Abstract
Using a large data set (n = 811), the relationship between acute respiratory infection illness severity and inflammatory biomarkers was investigated to determine whether certain symptoms are correlated more closely than others with the inflammatory biomarkers, interleukin‐8 (IL‐8) and nasal neutrophils. Participants with community acquired acute respiratory infection underwent nasal lavage for IL‐8 and neutrophil testing, in addition to multiplex polymerase chain reaction (PCR) methods for the detection and identification of respiratory viruses. Information about symptoms was obtained throughout the duration of the illness episode using the well‐validated Wisconsin Upper Respiratory Symptom Survey (WURSS‐21). Global symptom severity was calculated by the area under the curve (AUC) plotting duration versus WURSS total. Of the specimens tested, 56% were positively identified for one or more of nine different respiratory viruses. During acute respiratory infection illness, both IL‐8 and neutrophils positively correlate with AUC (rs = 0.082, P = 0.022; rs = 0.080, P = 0.030). IL‐8 and neutrophils correlate with nasal symptom severity: runny nose (r = 0.13, P = < 0.00001; r = 0.18, P = < 0.003), plugged nose (r = 0.045, P = 0.003; r = 0.14, P = 0.058), and sneezing (r = −0.02, P = < 0.0001; r = −0.0055, P = 0.31). Neutrophils correlate with some quality of life measures such as sleeping well (r = 0.15, P = 0.026). Thus, the study demonstrates that IL‐8 and neutrophils are correlated with severity of nasal symptoms during acute respiratory infection. Further research is necessary to determine if the concentration of these or other biomarkers can predict the overall duration and severity of acute respiratory infection illness. J. Med. Virol. 87:330–337, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: upper respiratory infection, biomarkers, interleukin‐8, neutrophils, signs and symptoms, rhinitis
INTRODUCTION
Acute respiratory infection illness (also known as the common cold, upper respiratory tract infection, or upper respiratory infection) is an acute infection, generally viral in etiology, involving structures of the upper airways [Simoes et al., 2006]. The most common causative agent is rhinovirus, but several other respiratory viruses are also known to cause acute respiratory illness [Gwaltney, 1985]. Acute respiratory infection is a common occurrence, yet detrimental to well‐being. Moreover, there is currently no cure or effective treatment option available [Cohen et al., 1993; Carter, 2002]. Symptoms of acute respiratory infection illness can be examined three‐dimensionally in the following groups: nasal, throat, and quality of life [Obasi et al., 2013]. These unpleasant, and sometimes, severe symptoms cause people to miss work and to utilize healthcare services, which places a notable burden on the individual and society [Fendrick et al., 2003; Eccles, 2005; Fiore et al., 2010; Ebell and Afonso, 2011].
For many years, it has been known that some inflammatory cytokines, such as interleukin‐8 (IL‐8), play a role in the pathophysiology of the common cold, as levels are found to increase in individuals sick with viral rhinitis compared to healthy individuals [Choi and Jacoby, 1992; Becker et al., 1993; Noah et al., 1995; Röseler et al., 1995; Gern et al., 1996; Johnston et al., 1997; Zhu et al., 1997; Kaul et al., 2000; Parry et al., 2000]. Within the immune system, IL‐8 acts to recruit and activate neutrophils, contributing to inflammation [Naclerio et al., 1988; Teran et al., 1997; Zeilhofer and Schorr, 2000; Turner, 2001]. However, little work has been done to correlate IL‐8 concentrations with specific acute respiratory infection symptoms and degree of illness. This paper presents important novel research boasting over 800 common cold cases with biomarkers and detailed records of acute respiratory infection severity making the breadth of our study unique. Additionally, not only is the relationship of the biomarkers with overall illness severity examined, but also more than 20 separate symptoms are considered one by one, and in meaningful clusters, in order to hone in on exactly what the relationship is between inflammatory biomarkers and certain cold symptoms. By investigating the relationship between acute respiratory infection illness severity and inflammatory biomarkers, the intent was to determine whether certain symptoms correlate more closely than others with inflammatory biomarkers such as IL‐8 and nasal neutrophils. An additional aim was to gain insight into the predictability of overall severity and duration of acute respiratory infection episodes based on change in IL‐8 and neutrophil levels during the first 3 days of illness.
In order to answer these questions, data sets from two studies [Barrett et al., 2010; Barrett et al., 2012] were combined to look at a total of 811 acute respiratory infection episodes. In both studies, a nasal lavage was performed within the first 72 hr of the onset of symptoms. In one of the studies, 685 participants returned about 48 hr later to provide a second nasal specimen during their acute respiratory infection. The nasal specimens were used to measure IL‐8 concentrations and neutrophil counts, and to undergo multiplex polymerase chain reaction (PCR) for the detection and identification of respiratory viruses. Throughout the duration of the acute respiratory infection episode participants provided information about their symptoms on a self‐report basis using the well‐validated Wisconsin Upper Respiratory Symptom Survey (WURSS‐21) [Barrett et al., 2002; Barrett et al., 2005; Barrett et al., 2009]. The WURSS scores were then used to statistically analyze correlations of symptom severity with IL‐8 and neutrophil levels.
IL‐8 and nasal neutrophils were hypothesized to have a significant positive correlation with overall acute respiratory infection severity, measured by both area under the curve (AUC) and WURSS total. Upon examination of the 21 WURSS items, the nasal group symptoms (runny nose, plugged nose and sneezing) [Obasi et al., 2013] were hypothesized to have the most significant correlations with the inflammatory biomarkers examined. Finally, it was hypothesized that higher IL‐8 and nasal neutrophil levels on Day 1 of the acute respiratory infection would be associated with lower WURSS total on Day 3 of the acute respiratory infection, due to the initial vigorous immune response leading to quicker amelioration of symptoms.
MATERIALS AND METHODS
For the purposes of this research, acute respiratory infection data from two randomized controlled trials were combined. Both trials' protocols and main results have been published elsewhere [Barrett et al., 2007; Barrett et al., 2010; Barrett et al., 2011; Barrett et al., 2012; Obasi et al., 2012; Rakel et al., 2013; Zgierska et al., 2013; Hayney et al., 2014]. Both trials from which the data came were approved and monitored by the University of Wisconsin Health Sciences Institutional Review Board and carried out in accordance with the ethical standards of the Declaration of Helsinki. All participants gave written informed consent.
Participants
In the PEP trial (Physician, Echinacea, and Placebo), which is a shortened version of the trial title “Placebo: Physician or Pill: A Randomized Controlled Trial in a Common Cold Model” [Barrett et al., 2007; Barrett et al., 2010; Barrett et al., 2011], n = 718 participants gave written informed consent, then were enrolled within the first 36 hr of new‐onset common cold symptoms and were followed until their colds resolved. The population consisted of community‐recruited 12 to 80 year olds. Enrollment and monitoring occurred over a period of about four and a half years from January 2004 until August 2008. Daily contact was made by telephone for the duration of participation.
In the MEPARI trial, Meditation or Exercise for Preventing Acute Respiratory Infection: A Randomized Controlled Trial [Barrett et al., 2012], written informed consent was obtained from n = 154 healthy individuals aged 50 years and older who were recruited from the community. The first cohort (n = 94) was enrolled in September 2009, while the second cohort (n = 60) began in January 2010. Participants from both cohorts were followed until exiting in May 2010. During the entire trial, participants were asked to start documenting symptoms anytime they thought they were getting a cold. Participants were contacted two times a week by telephone throughout the duration of the study.
Acute Respiratory Infection Monitoring
Both trials used the same criteria to define an acute respiratory infection illness episode: 1) Answer “Yes” to either: “Do you think you are coming down with a cold?” or “Do you think you have a cold?” 2) Exhibit one or more of four cold symptoms ‐ nasal discharge, nasal obstruction, sneezing, sore throat, and 3) Score two points or more on the Jackson scale [Jackson et al., 1962]. The Jackson score is calculated by summing eight symptom scores (sneezing, headache, malaise, chilliness, nasal discharge, nasal obstruction, sore throat, and cough), where each is rated 0 = absent, 1 = mild, 2 = moderate, 3 = severe. In the PEP trial, illness episode duration began at enrollment and continued through the last time the participant answered “yes” to, “Do you think you still have a cold?” To confirm that the illness had ended, the last “yes” answer had to be followed by a “no” answer for two days in a row. Monitoring was limited to a maximum of 14 days. In the MEPARI trial, the beginning of each acute respiratory infection illness episode was determined by the defining criteria described above. The last day of the illness episode was the last day the participant rated illness severity higher than 0 in response to “How sick do you feel today?” on the WURSS instrument. In both trials the date and time of self‐report questionnaires were recorded in order to assess illness duration.
Assessment of Symptoms
Acute respiratory infection severity was assessed using the Wisconsin Upper Respiratory Symptom Survey (WURSS) [Barrett et al., 2002; Barrett et al., 2005], which is a well validated illness specific quality of life outcome instrument. Items assess symptom severity and functional impairment, with a score of 1 considered to be very mild; 3, mild; 5, moderate; and 7, severe. Throughout the length of the illness, the WURSS‐21 [Barrett et al., 2009] was self‐administered twice daily by each PEP participant, and the WURSS‐24 daily by MEPARI participants. The WURSS‐21 contains one item rating overall illness severity (How sick do you feel today?); 10 items rating cold symptoms (runny nose, plugged nose, sneezing, sore throat, scratchy throat, cough, hoarseness, head congestion, chest congestion, feeling tired); nine items rating how much the cold episode has interfered with function and quality of life (think clearly; sleep well; breathe easily; walk, climb stairs, exercise; accomplish daily activities; work outside the home; work inside the home; interact with others; live your personal life); and one item rating daily change (Compared to yesterday, I feel that my cold is…). WURSS‐24 is a derivation of the WURSS‐44, and includes all WURSS‐21 items plus three items rating influenza‐like symptoms. These three items were not included in this study in order to make the WURSS scores between the two trials parallel.
Calculation of Outcomes
Illness duration was assessed in hours and minutes according to the times of self‐report and then converted to decimalized days. For the PEP trial, morning and evening scores for each item of the WURSS‐21 were first averaged. In both trials, for each day of illness, WURSS total was calculated by summing 19 items on the WURSS‐21, not including the first item assessing overall illness severity or the last item assessing change since the previous day. To calculate area under the curve (AUC) for global severity, the sum of WURSS totals across all time points of the illness episode was used, and trapezoidal approximation was applied. Nasal AUC was calculated by using the sum of the WURSS scores from only the nasal symptoms (runny nose, plugged nose, sneezing) for the duration of the acute respiratory infection episode. Individual symptoms from the WURSS were analyzed with respect to the main variables using the WURSS score from Day 1 of the acute respiratory infection episode, which corresponds to the day the nasal specimens were collected for biomarker analyses.
Biomarkers
Samples were collected at enrollment (Day 1) and again on Day 3 of participation in the PEP trial. In the MEPARI trial, when a participant reported acute respiratory infection symptoms equaling two or greater on the Jackson scale, samples were collected within the first 72 hr of onset of symptoms. Nasal specimens were obtained by performing nasal lavage, and were used for analysis of IL‐8 and neutrophils as indicators of nasal inflammation. Nasal lavage specimens were also used for PCR‐based viral identification. In both trials, IL‐8 concentrations were measured in picograms per milliliter (pg/ml) using an enzyme‐linked immunosorbent assay test (ELISA), according to manufacturer's protocol. Polymorphonuclear leukocytes were counted using a standard hemocytometer in both trials, and expressed as neutrophils per milliliter (PMN/ml). Multiplex PCR methods developed and validated at UW‐Madison were used to detect and identify respiratory viruses in nasal wash samples. With this accurate high‐throughput assay, over 100 rhinovirus serotypes, as well as serotypes of influenza, parainfluenza, coronavirus, respiratory syncytial virus, adenovirus, enterovirus, and metapneumovirus can be identified. Overall sensitivity is estimated at 94%, with 99% specificity [Lee et al., 2007].
Statistical Analyses
To assess the correlation among the variables, IL‐8, neutrophils, AUC, and acute respiratory infection duration, Spearman tests were done using SPSS version 20. Linear regression assessed the relationship between biomarkers and symptoms. Pearson correlation coefficients between biomarkers and certain symptoms were reported, and Student's t tests were used to test the significance of any specific symptom in the corresponding linear model (Table III). These tests were done using R version 3.0.2 (The R Foundation for Statistical Computing, http://r-project.org/). Preliminary analysis showed that the ranges of some variables were very wide and skewed, for example, the IL‐8 and neutrophil values, which ranged from zero to more than ten thousand. In this case, the Box–Cox transformation [Box and Cox, 1964] was used to transform these values in order to make the data more normally distributed, thus improving the validity of measures of association such as the Pearson correlation between variables and for other data stabilization procedures.
Table III.
IL‐8 and Neutrophil Correlations with ARI Symptoms
| Symptom score | Pearson correlation coefficient | Regression coefficient | P value |
|---|---|---|---|
| IL‐8 | |||
| *WURSS total | 0.068 | 0.0072 | <0.008 |
| *Nasal AUC | 0.057 | 0.0031 | 0.066 |
| Runny nose | 0.13 | 0.18 | <0.00001 |
| Plugged nose | 0.045 | 0.18 | 0.003 |
| Sneezing | −0.024 | −0.13 | <0.0001 |
| Scratchy throat | 0.017 | −0.12 | 0.017 |
| Daily change | 0.058 | −0.18 | <0.002 |
| Neutrophils | |||
| *WURSS total | 0.12 | 0.010 | <0.002 |
| *Nasal AUC | 0.073 | 0.0035 | 0.089 |
| Runny nose | 0.18 | 0.17 | <0.003 |
| Plugged nose | 0.14 | 0.11 | 0.058 |
| Sneezing | −0.0055 | −0.05 | 0.31 |
| Overall illness severity | 0.099 | 0.16 | 0.061 |
| Scratchy throat | −0.033 | −0.13 | 0.034 |
| Think clearly | 0.034 | −0.15 | 0.026 |
| Sleep well | 0.15 | 0.11 | 0.026 |
Pearson's correlations are calculated using non‐transformed data. Regression coefficients are calculated from the linear model with these variables as covariates, and logarithm of IL‐8/Neutrophils as response variable. P‐values are based on the Student's t tests for testing whether specific regression coefficient equals 0.
WURSS = Wisconsin Upper Respiratory Symptom Survey, AUC = area under curve.
Regression coefficient from separate linear models with variable as covariate and log (Biomarker) as response variable: log(Biomarker) ∼ Intercept + WURSStotal or log(Biomarker) ∼ Intercept + NasaIAUC.
For the results, regarding each model, the summary statistics are listed, including the estimated values for covariates, standard errors, t‐statistics and corresponding P‐values. The Pearson correlation coefficients between certain important variables are also reported to better explain the relationships of symptoms and biomarkers.
RESULTS
A total of 811 acute respiratory infection episodes were analyzed making up 7,124 days of acute respiratory infection illness, with a mean global severity score (AUC) of 275. Of these, 56% tested positive for PCR identifiable respiratory viruses, including adenovirus, bocavirus, coronavirus, enterovirus, influenza virus, metapneumovirus, parainfluenzavirus, rhinovirus, and respiratory syncytial virus. The biomarkers examined showed the mean IL‐8 was 1,139 pg/ml and mean neutrophils were 68 PMN/ml (Table I).
Table I.
Demographics of Study Population
| ARIs | 811 |
| Days of ARI illness | 7124 |
| Mean global severity (AUC) | 275 |
| Mean IL‐8 (pg/ml) | 1,139 |
| Mean neutrophils (PMN/ml) | 68 |
| Mean Age (years) | 36 |
| Gender | |
| Female | 514 |
| Male | 270 |
| Other | 0 |
| Virus detected | |
| Negative | 357 |
| Rhinovirus | 315 |
| Coronavirus | 53 |
| Parainfluenza virus | 19 |
| Influenza virus | 18 |
| Metapneumovirus | 17 |
| Respiratory syncytial virus | 17 |
| Enterovirus | 10 |
| Bocavirus | 8 |
| Adenovirus | 7 |
ARI = acute respiratory infection, AUC = area under curve (calculated as trapezoidal approximation using daily scores from Wisconsin Upper Respiratory Symptom Survey on y‐axis and duration of ARI illness on x‐axis), IL‐8 = interleukin 8, pg = picogram, ml = milliliter, PMN = polynorphonuclear leukocyte.
Originally called neutrophil activating factor‐1 (NAF‐1), IL‐8 is a chemokine that recruits and activates neutrophils [Marsh and Kendall, 1996; Zeilhofer and Schorr, 2000]. As physiologically expected, IL‐8 correlated significantly with neutrophils in the positive direction (r = 0.58, P < 0.001) [Teran et al., 1997; Turner et al., 1998]. Participants who had higher levels of IL‐8 had consistently more nasal neutrophils (Fig. 1).
Figure 1.
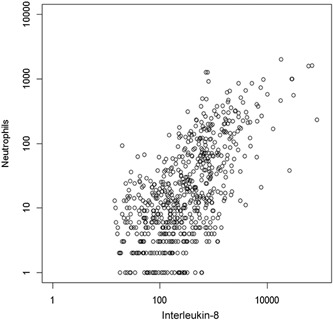
Correlation of interleukin‐8 with nasal neutrophils. Interleukin‐8 positively correlates with the number of nasal neutrophils present in a nasal lavage during an acute respiratory infection. (P < 0.001; rs = 0.583)
Significant positive correlation of both IL‐8 and neutrophils with AUC and acute respiratory infection duration was found. Participants who had a higher concentration of IL‐8 had colds that lasted longer and were more severe. Similarly, participants who had more nasal neutrophils had longer lasting and more severe colds (Table II). The correlation between IL‐8 and global severity of nasal specific symptoms, or nasal AUC, for the entire illness episode showed a positive trend (r = 0.0031, P = 0.066). Neutrophils also trended positively with nasal AUC (r = 0.0035, P = 0.088). On Day 1, IL‐8 significantly correlated with each of the three symptoms from the nasal group of WURSS: rhinorrhea, nasal obstruction, and sternutation (sneezing). Interestingly, IL‐8 on Day 1 of the illness also correlated with scratchy throat and with how participants responded to a question gauging overall symptom improvement. Nasal neutrophils correlated strongly with global severity measured by WURSS total, although the correlation was not maintained when nasal symptoms were considered individually, with the exception of runny nose. In addition to correlating with runny nose and global severity, neutrophils also correlated with scratchy throat, thinking clearly, and sleeping well (Table III).
Table II.
Spearman Correlation Tests of Main Variables
| Correlation coefficient | P value | |
|---|---|---|
| IL‐8/AUC | 0.082 | 0.022 |
| IL‐8/ARI duration | 0.141 | <0.001 |
| Neutrophils/AUC | 0.080 | 0.030 |
| Neutrophils/ARI duration | 0.080 | 0.028 |
IL‐8 = Interleukin‐8, AUC = area under curve (calculated as trapezoidal approximation using daily scores from Wisconsin Upper Respiratory Symptom Survey on y‐axis and duration of ARI illness on x‐axis), ARI = acute respiratory infection.
In order to investigate whether the IL‐8 and neutrophil levels from Day 1 of an acute respiratory infection episode can predict characteristics of the illness a couple days later, a time points analysis was performed using biomarkers on Day 1 and WURSS total on Day 3 of the PEP trial. It was hypothesized that high levels of IL‐8 and neutrophils on Day 1 would predict low WURSS score on Day 3. However, the findings were insignificant.
DISCUSSION
The results of this study show there is a significant correlation between nasal IL‐8 and neutrophils with the severity of rhinorrhea, nasal obstruction, and sternutation during acute respiratory infection illness. These data support the hypothesis that IL‐8 and neutrophils play a significant role in respiratory infection‐related nasal symptoms. Looking at the interconnectedness between biomarkers, IL‐8, a pro‐inflammatory cytokine, was found to significantly correlate with neutrophils in the positive direction, which makes sense physiologically as IL‐8 is a chemoattractant for neutrophils [Teran et al., 1997]. Several cytokine genes and gene products, including those for IL‐8, are known to play significant roles in the induction and modulation of inflammation in human nasal epithelium [Becker et al., 1993]. A clear correlation of the biomarkers, IL‐8 and nasal neutrophils, with symptoms of the common cold is shown using this sizeable and diverse set of data.
Many in vitro studies have found a relationship between viral respiratory infection and IL‐8 expression and/or concentrations. One study found a 4‐ to 10‐fold increase in IL‐8 mRNA expression after infecting human nasal epithelium with respiratory syncytial virus (RSV) or stimulation with tumor necrosis factor or interleukin‐1 [Becker et al., 1993]. They determined that IL‐8 is a major cytokine of human nasal epithelium, constitutively expressed and readily produced upon virus infection. RSV has also been found to increase both IL‐8 gene expression in human lung epithelial A549 cells [Fiedler et al., 1995], and IL‐8 release by buffy coat cells, especially in the presence of lung surfactant protein A [Hickling et al., 2000]. Another study showed that adenovirus‐induced Raf/MAPK activation contributes to IL‐8 production [Bruder and Kovesdi, 1997]. Additionally influenza virus A has been determined to induce interleukin‐8 expression in human airway epithelial cells [Choi and Jacoby, 1992].
Cytokine levels have also been examined in naturally acquired viral rhinitis. One group of investigators found elevated IL‐8 levels in patients with viral rhinitis when compared to control subjects, which suggests that IL‐8, among other cytokines, play a role in the pathophysiology of the common cold [Röseler et al., 1995]. The current findings support and extend the known role of IL‐8 in acute respiratory infection. In addition to showing IL‐8 concentrations are high in individuals with acute respiratory infection, the current results show that the IL‐8 concentration correlates with symptom severity. While the previous study examined specimens from 20 patients with naturally acquired viral rhinitis, this study's sample size is much larger with nasal specimens for over 800 community‐acquired acute respiratory infections.
The elevation of certain biomarkers, such as IL‐8, during acute respiratory infection has been most studied in rhinovirus infection. Several authors have reported a direct correlation between the severity of symptoms associated with in vitro rhinovirus infection and the concentration of interleukin‐8 in nasal secretions. Biagioli et al. examined the mechanism of rhinovirus‐induced IL‐8 response and suggested that mediation occurs through production of oxidative species and the subsequent activation of NF‐kappaB [Biagioli et al., 1999]. That study was carried out in vitro. The current study supports that study's findings and is more clinically applicable, as infections were naturally community‐acquired and are correlated with symptom severity. Global severity, measured by AUC, was found to correlate with biomarker concentrations of IL‐8 and neutrophils. Gern and colleagues report IL‐8 production contributes to acute respiratory infection symptom severity in children with rhinovirus or influenza A infection [Gern et al., 2002]. While this study used a different symptom scale, it too found that IL‐8 correlated with acute respiratory infection severity when all the acute respiratory infection episodes reported in the studied adult population are considered. Among these 811 episodes, nine types of viruses were identified. An analysis by viral type was tried, but once divided by virus the number of acute respiratory infection episodes in each group had insufficient power to draw conclusions.
A general correlation has been shown between IL‐8 and overall acute respiratory infection severity. The recent goal, however, was to hone in on the specific symptoms, or groups of symptoms that correlate most closely with IL‐8 and neutrophils. The results highlight rhinitis related symptoms, and these findings are supported by the literature. One study examined the association between experimental rhinovirus infection and the elaboration of IL‐8 into nasal secretions, and also looked at rhinovirus‐associated common cold symptoms [Turner et al., 1998]. They found IL‐8 concentrations were significantly higher in infected symptomatic subjects compared to infected asymptomatic or placebo‐infected subjects. More specifically, there was a correlation between severity of nasal obstruction and rhinorrhea with the change in IL‐8 concentration from baseline to 2–4 days after virus challenge.
In addition to correlating with nasal symptoms, IL‐8 correlated with scratchy throat, and with the item gauging overall symptom improvement. Neutrophils correlated with scratchy throat and two quality of life symptoms: thinking clearly and sleeping well. The scratchy throat correlation with IL‐8 and neutrophils could be explained as a result of postnasal drip triggered by acute respiratory infection. The correlation between neutrophils and sleep quality is an interesting issue. Healthy individuals who are sleep deprived are known to have increased neutrophils [Boudjeltia et al., 2008; Ruiz et al., 2012]. Similarly, night shift workers have been found to have increased levels of inflammatory markers, including neutrophils, compared to individuals who work day shift [Alireza et al., 2011]. Additionally, individuals with obstructive sleep apnea syndrome (OSAS) have a higher percentage of sputum neutrophils compared to normal controls [Lacedonia et al., 2011]. All of these examples imply that the poor sleep leads to the neutrophil increase. However, during an acute respiratory infection the neutrophils are likely due to the infection, which leads to poor sleep quality. Nasal symptoms due to neutrophil infiltrated non‐allergic rhinitis have been shown to have a large effect on sleep quality and quality of life [Gelardi et al., 2008]. Even though the participants we studied exhibit a different disease state than sleep apnea, it shows an important and consistent relationship between nasal symptoms and sleep quality. Likely, the poor sleep quality is due to nasal neutrophils, but our study does not allow us to determine if the poor sleep quality is a result of the nasal neutrophils, or if the nasal neutrophils are a result of the poor sleep quality.
Previously, no biomarkers have correlated well with acute respiratory infection illness domains or specific symptoms, nor have they been shown to predict outcomes [Barrett et al., 2006; Barrett et al., 2009]. The current study's results show a strong correlation with the nasal domain of acute respiratory infection illness, namely non‐allergic rhinitis, and in fact most strongly with three specific symptoms: rhinorrhea, nasal obstruction, and sternutation. However, a biomarker or panel of biomarkers that correlate well with biological symptoms and quality of life measures remains elusive.
An important remaining question concerns the role of cytokines and cells in the clearance of acute respiratory infection virus in humans compared to the mediation of symptoms. IL‐8 has been shown to be linked to clearance of a porcine respiratory virus [Lunney et al., 2010], and one study suggests that neutrophils do not play an important role in viral clearance during influenza virus infection in mice [Wareing et al., 2007]. Furthermore, investigators have run human experiments that attempt to determine predictability of acute respiratory infection severity or duration. One group of researchers asked clinicians and patients to predict the severity and duration of acute respiratory infection at an initial visit and had the patient complete the WURSS [Longmier et al., 2013]. There was no significant association found between participant and clinician predictions of severity or duration with the actual severity and duration of acute respiratory infection. In order to answer a similar question about predictability of cold severity, time‐points analysis that looked at IL‐8 and neutrophil levels on Day 1 of the acute respiratory infection episode was performed in relationship to WURSS score on Day 3 of the acute respiratory infection episode. The analysis gave insignificant results. Therefore, while IL‐8 is indicative of inflammation, it may not be a mediator affecting the chronological course of colds. Further research is necessary to determine if the concentration or change in concentration of cytokines throughout an acute respiratory infection episode can predict the overall duration and severity of the illness. For example, specimens from additional time points could be assayed, and a larger array of cytokines could be measured in order to create a better model of the importance of cells and cytokines for the mediation of symptoms and the control of viral replication. This insight could be useful for prevention and treatment options focusing on shortening the length of a cold, and abating symptoms.
In conclusion, the following nasal symptoms: rhinorrhea, nasal obstruction and sternutation, have been determined to correlate more closely with the biomarkers IL‐8 and neutrophils, than other symptoms on the WURSS‐21 item assessment tool. Additionally, both correlate with scratchy throat. Finally, within the quality of life group, neutrophils correlated with thinking clearly and sleeping well, while IL‐8 negatively correlated with how participants responded to a question gauging overall symptom improvement. While it has been known for years that cytokines like IL‐8 are involved in the disease process of acute respiratory infection illnesses, and while it is not surprising that the symptoms that correlate most closely with IL‐8 and neutrophils from nasal specimens are nasal symptoms, this relationship has not been well documented in detail, until now. With these findings, knowing more about acute respiratory infection symptomology and its relationship with inflammatory biomarkers opens doors to discovering more effective curative treatment methods and potential medications for the amelioration of unpleasant and burdensome symptoms.
ACKNOWLEDGEMENTS
The PEP trial was supported by grant R01AT001428 from the National Institutes of Health (NIH), National Center for Complementary and Alternative Medicine (NCCAM). The MEPARI trial was supported by NIH, NCCAM grant 1R01AT004313, and by NIH grant UL1RR025011 from the Clinical and Translational Science Award Program of the National Center for Research Resources. Dr. Barrett has been supported by career development grants from NIH NCCAM (K23AT00051; K24AT006543), and the Robert Wood Johnson Foundation Generalist Physician Faculty Scholars Program.
The authors would like to acknowledge and thank Tola Ewers, MS for managing the data sets and combining them for the purposes of this study; Daniel Muller, MD, PhD and medical student Paul Dorresteijn for writing assistance with technical editing and proofreading; Christopher L. Coe, PhD, David Rakel, MD, Aleksandra Zgierska, MD, PhD, Shari Barlow, BA, Rachel Sippy, MPH, Chidi Obasi, MD, Michele Gassman, MA, Mary Checovich, Amber Schemmel, and Supriya Hayer, MD for providing support and encouragement all along the way.
No competing financial interests exist.
REFERENCES
- Alireza S, Forough S, Khosro S, Omid A. 2011. Night work and inflammatory markers. Indian J Occup Environ Med 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett B, Brown R, Mundt M, Safdar N, Dye L, Maberry R, Alt J. 2005. The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid. J Clin Epidemiol 58:609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett B, Brown R, Rakel D, Mundt M, Bone K, Barlow S, Ewers T. 2010. Echinacea for treating the common cold: A randomized trial. Ann Intern Med 153:769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett B, Brown R, Rakel D, Rabago D, Marchand L, Scheder J, Mundt M, Thomas G, Barlow S. 2011. Placebo effects and the common cold: A randomized controlled trial. Ann Fam Med 9:312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett B, Brown R, Voland R, Maberry R, Turner R. 2006. Relations among questionnaire and laboratory measures of rhinovirus infection. Eur Respir J 28:358–361. [DOI] [PubMed] [Google Scholar]
- Barrett B, Brown RL, Mundt MP, Thomas GR, Barlow SK, Highstrom AD, Bahrainian M. 2009. Validation of a short form Wisconsin Upper Respiratory Symptom Survey (WURSS‐21). Health Qual Life Outcomes 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett B, Hayney MS, Muller D, Rakel D, Ward A, Obasi CN, Brown R, Zhang Z, Zgierska A, Gern J, West R, Ewers T, Barlow S, Gassman M, Coe CL. 2012. Meditation or exercise for preventing acute respiratory infection: A randomized controlled trial. Ann Fam Med 10:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett B, Locken K, Maberry R, Schwamman J, Brown R, Bobula J, Stauffacher EA. 2002. The Wisconsin Upper Respiratory Symptom Survey (WURSS): A new research instrument for assessing the common cold. J Fam Pract 51. [PubMed] [Google Scholar]
- Barrett B, Rakel D, Chewning B, Marchand L, Rabago D, Brown R, Scheder J, Schmidt R, Gern JE, Bone K, Thomas G, Barlow S, Bobula J. 2007. Rationale and methods for a trial assessing placebo, echinacea, and doctor‐patient interaction in the common cold. EXPLORE: J Sci Healing 3:561–572. [DOI] [PubMed] [Google Scholar]
- Becker S, Koren HS, Henke DC. 1993. Interleukin‐8 expression in normal nasal epithelium and its modulation by infection with respiratory syncytial virus and cytokines tumor necrosis factor, interleukin‐1, and interleukin‐6. Am J Respir Cell Mol Biol 8:20–27. [DOI] [PubMed] [Google Scholar]
- Biagioli MC, Kaul P, Singh I, Turner RB. 1999. The role of oxidative stress in rhinovirus induced elaboration of IL‐8 by respiratory epithelial cells. Free Radic Biol Med 26:454–462. [DOI] [PubMed] [Google Scholar]
- Boudjeltia KZ, Faraut B, Stenuit P, Esposito MJ, Dyzma M, Brohee D, Ducobu J, Vanhaeverbeek M, Kerkhofs M. 2008. Sleep restriction increases white blood cells, mainly neutrophil count, in young healthy men: A pilot study. Vasc Health Risk Manag 4:1467–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box GEP, Cox DR. 1964. An analysis of transformations. J R Stat Soc Series B Stat Methodol 26:211–252. [Google Scholar]
- Bruder JT, Kovesdi I. 1997. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin‐8 expression. J Virol 71:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JM. 2002. Hand washing decreases risk of colds and flu. J Natl Med Assoc 94. [PMC free article] [PubMed] [Google Scholar]
- Choi AM, Jacoby DB. 1992. Influenza virus A infection induces interleukin‐8 gene expression in human airway epithelial cells. FEBS Lett 309:327–329. [DOI] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DA, Russell MA, Jarvis MJ, Smith AP. 1993. Smoking, alcohol consumption, and susceptibility to the common cold. Am J Public Health 83:1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebell MH, Afonso A. 2011. A systematic review of clinical decision rules for the diagnosis of influenza. Ann Fam Med 9:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. 2005. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis 5:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrick A, Monto AS, Nightengale B, Sarnes M. 2003. The economic burden of non‐influenza‐related viral respiratory tract infection in the united states. Arch Intern Med 163:487–494. [DOI] [PubMed] [Google Scholar]
- Fiedler MA, Wernke‐Dollries K, Stark JM. 1995. Respiratory syncytial virus increases IL‐8 gene expression and protein release in A549 cells. Am J Physiol 269:L865–872. [DOI] [PubMed] [Google Scholar]
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, Cox NJ. Centers for Disease Control and P . 2010. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR RecommRep: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control 59:1–62. [PubMed] [Google Scholar]
- Gelardi M, Maselli Del Giudice A, Fiorella ML, Soleti P, Di Gioacchino M, Conti CM, Fulcheri M, Doyle R, Ciprandi G. 2008. Quality of life in non‐allergic rhinitis depends on the predominant inflammatory cell type. J Biol Regul Homeost Agents 22:73–81. [PubMed] [Google Scholar]
- Gern JE, Martin MS, Anklam KA, Shen K, Roberg KA, Carlson‐Dakes KT, Adler K, Gilbertson‐White S, Hamilton R, Shult PA, Kirk CJ, Da Silva DF, Sund SA, Kosorok MR, F. Lemanske R. 2002. Relationships among specific viral pathogens, virus‐induced interleukin‐8, and respiratory symptoms in infancy. Pediatr Allergy Immunol 13:386–393. [DOI] [PubMed] [Google Scholar]
- Gern JE, Vrtis R, Kelly EA, Dick EC, Busse WW. 1996. Rhinovirus produces nonspecific activation of lymphocytes through a monocyte‐dependent mechanism. J Immunol 157:1605–1612. [PubMed] [Google Scholar]
- Gwaltney JM Jr. 1985. Virology and immunology of the common cold. Rhinology 23:265–271. [PubMed] [Google Scholar]
- Hayney MS, Coe CL, Muller D, Obasi CN, Backonja U, Ewers T, Barrett B. 2014. Age and psychological influences on immune responses to trivalent inactivated influenza vaccine in the meditation or exercise for preventing acute respiratory infection (MEPARI) trial. Hum Vaccin Immunother 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickling TP, Malhotra R, Bright H, McDowell W, Blair ED, Sim RB. 2000. Lung surfactant protein A provides a route of entry for respiratory syncytial virus into host cells. Viral Immunol 13:125–135. [DOI] [PubMed] [Google Scholar]
- Jackson GG, Dowling HF, Muldoon RL. 1962. VII. Present concepts of the common cold. Am J Public Health Nations Health 52:940–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SL, Papi A, Monick MM, Hunninghake GW. 1997. Rhinoviruses induce interleukin‐8 mRNA and protein production in human monocytes. J Infect Dis 175:323–329. [DOI] [PubMed] [Google Scholar]
- Kaul P, Biagioli MC, Singh I, Turner RB. 2000. Rhinovirus‐induced oxidative stress and interleukin‐8 elaboration involves p47‐phox but is independent of attachment to intercellular adhesion molecule‐1 and viral replication. J Infect Dis 181:1885–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacedonia D, Salerno FG, Carpagnano GE, Sabato R, Depalo A, Foschino‐Barbaro MP. 2011. Effect of CPAP‐therapy on bronchial and nasal inflammation in patients affected by obstructive sleep apnea syndrome. Rhinology 49:232–237. [DOI] [PubMed] [Google Scholar]
- Lee W‐M, Grindle K, Pappas T, Marshall DJ, Moser MJ, Beaty EL, Shult PA, Prudent JR, Gern JE. 2007. High‐throughput, sensitive, and accurate multiplex PCR‐microsphere flow cytometry system for large‐scale comprehensive detection of respiratory viruses. J Clin Microbiol 45:2626–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmier E, Barrett B, Brown R. 2013. Can patients or clinicians predict the severity or duration of an acute upper respiratory infection? Fam Pract 30:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunney JK, Fritz ER, Reecy JM, Kuhar D, Prucnal E, Molina R, Christopher‐Hennings J, Zimmerman J, Rowland RRR. 2010. Interleukin‐8, interleukin‐1β, and interferon‐γ levels are linked to PRRS virus clearance. Viral Immunol 23:127–134. [DOI] [PubMed] [Google Scholar]
- Marsh JA, Kendall MD. 1996. The physiology of immunity: CRC Press 464 p. [Google Scholar]
- Naclerio RM, Proud D, Lichtenstein LM, Kagey‐Sobotka A, Hendley JO, Sorrentino J, Gwaltney JM. 1988. Kinins are generated during experimental rhinovirus colds. J Infect Dis 157:133–142. [DOI] [PubMed] [Google Scholar]
- Noah TL, Henderson FW, Wortman IA, Devlin RB, Handy J, Koren HS, Becker S. 1995. Nasal cytokine production in viral acute upper respiratory infection of childhood. J Infect Dis 171:584–592. [DOI] [PubMed] [Google Scholar]
- Obasi CN, Brown R, Ewers T, Barlow S, Gassman M, Zgierska A, Coe CL, Barrett B. 2012. Advantage of meditation over exercise in reducing cold and flu illness is related to improved function and quality of life. Influenza Other Respir Viruses no‐no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obasi CN, Brown RL, Barrett BP. 2013. Item reduction of the Wisconsin Upper Respiratory Symptom Survey (WURSS‐21) leads to the WURSS‐11. Qual Life Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry DE, Busse WW, Sukow KA, Dick CR, Swenson C, Gern JE. 2000. Rhinovirus‐induced PBMC responses and outcome of experimental infection in allergic subjects. J Allergy Clin Immunol 105:692–698. [DOI] [PubMed] [Google Scholar]
- Rakel D, Mundt M, Ewers T, Fortney L, Zgierska A, Gassman M, Barrett B. 2013. Value associated with mindfulness meditation and moderate exercise intervention in acute respiratory infection: The MEPARI Study. Fam Pract 30:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz FS, Andersen ML, Martins RCS, Zager A, Lopes JD, Tufik S. 2012. Immune alterations after selective rapid eye movement or total sleep deprivation in healthy male volunteers. Innate Immunity 18:44–54. [DOI] [PubMed] [Google Scholar]
- Röseler S, Holtappels G, Wagenmann M, Bachert C. 1995. Elevated levels of interleukins IL‐1 beta, IL‐6 and IL‐8 in naturally acquired viral rhinitis. Eur Arch Otorhinolaryngol 252(Suppl 1):S61–63. [DOI] [PubMed] [Google Scholar]
- Simoes EAF, Cherian T, Chow J, Shahid‐Salles SA, Laxminarayan R, John TJ. 2006. Acute respiratory infections in children. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Disease control priorities in developing countries. 2nd ed. Washington (DC): World Bank. [Google Scholar]
- Teran LM, Johnston SL, Schröder JM, Church MK, Holgate ST. 1997. Role of nasal interleukin‐8 in neutrophil recruitment and activation in children with virus‐induced asthma. Am J Respir Crit Care Med 155:1362–1366. [DOI] [PubMed] [Google Scholar]
- Turner RB. 2001. The treatment of rhinovirus infections: Progress and potential. Antiviral Res 49:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RB, Weingand KW, Yeh C‐H, Leedy DW. 1998. Association between interleukin‐8 concentration in nasal secretions and severity of symptoms of experimental rhinovirus colds. Clin Infect Dis 26:840–846. [DOI] [PubMed] [Google Scholar]
- Wareing MD, Shea AL, Inglis CA, Dias PB, Sarawar SR. 2007. CXCR2 is required for neutrophil recruitment to the lung during influenza virus infection, but is not essential for viral clearance. Viral Immunol 20:369–378. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Schorr W. 2000. Role of interleukin‐8 in neutrophil signaling. Curr Opin Hematol 7:178–182. [DOI] [PubMed] [Google Scholar]
- Zgierska A, Obasi CN, Brown R, Ewers T, Muller D, Gassman M, Barlow S, Barrett B. 2013. Randomized controlled trial of mindfulness meditation and exercise for the prevention of acute respiratory infection: Possible mechanisms of action. Evid Based Complement Alternat Med 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Tang W, Gwaltney JM, Wu Y, Elias JA. 1997. Rhinovirus stimulation of interleukin‐8 in vivo and in vitro: Role of NF‐κB. Am J Physiol Lung Cell Mol Physiol 273:L814–L824. [DOI] [PubMed] [Google Scholar]


