Monograph
An extensive set of data was collected to characterise START participants prior to randomisation. This monograph includes 15 papers: a community perspective on the START trial (27); a description of the challenges and keys to success of the enrolment effort by 215 sites in 35 countries (28); a description of the informed consent process in START, including the design of a nested cluster-randomised study comparing a “standard” with a “concise” consent (29); and 12 other papers either describing baseline characteristics of all 4685 HIV-positive participants or describing the participants in the substudies of START. Below we highlight some characteristics of this unique cohort of individuals who were ART naïve and had CD4 cell counts >500 cells/μL at study entry.
Median age of the cohort was 36 years and 27% were women. Median CD4 cell count was 651 cells/μL and median HIV RNA was 12,754 copies/mL (30).
For 2.4% of participants the HIV RNA level was 50 copies/mL or lower (31).
Approximately 19% of participants had two or more cardiovascular risk factors; 32% of participants reported smoking (32).
Chronic kidney disease defined as the eGFR < 60 mL/min/1.73m2 or dipstick proteinuria 1+ or greater was present in 6.2% of participants (33).
Approximately 17% of participants reported condomless sex with a serodiscordant partner in the two months prior to randomisation (34).
There was substantial geographic diversity in carrying out routine screening for transmitted ART-resistant virus, with screening much more likely to occur in resource-rich areas of the world (35).
Quality of life was mostly favourable; the average of the visual analogue scale of overall current health was 80.9 out of 100 (36).
Changes in neurocognitive test performance will be compared for the immediate and deferred ART groups in 608 participants. At entry about 20% of participants had at least mild neurocognitive impairment (37).
Changes in arterial elasticity will be compared for the immediate and deferred ART groups in 331 participants. At entry, impaired (i.e. lower) small and large artery elasticity was associated with increased age, female gender and increased systolic and diastolic blood pressure. Small arterial elasticity was also impaired among those with prior CVD, whereas it was higher for those on lipid-lowering therapy (38).
Changes in pulmonary function will be compared for the immediate and deferred ART groups in 1026 participants. At entry the prevalence of chronic obstructive pulmonary disease (defined as forced expiratory volume in one second divided by forced vital capacity less than the lower limit of normal) was 6.8% (39).
Changes in liver fibrosis as measured by transient elastography (FibroScan®) will be compared for the immediate and deferred group in 221 participants. At entry the median FibroScan® score was 4.9 kPa and 7.8% had evidence of significant liver fibrosis (40).
Changes in bone mineral density (BMD) by dual-energy x-ray absorptiometry will be compared for the immediate and deferred ART groups in 424 participants. At entry, about 2% had osteoporosis and 35% had a low BMD (41).
Concluding remarks
The goal of this monograph is to provide a detailed description of a unique cohort of individuals – HIV-positive adults who are ART naïve and have CD4 cell counts >500 cells/μL. Because of the support we have received to do this study and the time commitments of so many study participants, much of the data presented here is unprecedented. Like in any clinical trial, it is important to understand the target population to whom the findings apply. In that regard, this monograph will be very informative and will surely be used as a reference once follow-up information becomes unblinded and those results are communicated.
Acknowledgments
The INSIGHT Scientific Steering Committee (A Babiker, J Baker, W Belloso, C Cohen [vice-chair], S Collins, D Cooper, R Davey, D Dwyer, JM Molina, C Lane, J Lundgren [chair], J Neaton, A Phillips [vice-chair], J Rockstroh) peer-reviewed all sections of this monograph, and Sue Meger copyedited the content.
We would like to thank the START participants without whom this work would not be possible and the following individuals for their work on the START trial.
Protocol Team: James D. Neaton (INSIGHT PI), Abdel G. Babiker (cochair), Fred M. Gordin (cochair), Jens D. Lundgren (cochair), Brian K. Agan, Beverly Alston-Smith, Alejandro Arenas-Pinto, Jose R. Arribas, Jason V. Baker, John Baxter, Waldo H. Belloso, Kate Brekke, Bruce Brew, Susan W. Brobst, William Burman, Richard Clark, David A. Cooper, Richard T. Davey Jr., Guy De La Rosa, Eileen T. Denning, Matthew Dolan, Gregory Dore, Daniel Duprez, Ezekiel Emanuel, Sean Emery, Gerd Fätkenheuer, Christine Grady, Birgit Grund, Bernard Hirschel, Bruno Hoen, Margaret A. Johnson, Chrispin Kambili, Karin Klingman, Ken M. Kunisaki, Alan Landay, Bruno Ledergerber, Eric Lafebvre, Sandra N. Lehrman, Ana Martinez, Sue Meger, Kelly Misar, Ronald T. Mitsuyasu, Amanda Mocroft, Jean-Michel Molina, David Munroe, Michael Norton, Sarah L. Pett, Andrew Phillips, Deenan Pillay, Danielle Porter, Richard W. Price, Michael Proschan, Claire Rappoport, Peter Reiss, Boris Renjifo, Kevin Robertson, Jürgen Rockstroh, James F. Rooney, Michael J. Ross, Mauro Schechter, Siegfried Schwarze, Daniel Seekins, Shweta Sharma, Wendy Snowden, Amalio Telenti, Jeff Tryon, Michael J. Vjecha, Edwina Wright.
Substudy Chairs: Jason V. Baker, Daniel Duprez (arterial elasticity); Andrew Carr, Jennifer Hoy (bone mineral density); Matthew Dolan, Amalio Telenti (genomics); Christine Grady (informed consent); Gail Matthews, Jürgen Rockstroh (liver fibrosis progression); Waldo H. Belloso, Jonathan M. Kagan (monitoring); Edwina Wright, Bruce Brew, Richard W. Price, Kevin Robertson (neurology); Ken M. Kunisaki, John E. Connett, Dennis E. Niewoehner (pulmonary).
Community Advisory Board: Claire Rappoport (INSIGHT community liaison), Peer D. Aagaard, Simon Collins, Jae Condon, Giulio Maria Corbelli, Nathan Geffen, Michael Meulbroek, David Munroe, Moses Supercharger Nsubuga, Dwight E. Peavey, Siegfried Schwarze, Nimit Tienudom, Mirta Valdez, Jo Watson.
Endpoint Review Committee: Alan Lifson (chair), Waldo H. Belloso, Richard T. Davey Jr., Daniel Duprez, Jose M. Gatell, Jennifer Hoy, Ronald T. Mitsuyasu, Court Pedersen, Richard W. Price, Ronald Prineas, John Worley.
Drug Distribution: Sandy McBurnett (Almac Clinical Trials Services), Julie Eckstrand.
Specimen Repositories: Emily Flowers, Marie Hoover, Kenneth Smith (Advanced BioMedical Laboratories, LLC); Debra Garcia (AIDS and Cancer Specimen Resource).
ECG Reading Center: Marry Barr, Charles Campbell, Susan Hensley, Julie Hu, Lisa Keasler, Yawing Li, Elsayed Z. Soliman, Tonya Taylor, Zhu-Ming Zhang (Epidemiological Cardiology Research Center [EPICARE]).
Division of AIDS, National Institute of Allergy and Infectious Diseases: Beverly Alston-Smith, Ellen DeCarlo, Karin Klingman.
Minnesota Coordinating Center: Kate Brekke, Gary Collins, Eileen T. Denning, Alain DuChene, Nicole Wyman Engen, Michelle George, Birgit Grund, Merrie Harrison, Katherine Huppler Hullsiek, Eric Krum, Sue Meger, Ray Nelson, Jacqueline Neuhaus, Kien Quan, Siu-Fun Quan, Terri Schultz, Shweta Sharma, Gregory Thompson.
International Coordinating Centers
Copenhagen HIV Programme, University of Copenhagen, Denmark: Bitten Aagaard, Alvaro Humberto Diniz Borges, Marius Eid, Jesper Grarup, Per Jansson, Zillah Joensen, Birgit Nielsen, Mary Pearson, Ruth Pedersen.
The Kirby Institute, University of New South Wales, Sydney, Australia: Cate Carey, Lara Cassar, David Courtney-Rodgers, Megan Evans, Sally Hough, Simone Jacoby, Joe Levitt, Rose Robson, Nisha Seneviratne, Alexis Shambrook.
Medical Research Council Clinical Trials Unit at UCL, London, United Kingdom: Brian Angus, Alejandro Arenas-Pinto, Rachel Bennett, Nafisah Braimah, Emily Dennis, Nicki Doyle, Michelle Gabriel, Fleur Hudson, Brooke Jackson, Adrian Palfreeman, Babasola Popoola, Charlotte Russell.
Veterans Affairs Medical Center, Washington, DC, United States: Donna Conwell, Haidee Elvis, Elizabeth Finley, Fred M. Gordin, Virginia Kan, Laura Lynch, Janet Royal, Adriana Sánchez, Barbara Standridge, Douglas Thomas, Melissa Turner, Michael J. Vjecha.
Site Coordinating Centers and Clinical Site Investigators by Country
Argentina: Emiliano Bissio, Claudia Checa Cabot, Liliana Calanni, Arnaldo D. Casiro, Ana Alfonsina Crinejo, Lucia Daciuk, Daniel Omar David, Marisa del Lujan Sanchez, Marisa Garofalo, Graciela Guaragna, Isabel Lanusse, Hector Enrique Laplume, Gustavo Daniel Lopardo, Marcelo H. Losso, Sergio Lupo, Horacio Mingrone, Alejandra Moricz, Ines Otegui, Luciana Peroni, Lucas Roby, Eduardo Warley.
Australia: David Baker, Mark Bloch, David A. Cooper, Jessica Costa, Nicholas Doong, Dominic Dwyer, Martyn French, Helen Lau, Catherine Pell, Isabel Prone, Tim Read, Norman Roth, Diane Rowling, David Shaw, Julie Silvers, Kate Sinn, Ban Kiem Tee, Robyn Vale, Emanuel Vlahakis.
Austria: Armin Rieger, Veronique Romer Touzeau, Brigitte Schmied, Norbert Vetter.
Belgium: Helga Ceunen, Nathan Clumeck, Eric Florence, Tessa James, Kabamba Kabeya, Lida van Petersen, Eric H. Van Wijngaerden, Linos Vandekerckhove.
Brazil: Carlos Brites, Jorge Casseb, Ricardo Braga de Castro, Elisânagela Gois, Beatriz Grinsztejn, Estela Luz, Jose Valdez Madruga, Gustavo Mizuno, Anna Mostachio, Luciana Passos, Luiz Carlos Pereira Jr., Tânia Reuter, Mauro Schechter, Tâmara Newman Souza, Sandra Wagner, Carina Beppu Yoshida.
Chile: Gladys Allendes, Marcelo J. Wolff.
Czech Republic: David Jilich, Dalibor Sedlacek.
Denmark: Philippa Collins, Jan Gerstoft, Lene Hergens, Lene P. Jensen, Iben Rose Lofthiem, Lars Mathiesen, Lars Oestergaard, Court Pedersen.
Estonia: Kai Zilmer.
Finland: Outi Debnam, Matti Ristola.
France: Jean-Pierre Aboulker, Raphael Biekre, Francois Boue, Catherine Capitant, Catherine Chakvetadze, Antoine Cheret, Catherine Chirouze, Sylvie Dargere, Jade Ghosn, Cécile Goujard, Bruno Hoen, Imad Kansau, Marina Karmochkine, Christine Katlama, Barbara Lebas, Benedicte Lefebvre, Jean-Daniel Lelievre, Nicolas Leturque, Yves Levy, Catherine Majerholc, Laurence Meyer, Jean-Michel Molina, Emmanuelle Netzer, Laurence Niedbalski, Jerome Pacanowski, Marc-Antoine Valantin, Renaud Verdon, Jean-Paul Viard, Laurence Weiss, David Zucman.
Germany: Keikawus Arastéh, Claudia Bachmann, Brigitta Becker, Renate Bieder, Johannes R. Bogner, Stefan Esser, Cecilie Feind, Ellen Harrer, Thomas Harrer, Martin Hartmann, Susanne Heesch, Christian Hoffmann, Martin Hower, Erika Jäger, Björn Jensen, Heiko Jessen, Hartwig Klinker, Gudrun Mark, Torsten Meier, Vera Müller, Ilse Ott, Andreas Plettenberg, Jürgen Rockstroh, Renate Röger, Bernd Salzberger, Geetha Sarrach, Christoph Stephan, Albrect Stoehr, Matthias Stoll, Eleonore Thomas, Jan van Lunzen, Heidi Wiehler, Carmen Zedlack, Nadine Zerche.
Greece: Olga Anagnostou, Maria Chini, Vicky Gioukari, Angelos Hatzakis, Andreas Katsambas, Marios K. Lazanas, Ilias Mariolis, Simeon Metallidis, Pavlos Nikolaidis, Antonios Papadopoulos, Vassilios Paparizos, Vassilis Papastamopoulos, Konstantinos Protopapas, Helen Sambatakou, Athanasios Skoutelis, Giota Touloumi.
India: Nagalingeswaran Kumarasamy, Sanjay Pujari.
Ireland: Elizabeth Coghlan, Patrick Mallon.
Israel: Eynat Kedem, Hadar Lender, Shimon Pollack, Eduardo Shahar, Dan Turner.
Italy: Andrea Antinori, Raffaella Libertone, Patrizia Morelli, Silvia Nozza, Maria Rita Parisi, Giuseppe Tambussi.
Luxembourg: Charlotte Lieunard, Therese Staub.
Malaysia: Raja Iskandar Shah Raja Azwa.
Mali: Mamadou Cissé, Sounkalo Dao, Susan Orsega, Sophia Siddiqui, Christian Yoder.
Mexico: Juan Sierra Madero.
Morocco: Ikbale Erradey, Hakima Himmich, Kamal Marhoum El Filali.
Nigeria: Christopher Akolo.
Norway: Vidar Ormaasen, Linda Skeie.
Peru: Rosemarie Ayarza, Romina Chinchay, Mónica del Portal, Fanny García, María Esther Guevara, José Hidalgo, Rosa Infante, Alberto La Rosa, Javier Lama, Fernando Mendo, Raúl Salazar, Mónica Sánchez.
Poland: Elzbieta Bakowska, Robert Flisiak, Anna Grzeszczuk, Andrzej Jerzy Horban, Brygida Knysz, Aleksandra Szymczak.
Portugal: Teresa Bapista, Rui Sarmento E. Castro, Manuela Doroana, Sara Lino, Fernando Maltez, Kamal Mansinho, Josefina Mendez, Ana Sequeira.
South Africa: Sharlaa Badal-Faesen, Mmabatho Ngoananoka Portia Dintwe, Elizabeth Fielder, Jaynthie Govender, Christie Hieberg, Melissa Hero, Nicola Killa, Rosie Mngqibisa, Sundrapragasen Pillay, Maureen Rattley, Robin Wood.
Spain: José López Aldeguer, Jose R. Arribas, Bonaventura Clotet, Patricia Cobarsi, Sandra Cuellar, David Dalmau, Mariano Matarranz del Amo, Pere Domingo, Vicente Estrada, Jose Maria Gatell, Ignacio de los Santo Gil, Alicia Gonzalez, Ana Gonzalez, Mar Gutierrez, Antonio Ocampo Hermida, Patricia Herrero, Hernando J. Knobel, José Sanz Moreno, Maria Luisa Montes Ramirez, Maria Rodrigo, Rafael Rubio, Maite Sanchez, Jesus Sanz Sanz, Fernando Warncke.
Sweden: Magnus Gisslén, Camilla Håkangård, Lissie Johansson, Kristina Törqvist.
Switzerland: Christine Bruelisauer, Alexandra L. Calmy, Hansjakob Furrer, Melanie Lacalamita, Thanh Lecompte, Nicolas Müller, Marion Rizo-Oberholzer, Marcel Stoeckle.
Thailand: Anchalee Avihingsanon, Chureeratana Bowonwatanuwong, Kanlaya Charoentonpuban, Ploenchan Chetchotisakd, Manassinee Horsakulthai, Thidarat Jupimai, Pacharee Kantipong, Sasisopin Kiertiburanakul, Virat Klinbuayaem, Kobkeaw Laohajinda, Wisit Prasithsirikul, Winai Ratanasuwan, Kiat Ruxrungtham, Khuanchai Supparatpinyo, Wanida Thiansanguankul, Sasiwimol Ubolyam.
Uganda: Zacchaeus Anywaine, Bernard Kikaire, Cissy Kityo, Joseph Lutaakome, Henry Mugerwa, Peter Mugyenyi, Paula Munderi.
United Kingdom: Jocelyn Ablorde, Asma Ashraf, Andrew Bexley, Christine Bowman, Andrew de Burgh-Thomas, David R. Chadwick, Fabian Chen, Amanda Clarke, Yvonne Clowes, Satyajit Das, Carl De Souza, David Dockrell, Carol Emerson, Martin Fisher, Julie Fox, Brian Gazzard, Mark Gompels, James Hand, Jan Harding, Phillip Hay, Elbushra Herieka, Christopher Higgs, Akil Jackson, Louise Jennings, Margaret A. Johnson, Indra Karunaratne, Stephen Kegg, Pauline Lambert, Siobhan Lynch, Linda Mashonganyika, John Masterson, Siobhan McKenna, Sinead McKernan, Borja Mora-Peris, Tarik Moussaoui, Olanike Okolo, Chloe Orkin, Say Pheng Quah, Jonathan Ross, Amandip Sahota, Cathy Stretton, Alastair Teague, Juan Manuel Tiraboschi, David White, Edmund Wilkins, Ian Williams, Alan Winston, Martin Joseph Wiselka, Mike Youle.
United States: Midnela Acevedo-Flores, Laurie Andrews, Lourdes Angeli, Jorge Santana Bagur, Susan Banks, Irma Barahona, Mary Bavaro, John Baxter, Ioana Bica, Ann Brown, William Burman, Michele Carter, Willie Carter, Mable Chow, Richard Cindrich, Jonathan Clemmer, Patricia Coburn, Calvin Cohen, Nancy Crum-Cianflone, Gabriel Culbert, Sylvia Dávila, Edwin DeJesus, Nila Desai, Jaime Deville, Jeffrey Dinsmore, Sara Echols, Wafaa El-Sadr, Kim Epperson, Marti Farrough, Irma Febo, Patricia Flynn, Michael Frank, Susan Fraser, Gerald Friedland, Luis Fuentes, Charlene Gaca, Anuradha Ganesan, Edward Gardner, Tabetha Gayton, Christiane Geisler, Rachel Givot, Matthew Goetz, Julia Green, Edie Gunderson, W. Keith Henry, Sally Hodder, Gerald Horton, Valery Hughes, Chivon McMullen Jackson, Mamta Jain, Mary Johnson, Rohit Kalra, Michael Kolber, Susan Koletar, Andrea Kovacs, Princy Kumar, Ann Labriola, Tahaniyat Lalani, Laurie Luna, Rodger MacArthur, Linda Makohon, Cheryl Marcus, Norman Markowitz, Norma Martinez, Rose McConnell, Jason McGuire, Maximiliano Menna, Daniela Morales, Libyadda Mosley, Melissa Murphy, David Mushatt, Daniel Nixon, Kimberly Norris, Richard Novak, Jason Okulicz, Eva Operskalski, Marta Paez-Quinde, David Parenti, Sonja Parker, Triniece Pearson, Vicky Peña, Tianna Petersen, Gerald Pierone Jr., Mobeen Rathore, Nancy Reilly, Carol Rosario, Wendy Rossen, Ann Sanders, Michael Sands, Ruth Santos, Connie Scott, James Scott, William Shearer, Jonathan Shuter, Terry Sjoberg, Charurut Somboonwit, Rita Sondengam, Stephen Spector, Jessica Springer, Shoba Swaminathan, Nicole Swanson, Suzanne Sweek, Tom Tanner, Ellen Tedaldi, Edward Telzak, Zelalem Temesgen, Nathan Thielman, Douglas Thomas, Jill Utech, Cornelius Van Dam, Luz Marina Vasco, Isabel Vecino, Barbara Wade, David Wallace, Kathy Watson, Vicky Watson, Amy Weintrob, Stephen Weis, Mona White, Aimee Wilkin, Timothy Wilkin, Naja Wilson, David Wohl, Amy Yi, Ram Yogev.
The START study is registered at clinicaltrials.gov (NCT00867048).
Funding
The START study is primarily funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1-AI068641, the Department of Bioethics at the NIH Clinical Center and five NIH institutes: the National Cancer Institute, the National Heart, Lung, and Blood Institute, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke and the National Institute of Arthritis and Musculoskeletal disorders. Financial support is also provided by the French Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS), the German Ministry of Education and Research, the European AIDS Treatment Network (NEAT), the Australian National Health and Medical Research Council, and the UK Medical Research Council and National Institute for Heath Research. Six pharmaceutical companies (AbbVie, Inc., Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, Janssen Scientific Affairs, LLC, and Merck Sharp and Dohme Corp.) donate antiretroviral drugs to START.
Footnotes
Disclosures
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The University of Minnesota, the sponsor of START, receives royalties from the use of abacavir, one of the HIV medicines that can be used in START.
References
- Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–265. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14:281–290. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASCADE Collaboration. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171:1560–1569. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain LE, Logan R, Robins JM, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154:509–515. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin CA, Cooper DA, Collins S, Schechter M. Rating evidence in treatment guidelines: a case example of when to initiate combination antiretroviral therapy (cART) in HIV-positive asymptomatic persons. AIDS. 2013;27:1839–1846. doi: 10.1097/qad.0b013e328360d546. [DOI] [PubMed] [Google Scholar]
- Lundgren JD, Babiker AG, Gordin FM, Borges ÁH, Neaton JD. When to start antiretroviral therapy: the need for an evidence base during early HIV infection. BMC Med. 2013;11:148. doi: 10.1186/1741-7015-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco RA, Saag MS. When to start antiretroviral therapy: as soon as possible. BMC Med. 2013;11:147. doi: 10.1186/1741-7015-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sadr WM, Lundgren J, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- El-Sadr WM, Grund B, Neuhaus J, et al. Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy: a randomized trial. Ann Intern Med. 2008;149:289–299. doi: 10.7326/0003-4819-149-5-200809020-00003. [DOI] [PubMed] [Google Scholar]
- Lundgren JD, Babiker A, El-Sadr W, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008;197:1145–1155. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- Pant Pai N, Tulsky JP, Lawrence J, Colford JM, Reingold AL. Structured treatment interruptions (STI) in chronic suppressed HIV infection in adults. Cochrane Database of Syst Rev. 2005 doi: 10.1002/14651858.CD005482. [DOI] [PubMed] [Google Scholar]
- Neaton JD, Babiker A, Bohnhorst M, et al. Regulatory impediments jeopardizing the conduct of clinical trials in Europe funded by the National Institutes of Health. Clin Trials. 2010;7:705–718. doi: 10.1177/1740774510376547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grund B, Baker JV, Deeks S, et al. Combined effect of interleukin-6 and D-dimer on the risk of serious non-AIDS conditions: data from 3 prospective cohorts. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, USA. March 2013. [Google Scholar]
- Borges ÁH, Silverberg MJ, Wentworth D, et al. Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS. 2013;27:1433–1441. doi: 10.1097/QAD.0b013e32835f6b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordell AD, McKenna M, Borges ÁH, et al. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc. 2014;3:e000844. doi: 10.1161/JAHA.114.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges AH, Weitz JI, Collins G, et al. Markers of inflammation and activation of coagulation are associated with anaemia in antiretroviral-treated HIV disease. AIDS. 2014;28:1791–1796. doi: 10.1097/QAD.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware DR, Hullsiek KH, Puronen CE, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203:1637–1646. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–1224. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Lűscher T. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35:1782–1791. doi: 10.1093/eurheartj/ehu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiker AG, Emery S, Fätkenheuer G, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials. 2013;10(1 Suppl):S5–S36. doi: 10.1177/1740774512440342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffen N. 2015, this supplement.
- Grarup J. 2015, this supplement.
- Denning E. 2015, this supplement.
- Sharma S. 2015, this supplement.
- Law MG. 2015, this supplement.
- Soliman EZ. 2015, this supplement.
- Achhra AC. 2015, this supplement.
- Rodger AJ. 2015, this supplement.
- Baxter JD. 2015, this supplement.
- Lifson AR. 2015, this supplement.
- Wright EJ. 2015, this supplement.
- Baker JV. 2015, this supplement.
- Kunisaki KM. 2015, this supplement.
- Matthews GV. 2015, this supplement.
- Carr A. 2015, this supplement.


