INTRODUCTION
Fibrosis is a feature of many cardiomyopathies and the failing heart and is a major independent predictor of adverse cardiac outcomes. Replacement fibrosis is typically the result of myocardial infarction. Diffuse interstitial fibrosis results from common cardiovascular risk factors; interstitial fibrosis has been shown to be reversible and treatable with early intervention. Non –invasive imaging methods to detect fibrosis are in development. Recent advances have been made in the fields of cardiac magnetic resonance imaging (CMR), computed tomography (CT) and nuclear medicine. Our review particularly focuses on the field of CMR and the techniques of Late Gadolinium Enhancement (LGE) and T1 mapping, useful in the detection of myocardial scar and diffuse myocardial fibrosis respectively.
PATHOLOGICAL BASIS OF FIBROSIS
The extracellular matrix (ECM) is a dynamic molecular network that is essential in giving strength to the heart and coordinated signaling between cells in the tissue. It anchors cardiac muscle cells (myocytes), regulates tissue mechanics, and stores growth factors. [1–3] The ECM is composed of collagens and elastic fibers buried in a gel of proteoglycans, polysaccharides and glycoproteins. Aberrant healing processes result in the common pathological feature called fibrosis. Fibrosis forms from an increased amount of collagen (fibrosis) resulting from altered collagen turnover, in which net collagen deposition exceeds net collagen breakdown. Diffuse myocardial fibrosis is known to increase with age and other cardiovascular risk factors [4]. At a molecular level, matrix metalloproteinases also play a key role in the development of myocardial fibrosis.
Increased myocardial collagen deposition is the common endpoint for a wide variety of cardiomyopathies. Collagen deposition results in abnormal myocardial stiffness and contractility, which leads to progression of heart failure and disruption of the intercellular signaling. These disruptive processes may lead to malignant arrhythmias and sudden death. Indeed, multiple clinical studies have shown fibrosis to be a major independent predictor of adverse cardiac outcomes [5–8]. It is always present in end-stage heart failure [9]. Diastolic function is initially affected and followed by deterioration of systolic function [10].
The two distinct types of fibrosis in the heart are replacement fibrosis and interstitial fibrosis. Replacement fibrosis is focal development of scar that replaces dead cardiomyocytes from injury and is only seen when the integrity of the cell wall is affected [11]. Depending on the etiology, both regional and diffuse patterns can be seen. Scarring from myocardial infarction is the most common cause of replacement fibrosis. Hypertrophic cardiomyopathy, sarcoidosis, myocarditis, chronic renal insufficiency, and toxic cardiomyopathies are other conditions associated this type of fibrosis [12, 13].
Interstitial fibrosis is generally a diffuse process. It has two subtypes, reactive and infiltrative interstitial. Reactive fibrosis is present in a variety of common conditions, including aging and hypertension. It is caused by an increase in collagen production and deposition by stimulated myofibroblasts. Infiltrative interstitial fibrosis is much rarer and is caused by progressive deposition of insoluble proteins or glycosphingolipids in the interstitium. Examples of infiltrative fibrosis include amyloidosis and Anderson-Fabry disease [14, 15]. Eventually, both interstitial and infiltrative fibrosis lead to cardiomyocyte apoptosis and replacement fibrosis [10]. Unlike replacement fibrosis, interstitial fibrosis may be reversible and is a target for treatment [16, 17].
The ability to noninvasively image fibrosis could be very useful for diagnostic and therapeutic purposes in cardiomyopathy treatment. Tissue biopsy has been the gold standard for fibrosis assessment, but it is invasive in nature and prone to sampling error. The emergence of noninvasive imaging modalities like cardiovascular magnetic resonance (CMR) imaging and computed tomography (CT), have led to development of novel imaging methods for a range of cardiomyopathies.
DETECTION OF FIBROSIS WITH ENDOMYOCARDIAL BIOPSY
The gold standard for the detection of myocardial fibrosis is endomyocardial fibrosis. A small (<1mm3) sample is taken, typically from the right ventricular side of the distal myocardial septum. The sample is assessed using Masson trichome staining. Quantitative absolute assessment of the collagen volume fraction in tissue samples is measured by quantitative morphometry with picrosirius red.
Being an invasive technique, this carries a risk of complications. In cases of localized fibrosis, sampling error restricts the accuracy. It is also not possible to determine fibrotic involvement of the whole LV.
DETECTION OF REPLACEMENT/ FOCAL FIBROSIS WITH LATE GADOLINIUM ENHANCEMENT (LGE) CARDIAC MAGNETIC RESONANCE (CMR)
CMR imaging demonstrates safe, high resolution imaging without ionizing radiation. CMR is well established as a standard of reference for the evaluation of myocardial structure and function. Pixel signal intensity of CMR images is based on the magnetic properties of hydrogen nuclei in the magnetic field. The two most common parameters from CMR are longitudinal relaxation time, T1, and transverse relaxation time, T2.
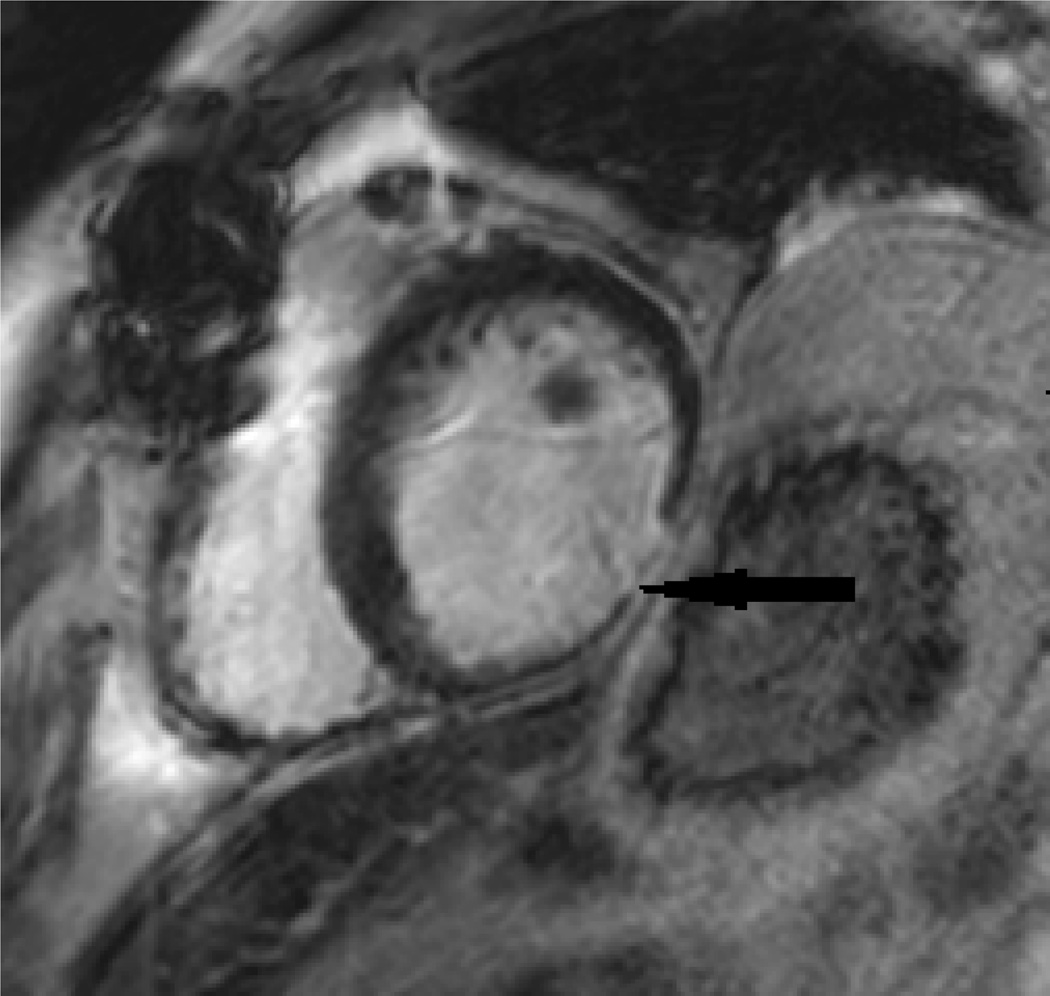
A unique clinical role of CMR (in comparison to echocardiography) is the use of late gadolinium enhancement (LGE) to define the presence of focal fibrosis or myocardial scar. For the evaluation of focal fibrosis, for example, from myocardial infarction (MI), late gadolinium enhancement (LGE) imaging has been a gold standard for visualization and quantification of scar. Figure 1 below shows scar from an inferior wall MI.
Figure 1.
Inferior wall MI (black arrow). Wall thinning and Late Gadolinium Enhancement is seen.
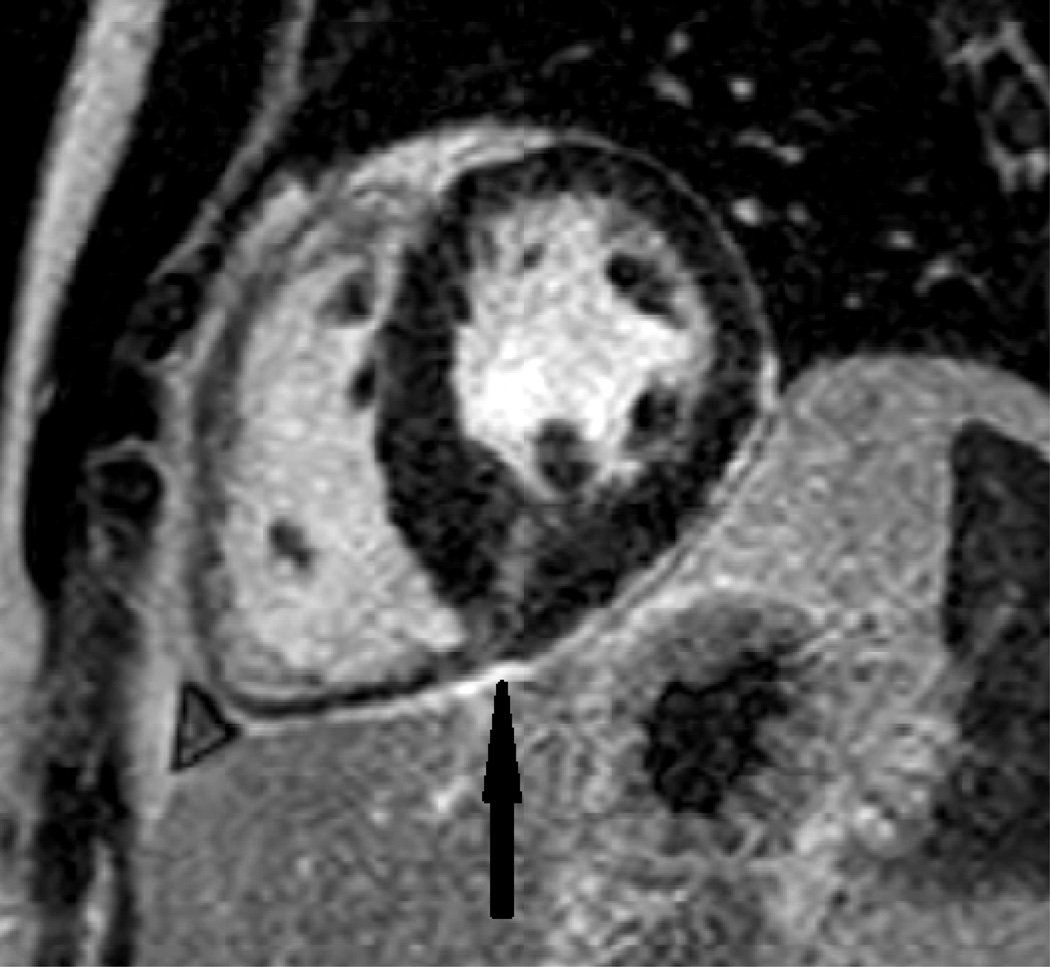
Myocardial scar is most commonly observed in fact as a result of MI. However, nonischemic cardiomyopathies are also frequently associated with LGE. CMR can be used to classify patients with myocardial dysfunction as ischemic versus nonischemic etiology based on LGE images. This distinction is very meaningful for clinical treatment. Figure 2 below shows LGE at the inferior right ventricular insertion point, a typical location for scar in hypertrophic cardiomyopathy patients.
Figure 2.
Delayed post contrast image showing LGE (black arrow) at the inferior right ventricular insertion point in a patient with hypertrophic cardiomyopathy.
The physiological basis of the LGE of myocardial fibrosis is based on the combination of an increased volume of distribution for the contrast agent and a prolonged wash-out related to the decreased capillary density within the myocardial fibrotic tissue [18, 19].
LGE CMR TECHNIQUE
The LGE technique to detect myocardial scar has a major advantage in its simplicity and robustness: an inversion pulse is used to suppress normal myocardium, followed by a standard gated T1-weighted gradient echo acquisition.
LGE sequences are based on distribution difference of the gadolinium based contrast agent in normal and fibrotic tissue. In areas of higher gadolinium chelate concentration, T1 time is shorter than in adjacent issue and shows high signal intensity on LGE image.
The discrimination between scarred/fibrotic myocardium and normal myocardium relies on contrast concentration differences combined with the chosen setting of the inversion-recovery sequence parameters. These parameters are set to “null” the normal myocardial signal that appears dark in the final image relative to the bright signal of the scarred/fibrotic myocardium.
Given various specific properties of the tissue, the T1 shortening induced by the gadolinium contrast agent generates specific differences in signal intensity. The major tissue parameters that influence the final voxel signal intensity in the contrast-enhanced images are local perfusion; extracellular volume of distribution; water exchange rates among the vascular; interstitial, and cellular spaces; and wash-in and wash-out kinetics of the contrast agent [18, 20].
The myocardial “gray zone” is increasingly being defined on LGE clinical studies. The “gray zone” has been conceptually defined as myocardium with intermediate signal intensity enhancement between normal and scarred/fibrotic myocardium [21]. This area reflects tissue heterogeneity within the infarct periphery and has been shown to strongly correlate with ventricular arrhythmia inducibility and postmyocardial infarction mortality in ischemic cardiomyopathy [21, 22].
LGE CMR: CLINICAL APPLICATIONS
LGE-CMR has become a first-line noninvasive exam for the etiologic assessment of new-onset myocardial dysfunction [13, 23]. LGE with CMR came to the clinical forefront in the setting of ischemic cardiomyopathy. Subendocardial or transmural LGE is the typical pattern seen in myocardial infarcts using LGE-CMR.
Kim et al. [24] showed that regional differences in signal intensity were correlated to the extent and severity of myocardial injury. Subsequently they reported in experimental studies that the spatial extent of hyperenhancement was the same as the spatial extent of the collagenous scar at 8 weeks with highly significant correlations. In ischemic cardiomyopathy, the transmural extent of LGE is predictive of myocardial wall recovery after revascularization, but it is also predictive of adverse LV remodeling [25, 26].
LGE-CMR also provides prognostic information in nonischemic cardiomyopathies. LGE is significantly and independently associated with adverse cardiac events in patients with cardiac amyloidosis [27] and in patients undergoing aortic valve replacement [28]. In hypertrophic cardiomyopathy, Rubinshtein et al. [29] and Kwon et al. [30] reported that LGE was strongly associated with arrhythmia and subsequent sudden cardiac death.
Different patterns of enhancement have been reported according to the underlying etiology, whether ischemic or non-ischemic. Typical LGE enhancement patterns [31] for different cardiomyopathies are summarized in Table 1.
Table 1.
CMR LGE enhancement patterns for different cardiomyopathies [31]
| Mid myocardial : Hypertrophic cardiomyopathy, Dilated cardiomyopathy, Pulmonary hypertension |
| Patchy: Sarcoid, Amyloid, Myocarditis |
| Transmural: Infarction (most common), Sarcoid (chronic), Myocarditis (Severe) |
| Subendocardial: Infarction, Amyloid, Hypereosinophilic syndrome, Cardiac Transplant |
| Subepicardial: Myocarditis (most common), Sarcoid |
LGE CMR LIMITATIONS
Recently the CMR community has come to realize a disadvantage of the LGE method: the “normal” myocardium that is suppressed by the inversion pulse may actually contain low levels of diffuse fibrotic tissue in many diseases. Although LGE CMR is well validated and clinically accepted for the evaluation of focal myocardial scar, it has inherent disadvantages for assessment of diffuse myocardial fibrosis. LGE relies on the differences in signal intensity between scarred and adjacent normal myocardium to generate image contrast. As this method needs a normal myocardium reference value, the LGE CMR method is unlikely to detect the presence of fibrosis in diffuse cases where there is no clear distinction between fibrotic tissue and normal myocardium.
There are limitations of the LGE CMR method in its precise classification of myocardial fibrosis as present or absent. With conventional LGE imaging sequences, signal intensity is expressed on an arbitrary scale that differs from one imaging study to another and therefore is challenging to assess for direct signal quantification in cross-sectional or longitudinal comparisons. The late gadolinium-enhanced myocardial fibrotic tissue is influenced not only by technical parameters set during image acquisition (inversion time [32], slice thickness, and so on), but also according to the intensity threshold that is arbitrarily set during post-processing to differentiate normal from fibrotic myocardium [33]. Presently, there is no single consensus on the intensity threshold settings to use for clinical assessment of myocardial fibrosis.
Various methods have been reported to define late enhanced myocardium, with significantly different results [33], perhaps explaining the variation in frequency of myocardial fibrosis by LGE CMR in in different studies [34]. Thus while the LGE CMR method has been widely adopted in the clinical setting, wide variation in quantification of focal fibrosis and lack of detection of diffuse myocardial fibrosis have led to additional CMR approaches.
DETECTION OF DIFFUSE FIBROSIS WITH CMR BY T1 MAPPING
T1 mapping is an imaging method which can provide a quantitative assessment of tissue characterization on CMR [35]. T1 mapping enables identification of early myocardial fibrosis at a treatable stage, when it cannot be otherwise detected by circulating biomarkers [36]. There is now a growing body of evidence that T1 mapping can detect early fibrosis that is not otherwise detectable by LGE method. [37]
In comparison to LGE images, T1 mapping reduces the influences of windowing and variations in signal enhancement by directly measuring the underlying T1 relaxation times. T1 relaxation time is measured in milliseconds, and represents a magnetic property of the tissue, also referred to as longitudinal or spin lattice relaxation. The T1 relaxation time of the normal myocardium is on the order of 1000 msec. In the presence of an MRI contrast agent, the T1 relaxation time can be substantially reduced and thus the T1 time is reflective of the concentration of the MRI contrast agent in the tissue. The actual T1 times of each element of myocardium are determined on a pixel by pixel basis.
Before gadolinium contrast agent administration (“native” T1 values), areas of diffuse myocardial fibrosis have greater T1 values (by about 10–20%) than normal tissue. After gadolinium administration, T1 values are lower than normal in diffuse myocardial fibrosis. The expanded extracellular space in diffuse fibrosis accumulates more gadolinium-based contrast than healthy tissue which is compact with myocytes. Reduction in T1 values is not specific for diffuse myocardial fibrosis, however. T1 time reduction may also occur with cardiomyopathies where the extracellular space is expanded, such as with amyloid depositions. [38]
T1 MAPPING TECHNIQUE
By reconstructing a sequence of images, T1 maps are generated in which every pixel represents T1 relaxation time of the corresponding section of myocardium. The Modified Look-Locker Inversion-recovery (MOLLI) sequence, described by Messroghli et al. [39–42] is a frequently used T1 mapping sequence. A currently favored approach to T1 mapping is a short MOLLI (modified Look-Locker inversion recovery) sequence. Using the ShMOLLI sequence, the average breath hold decreases from 18 to 9.1 seconds, and the number of required heartbeats decreases from 17 to nine [43]. This is particularly useful for dyspneic patients.
High-resolution native and post-contrast T1 maps may be obtained within a single breath hold. This MOLLI sequence has been thoroughly described, optimized, and tested in phantom studies, on healthy volunteers, and ischemic cardiomyopathy patients. ECG gated images are acquired at end-diastole. Images from multiple consecutive inversion-recovery acquisitions are then merged into one data set. A T1 map of the myocardium is created which is a parametric reconstructed image.
T1 maps can be obtained before or after gadolinium contrast administration. The pre-contrast T1 map is a baseline reference. The post-contrast T1 maps are assessed at different time points after contrast administration. A T1 distribution histogram may be created to analyze the composition of each myocardial slice. A curve of myocardial T1 recovery which reflects the contrast agent wash-out can be obtained using post contrast maps [44]. The MOLLI technique is sensitive to heart rate extreme values. It may also underestimate T1 times before gadolinium (native t1) and is best used for post gadolinium images. It does, however, produce a highly reproducible and fast T1 map of the heart. Intra and inter-observer agreement levels range is on the order of approximately 10% [44].
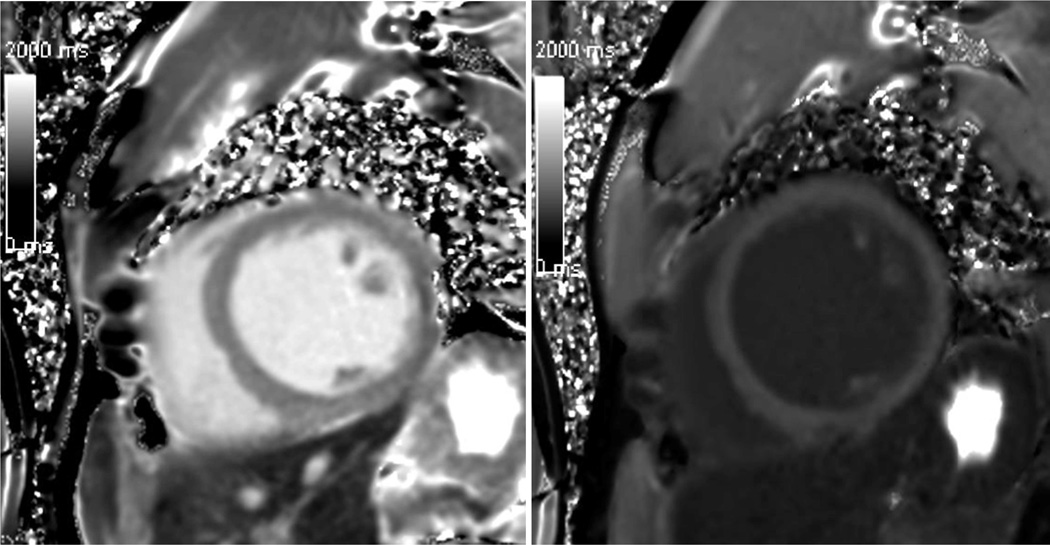
Besides MOLLI and shMOLLI T1 mapping, other CMR techniques are also available to obtain CMR T1 maps. [39, 42, 45–47]. These T1 mapping variants have been designed to have varying sensitivities to motion artifacts, heart rate, and intrinsic T1 value ranges [42]. The accuracy and reproducibility of the final T1 measurements is directly affected by the acquisition sequence. When comparing results of different studies it is thus important to note the particular technique used. Figure 3 below shows grey scale images of pre-contrast and 25 min post contrast T1 maps in a healthy volunteer acquired using the SMOLLI technique.
Figure 3.
Grey scale images of pre-contrast (left) and 25 min post contrast (right) T1 maps in a healthy volunteer acquired using the SMOLLI technique.
QUANTIFYING T1 MAPPING RESULTS: PARAMETERS AVAILABLE FROM T1 MAPS
There are three general approaches to obtaining T1 values to describe the tissue composition of the myocardium: “native” T1 values (no gadolinium contrast administered), post-gadolinium T1 time and normalized values (such as extracellular volume fraction or partition coefficient). Post gadolinium T1 times vary, depending on renal excretion of the contrast agent and delay time in measurement after gadolinium administration. The impact of those confounding variables might be reduced by calculating relative T1 mapping indices including the partition coefficient (λ) and extracellular volume fraction (ECV); both parameters are derived from the ratio of T1 change in blood and myocardium [45, 48] and are expressed as percentages. Calculation of ECV requires concurrent measurement of hematocrit (HCT). The ECV and λ are calculated using the following formulas [49]:
The ECV expresses the proportion of the myocardium representing interstitial space versus cellular space. With greater fibrosis, the interstitial component increases relatively to the cellular space. Gadolinium distributes only to the extracellular space and appears to be retained preferentially in areas of collagen/ scar. Thus in the presence of disease, native T1 is increased, post gadolinium T1 is decreased and ECV is increased. ‘ECV maps’ can be created from co-registration of T1 maps to locate the diffuse fibrosis [50, 51].
A relatively small number of studies are available to identify the ‘best’ T1 parameter (e.g., native T1, post gadolinium T1 or ECV) to detect various disease states. ECV has been a particularly attractive variable to quantify myocardial fibrosis since it normalizes for blood pool and hematocrit. However, ECV incorporates five different variables into its computation, each with an associated measurement error. If the errors in these variables accumulate, ECV could be insensitive for disease detection.
T1 MAPPING CLINICAL APPLICATIONS
Patient studies using T1 mapping have varied in study and design. The acquisition sequences have varied. Some studies have evaluated both native and post contrast T1 maps whereas others have only evaluated native T1 maps. Despite the differences in technique, a clear pattern that has been seen is that in cardiac disease post contrast T1 times are shorter. Table 2 summarizes ten key articles where cardiovascular diseases have been assessed with T1 mapping.
Table 2.
Clinical studies using T1 mapping to evaluate myocardial fibrosis
| Author and Date |
Disease | Technique | Sample size (Cases/ Controls) |
Conclusions |
|---|---|---|---|---|
| Messroghli 2007 [42] | Acute or chronic | MOLLI | 24/24 | In acute and chronic infarction, pre contrast T1 values were higher than T1 values in remote myocardium |
| Maceira 2005 [46] | Amyloidosis | LL | 22/16 | Subepicardial post contrast T1 values were significantly reduced in amyloid compared with controls |
| Broberg 2010 [64] | Adult congenital heart disease | LL | 50/14 | Cases of adult congenital heart disease had elevated fibrosis index |
| Flett 2010 [65] | Aortic stenosis/HCM | LL | 18/8 (aortic stenosis). 8/8 (HCM) | A high correlation was seen between T1 mapping with equilibrium contrast cardiac imaging and histologic samples of aortic stenosis and HCM |
| Gai 2011 [66] | Type 1 Diabetes | LL | 19/13 | A significant difference was seen in post contrast T1 values between those at low risk for diabetes compared with those at high risk |
| Ugander 2012 [49] | NICM/ prior MI | MOLLI | 30/11, 36/11 | ECV is increased in those with prior MI and NICM |
| Bauner 2012 [52] | Chronic MI | MOLLI | 26/26 | A significant difference is seen in post contrast T1 values in chronically infarcted myocardium compared to healthy myocardium |
| Turkbey 2012 [67] | Myotonic muscular dystrophy | LL | 33/13 | Lower post contrast T values were seen in myotonic muscular dystrophy than in controls |
| Dass 2012 [68] | HCM/DCM | ShMOLLI | 28/12 (HCM), 18/12 (DCM) | Cases with HCM or DCM had higher pre contrast T1 times than controls |
| Messroghli 2003 [41] | Acute MI | LL | 8/8 | Post contrast T1 values in acute MI were significantly reduced compared to normal myocardium |
Note: MOLLI = modified look locker inversion, LL = look locker, ShMOLLI – short modified look locker version, MI = myocardial infarction, HCM = hypertrophic cardiomyopathy, DCM – dilated cardiomyopathy, NICM = non ischemic cardiomyopathy.
Multiple studies have also examined the use of ECV and cardiovascular diseases. A concern is that ECV has a wide range of normal values, ranging from about 23% to 30%. [37] This range may overlap with ECV values in early disease. Although ECV is less likely to be useful as a single cut-off value to identify abnormal versus normal patients, change in ECV within an individual may be a more promising approach to assess, for example, a therapeutic response.
The accuracy of myocardial T1 mapping has been recorded in a few studies. Bauner et al [52] and Messroghli [42] et al found sensitivities and specificities to be greater than 95% for detection of chronic myocardial infarction using contrast enhanced T1 mapping. Ferreira et al [53] reported that, in 21 patients with acute regional myocardial edema and no infarction and 21 healthy patients, unenhanced T1 mapping had sensitivity and specificity of 92%.
T1 MAPPING VALIDATION
Relatively few validation studies have been carried out comparing histology to T1 mapping values. Iles et al [47] studied a symptomatic heterogeneous heart failure population using post contrast MOLLI. On myocardial biopsy of transplanted hearts, they demonstrated an inverse correlation of T1 values with percentage fibrosis. They also found a reduction in T1 time with worsening diastolic function. Sibley et al. used a post-contrast Look-Locker technique. They also demonstrated an inverse correlation between T1 time and histological fibrosis on biopsy in patients with a broad range of cardiomyopathies [54].
A few other studies using post contrast Look-Locker, MOLLI and other T1 techniques have shown a correlation between T1 values and percentage myocardial fibrosis. Non-contrast T1 mapping techniques has been less well validated.
T1 mapping can accurately differentiate both interstitial and replacement fibrosis from normal myocardium, as demonstrated by Kehr et al. [55] They carried out an in vitro magnetic resonance study of selected human myocardium samples, and documented post-contrast T1 values for both diffuse and replacement fibrosis to be significantly different from T1 values for normal myocardium. There was no significant difference between the diffuse fibrosis and replacement fibrosis T1 values, however.
T1 MAPPING LIMITATIONS
T1 mapping of the heart is technically demanding and standardization of the methodology is required.
The accuracy is sensitive to several confounding factors [35]. These include the gadolinium myocardial washout rate (affected by glomerular filtration rate) and the properties of the gadolinium contrast agent (dose, concentration, injection rate, relaxitivity, water exchange rate). The time delay after gadolinium administration which post contrast times are measured and the type of acquisition sequence used should be recorded as they significantly affect the final T1 value. Areas of LGE significantly affect the mean slice T1 value and interfere with diagnosing diffuse fibrosis. These areas therefore need to be accounted for. Myocardial T1 distribution can be significantly scattered, and this might limit its sensitivity for disease states with less severe fibrosis. T1 maps are usually created at the mid-ventricular level. If the fibrosis is not homogenous, areas of disease may not be measured.
COMPUTED TOMOGRAPHY (CT)
CT scanning is increasingly having widespread clinical application for evaluation of coronary artery disease. The utility of cardiac CT for the evaluation of coronary artery stenosis has already been demonstrated in large, multi-center clinical trials [56–59]. Given the significant clinical advantages of CCTA for coronary artery lesion evaluation, it would be highly desirable if CT would also be useful for characterization of myocardial tissue abnormalities as well as myocardial function.
CT thus far has demonstrated initial utility primarily for the evaluation of myocardial scar. Lardo et al [60] demonstrated in an animal study that the spatial extent of acute and healed myocardial infarction could be determined and quantified accurately with contrast-enhanced CT. The CT findings were compared to histology. In a cohort of patients with intermediate-high pre test probability, Bettencourt et al found that CT delayed enhancement had good accuracy (90%) for ischemic scar detection with low sensitivity (53%) but excellent specificity (98%).
The use of MDCT for diffuse abnormalities of myocardial tissue is significantly more challenging than the evaluation of focal myocardial scar due to the low contrast resolution of CT scanning. The distribution of iodinated contrast agent in the myocardium may be used to assess the degree of fibrosis. Several new studies highlight the potential of MDCT in this regard.
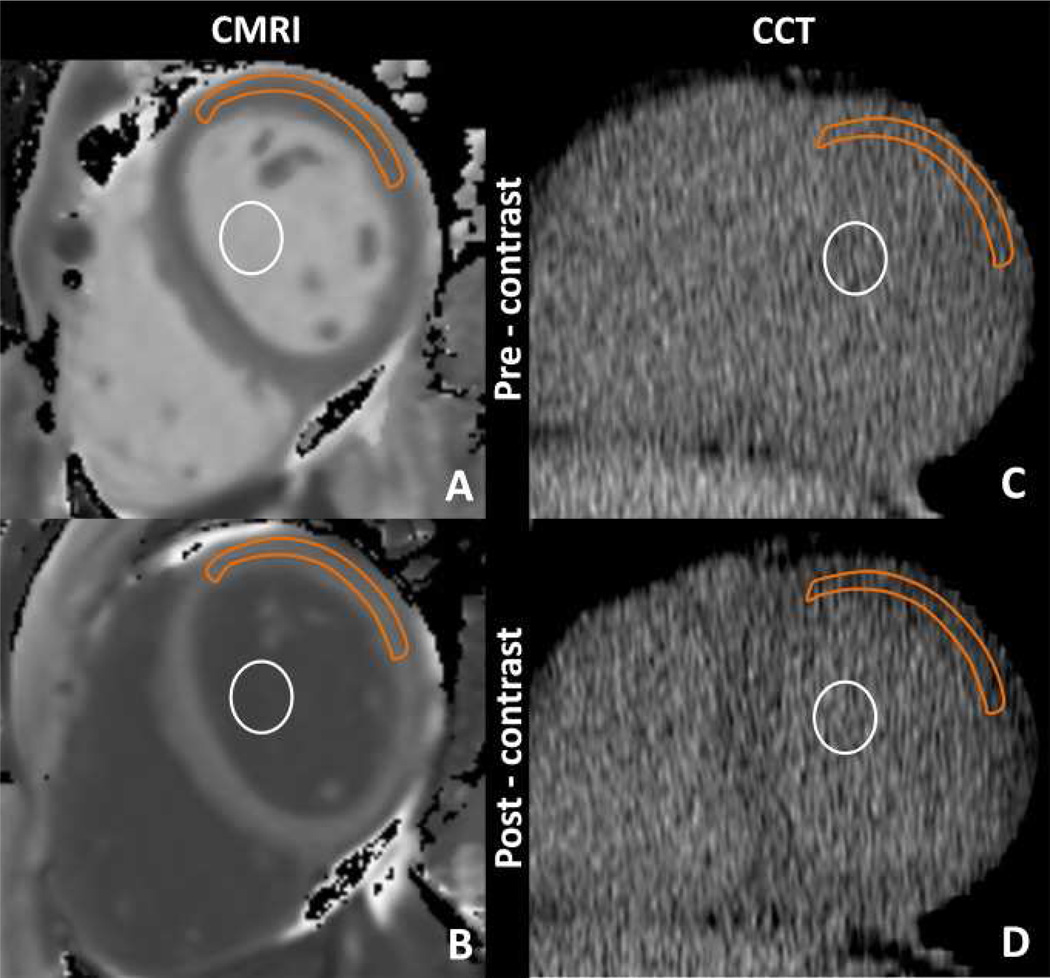
ECV measured with cardiac CT represents a new approach toward the clinical assessment of diffuse myocardial fibrosis. Nacif et al found [61] good correlation between myocardial ECV measured at cardiac CT and that measured at T1 mapping cardiac MR imaging in 24 subjects. A combination of healthy subjects and heart failure patients were studied. ECV was higher in patients with heart failure than in healthy control subjects for both cardiac CT and cardiac MR imaging, as expected. For both cardiac MR imaging and cardiac CT, ECV was positively associated with end diastolic and end systolic volume and inversely related to ejection fraction. Figure 4 below shows pre and post contrast MR and CT images. The antero-lateral segment was used as this segment was most reliably identified on the pre contrast CT.
Cardiac MR imaging region of interest measurements obtained, A, before and, B, after gadolinium chelate administration and reformatted cardiac CT region of interest measurements obtained, C, before and, D, after administration of an iodinated contrast agent. Orange outline = myocardium, white circle = blood pool.
From: Nacif et al (2012) Interstitial myocardial fibrosis assessed as extracellular volume fraction with low-radiation-dose cardiac CT.
The results of Nacif at el were confirmed in a subsequent study by Bandula et al [62] who demonstrated that ECV measured using a equilibrium CT technique in twenty four patients with aortic stenosis correlated well with histologic quantification of myocardial fibrosis and with ECV derived by using equilibrium MR imaging.
In terms of clinical application, Langer et al [63] studied patients with hypertrophic cardiomyopathy with MDCT. In that study, CT was able to reliably detect myocardial fibrosis as evident by late enhancement. Patient- and segment-based sensitivity was 100% and 68 % respectively compared to LGE-CMR. This technique could therefore be potentially used in cases with CMR contra-indications.
SUMMARY AND FUTURE PERSPECTIVES
CMR methods to noninvasively identify diffuse myocardial fibrosis have great potential to characterize and quantify early disease. Myocardial fibrosis is a common endpoint of many chronic myocardial and systemic diseases and is not available by other noninvasive tests. T1 mapping adds to the information provided by LGE-CMR and further improves our knowledge and the clinical assessment of myocardial diffuse fibrosis. This technique might help us to better stratify much larger and lower cardiovascular risk patient populations (diabetics, hypertensive), detecting subclinical myocardial changes before the onset of diastolic and systolic dysfunction.
The clinical value of T1 mapping remains to be seen, however. Two especially interesting applications for T1 mapping are amyloidosis and hypertrophic cardiomyopathy. In both cases, the extent of disease is otherwise difficult to quantify.
Further work in the field is ongoing to determine which disease processes may benefit by T1 mapping, and which parameter (s) (e.g., native T1, post gadolinium T1, ECV) are most sensitive and specific to identify the presence or absence of disease and its extent. Studies with large groups of patients and prospective studies are needed using standardized imaging protocols.
KEY POINTS.
LGE is a simple, robust, well validated method for the assessment of scar in acute and chronic myocardial infarction.
LGE is useful for distinguishing between ischemic and nonischemic cardiomyopathy. Specific LGE patterns are seen in nonischemic cardiomyopathy.
Patient studies using T1 mapping have varied in study, design and acquisition sequences.
Despite the differences in technique, a clear pattern that has been seen is that in cardiac disease post contrast T1 times are shorter.
ECV measured with cardiac CT represents a new approach toward the clinical assessment of diffuse myocardial fibrosis by evaluating the distribution of iodinated contrast.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David A. Bluemke, Email: bluemked@nih.gov, Radiology and Imaging Sciences, National Institutes of Health, Bethesda, MD, Phone: 3014021854, Fax: 3014021854.
Puskar Pattanayak, Email: puskar.pattanayak@nih.gov, Laboratory od Diagnostic Radiology Research. National Institutes of Health, Bethesda,,MD, Phone: 2023044226.
REFERENCES
- 1.Libby P, Lee RT. Matrix matters. Circulation. 2000;102(16):1874–1876. doi: 10.1161/01.cir.102.16.1874. [DOI] [PubMed] [Google Scholar]
- 2.Speiser B, Riess CF, Schaper J. The extracellular matrix in human myocardium: Part I: Collagens I, III, IV, and VI. Cardioscience. 1991;2(4):225–232. [PubMed] [Google Scholar]
- 3.Speiser B, Weihrauch D, Riess CF, Schaper J. The extracellular matrix in human cardiac tissue. Part II: Vimentin, laminin, and fibronectin. Cardioscience. 1992;3(1):41–49. [PubMed] [Google Scholar]
- 4.Caspari PG, Gibson K, Harris P. Changes in myocardial collagen in normal development and after beta blockade. Recent advances in studies on cardiac structure and metabolism. 1975;7:99–104. [PubMed] [Google Scholar]
- 5.Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113(23):2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 6.Kwong RY, Sattar H, Wu H, Vorobiof G, Gandla V, Steel K, Siu S, Brown KA. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation. 2008;118(10):1011–1020. doi: 10.1161/CIRCULATIONAHA.107.727826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. Journal of the American College of Cardiology. 2006;48(10):1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 8.Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. Journal of the American College of Cardiology. 2011;58(12):1271–1279. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 9.Schaper J, Speiser B. The extracellular matrix in the failing human heart. Basic research in cardiology. 1992;87(Suppl 1):303–309. doi: 10.1007/978-3-642-72474-9_26. [DOI] [PubMed] [Google Scholar]
- 10.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and rennin-angiotensin-aldosterone system. Circulation. 1991;83(6):1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 11.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 12.Bohl S, Wassmuth R, Abdel-Aty H, Rudolph A, Messroghli D, Dietz R, Schulz-Menger J. Delayed enhancement cardiac magnetic resonance imaging reveals typical patterns of myocardial injury in patients with various forms of non-ischemic heart disease. The international journal of cardiovascular imaging. 2008;24(6):597–607. doi: 10.1007/s10554-008-9300-x. [DOI] [PubMed] [Google Scholar]
- 13.Karamitsos TD, Francis JM, Myerson S, Selvanayagam JB, Neubauer S. The role of cardiovascular magnetic resonance imaging in heart failure. Journal of the American College of Cardiology. 2009;54(15):1407–1424. doi: 10.1016/j.jacc.2009.04.094. [DOI] [PubMed] [Google Scholar]
- 14.Hosch W, Kristen AV, Libicher M, Dengler TJ, Aulmann S, Heye T, Schnabel PA, Schirmacher P, Katus HA, Kauczor HU, et al. Late enhancement in cardiac amyloidosis: correlation of MRI enhancement pattern with histopathological findings. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis. 2008;15(3):196–204. doi: 10.1080/13506120802193233. [DOI] [PubMed] [Google Scholar]
- 15.Moon JC, Sachdev B, Elkington AG, McKenna WJ, Mehta A, Pennell DJ, Leed PJ, Elliott PM. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. European heart journal. 2003;24(23):2151–2155. doi: 10.1016/j.ehj.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. The New England journal of medicine. 1999;341(10):709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 17.Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation. 2000;102(22):2700–2706. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 18.Croisille P, Revel D, Saeed M. Contrast agents and cardiac MR imaging of myocardial ischemia: from bench to bedside. European radiology. 2006;16(9):1951–1963. doi: 10.1007/s00330-006-0244-z. [DOI] [PubMed] [Google Scholar]
- 19.Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation. 1996;94(12):3318–3326. doi: 10.1161/01.cir.94.12.3318. [DOI] [PubMed] [Google Scholar]
- 20.Judd RM, Atalay MK, Rottman GA, Zerhouni EA. Effects of myocardial water exchange on T1 enhancement during bolus administration of MR contrast agents. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1995;33(2):215–223. doi: 10.1002/mrm.1910330211. [DOI] [PubMed] [Google Scholar]
- 21.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114(1):32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marban E, Tomaselli GF, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115(15):2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. European heart journal. 2005;26(15):1461–1474. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 24.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100(19):1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 25.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343(20):1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 26.Orn S, Manhenke C, Anand IS, Squire I, Nagel E, Edvardsen T, Dickstein K. Effect of left ventricular scar size, location, and transmurality on left ventricular remodeling with healed myocardial infarction. The American journal of cardiology. 2007;99(8):1109–1114. doi: 10.1016/j.amjcard.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 27.Austin BA, Tang WH, Rodriguez ER, Tan C, Flamm SD, Taylor DO, Starling RC, Desai MY. Delayed hyper-enhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. JACC Cardiovascular imaging. 2009;2(12):1369–1377. doi: 10.1016/j.jcmg.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, Tarasoutchi F, Grinberg M, Rochitte CE. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. Journal of the American College of Cardiology. 2010;56(4):278–287. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 29.Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, Bos JM, Tajik AJ, Valeti US, Nishimura RA, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circulation Heart failure. 2010;3(1):51–58. doi: 10.1161/CIRCHEARTFAILURE.109.854026. [DOI] [PubMed] [Google Scholar]
- 30.Kwon DH, Smedira NG, Rodriguez ER, Tan C, Setser R, Thamilarasan M, Lytle BW, Lever HM, Desai MY. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: correlation with histopathology and prevalence of ventricular tachycardia. Journal of the American College of Cardiology. 2009;54(3):242–249. doi: 10.1016/j.jacc.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Cummings KW, Bhalla S, Javidan-Nejad C, Bierhals AJ, Gutierrez FR, Woodard PK. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics : a review publication of the Radiological Society of North America, Inc. 2009;29(1):89–103. doi: 10.1148/rg.291085052. [DOI] [PubMed] [Google Scholar]
- 32.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218(1):215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 33.Spiewak M, Malek LA, Misko J, Chojnowska L, Milosz B, Klopotowski M, Petryka J, Dabrowski M, Kepka C, Ruzyllo W. Comparison of different quantification methods of late gadolinium enhancement in patients with hypertrophic cardiomyopathy. European journal of radiology. 2010;74(3):e149–e153. doi: 10.1016/j.ejrad.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 34.Amado LC, Gerber BL, Gupta SN, Rettmann DW, Szarf G, Schock R, Nasir K, Kraitchman DL, Lima JA. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. Journal of the American College of Cardiology. 2004;44(12):2383–2389. doi: 10.1016/j.jacc.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 35.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. Journal of the American College of Cardiology. 2011;57(8):891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wynn TA. Cellular and molecular mechanisms of fibrosis. The Journal of pathology. 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S, Han J, Nacif MS, Jones J, Kawel N, Kellman P, Sibley CT, Bluemke DA. Diffuse myocardial fibrosis evaluation using cardiac magnetic resonance T1 mapping: sample size considerations for clinical trials. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2012;14:90. doi: 10.1186/1532-429X-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbers LF, Baars EN, Brouwer WP, Beek AM, Hofman MB, Niessen HW, van Rossum AC, Marcu CB. T1 mapping shows increased extracellular matrix size in the myocardium due to amyloid depositions. Circulation Cardiovascular imaging. 2012;5(3):423–426. doi: 10.1161/CIRCIMAGING.112.973438. [DOI] [PubMed] [Google Scholar]
- 39.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2004;52(1):141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 40.Messroghli DR, Greiser A, Frohlich M, Dietz R, Schulz-Menger J. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. Journal of magnetic resonance imaging : JMRI. 2007;26(4):1081–1086. doi: 10.1002/jmri.21119. [DOI] [PubMed] [Google Scholar]
- 41.Messroghli DR, Niendorf T, Schulz-Menger J, Dietz R, Friedrich MG. T1 mapping in patients with acute myocardial infarction. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2003;5(2):353–359. doi: 10.1081/jcmr-120019418. [DOI] [PubMed] [Google Scholar]
- 42.Messroghli DR, Walters K, Plein S, Sparrow P, Friedrich MG, Ridgway JP, Sivananthan MU. Myocardial T1 mapping: application to patients with acute and chronic myocardial infarction. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2007;58(1):34–40. doi: 10.1002/mrm.21272. [DOI] [PubMed] [Google Scholar]
- 43.Piechnik SK, Ferreira VM, Dall'Armellina E, Cochlin LE, Greiser A, Neubauer S, Robson MD. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2010;12:69. doi: 10.1186/1532-429X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Messroghli DR, Plein S, Higgins DM, Walters K, Jones TR, Ridgway JP, Sivananthan MU. Human myocardium: single-breath-hold MR T1 mapping with high spatial resolution--reproducibility study. Radiology. 2006;238(3):1004–1012. doi: 10.1148/radiol.2382041903. [DOI] [PubMed] [Google Scholar]
- 45.Flacke SJ, Fischer SE, Lorenz CH. Measurement of the gadopentetate dimeglumine partition coefficient in human myocardium in vivo: normal distribution and elevation in acute and chronic infarction. Radiology. 2001;218(3):703–710. doi: 10.1148/radiology.218.3.r01fe18703. [DOI] [PubMed] [Google Scholar]
- 46.Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, Sheppard MN, Poole-Wilson PA, Hawkins PN, Pennell DJ. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111(2):186–193. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 47.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. Journal of the American College of Cardiology. 2008;52(19):1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 48.Jerosch-Herold M, Sheridan DC, Kushner JD, Nauman D, Burgess D, Dutton D, Alharethi R, Li D, Hershberger RE. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. American journal of physiology Heart and circulatory physiology. 2008;295(3):H1234–H1242. doi: 10.1152/ajpheart.00429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, Sibley CT, Chen MY, Bandettini WP, Arai AE. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. European heart journal. 2012;33(10):1268–1278. doi: 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2012;14:63. doi: 10.1186/1532-429X-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kellman P, Wilson JR, Xue H, Bandettini WP, Shanbhag SM, Druey KM, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2012;14:64. doi: 10.1186/1532-429X-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bauner KU, Biffar A, Theisen D, Greiser A, Zech CJ, Nguyen ET, Reiser MF, Wintersperger BJ. Extracellular volume fractions in chronic myocardial infarction. Investigative radiology. 2012;47(9):538–545. doi: 10.1097/RLI.0b013e3182631c37. [DOI] [PubMed] [Google Scholar]
- 53.Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Choudhury RP, Friedrich MG, Robson MD, Neubauer S. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2012;14:42. doi: 10.1186/1532-429X-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sibley CT, Noureldin RA, Gai N, Nacif MS, Liu S, Turkbey EB, Mudd JO, van der Geest RJ, Lima JA, Halushka MK, et al. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology. 2012;265(3):724–732. doi: 10.1148/radiol.12112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kehr E, Sono M, Chugh SS, Jerosch-Herold M. Gadolinium-enhanced magnetic resonance imaging for detection and quantification of fibrosis in human myocardium in vitro. The international journal of cardiovascular imaging. 2008;24(1):61–68. doi: 10.1007/s10554-007-9223-y. [DOI] [PubMed] [Google Scholar]
- 56.Miller JM, Dewey M, Vavere AL, Rochitte CE, Niinuma H, Arbab-Zadeh A, Paul N, Hoe J, de Roos A, Yoshioka K, et al. Coronary CT angiography using 64 detector rows: methods and design of the multi-centre trial CORE-64. European radiology. 2009;19(4):816–828. doi: 10.1007/s00330-008-1203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, Nieman K, van Werkhoven JM, Pundziute G, Weustink AC, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. Journal of the American College of Cardiology. 2008;52(25):2135–2144. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 58.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. Journal of the American College of Cardiology. 2008;52(21):1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 59.Hausleiter J, Meyer T, Hadamitzky M, Zankl M, Gerein P, Dorrler K, Kastrati A, Martinoff S, Schomig A. Non-invasive coronary computed tomographic angiography for patients with suspected coronary artery disease: the Coronary Angiography by Computed Tomography with the Use of a Submillimeter resolution (CACTUS) trial. European heart journal. 2007;28(24):3034–3041. doi: 10.1093/eurheartj/ehm150. [DOI] [PubMed] [Google Scholar]
- 60.Lardo AC, Cordeiro MA, Silva C, Amado LC, George RT, Saliaris AP, Schuleri KH, Fernandes VR, Zviman M, Nazarian S, et al. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation. 2006;113(3):394–404. doi: 10.1161/CIRCULATIONAHA.105.521450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nacif MS, Kawel N, Lee JJ, Chen X, Yao J, Zavodni A, Sibley CT, Lima JA, Liu S, Bluemke DA. Interstitial myocardial fibrosis assessed as extracellular volume fraction with low-radiation-dose cardiac CT. Radiology. 2012;264(3):876–883. doi: 10.1148/radiol.12112458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bandula S, White SK, Flett AS, Lawrence D, Pugliese F, Ashworth MT, Punwani S, Taylor SA, Moon JC. Measurement of myocardial extracellular volume fraction by using equilibrium contrast-enhanced CT: validation against histologic findings. Radiology. 2013;269(2):396–403. doi: 10.1148/radiology.13130130. [DOI] [PubMed] [Google Scholar]
- 63.Langer C, Lutz M, Eden M, Ludde M, Hohnhorst M, Gierloff C, Both M, Burchert W, Faber L, Horstkotte D, et al. Hypertrophic cardiomyopathy in cardiac CT: a validation study on the detection of intramyocardial fibrosis in consecutive patients. The international journal of cardiovascular imaging. 2014;30(3):659–667. doi: 10.1007/s10554-013-0358-8. [DOI] [PubMed] [Google Scholar]
- 64.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circulation Cardiovascular imaging. 2010;3(6):727–734. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122(2):138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 66.Gai N, Turkbey EB, Nazarian S, van der Geest RJ, Liu CY, Lima JA, Bluemke DA. T1 mapping of the gadolinium-enhanced myocardium: adjustment for factors affecting interpatient comparison. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2011;65(5):1407–1415. doi: 10.1002/mrm.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turkbey EB, Gai N, Lima JA, van der Geest RJ, Wagner KR, Tomaselli GF, Bluemke DA, Nazarian S. Assessment of cardiac involvement in myotonic muscular dystrophy by T1 mapping on magnetic resonance imaging. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9(10):1691–1697. doi: 10.1016/j.hrthm.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dass S, Suttie JJ, Piechnik SK, Ferreira VM, Holloway CJ, Banerjee R, Mahmod M, Cochlin L, Karamitsos TD, Robson MD, et al. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circulation Cardiovascular imaging. 2012;5(6):726–733. doi: 10.1161/CIRCIMAGING.112.976738. [DOI] [PubMed] [Google Scholar]