Abstract
Objective
Mammalian target of rapamycin (mTOR) pathway is activated in malignant melanoma and in situ lesions as opposed to benign nevi. Inhibition of PI3K-Akt-mTOR signaling is implicated in sensitization of melanoma cells to alkylating agents [temozolomide (TMZ)] and inhibition of tumor angiogenesis.
Methods
We conducted a single-arm phase II multi-institution cooperative group study to assess the antitumor activity and safety profile of the combination of TMZ and the rapamycin derivative everolimus in patients with metastatic unresectable malignant melanoma. Patients received 10 mg/d of RAD001 for 5 of 7 days (ie, 50 mg/ wk) and 200 mg/m2/d of TMZ for 5 days each cycle.
Results
Of the first 39 eligible patients, 17 were PFS-9 successes, for a predetermined threshold of 18/39 patients for a positive trial. Overall, 21 of 48 patients were progression free at 9 weeks, for an event-free survival rate of 44% (95% confidence interval, 29%–59%). The median progression-free survival was 2.4 months and the median overall survival was 8.6 months. Four patients achieved a partial response; the median duration of response was 15.1 months. No complete remissions were observed. Treatment was in general well tolerated with only 1 patient discontinuing therapy due to toxicity (hyperlipidemia).
Conclusions
The combination of TMZ and RAD001 was well tolerated but failed to meet/exceed our study threshold for promising clinical activity in patients with metastatic melanoma.
Keywords: cancer, melanoma, mammalian target of rapamycin (mTOR), angiogenesis, vascular endothelial growth factor (VEGF)
The management of patients with metastatic melanoma remains a difficult problem. Although potentially curable when diagnosed early, the median survival of patients with metastatic disease is generally <1 year, with <5% of patients being alive at 5 years.1 One of the major impediments to successful treatment of advanced melanoma is the rapid development of chemotherapy resistance. A number of mechanisms have been described including impaired drug transport, multidrug resistance–associated protein,2 detoxification, or enhanced DNA repair. In addition, recent studies have shown that repeated in vitro exposure of melanoma cells to dacarbazine (DTIC)3 results in the selection of resistant cell lines with increased tumorigenic and metastatic potential. A recently described escape mechanism of melanoma cells exposed to alkylating agents is the increased production of prosurvival angiogenic factors, such as vascular endothelial growth factor (VEGF) and interleukin-8 by the tumor,4 which correlates with tumor progression and decreased survival in metastatic melanoma.5,6 Although the specific signaling pathways contributing to cytokine upregulation have not yet been completely elucidated, several lines of evidence suggest a link between phosphatidylinositol 3-kinase (PI3K)/Akt7,8 and the downstream mammalian target of rapamycin (mTOR)9 pathway and angiogenesis. Deregulation of mTOR is emerging as a common theme in the pathogenesis of many tumor types. In melanoma, it is activated in approximately 70% of malignant lesions, as opposed to benign nevi.10 In addition, preclinical studies have shown that tumors with loss of PTEN (phosphatase and tensin homolog) gene function and therefore with hyperactive Akt/PKB (protein kinase B) signaling are preferentially sensitive to mTOR inhibition.11 Loss of PTEN has been implicated in 20% to 40% of primary cutaneous melanomas.12
Addition of rapamycin to chemotherapy has been shown to sensitize melanoma cells to temozolomide (TMZ), exert a synergistic effect on drug-induced tumor apoptosis,13 and inhibit angiogenesis by decreasing VEGF secretion by the tumor.9,14 Preclinical melanoma animal models also suggest that addition of the mTOR inhibitor temsirolimus to DTIC15 or cisplatin16 has the potential to increase chemotherapeutic efficacy. In the presented study, we hypothesized that addition of the orally active derivative of rapamycin, everolimus (RAD001; Novartis, Basel, Switzerland) to TMZ would provide an additive therapeutic benefit in metastatic melanoma. We have previously noted instances of response to TMZ in patients who progressed on single-agent RAD001 and were treated with TMZ immediately after discontinuation of the drug, suggesting synergy between these 2 agents (Markovic SN, unpublished data, 2007). For this reason, on cycle 1, RAD001 was begun 1 week before initiation of chemotherapy. Once mTOR inhibition was achieved, combined therapy was begun concomitantly for all subsequent cycles.
METHODS
Patient Eligibility and Study Design
This was a single-arm phase II multi-institution study conducted through the North Central Cancer Treatment Group to assess the antitumor activity and safety profile of TMZ/ RAD001 combination in patients with unresectable metastatic melanoma. All patients provided written informed consent and the study was approved by the institutional review boards of all participating institutions. Eligible patients had to be above the age of 18 years and have histologically confirmed melanoma. Other key eligibility criteria included measurable disease as defined by the Response Evaluation Criteria in Solid Tumors (RECIST), performance status of 0 to 2, life expectancy of ≥12 weeks, adequate hematologic, renal, and hepatic function, and ≥4 weeks since the last immunotherapy, investigational agent, radiation therapy, or chemotherapy treatment, with the exception of biological agents for which ≥6 weeks were required. Major exclusion criteria were prior therapy with TMZ/DTIC, rapamycin or their analogs, untreated metastatic melanoma to the brain or progression of brain metastases within 3 months of study entry, active uncontrolled infections, immunosuppression from any cause, planned use of live vaccines, congestive heart failure, uncontrolled diabetes, gastrointestinal disease that may alter the absorption of everolimus, presence of a known bleeding diathesis or therapy with Coumadin, and history of other malignancy in the past 5 years with the exception of nonmelanoma skin cancer treated with local resection only. Patients who were using drugs inducing CYP3A4 activity were allowed on the study only if such drugs were discontinued ≥3 days before starting everolimus therapy. In addition, patients had to be willing to forgo foods high in fat content 2 hours before and 2 hours after administration of everolimus therapy and abstain from eating grapefruit or drinking grapefruit juice for the duration of the study. Women who were pregnant or breastfeeding were not enrolled.
Eligible patients began therapy with RAD001 1 week before initiation of TMZ. Cycle 1 consisted of 5 weeks of treatment (35 d) in which patients received 10 mg/d of RAD001 on days 1 to 5, 8 to 12, 15 to 19, 22 to 26, 29 to 33, and 200 mg/m2/d of TMZ on days 8 to 12. For all subsequent cycles, patients were treated with 10 mg/d of RAD001 on days 1 to 5, 8 to 12, 15 to 19, 22 to 26, and 200 mg/m2/d of TMZ on days 1 to 5 of each 28-day cycle. Patients were eligible for retreatment until disease progression, unacceptable toxicity, or refusal. Tumor assessment with conventional computed tomography or magnetic resonance imaging or spiral computed tomography was done at baseline (≤28 d before registration) and every other cycle thereafter. Before each cycle, patients underwent a physical examination, toxicity assessment, and assessment of hematologic, chemistry, and lipid panels. A complete blood cell count with differential was checked weekly for the first 2 cycles, and every other week thereafter for the duration of the study. Treatment was held on day 1 of each treatment cycle if the absolute neutrophil count (ANC) was <1000/mm3 or the platelet count (PLT) was <100,000/mm3, or the patient developed ≥grade 3 elevations in liver enzymes, ≥grade 2 mucositis, or any other ≥grade 3 non-hematologic toxicity. When patients had recovered to ≤grade 1 toxicity, treatment was restarted with 25% dose reduction in TMZ and reduction in RAD001 dose by 10 mg/wk (ie, to 4 d/ wk). If ANC ≤500/mm3, PLT≤50,000/mm3, or development of ≥grade 4 liver toxicity, ≥grade 3 mucositis that recurred despite maximal supportive care, or ≥grade 4 nonhematologic toxicity, TMZ dose was decreased by 50% and RAD001 dose by 20 mg/wk (ie, to 3 d/wk) upon recovery to ≤grade 1 toxicity. A maximum of 2 dose reductions and ≤3 weeks on hold were allowed. For patients experiencing grade 2 pneumonitis, RAD001 was interrupted if symptoms were bothersome. Treatment was omitted for patients diagnosed with grade 3 pneumonitis until recovery to ≤grade 1, after which treatment was restarted within 2 weeks at a reduced dose (ie, decreased by 10 mg/wk) if evidence of clinical benefit. Treatment was permanently discontinued in patients experiencing grade 4 pneumonitis.
All patients received standard supportive care at the discretion of their treating physician. Pneumocystis jiroveci pneumonia prophylaxis was advised for patients who developed lymphopenia (absolute lymphocyte count <500/μL) and those who were likely to be on therapy for >2 months. Patients experiencing noninfectious pneumonitis associated with RAD001 were treated with systemic corticosteroids after an infectious etiology had been ruled out.
Plasma VEGF Quantification
Blood samples were collected at baseline (before initiation of therapy) and before each cycle. Plasma levels of VEGF-A, VEGF-C, and VEGF-D were measured using Duoset antibodies from R and D Systems (Minneapolis, MN) as per manufacturer’s instructions. Briefly, 96-well plates were coated overnight at 4°C with capture antibodies at 1.0 μg/mL for VEGF and 2.0 μg/mL for VEGF-C and VEGF-D. The plates were washed and blocked for 1 hour at room temperature with reagent diluent, and 50 μL of undiluted plasma was added to wells in duplicate and incubated for 2 hours at room temperature. The plates were washed with phosphate-buffered saline (PBS) and 0.5% Tween-20, and 100 ng/mL VEGF and 200 ng/mL VEGF-C and VEGF-D biotinylated detection antibodies were added for 1 hour at RT and followed by incubation with a streptavidin-horseradish peroxidase complex. Color was developed with Thermo Scientific Pierce (TMB) substrate and optical density was measured at 450 nm. VEGF and VEGF-D concentration was determined using a standard curve ranging from 0 to 2000 pg/mL. VEGF-C concentration was determined using a standard curve ranging from 0 to 6000 pg/mL.
Quantitative Real-time Polymerase Chain Reaction (PCR)
Peripheral blood mononuclear cells (PBMCs) collected before and after treatment were thawed and prepared for RNA extraction using RNeasy Mini Kit (QIAGEN, Valencia, CA) as per manufacturer’s instructions. Three patients were selected based on the number of treatment cycles received on study as a measure of response to treatment and clinical outcome: >9 cycles (patient 4), <5 cycles (patient 8), >5 cycles, but <8 cycles (patient 25). Cells were lysed in RLT buffer and homogenized using a 20-G needle. The lysate was precipitated using ethanol and separated using an RNeasy spin column and centrifugation. RNA was resuspended in RNase-free water. RNA concentration was determined by nanospectroscopy at 260 and 280 nm. Complementary DNA (cDNA) was prepared using RT2 First Stand Kit (SABioscience, Frederick, MD) as per manufacturer’s guidelines. 1 μg of RNA from 6 samples was added to each reverse-transcription (RT) reaction. The RT cocktail was prepared and incubated at 42°C for 15 minutes and was terminated by heating to 95°C for 5 minutes. RT2 Profiler PCR Array (96-well format) of human mTOR signaling was chosen for pathway analysis (SABioscience) and prepared as per manufacturer’s instructions. This plate analyzes 83 genes, 5 housekeeping genes and 8 controls. PCR cocktail was made up for each sample containing RT2 qPCR Master Mix (SABioscience) and the cDNA synthesized as above to a total volume of 25 μL. The 6 plates were run on an ABI 7900HT real-time instrument and set to detect the standard curve and dissociation curve using SYBR Green fluorescence. Samples were run in duplicate and analyzed using SDS 2.4 Software (Syntex Solutions and SDS 2.3; Applied Biosystems, Carlsbad, CA).
Statistical Considerations
The primary endpoint of this trial was the 9-week event-free survival (EFS) rate. The EFS rate was defined as the number of PFS-9 successes (patients who were progression free at 9 wk) divided by the number of eligible patients who had begun treatment. A phase III trial comparing the impact of TMZ to DTIC in the treatment of patients with advanced metastatic melanoma17 reported that the 9-week progression-free survival (PFS) rate was 35% with TMZ and 30% with DTIC. For our study, a single-stage phase II trial was designed based on the properties of the binomial distribution. The chosen design required 39 eligible patients to test that the true 9-week EFS rate was at most 35%17 versus the alternative that it is at least 55%. If there were at least 18 PFS-9 successes, the regimen would be considered promising for future study, otherwise the regimen would be considered ineffective in this patient population. As designed, this single-stage phase II trial had a significance level of 0.10 when the true 9-week EFS rate was 35%, and a power of 90% for detecting a true 9-week EFS rate of 55%. A 95% confidence interval for the 9-week EFS rate was constructed using the properties of the binomial distribution. Tumor response rate was defined as the number of patients who achieved a confirmed partial response (PR) or complete response (CR) divided by the total number of eligible patients who started the study treatment. RECIST criteria were used for determining tumor response, which are different than the WHO criteria used in the Middleton and colleagues phase III trial. Although the disagreement between the 2 methods of response assessment could influence an accurate comparison with historical trials,18 other studies find a high concordance.19–21 PFS time was defined as the time between registration and documented progression or death. Overall survival (OS) time was defined as the time between registration and death due to any cause. Both PFS and OS distributions were estimated using the Kaplan-Meier method.
The trial design included an adverse event stopping rule to account for excessive toxicity. After the first 6 patients were enrolled, accrual was stopped and the toxicity profile was examined. If 3 or more patients experienced a grade 3 + toxicity deemed at least possibly related to treatment in the first cycle, a regimen change would be considered. In addition, if 3 or more out of the first 10 treated patients (or 30% of all patients after the first 10) experienced a grade 3 + non-hematologic toxicity deemed at least possibly related to study treatment, accrual would be suspended so that the study team could evaluate the toxicity profile. Toxicities were graded and attribution assigned by treating physicians using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
RESULTS
Patient Characteristics
Forty-nine patients were accrued into this study between January and October of 2008. One patient was deemed ineligible due to active brain metastases diagnosed before receiving any study treatment. All forthcoming analyses are based on the remaining 48 patients unless stated otherwise. The clinical and demographic characteristics of the study population are presented in Table 1. The median number of cycles administered was 2 (range, 1 to 27 +). Reasons for study discontinuation included: disease progression (39 patients), patient refusal (5 patients), adverse events (1 patient, hyperlipidemia), alternative treatment (1 patient, surgery), and comorbid medical problems (1 patient, congestive heart failure).
TABLE 1.
Clinical and Demographic Characteristics of the Study Cohort
| n = 48 | |
|---|---|
| Age (median; range) | 60 (29–84) |
| Male/female ratio; % | 32/16 (66/33) |
| ECOG PS, n (%) | |
| 0 | 36 (75) |
| 1 | 11 (23) |
| 2 | 1 (2) |
| M stage, n (%) | |
| M1a (soft tissue) | 9 (19) |
| M1b (lung) | 10 (21) |
| M1c (all viscera) | 28 (60) |
| Metastatic site, n (%) | |
| Lung | 21 (44) |
| Liver | 19 (40) |
| Lymph nodes | 14 (30) |
| Soft tissue | 9 (19) |
| Brain | 2 (4) |
| Baseline LDH, n (%) | |
| Within ULN | 29 (64) |
| > ULN | 16 (36) |
| Prior therapy type, n (%) | |
| Vaccine therapy | 13 (27) |
| Systemic chemotherapy | 2 (4) |
| Radiation therapy | 14 (30) |
ECOG PS indicates Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Clinical Efficacy
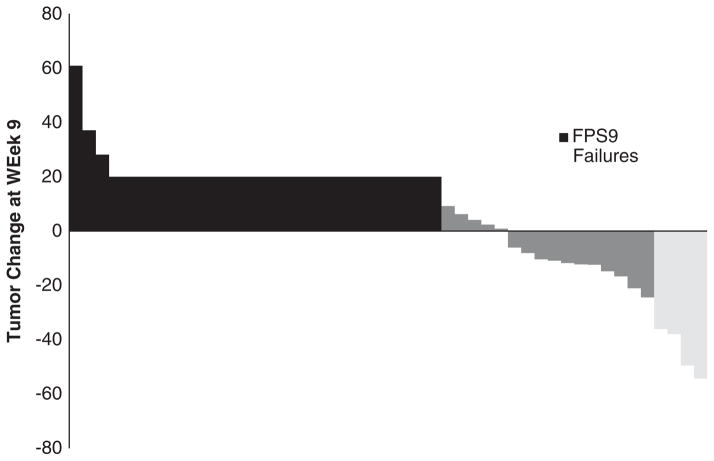
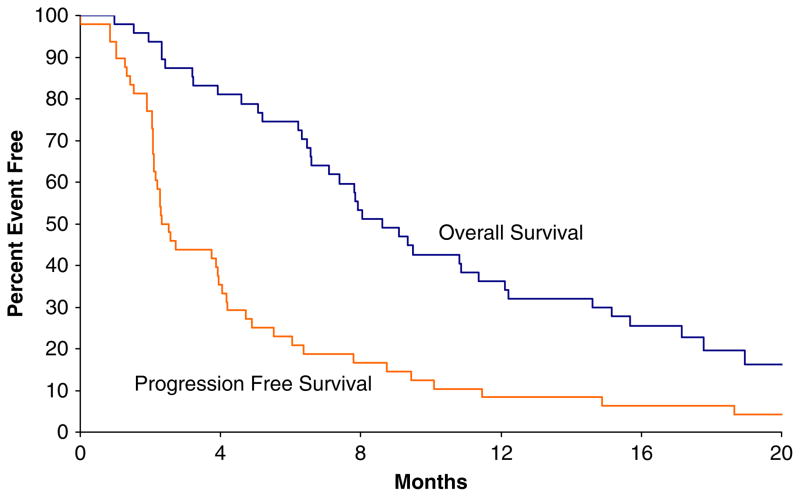
Patients were followed for a median of 25 months (range, 2 to 30 mo). Of the first 39 eligible patients, 17 [44%; 95% confidence interval (CI), 28%–60%] were PFS-9 successes, which did not meet the predetermined 18 patients threshold for a positive trial. Overall, 21 of 48 patients were progression free at 9 weeks, thus making the overall EFS rate 44% (95% CI, 29%–59%). Among the 48 patients enrolled, 4 patients achieved a PR, for an objective response rate of 8.3% (95% CI, 2.3%–20.0%) (Fig. 2). The median duration of response for these patients was 15.1 months (range, 9 to 39 mo). No complete remissions were observed. Of the 4 patients with a confirmed PR, 3 have subsequently progressed at 8.7, 11.5, and 18.7 months, respectively, after first indication of response. There were also 3 patients who achieved an unconfirmed PR but failed to achieve a confirmed response. At last follow-up, 7 patients (15%) were alive, 1 of which remains progression free 39 months after study entry, and 41 patients (85%) have died of disease. The median PFS was 2.4 months (95% CI, 2.1–4.0 mo) and the median OS was 8.6 months (95% CI, 7.1–12.2 mo) (Fig. 1). Using Cox Proportional Hazards models, it was determined that neither PFS nor OS rates were different with respect to lactate dehydrogenase status (elevated vs. normal not shown).
FIGURE 2.
Waterfall plot illustrating tumor size changes after 2 cycles (week 9).
FIGURE 1.
Progression-free and overall survival times for the study cohort.
Safety and Tolerability
Fourteen (29%) patients required at least 1 dose modification. The most common reason for dose reduction/omission was hematologic toxicity (9 patients). Twenty-one (43%) patients experienced at least 1 severe (grade 3 +) toxicity (possibly, probably, or definitely related to treatment), the most common being: decreased lymphocyte count (19%), leukopenia (13%), decreased neutrophil count (12%), and decreased PLT (10%). The grade 2 and higher nonhematologic toxicities that were seen in >1 patient were fatigue, nausea, and elevated liver function tests (Table 2).
TABLE 2.
Grade ≥2 Toxicities Reported as Possibly, Probably, or Definitely Related to Treatment
| Event | n | ||
|---|---|---|---|
|
| |||
| Grade 2 | Grade 3 | Grade 4 | |
|
|
|||
| Neutropenia | 12 | 3 | 3 |
| Lymphopenia | 12 | 8 | 1 |
| Thrombocytopenia | 5 | 1 | 4 |
| Anemia | 5 | 2 | 0 |
| Fatigue | 11 | 2 | 0 |
| Nausea | 5 | 2 | 0 |
| Vomiting | 2 | 1 | 0 |
| Anorexia | 4 | 1 | 0 |
| Constipation | 4 | 0 | 0 |
| Mucositis | 6 | 0 | 0 |
| Taste changes | 2 | 0 | 0 |
| Pneumonitis | 2 | 0 | 0 |
| Myalgia | 2 | 1 | 0 |
| Muscle weakness | 2 | 1 | 0 |
| Arthralgia | 1 | 1 | 0 |
| Back pain | 0 | 1 | 0 |
| Extremity pain | 0 | 1 | 0 |
| Rash | 3 | 0 | 0 |
| Urticaria | 1 | 0 | 0 |
| Erythema multiforme | 1 | 0 | 0 |
| Hypersensitivity | 0 | 0 | 1 |
| Cellulitis | 0 | 0 | |
| Elevated liver function tests | 0 | 2 | 0 |
| Hypertriglyceridemia | 1 | 1 | 0 |
| Hypercholesterolemia | 2 | 0 | 1 |
| Hypercalcemia | 1 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 1 |
| Elevated creatinine | 1 | 0 | 0 |
Changes in Plasma VEGF Levels
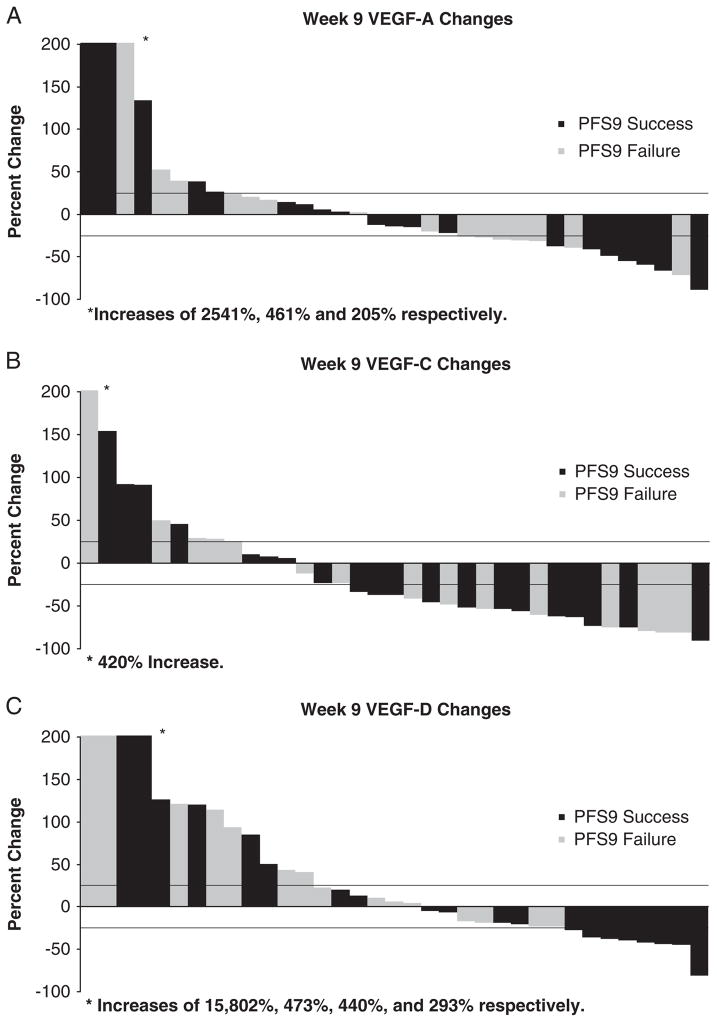
Plasma VEGF levels were available at baseline for 47 patients, before cycle 2 for 46 patients, and before cycle 3 for 35 patients. Changes from baseline were computed by subtracting the baseline value from the current value and dividing the difference by the baseline value. Changes of <25% in either direction were categorized as no change. Changes of at least 25% higher or lower than baseline were declared significant increases or decreases. There was no significant difference between baseline VEGF-A, VEGF-C, and VEGF-D levels of PFS-9 success patients versus failures (Table 3). The percent change in VEGF-A and VEGF-C between baseline and cycle 3 was also not significantly different based on PFS status (Table 3; Fig. 3). Plasma VEGF-D levels were found to be decreased before cycle 3 in 8/20 (40%) patients who remained progression free at 9 weeks versus 0/15 (0%) patients who progressed. At the time of first documented treatment response, plasma VEGF-D levels decreased in the majority of patients 6/7 (86%), plasma VEGF-C levels decreased in 4/7 patients (57%), whereas plasma levels of VEGF-A decreased in only a minority 1/7 patients (15%).
TABLE 3.
Plasma Levels of VEGF-A, VEGF-C, and VEGF-D Levels at Baseline, 5 Weeks, 9 Weeks, the Time of Response, and the Time to Progression by PFS-9 Status
| VEGF-A | VEGF-C | VEGF-D | |
|---|---|---|---|
| Baseline Values, Median (Range) | |||
| Successes (N = 21) | 157 (2–639) | 1782 (221–5159) | 215 (10–4965) |
| Failures (N = 26) | 159 (14–586) | 1712 (272–8550) | 138 (0.2–6466) |
| CFB* at 5 wk | |||
| Up/no change/down† | |||
| Successes (N = 21) | 5/10/6 | 0/6/15 | 7/6/8 |
| Failures (N = 25) | 1/14/10 | 3/3/19 | 8/11/6 |
| CFB* at 9 wk | |||
| Up/no change/down† | |||
| Successes (N = 20) | 5/8/7 | 4/4/12 | 6/6/8 |
| Failures (N = 15) | 3/5/7 | 5/2/8 | 7/8/0 |
| CFB1 at time of response‡ | 4/2/1 | 2/1/4 | 0/1/6 |
| Up/no change/down† | |||
| CFB1 at time of progression§ | 8/13/11 | 7/4/21 | 10/15/7 |
| Up/no change/down† | |||
CFB indicates change from baseline.
Up ≥25% increase, down ≥25% decrease, no change ≤25% change.
There were 7 patients with at least an unconfirmed PR.
Data were available on 32 patients that the time of their progression.
PR indicates partial response; VEGF, vascular endothelial growth factor.
FIGURE 3.
Waterfall plots illustrating changes from baseline to week 9 for VEGF-A (A), VEGF-C (B), and VEGF-D (C) levels by PFS-9 status (PFS-9 success or failure). VEGF indicates vascular endothelial growth factor.
Markers Of mTOR Inhibition
The effects of RAD001 treatment on mTORC1 (raptor-containing mTOR complex 1) signaling showed a consistent decrease in phosphorylated ribosomal S6 kinase (pS6K1) in the 2 patients with a favorable response to treatment (patients 4 and 25), as opposed to the patient who responded poorly (patient 8). Inhibition of eukaryotic translation initiation factor 4E-binding protein (4EBP1) was only seen in the best responder (patient 4). Akt but not Rictor showed a significant decrease in patient 4, implying that both mTORC1 and mTORC2 (rictor-containing mTOR complex 2) were affected by the treatment. Akt increased in both the intermediate and poor responders, whereas the effect on Rictor was inconsistent (Table 4).
TABLE 4.
mTOR Quantitative Real-time PCR Array on Before and After Treatment PBMCs
| Gene | Fold Change
|
||
|---|---|---|---|
| Patient 4 | Patient 8 | Patient 25 | |
| Akt | −1.5657 | 7.8517 | 2.5099 |
| EIF4EBP1 | −1.1265 | 1.9575 | 1.7659 |
| mTOR | 1.0983 | 4.3229 | −2.0378 |
| Rictor | 1.1469 | 1.2271 | −2.203 |
| PS6K1 | −1.1783 | 7.4013 | −4.2668 |
| Raptor | −1.3409 | 9.0105 | 1.9856 |
Key players in the mTOR pathway are listed.
Three patients were selected based on the number of treatment cycles received on study as a measure of response to treatment and clinical outcome: >9 cycles (patient 4), <5 cycles (patient 8), >5 cycles but <8 cycles (patient 25).
EIF4EBP1 indicates eukaryotic translation initiation factor 4E-binding protein; mTOR, mammalian target of rapamycin, PS6K1-ribosomal S6 kinase; PBMC, peripheral blood mononuclear cell; PCR, polymerase chain reaction.
DISCUSSION
The results of the presented study show that the combination of TMZ and RAD001 does not seem to offer a significant therapeutic advantage over TMZ-treated controls.1 The study failed to meet/exceed our threshold for promising clinical activity. Of the first 39 eligible patients, 17 were PFS-9 successes, for a predetermined threshold of 18/39 patients for a positive trial. Overall, treatment was well tolerated with only 1 patient discontinuing therapy due to toxicity (hyperlipidemia). The most common toxicities were fatigue, nausea, elevated liver function tests, and cytopenia. A phase II trial of single-agent RAD001 in metastatic melanoma has been conducted by the North Central Cancer Treatment Group (N0377).22 The dose initially used in that study of 30 mg/wk was subsequently increased in the second portion of the trial, in view of phase I data suggesting that 10 mg/d was safe and well tolerated.23 However, at this increased dose level (70 mg/wk) there were a number of dose reductions and therapy delays.
It is uncertain whether the dose and selected schedule for our study did not allow adequate inhibition of mTOR pathway and consistent suppression of VEGF secretion, or whether there were other mechanisms in the biology of melanoma that allowed this pathway to be bypassed. In a CA20498 syngeneic rat pancreatic tumor model, RAD001 dose-dependent inhibition of tumor growth correlated with decreased phosphorylation of p4EBP1 and pS6K1 in tumors24 suggesting that inhibition of mTOR pathway may be used as a surrogate for treatment efficacy. Modeling studies of S6K1 inhibition indicated that daily dosing resulted in more complete and sustained effect on target inhibition than one 20 to 30 mg weekly dose, despite similar total exposure.25 This model was further refined in a phase I study, and pS6K1 was found to be completely inhibited at 5 and 10 mg daily and at 20, 50, and 70 mg weekly doses.26 In our trial, we used RAD001 at 10 mg/d for 5 of 7 days (ie, 50 mg/wk). Our studies on PBMCs collected at baseline and posttreatment suggested that this dose probably achieved adequate downregulation of mTORC1 pathway markers (S6K1 and/or 4EBP1) in those patients who had a favorable or intermediate response to therapy. However, inhibition of 4EBP1 was only seen in one of the best responder patients (patient #4, Table 4), which is in line with previous observations suggesting that 4EBP1 may be a less reproducible marker of mTOR inhibition.26 We used PBMCs as a source for biomarker analysis given that preclinical studies suggest a correlation between the antitumor efficacy of RAD001 and S6K1 inactivation in PBMCs, therefore implying that long-term monitoring of PBMC-derived S6K1 activity levels could be used for assessing RAD001 treatment schedules in cancer patients.24
Like rapamycin, everolimus acts by binding to a ubiquitous intracellular receptor, the immunophilin FKBP-12 to inhibit the kinase activity of mTOR.27 Because the rictor-containing mTORC2 complex is not bound by FKBP-12-rapamycin, RAD001 was thought to inhibit only mTORC1.28 However, in certain cell lines (eg, HeLa), prolonged rapamycin treatment reduces the levels of mTORC2 below those needed to maintain the phosphorylation of Akt.27 In contrast, others have showed that mTOR inhibition by rapamycin triggers a feedback mechanism resulting in activation of Akt signaling29,30 and that this could be tissue and/or dose specific.30,31 In our study, downregulation of Akt was seen in the patient who had a favorable response to therapy, whereas the others showed an increase in Akt signaling after treatment, suggesting a potential mechanism of rapamycin resistance. Clinical responses to mTOR inhibitors in other malignancies,32–34 although promising, are often partial and transient, and there has been significant interest in enhancing the antineoplastic effects of these agents and potentially overcoming resistance by use of combination therapies30 or use of dual mTORC1/ mTORC2 inhibitors.35,36 It will therefore become important to identify biomarkers that can predict sensitivity to rapalogs, as well as Akt/PKB inhibition in individual patients, and to design dosing regimens optimal for antitumor efficacy.
Our data did not reproduce the studies of rapamycin-induced VEGF inhibition,10 although inhibition of VEGF-D secretion showed some correlation with the response to treatment. Inhibition of VEGF-C/VEGF-D/VEGF-receptor 3 axis is thought to reduce tumor lymphangiogenesis and lymphatic metastasis in animal models.37,38 mTOR, the target of everolimus, can induce expression of VEGF-C, and inhibition of mTOR with rapamycin has been shown to potently reduce VEGF-C expression in a murine skin flap model39 and murine tumor xenografts.40 However, because VEGF-C is typically produced by pericytes of blood vessels,41 and the effects of VEGF are on immune function rather than tumor growth in isolation, in vitro models of blockade of VEGF-C are unlikely to provide a truly representative model for antitumor effects against melanoma in patients. It is unclear to what extent inhibition of VEGF-D versus VEGF-C40 secretion in vivo by everolimus or the modulation of VEGF secretion by addition of TMZ5 played a role in our study. Pending biomarker analysis may offer insights into potential combinations of RAD001 or the current regimen with other treatments in advanced melanoma, and a clinical trial exploring the combination of anti-VEGF therapy (bevacizumab) with everolimus and cytotoxic chemotherapy (carboplatin/paclitaxel) is currently underway (NCT00976573).
Acknowledgments
Conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-35195, CA-35103, CA-35267, CA-35431, CA-35269, CA-35101, CA-37417, and CA-63849.
Footnotes
Additional participants: Carle Cancer Center CCOP, Urbana, IL (Kendrith M. Rowland, Jr, MD); Meritcare Hospital CCOP, Fargo, ND 58122 (Preston D. Steen, MD); Rapid City Regional Oncology Group, Rapid City, SD (Richard C. Tenglin, MD); CentraCare Clinic, St Cloud, MN (Donald Jurgens, MD); Missouri Valley Cancer Consortium, Omaha, NE (Gamini S. Soori, MD); Cancer Care Associates, Tulsa, OK (Allan Keller, MD); Lehigh Valley Hospital, Allentown, PA (Suresh Nair, MD); Sioux Community Cancer Consortium, Sioux Falls, SD (Miroslaw Mazurczak, MD); Wichita Community Clinical Oncology Program, Wichita, KS (Shaker R. Dakhil, MD); Colorado Cancer Research Program, Denver, CO (Eduardo R. Pajon, Jr, MD); Essentia Duluth CCOP, Duluth, MN (Daniel A. Nikcevich, MD); Iowa Oncology Research Association CCOP, Des Moines, IA (Robert J. Behrens, MD); St. Vincent Regional Cancer Center CCOP, Green Bay, WI (Anthony J. Jaslowksi, MD); Northern Indiana Cancer Research Consortium CCOP, South Bend, IN (Robin T. Zon, MD).
The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
The authors declare no conflicts of interest.
References
- 1.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 2.Berger W, Hauptmann E, Elbling L, et al. Possible role of the multidrug resistance-associated protein (MRP) in chemoresistance of human melanoma cells. Int J Cancer. 1997;71:108–115. doi: 10.1002/(sici)1097-0215(19970328)71:1<108::aid-ijc18>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Lev DC, Onn A, Melinkova VO, et al. Exposure of melanoma cells to dacarbazine results in enhanced tumor growth and metastasis in vivo. J Clin Oncol. 2004;22:2092–2100. doi: 10.1200/JCO.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 4.Lev DC, Ruiz M, Mills L, et al. Dacarbazine causes transcriptional up-regulation of interleukin 8 and vascular endothelial growth factor in melanoma cells: a possible escape mechanism from chemotherapy. Mol Cancer Ther. 2003;2:753–763. [PubMed] [Google Scholar]
- 5.Rofstad EK, Halsor EF. Vascular endothelial growth factor, interleukin 8, platelet-derived endothelial cell growth factor, and basic fibroblast growth factor promote angiogenesis and metastasis in human melanoma xenografts. Cancer Res. 2000;60:4932–4938. [PubMed] [Google Scholar]
- 6.Ugurel S, Rappl G, Tilgen W, et al. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 7.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 8.Dimmeler S, Zeiher AM. Akt takes center stage in angiogenesis signaling. Circ Res. 2000;86:4–5. doi: 10.1161/01.res.86.1.4. [DOI] [PubMed] [Google Scholar]
- 9.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 10.Karbowniczek M, Spittle CS, Morrison T. mTOR is activated in the majority of malignant melanomas. J Invest Dermatol. 2008;128:980–987. doi: 10.1038/sj.jid.5701074. [DOI] [PubMed] [Google Scholar]
- 11.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA. 2001;98:10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsao H, Mihm MC, Jr, Sheehan C. PTEN expression in normal skin, acquired melanocytic nevi, and cutaneous melanoma. J Am Acad Dermatol. 2003;49:865–872. doi: 10.1016/s0190-9622(03)02473-3. [DOI] [PubMed] [Google Scholar]
- 13.Sinnberg T, Lasithiotakis K, Niessner H, et al. Inhibition of PI3K-AKT-mTOR signaling sensitizes melanoma cells to cisplatin and temozolomide. J Invest Dermatol. 2009;129:1500–1515. doi: 10.1038/jid.2008.379. [DOI] [PubMed] [Google Scholar]
- 14.Dormond O, Contreras AG, Meijer E, et al. CD40-induced signaling in human endothelial cells results in mTORC2- and Akt-dependent expression of vascular endothelial growth factor in vitro and in vivo. J Immunol. 2008;181:8088–8095. doi: 10.4049/jimmunol.181.11.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thallinger C, Werzowa J, Poeppl W, et al. Comparison of a treatment strategy combining CCI-779 plus DTIC versus DTIC monotreatment in human melanoma in SCID mice. J Invest Dermatol. 2007;127:2411–2417. doi: 10.1038/sj.jid.5700872. [DOI] [PubMed] [Google Scholar]
- 16.Thallinger C, Poeppl W, Pratscher B, et al. CCI-779 plus cisplatin is highly effective against human melanoma in a SCID mouse xenotranplantation model. Pharmacology. 2007;79:207–213. doi: 10.1159/000101008. [DOI] [PubMed] [Google Scholar]
- 17.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 18.Mazumdar M, Smith A, Schwartz LH. A statistical simulation study finds discordance between WHO criteria and RECIST guideline. J Clin Epidemiol. 2004;57:358–365. doi: 10.1016/j.jclinepi.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe H, Yamamoto S, Kunitoh H, et al. Tumor response to chemotherapy: the validity and reproducibility of RECIST guidelines in NSCLC patients. Cancer Sci. 2003;94:1015–1020. doi: 10.1111/j.1349-7006.2003.tb01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad SR, Saini S, Sumner JE, et al. Radiological measurement of breast cancer metastases to lung and liver: comparison between WHO (bidimensional) and RECIST (unidimensional) guidelines. J Comput Assist Tomogr. 2003;27:380–384. doi: 10.1097/00004728-200305000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Park JO, Lee SI, Song SY, et al. Measuring response in solid tumors: comparison of RECIST and WHO response criteria. Jpn J Clin Oncol. 2003;33:533–537. doi: 10.1093/jjco/hyg093. [DOI] [PubMed] [Google Scholar]
- 22.Rao R, Windschitl H, Allred J. Phase II trial of the mTOR inhibitor everolimus (RAD-001) in metastatic melanoma. J Clin Oncol. 2006;24(suppl):8043. meeting abstracts. [Google Scholar]
- 23.O’Donnell A, Faivre S, Burris HA, III, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–1595. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 24.Boulay A, Zumstein-Mecker S, Stephan C, et al. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64:252–261. doi: 10.1158/0008-5472.can-3554-2. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka C, O’Reilly T, Kovarik JM, et al. Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol. 2008;26:1596–1602. doi: 10.1200/JCO.2007.14.1127. [DOI] [PubMed] [Google Scholar]
- 26.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 27.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 28.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 29.Wan X, Harkavy B, Shen N, et al. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 30.Gupta M, Ansell SM, Novak AJ, et al. Inhibition of histone deacetylase overcomes rapamycin-mediated resistance in diffuse large B-cell lymphoma by inhibiting Akt signaling through mTORC2. Blood. 2009;114:2926–2935. doi: 10.1182/blood-2009-05-220889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Yue P, Kim YA, et al. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/ raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008;68:7409–7418. doi: 10.1158/0008-5472.CAN-08-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witzig TE, Reeder CB, LaPlant BR, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia. 2011;25:341–347. doi: 10.1038/leu.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zent CS, LaPlant BR, Johnston PB, et al. The treatment of recurrent/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) with everolimus results in clinical responses and mobilization of CLL cells into the circulation. Cancer. 2010;116:2201–2207. doi: 10.1002/cncr.25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 35.Carayol N, Vakana E, Sassano A, et al. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci USA. 2010;107:12469–12474. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhagwat SV, Gokhale PC, Crew AP, et al. Preclinical characterization of OSI-027, a potent and selective inhibitor of mTORC1 and mTORC2: distinct from rapamycin. Mol Cancer Ther. 2011;10:1394–1406. doi: 10.1158/1535-7163.MCT-10-1099. [DOI] [PubMed] [Google Scholar]
- 37.He Y, Kozaki K, Karpanen T, et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 38.Lin J, Lalani AS, Harding TC, et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005;65:6901–6909. doi: 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- 39.Huber S, Bruns CJ, Schmid G, et al. Inhibition of the mammalian target of rapamycin impedes lymphangiogenesis. Kidney Int. 2007;71:771–777. doi: 10.1038/sj.ki.5002112. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi S, Kishimoto T, Kamata S, et al. Rapamycin, a specific inhibitor of the mammalian target of rapamycin, suppresses lymphangiogenesis and lymphatic metastasis. Cancer Sci. 2007;98:726–733. doi: 10.1111/j.1349-7006.2007.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yonekura H, Sakurai S, Liu X, et al. Placenta growth factor and vascular endothelial growth factor B and C expression in microvascular endothelial cells and pericytes. Implication in autocrine and paracrine regulation of angiogenesis. J Biol Chem. 1999;274:35172–35178. doi: 10.1074/jbc.274.49.35172. [DOI] [PubMed] [Google Scholar]