Abstract
Necrosis, an inflammatory form of cell death, has been considered to be an accidental death and/or cell death due to injury. However, the literature in the last decade has established that necrosis is a regulated form of cell death, and that inhibition of specific molecular pathways leading to necrosis can block it and reduce inflammation. Since necrotic lesions are observed in several immune mediated human pathologies, in this review we will discuss the impact that this form of programmed cellular demise has in the pathology of immune mediated nephropathies.
Keywords: Nephritis, Autoimmunity, Cell Death, Necrosis
1. Cell death
Classically cell death has been divided into two categories: 1) Apoptosis or programmed cell death, a regulated form of cell death, and 2) Necrosis, accidental and pro-inflammatory cell death. The term ‘programmed cell death’ was first used by Lockshin et al. while describing the breakdown of the intersegmental muscles of silkworms [1]. With histological studies of ischemic liver, Kerr et al distinguished two types of cell death: classical necrosis, and “apoptosis”, which involved a process of formation of cytoplasmic bodies that often contained fragmented nuclear material. The latter was suggested to be a basic programmed phenomenon [1; 2; 3; 4]. Apoptosis and necrosis were thought of as two opposing mechanisms, necrosis being a purely accidental and passive cell death characterized by swelling of cytoplasm without nuclear disintegration, whereas apoptosis as a highly regulated form of cell death.
Laster et al. [5] observed that tumor necrosis factor induced two different forms of cell death in various cell types. One form had classical features of apoptosis and the second had characteristics of necrosis. This observation suggested that necrosis could in fact be a programmed form of cell death, regulated by defined set of signaling molecules. Following this initial observation, several studies have provided evidence that necrosis could be in fact a regulated process, and led to the concept of ‘regulated necrosis’. In 2005 Degterev et al [6] coined the term ‘Necroptosis’ to define a form of regulated non-apoptotic cell death with characteristic morphological features of necrosis.
Recently a new form of cell death in neutrophils has been reported. Neutrophils release chromatin in the form of neutrophil extracellular traps, and the form of cell death is known as ‘NETosis’ [7; 8]. NETosis has been reported to be distinct from necrosis and apoptosis and as independent of caspase activation, however the exact molecular pathways leading to this form of cell death are not yet completely understood [8]. As mast cells [9] have also been reported to undergo this form of cell death, it is sometimes referred to as ‘ETosis’ to include both cell types [10].
2. Regulated necrosis: Molecular pathways
Several enzymes such as Receptor Interacting Protein kinase (RIP) and Poly (ADP Ribose) polymerase (PARP-1) have been shown to play a role in the induction and execution of programmed necrosis (Figure 1 and Figure 2 illustrate their respective molecular pathways). The resulting cell death is a consequence of complex interactions between several molecular and biochemical processes occurring within the cell. The major executioners are believed to be calcium and reactive oxygen species (ROS), which cause damage to proteins, lipids, DNA, and cause loss of cell integrity and death [11].
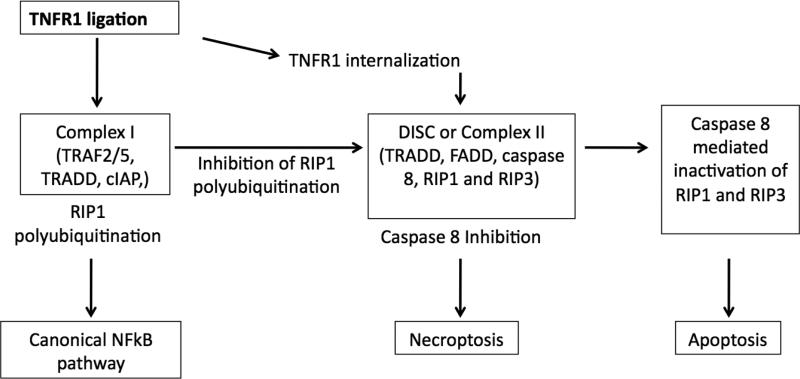
Figure 1. Molecular mechanisms of necroptosis. Complex I.
Ligation of TNFR1 leads to formation of Complex I consisting of TRAF2/5, TRADD, cellular inhibitor of apoptosis proteins (cIAP) and polyubiquitination of RIP1, followed by activation of canonical NFkB pathway. Complex II. Internalization of TNFR1 leads to assembly of complex II consisting of TRADD, FADD, caspase 8, RIP1 and RIP3. Caspase 8 activates the classical caspase pathway and also inactivates RIP1 and RIP3, this leads to apoptosis. In the absence or inhibition of caspase 8, RIP1 and RIP3 are activated by phoshporylation and induce necroptosis.
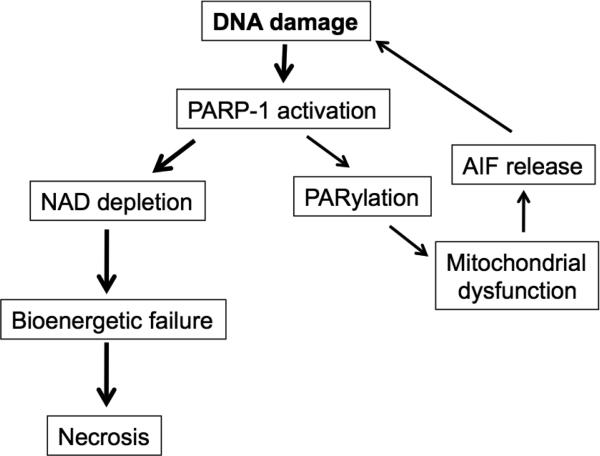
Figure 2. Molecular mechanisms of PARP-1 induced necrosis.
Extensive DNA damage caused by radiation or during inflammation activates PARP-1, a DNA sentinel. PARP1 uses its enzymatic substrate NAD to generate Poly-(ADP-Ribose) polymers (PARs) through a process termed PARylation. PARylation transmits signals from the nucleus to the mitochondra, which in turn release apoptosis inducing factor (AIF). AIF tranlocates to the nucleus and induces more DNA damage, which further activates PARP-1. Depletion of NAD as a result of extensive PARP-1 activation leads to bioenergetic collapse and necrosis.
Death receptor ligands stimulate apoptosis by default. However, if caspase activation is hampered, those ligands can induce necrosis. Ligation of TNF receptor 1 (TNFR1) in the absence of caspase activity induces the assembly of a complex consisting of Receptor interacting protein kinase 1 and 3 (RIP1 and RIP3), which has recently been referred to as ‘necrosome’. This complex results in a regulated form of necrosis- Necroptosis, characterized by the involvement of RIP1 and/or RIP3 [12]. Although the role of Fas associated death domain adaptor protein (FADD) in TNFα induced necrosis is not clear yet, using TNFR1-associated death domain protein (TRADD) deficient mice Pobezinskaya et al [13] showed that TRADD is required for TNFα induced necrosis. TRADD is necessary to recruit RIP1 to TNFR complex in mouse embryonic fibroblasts. During this form of cell death, RIP1 and RIP3 interact through their RIP homotypic interaction motifs (RHIM) [12; 14]. The kinase activity of RIP1 is indispensible for the interaction. RIP1 is shown to be an important mediator of regulated necrosis. However, virus associated necrosis has been shown to proceed independently of RIP1 [15]. It is possible that viruses induce a form of cell death different from necroptosis observed with TNF stimulation. Further evidence for the essential role of RIP-1 is provided by the observation that inhibition of enzyme activity of RIP1 by small molecule inhibitor Necrostatin inhibits TNFα-induced necrosis [6].
Another mediator of regulated necrosis is Poly (ADP-Ribose) Polymerase-1 (PARP-1), which is activated in response to moderate DNA damage and facilitates repair. PARP-1 catalyzes the conversion of NAD+ into nicotinamide and poly(ADP-ribose) polymers (PAR). The poly (ADP-ribosyl-)ation of nuclear proteins allows DNA repair, and cell survival is the outcome. Extensive DNA damage, however, leads to over-activation of PARP-1. The excessive activation of PARP-1 causes NAD+ depletion. Subsequent ATP depletion results in irreversible bioenergetic failure and necrosis [16]. Inhibition or deletion of PARP-1 inhibits necrosis [16; 17], and we showed previously that inhibition or absence of PARP-1 protects mice from immune mediated nephritis [18]. PARP-1 also acts as a co-activator of transcription factor NFκB. Indeed, NFκB activation during septic shock, ischemia/reperfusion injury or collagen-induced arthritis is greatly reduced after inhibition or in the absence of PARP-1 [19; 20; 21; 22].
Several mediators have been implicated in the execution phase of necrosis. Reactive oxygen species (ROS) generated during inflammation cause damage to cellular macromolecules including DNA. Oxidative stress will, therefore, lead to necrosis via a PARP-1 dependent pathway. ROS also modify proteins including oxidation of membrane phospholipids. Lipid peroxidation leads to leakage of Ca2+ and proteases by destabilizing mitochondrial, lysosomal, and endoplasmic reticular membranes. Increased intracellular Ca2+ leads to the opening of mitochondrial permeability transition (MPT) pore (MPTP), resulting in loss of mitochondrial membrane potential, swelling, and rupture of mitochondrial outer membrane. To date, three proteins are known to comprise the MPTP; the voltage-dependent anion channel (VDAC), the adenine nucleotide translocator (ANT), cyclophilin D (Cyp D: a mitochondrial peptidyl prolyl-cis, trans-isomerase) [23]. The role of Cyp D in MPT and induction of necrosis has been established convincingly with the use of Cyp D deficient mice [24; 25]. Cyp D dependent MPT also plays a major role in ischemia/reperfusion injury [26]. TNFα and zVAD.fmk treatment results in a mitochondrial defect in ADP transport through ANT, which is dependent on RIP1 [27]. Increased intracellular Ca2+ also activates calpains, which leads to release of cathepsins in the cytoplasm, and further contribute to the execution of necrotic cell death.
Interestingly inhibition of PARP-1 does not block TNF mediated RIP dependent necrosis and inhibition of RIP1/RIP3 does not prevent DNA damage induced PARP-1 mediated cell death [28]. The cell death pathways mediated by RIP-1/RIP3 and PARP-1, although apparently independent, may in fact be linked under certain circumstances. Xu et al showed that PARP-1 activation and necrosis requires activation of RIP-1 [29]. The mechanisms by which RIP-1 leads to activation of PARP-1, however, are not yet understood.
In addition to regulating necrosis, PARP-1 can form stable complexes with transcription factors such as p53 and fos. PARP-1 can act as a co-activator of transcriptional factor NFκB. Over-activation of PARP-1 enhances inducible nitric oxide synthase (iNOS) expression in an NFκB-dependent manner [30]. Inhibitors of PARP prevent the expression of pro-inflammatory agents such as iNOS, adhesion molecules, and neutrophil migration to inflammatory sites [30]. Furthermore, NFκB activity is impaired and iNOS, TNFα and IFNγ expression is reduced in PARP-1 deficient mice subjected to endotoxic shock [19]. Activation of pro-inflammatory cytokines and induction of reactive oxygen/nitrogen species following NFκB activation may in turn lead to necrosis and amplification of local inflammatory response. Inhibition of PARP-1 activity reduces secretion of pro-inflammatory cytokines as well as neutrophil migration to the inflammatory sites [16; 19; 31]. Moreover, our recent unpublished data show that PARP-1 also regulates the active release of HMGB1 in both macrophages and mouse mesangial cells. PARP-1 may, therefore, have profound modulatory effect on inflammatory response.
3. Immune Mediated Nephropathies
3.1 Lupus nephritis
Lupus GN is among the most devastating chronic effects of lupus disease. It is the leading cause of long-term disability, and ranks high as a cause of morbidity and mortality in SLE patients. The pathological manifestations of lupus GN are very diverse and have been re-classified [32]. Of the six classes defined by WHO, classes III-V are characterized by the presence of necrotic lesions, either segmental or global with distinct signs of necrotic cell death [32].
Glomerular necrosis is a feature of class III and IV lupus nephritis although not observed in pure mesangial proliferative (class II) or membranous (class V) lupus nephritis. It consists of a focus of smudgy fibrinoid obliteration of the glomerular tuft, which is often associated with any or all of the following: deposition of intracapillary fibrin, glomerular basement membrane rupture or gap formation, and apoptosis of infiltrating neutrophils forming pyknotic or karyorrhectic nuclear debris. Necrotizing lesions are typically segmental, but more than one glomerular lobule may be affected, particularly in diffuse proliferative lupus nephritis class IV. As in other forms of glomerulonephritis in which necrotizing lesions are common (e.g., pauci-immune crescentic glomerulonephritis), early cellular crescents frequently directly overlie the affected lobules. The lesions of active glomerular disease for class III and class IV are similar and the two classes are distinguished based on the percentage of glomeruli affected [32; 33].
3.2 IgA Nephropathy
The characteristic feature is the deposition of IgA1 subclass (with or without complement C3) in the glomeruli. A defective O-glycosylation of serine threonine and proline-rich hinge region of IgA1 subclass appears to play a central role. IgA nephropathy occurs most commonly between the ages of 10 and 40 years. Most studies show a male predominance, with an overall average male:female ratio of approximately 2:1 [34; 35]. Another disease associated with glomerular IgA deposits is Henoch-Schönlein purpura (HSP). HSP is a more systemic form of disease involving IgA mediated vasculitis and is often associated with infectious agents such as streptococci and mycoplasma [36].
Classes III, IV, V of IgA nephropathy are characterized by necrotic lesions along with glomerular hypercellularity (mesangial or endocapillary) and cresent formation. The renal histopathology in HSP is virtually indistinguishable from IgA nephropathy. The glomerulonephritis follows the deposition of IgA containing immune complexes on mesangial cells or through the binding of IgA to specific receptors on mesangial cells in absence of antigen. The binding of IgA activates mesangial cells to secrete cytokines such as IL-6, platelet aggregating factor, fibronectin and transforming growth factor [37; 38; 39]. Enhanced TGF-β release and matrix protein synthesis favor progression toward sclerosis.
3.3 Anti-glomerular basement membrane nephritis
Anti-glomerular basement membrane disease/Goodpasture's disease is a rare pulmonary-renal syndrome. The pathogenic features incorporated in the diagnostic criteria include cresentic glomerulonephritis with the majority of glomeruli showing crescents and linear IgG deposits often accompanied by complement C3. Half of the patients present with alveolitis and pulmonary hemorrhage. The circulating auto-antibodies (autoAbs) can be detected by ELISA and are directed against the non-collagenous domain of alpha 3 chain of type IV collagen [α3(IV)NC1] [40]. The antigen is found only in specialized basement membrane such as kidney, lung, choroids plexus, retina and cochlea. The autoAbs react with a conformational epitope which is most likely formed from the combined amino and carboxyl epitopes. The disease can be transferred from patients to animals by transferring antibodies [41]. Serum transfer from diseased mice to syngenic recipients leads to development of disease [42]. There is a well established experimental autoimmune glomerulonephritis in which a anti-basement glomerulonephritis animal model can be induced by active immunization of susceptible strains of rat/mice with glomerular basement membrane or α3(IV)NC1 [43; 44]. Autoreactive T cells have been shown to be both necessary and sufficient for disease development. Th1 cytokines (IFNγ, IL12), but not Th2 (IL10, IL4) cytokines play a major role in susceptibility [42; 44; 45]. Susceptibility to disease depends on both genetic and environmental factors.
Glomerular fibrinoid necrosis and crescent formation are the histologic hallmarks of acute anti-GBM glomerulonephritis [46; 47]. Even though the glomerular binding of anti-GBM antibodies is always diffuse and global, at the time of biopsy, glomerular tuft necrosis more often is segmental rather than global [48].
3.4 Pauci-immune glomerulonephritis
Pauci-Immune glomerulonephritis is a form of rapidly progressive glomerulonephritis that is characterized by almost complete absence of immunoglobulin deposits (as assessed by immunofluorescence). Pauci-immune necrotizing glomerulonephritis can be broadly divided into two subgroups: 1) associated with Anti-Neutrophil Cytoplasmic Antibodies (ANCAs), and 2) ANCA independent (also called idiopathic crescentic GN). ANCA associated GN includes granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and Churg-Strauss syndrome (CSS) [49]. GPA; this form is characterized by granulomatous lesions and vasculitic disease manifestations. Granulomatous lesions are found in respiratory tract, while vasculitic manifestations are found primarily in lung and kidney (glomerulonephritis). In particular GN is reported in 38% to 70% of patients [50].
MPA: Rapidly progressive glomerulonephritis (RPGN) is the major clinical feature of MPA with 80% to 100% of patients presenting with renal manifestations [51; 52]. The hallmark finding on biopsy is focal segmental necrotizing glomerulonephritis. Glomerular crescents are seen in approximately 80% patients [51].
CSS: Renal involvement in CSS is very infrequent (less than quarter of CSS patients). Similar to other forms of ANCA associated GN, the characteristic glomerular lesion of CSS is focal segmental glomerulonephritis with necrotizing features. Renal disease, however, is considered milder and rarely causes renal failure [53; 54].
The hallmark histologic lesions of acute pauci-immune ANCA glomerulonephritis are crescents and fibrinoid necrosis, which occur at the same frequency irrespective of the presence or absence of associated systemic vasculitis [55; 56; 57; 58]. Foci of fibrinoid necrosis often contain neutrophil granule constituents indicating neutrophil activation and degranulation at these sites [59]. Crescent formation appears to begin adjacent to foci of segmental necrosis. This extremely lytic necrosis is similar to focal lytic lesions in many other small vessels in ANCA-associated vasculitis [59].
ANCA negative GN: Approximately 10-30% patients with Pauci-immune are negative for ANCA. Although the exact mechanism of crescentic glomerulonephritis observed in these patients is not known, neutrophils seem to play an important role. [60; 61]. The subsequent activation and degranulation of neutrophils may result in necrotic cell death of glomerular cells. Although, the ANCA negative GN shows higher incidence of chronic glomerular lesions as opposed to acute lesions in ANCA positive, a clear distinction in renal pathologies in these two forms of pauci immune GN has not been established yet.
Both ANCA glomerulonephritis and anti-GBM glomerulonephritis have extensive fibrinoid necrosis, focal destruction of Bowman's capsule, and disordered crescents. Pauci-immune biopsies especially those with Wegener's granulomatosis, have focal hemorrhagic papillary necrosis that is caused by leukocytoclastic angiitis affecting the medullary vasa recta [49].
3.5 Membranous Glomerulonephritis
Although glomerular lobulation, mesangial hypercellularity, segmental scars, inflammation, and necrosis are not common features of idiopathic membranous Glomerulonephritis (MGN), necrotic lesions are often seen in secondary forms of MGN, and more commonly are seen with a systemic disease [62].
4. Programmed necrosis in Nephritis
The nephropathies described above are distinct forms of GN. However the pathophysiological features such as fibrinoid necrosis are shared by the majority of these diseases. Crescent formation is often observed close to necrotic foci. The forms of GN show presence of clear signs of necrotic cell death, pointing to the notion that regulated necrosis may play an important pathogenic role. Several immunological and pathological features of renal inflammation can mediate or induce necrosis in the kidney (Table 1). We describe below some of the pathways leading to necrotic cell death in the kidney.
Table 1.
| Nephritides | Pathological findings | Possible initial triggers of necrosis |
| Lupus Nephritis | -Mesangial proliferation, crescent formation, glomerular necrosis | -Complement activation |
| -ROS generation by infiltrating cells | ||
| -Granular mesangial orsubendothelial or subepithelial IgG deposits | -Cytokine secretion | |
| IgA Nephropathy | -Glomerular hypercellularity (mesangial or endocapillary), acute tubular necrosis, and crescent formation | -Cytokine secretion by mesangial cells |
| -Enhanced TGF-(3 release by mesangial cells leading to sclerosis | -ROS generation by infiltrating cells. | |
| -Linear IgA deposits | -Complement activation | |
| Anti-GBM nephritis | -Wide spread glomerular crescents | - Complement activation |
| -Glomerular fibrinoid necrosis | ||
| -Linear IgG deposits | ||
| Pauci immune glomerulonephritis | - Crescents and fibrinoid necrosis often containing neutrophil granule constituents | -ROS generated by neutrophil activation |
| -Cytokines and proteases released by neutrophils |
4.1 Complement system
Renal inflammation in autoimmune diseases results from deposition of immune complexes in the kidney or binding of autoantibodies to antigens on the renal intrinsic cells. These immune-complexes fix complement C1q further leading to activation of the complement cascade. The complement activation results in the release of several components such as complement C5a that act as chemoattractants for leukocytes and amplify inflammation. The final step of complement activation is the formation of complement C5-9 membrane attack complex (MAC). MAC binds to cell surface and creates pores in the cell membrane. The cell loses membrane integrity and undergoes necrotic cell death [63]. The complement cascade induces cellular injury, rupture of plasma membrane and lysosomal membranes, leading to necrosis. This necrosis increases inflammation. Complement activation and deposition is a common phenomenon in lupus nephritis, ANCA vasculitits, Goodpasture, HSP, and IgA nephropathy [64; 65; 66; 67; 68]. The autoAb deposition in the kidney either passive or through the binding of Abs to the autoAg expressed in the kidney, leads to complement activation. Lower C3 levels are consistently used in classification or activation criteria features of systemic lupus erythematosus with nephritis [69], further exemplifying the importance of complement activation.
4.2 ROS
Reactive oxygen species are generated by immune cells as well as tissue cells such as mesangial cells in the glomeruli [70; 71]. ROS act as a source of stimulation for immune cells. ROS induce cell death in cells either through the apoptotic or necrotic pathways. ROS can also act as second messengers and play an important role in regulating signal transduction pathways in immune cells. For example, ROS can regulate calcium response in endothelial cells [72; 73]. ROS can induce necrosis and further amplify inflammation. The activation of complement also recruits inflammatory cells such as neutrophils and macrophages, which generate inflammatory mediators including reactive oxygen species and reactive nitrogen species [74]. This initiates an extensive inflammatory response in the kidney. Reactive oxygen and nitrogen species cause damage to DNA, which activates PARP-1. Excessive PARP-1 activation then causes pro-inflammatory necrotic cell death through bioenergetic failure and thus serving as a positive feedback signal to inflammation. The recruitment of inflammatory cells as well as proliferation of mesangial cells results in crescent formation. Cellular crescents containing predominantly macrophages are associated with Bowman's capsule rupture, and are prone to progress to fibrosis [75].
The loss of vascular permeability following infiltration of immune cells usually leads to fibrin deposition, generation of micro-thrombi and fibrinoid necrosis [76; 77; 78]. Due to lack of aerobic respiration, thrombi might create an environment ischemic and therefore with limited energy. Caspase activation is an energy dependent process and therefore, might not be favored during ischemia because of the energy-depleted environment. The lack (or reduced) activation of caspases including caspase 8, which is a negative regulator of necroptosis may facilitate necrosis rather than apoptosis, and result in inflammation.
4.3 Pro-inflammatory cytokines in the kidney
TNFα and IL1 are two major cytokines implicated in the pathogenesis of kidney disease [79; 80; 81; 82; 83; 84]. Although initially the source for TNFα was considered to be infiltrating leukocytes, Timoshanko et al showed that the source of TNFα is in fact the intrinsic renal cells [85]. TNF-α plays a key role in the development of GN [81; 82; 84], which has further been confirmed by the inability to induce anti-GBM GN in TNF-αβ-deficient mice [83]. Blockade of TNF-α has also been shown to reduce inflammation and scarring in experimental crescentic GN [82]. Treatment of lupus prone New Zealand Black x New Zealand White F1 (NZBxNZW F1) mice with TNF receptor type II (TNFRII) Ig prolonged survival and inhibited secretion of proinflammatory cytokines by renal cells [86]. TNF receptor triggering in the absence of caspase activation is conducive of necrosis, therefore the micro-thrombi formed in the kidney, the low energy availability during inflammation and a high local concentration of TNFα may lead to cell death through the necrotic pathways rather than apoptosis, and thus necrosis may play a potential pathogenic role in nephritis.
5. Necrosis in amplification of inflammation
Necrosis is a highly pro-inflammatory form of cell death, and results in the release of ‘alarmins’ or ‘danger signals’ such as heat shock proteins, uric acid, ATP, DNA, and nuclear proteins that alert and activate the innate immune system [11; 87]. These alarmins activate the immune system through several pattern recognition molecules such as Toll-like receptors (TLR) and Nod-like receptors (NOD). Heat shock protein 60 (Hsp60) is released by nephritic kidneys and exogenous administration of Hsp60 results in increased severity of nephrotoxic serum induced nephritis in mice [88]. Another such danger signal is High Mobility Group Box I (HMGB1) protein. Receptor of Advanced Glycation End Products (RAGE) and TLR4 are the putative receptors for HMGB1 [89; 90].
HMGB1 expression has been associated with the formation of granulomas in adenine-induced nephropathy in rats and mice [91]. In patients with chronic kidney disease (CKD) HMGB1 levels negatively correlated with glomerular filtration rate [92]. HMGB1 levels correlated with levels of pro-inflammatory cytokines/markers such as TNFα and IL 6, suggesting HMGB1 as predictor of disease severity. Serum levels of HMGB1 were significantly higher in patients with GPA that had active disease rather than patients in remission [93]. Therefore HMGB1 may be a useful marker for disease activity in GPA. In contrast patients with microscopic polyangiitis had comparable levels of HMGB-1 regardless of disease activity, suggesting that HMGB1 may be useful in discriminating between different forms of ANCA associated vasculitides [93]. However more studies are needed as Bruchfeld et al did not detect any differences in HMGB1 levels between different forms of ANCA-associated vasculitis (AAV) [94]. Interestingly the latter study also showed that HMGB1 is increased in AAV patients with renal manifestations, and the levels decrease during remission. HMGB1 has also been shown to be upregulated in the sera of patients with Henoch-Schönlein purpura nephritis and IgA nephritis [95]. Other danger signals released by dying cells that have been implicated in renal inflammation include S100 family of proteins and uric acid [96; 97; 98].
The identification of danger signals released by necrotic cells as mediators of renal inflammation strongly supports the notion that regulated necrosis plays an important role in the pathogenesis of immune mediated nephritides.
Lupus patients that lacked DNase-1 activity and therefore, the ability to degrade NETs, had severe renal disease [99], suggesting NETosis as a contributing factor in lupus nephritis. NETs have been reported in kidney biopsies from ANCA glomerulonephritis and lupus nephritis patients [100; 101]. NETosis is generally considered pro-inflammatory and may contribute to renal inflammation by providing accessible nuclear antigens for IC deposition. Recently IgA was shown to enhance NETosis through FcαRI ligation [102], although relevance of this activation in nephropathies was not investigated.
Finally there has been a growing interest in this form of cell demise and investigators have demonstrated in both animal models and patients that regulated necrosis is implicated in the pathophysiology of several other inflammatory diseases. During pancreatitis, most cell death was found to be necrotic rather than apoptotic and the degree of necrosis correlates with the severity of the disease [103]. In a murine model of pancreatitis, He, S et al showed that RIP3 deficient mice are resistant to cerulein-induced pancreatitis [104]. Nec-1 treatment protects against ischemia reperfusion (IR) injury in vivo, suggesting a possible role for Necroptosis in IR injury. Gunther et al showed a direct evidence for regulation of necroptosis by caspase 8 in inflammatory bowel disease (IBD) [105]. The authors show that TNFα induces epithelial necroptosis in a RIP3 dependent manner, and this cell death is inhibited by activation of caspase 8, thus suggesting a role for necroptosis in the pathogenesis of IBD [105]. Mice deficient in FADD develop chronic skin inflammation, which is triggered by RIP3, induced necroptosis [106].
6. Conclusions
Necrosis plays an important role in the pathogenesis of several inflammatory diseases, including immune mediated nephritides. The immune response to initial damage to the tissue induces necrosis and this necrotic cell death further amplifies the inflammatory response through the release of mediators of inflammation, thus forming a pathogenic feedback loop (Figure 3). Although a significant progress has been made in identifying the danger signals released by injured/dying/dead cells and their role in amplifying the inflammatory response, little effort has been made to regulate cell death to curb inflammation. Due the regulated nature of necrosis as opposed to accidental death, novel target are emerging to inhibit this form of cellular demise. Therefore, targeting necrotic cell death may prove to be an effective treatment for immune mediated nephropathies.
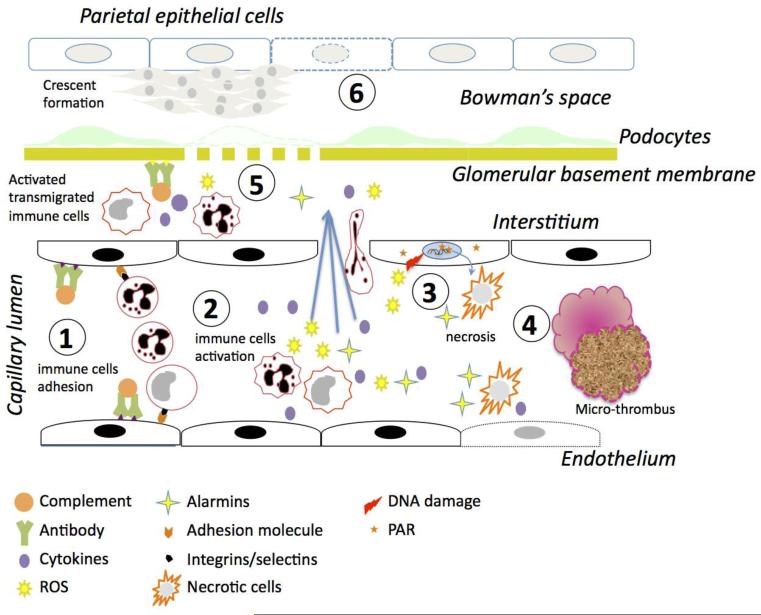
Figure 3. Role of necrosis in renal tissue injury.
1. Deposition of immune complexes on the endothelial cells fixes complement and allows for the adherence and activation of monocytes and neutrophils through interactions between adhesion molecules and selectins/integrins. 2. Activation of monocytes and neutrophils leads to release of pro-inflammatory cytokines, reactive oxygen species (ROS) activate endothelial cells and lead to endothelial dysfunction. The neutrophils and monocytes then migrate into the interstitium. 3. The ROS generated causes DNA damage, leading to PARP-1 activation and necrosis of both leukocytes and endothelial cells. The necrotic cells release alarmins further activating leukocytes and endothelial cells. 4. Endothelial dysfunction, leukocyte infiltration, and necrosis finally lead to micro-thrombi causing areas of local ischemia. Necrosis resulting from all these processes leads to a feed back loop that exacerbates the inflammatory response and induces further tissue damage. 5. The leukocytes migrated into the interstitium get further activated by the immune complexes deposited on the glomerular basement membranes. Cytokines and inflammatory mediators released by the immune cells in the interstitium cause glomerular membrane rupture and loss of podocyte foot processes. 6. The inflammatory mediators enter the Bowman's space, activate epithelial cells and finally lead to crescent formation.
Highlights.
The mechanisms of necrotic cell death are discussed.
The pathogenesis of glemerulonephritides is discussed.
The interplay between renal inflammatory triggers and necrotic cell death are discussed.
The potential benefits of inhibiting necrosis are proposed.
Abbreviations
- ANT
adenine nucleotide translocator (ANT)
- ANCA
Anti-Neutrophil Cytoplasmic Antibodies
- AAV
ANCA-associated vasculitis
- CSS
Churg-Strauss syndrome
- CypD
cyclophilin D
- FADD
Fas associated death domain adaptor protein
- GPA
granulomatosis with polyangiitis
- GBM
glomerular basement membrane
- GN
Glomerulonephritis
- HSP
Henoch-Schönlein purpura
- HMGB1
High Mobility Group Box-1
- iNOS
inducible nitric oxide synthase
- MGN
membranous Glomerulonephritis
- MAC
membrane attack complex
- MPA
microscopic polyangiitis
- MPTP
mitochondrial permeability transition pore
- NAD
Nicotinamide adenine dinucleotide
- α3(IV)NC1
noncollagenous domain of alpha 3 chain of type IV collagen
- PAR
poly(ADP-ribose) polymers
- PARP-1
Poly (ADP-Ribose) polymerase
- ROS
reactive oxygen species
- RIP
Receptor Interacting Protein kinase
- RHIM
RIP homotypic interaction motifs
- TNFα
Tumor necrosis factor alpha
- TNFR1
TNF receptor 1
- TRADD
Tumor necrosis factor receptor type 1-associated death domain protein
- VDAC
voltage-dependent anion channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lockshin RA, Williams CM. Programmed Cell Death--I. Cytology of Degeneration in the Intersegmental Muscles of the Pernyi Silkmoth. J Insect Physiol. 1965;11:123–33. doi: 10.1016/0022-1910(65)90099-5. [DOI] [PubMed] [Google Scholar]
- 2.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lockshin RA, Zakeri Z. Programmed cell death and apoptosis: origins of the theory. Nat Rev Mol Cell Biol. 2001;2:545–50. doi: 10.1038/35080097. [DOI] [PubMed] [Google Scholar]
- 4.Zakeri Z, Lockshin RA, Martinez AC. Meeting report: mechanisms of cell death 2000. Apoptosis. 2001;6:403–4. doi: 10.1023/a:1011346421529. [DOI] [PubMed] [Google Scholar]
- 5.Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988;141:2629–34. [PubMed] [Google Scholar]
- 6.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–80. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 10.Wartha F, Henriques-Normark B. ETosis: a novel cell death pathway. Sci Signal. 2008;1:pe25. doi: 10.1126/stke.121pe25. [DOI] [PubMed] [Google Scholar]
- 11.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–87. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–14. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 13.Pobezinskaya YL, Kim YS, Choksi S, Morgan MJ, Li T, Liu C, Liu Z. The function of TRADD in signaling through tumor necrosis factor receptor 1 and TRIF-dependent Toll-like receptors. Nat Immunol. 2008;9:1047–54. doi: 10.1038/ni.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22:263–8. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–13. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–28. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 17.Jog NR, Caricchio R. Differential regulation of cell death programs in males and females by Poly (ADP-Ribose) Polymerase-1 and 17beta estradiol. Cell Death Dis. 2013;4:e758. doi: 10.1038/cddis.2013.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jog NR, Dinnall JA, Gallucci S, Madaio MP, Caricchio R. Poly(ADP-ribose) polymerase-1 regulates the progression of autoimmune nephritis in males by inducing necrotic cell death and modulating inflammation. J Immunol. 2009;182:7297–306. doi: 10.4049/jimmunol.0803565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliver FJ, Menissier-de Murcia J, Nacci C, Decker P, Andriantsitohaina R, Muller S, de la Rubia G, Stoclet JC, de Murcia G. Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly (ADP-ribose) polymerase- 1 deficient mice. EMBO J. 1999;18:4446–54. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng J, Devalaraja-Narashimha K, Singaravelu K, Padanilam BJ. Poly(ADP-ribose) polymerase-1 gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol. 2005;288:F387–98. doi: 10.1152/ajprenal.00436.2003. [DOI] [PubMed] [Google Scholar]
- 21.Garcia S, Bodano A, Gonzalez A, Forteza J, Gomez-Reino JJ, Conde C. Partial protection against collagen antibody-induced arthritis in PARP-1 deficient mice. Arthritis Res Ther. 2006;8:R14. doi: 10.1186/ar1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Rey E, Martinez-Romero R, O'Valle F, Aguilar-Quesada R, Conde C, Delgado M, Oliver FJ. Therapeutic Effect of a Poly(ADP-Ribose) Polymerase-1 Inhibitor on Experimental Arthritis by Downregulating Inflammation and Th1 Response. PLoS ONE. 2007;2:e1071. doi: 10.1371/journal.pone.0001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–40. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–44. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–8. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 26.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–62. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 27.Temkin V, Huang Q, Liu H, Osada H, Pope RM. Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol Cell Biol. 2006;26:2215–25. doi: 10.1128/MCB.26.6.2215-2225.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sosna J, Voigt S, Mathieu S, Lange A, Thon L, Davarnia P, Herdegen T, Linkermann A, Rittger A, Chan FK, Kabelitz D, Schutze S, Adam D. TNF-induced necroptosis and PARP-1-mediated necrosis represent distinct routes to programmed necrotic cell death. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-013-1381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, Huang S, Liu ZG, Han J. Poly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK1 activation. J Biol Chem. 2006;281:8788–95. doi: 10.1074/jbc.M508135200. [DOI] [PubMed] [Google Scholar]
- 30.Le Page C, Sanceau J, Drapier JC, Wietzerbin J. Inhibitors of ADP-ribosylation impair inducible nitric oxide synthase gene transcription through inhibition of NF kappa B activation. Biochem Biophys Res Commun. 1998;243:451–7. doi: 10.1006/bbrc.1998.8113. [DOI] [PubMed] [Google Scholar]
- 31.Shall S, de Murcia G. Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat Res. 2000;460:1–15. doi: 10.1016/s0921-8777(00)00016-1. [DOI] [PubMed] [Google Scholar]
- 32.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–50. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 33.Makino H, Hayashi Y, Yamasaki Y, Shikata K, Kashihara N, Kira S, Ota Z. Clinical significance of necrosis in lupus nephritis. Intern Med. 1994;33:461–5. doi: 10.2169/internalmedicine.33.461. [DOI] [PubMed] [Google Scholar]
- 34.Ibels LS, Gyory AZ. IgA nephropathy: analysis of the natural history, important factors in the progression of renal disease, and a review of the literature. Medicine (Baltimore) 1994;73:79–102. [PubMed] [Google Scholar]
- 35.Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29:829–42. doi: 10.1016/s0272-6386(97)90456-x. [DOI] [PubMed] [Google Scholar]
- 36.Kraft DM, McKee D, Scott C. Henoch-Schonlein purpura: a review. Am Fam Physician. 1998;58:405–8. 411. [PubMed] [Google Scholar]
- 37.Chen A, Chen WP, Sheu LF, Lin CY. Pathogenesis of IgA nephropathy: in vitro activation of human mesangial cells by IgA immune complex leads to cytokine secretion. J Pathol. 1994;173:119–26. doi: 10.1002/path.1711730208. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Zhao MH, Zhang YK, Li XM, Wang HY. Binding capacity and pathophysiological effects of IgA1 from patients with IgA nephropathy on human glomerular mesangial cells. Clin Exp Immunol. 2004;136:168–75. doi: 10.1111/j.1365-2249.2004.02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Armada MJ, Gomez-Guerrero C, Egido J. Receptors for immune complexes activate gene expression and synthesis of matrix proteins in cultured rat and human mesangial cells: role of TGF-beta. J Immunol. 1996;157:2136–42. [PubMed] [Google Scholar]
- 40.Leinonen A, Netzer KO, Boutaud A, Gunwar S, Hudson BG. Goodpasture antigen: expression of the full-length alpha3(IV) chain of collagen IV and localization of epitopes exclusively to the noncollagenous domain. Kidney Int. 1999;55:926–35. doi: 10.1046/j.1523-1755.1999.055003926.x. [DOI] [PubMed] [Google Scholar]
- 41.Lerner RA, Glassock RJ, Dixon FJ. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967;126:989–1004. doi: 10.1084/jem.126.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalluri R, Danoff TM, Okada H, Neilson EG. Susceptibility to anti-glomerular basement membrane disease and Goodpasture syndrome is linked to MHC class II genes and the emergence of T cell-mediated immunity in mice. J Clin Invest. 1997;100:2263–75. doi: 10.1172/JCI119764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbate M, Kalluri R, Corna D, Yamaguchi N, McCluskey RT, Hudson BG, Andres G, Zoja C, Remuzzi G. Experimental Goodpasture's syndrome in Wistar-Kyoto rats immunized with alpha3 chain of type IV collagen. Kidney Int. 1998;54:1550–61. doi: 10.1046/j.1523-1755.1998.00153.x. [DOI] [PubMed] [Google Scholar]
- 44.Kitching AR, Turner AL, Semple T, Li M, Edgtton KL, Wilson GR, Timoshanko JR, Hudson BG, Holdsworth SR. Experimental autoimmune anti-glomerular basement membrane glomerulonephritis: a protective role for IFN-gamma. J Am Soc Nephrol. 2004;15:1764–74. doi: 10.1097/01.asn.0000128968.27705.5e. [DOI] [PubMed] [Google Scholar]
- 45.Hopfer H, Maron R, Butzmann U, Helmchen U, Weiner HL, Kalluri R. The importance of cell-mediated immunity in the course and severity of autoimmune anti-glomerular basement membrane disease in mice. FASEB J. 2003;17:860–8. doi: 10.1096/fj.02-0746com. [DOI] [PubMed] [Google Scholar]
- 46.Teague CA, Doak PB, Simpson IJ, Rainer SP, Herdson PB. Goodpasture's syndrome: an analysis of 29 cases. Kidney Int. 1978;13:492–504. doi: 10.1038/ki.1978.72. [DOI] [PubMed] [Google Scholar]
- 47.McPhaul JJ, Jr., Mullins JD. Glomerulonephritis mediated by antibody to glomerular basement membrane. Immunological, clinical, and histopathological characteristics. J Clin Invest. 1976;57:351–61. doi: 10.1172/JCI108286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson CB, Dixon FJ. Anti-glomerular basement membrane antibody-induced glomerulonephritis. Kidney Int. 1973;3:74–89. doi: 10.1038/ki.1973.14. [DOI] [PubMed] [Google Scholar]
- 49.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 50.Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63:1164–77. doi: 10.1046/j.1523-1755.2003.00843.x. [DOI] [PubMed] [Google Scholar]
- 51.Guillevin L, Durand-Gasselin B, Cevallos R, Gayraud M, Lhote F, Callard P, Amouroux J, Casassus P, Jarrousse B. Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum. 1999;42:421–30. doi: 10.1002/1529-0131(199904)42:3<421::AID-ANR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 52.Lhote F, Cohen P, Guillevin L. Polyarteritis nodosa, microscopic polyangiitis and Churg-Strauss syndrome. Lupus. 1998;7:238–58. doi: 10.1191/096120398678920055. [DOI] [PubMed] [Google Scholar]
- 53.Sinico RA, Di Toma L, Maggiore U, Tosoni C, Bottero P, Sabadini E, Giammarresi G, Tumiati B, Gregorini G, Pesci A, Monti S, Balestrieri G, Garini G, Vecchio F, Buzio C. Renal involvement in Churg-Strauss syndrome. Am J Kidney Dis. 2006;47:770–9. doi: 10.1053/j.ajkd.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 54.Pagnoux C, Guillevin L. Churg-Strauss syndrome: evidence for disease subtypes? Curr Opin Rheumatol. 2010;22:21–8. doi: 10.1097/BOR.0b013e328333390b. [DOI] [PubMed] [Google Scholar]
- 55.Savage CO, Winearls CG, Evans DJ, Rees AJ, Lockwood CM. Microscopic polyarteritis: presentation, pathology and prognosis. Q J Med. 1985;56:467–83. [PubMed] [Google Scholar]
- 56.Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–7. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 57.Ferrario F, Tadros MT, Napodano P, Sinico RA, Fellin G, D'Amico G. Critical re-evaluation of 41 cases of “idiopathic” crescentic glomerulonephritis. Clin Nephrol. 1994;41:1–9. [PubMed] [Google Scholar]
- 58.Vizjak A, Rott T, Koselj-Kajtna M, Rozman B, Kaplan-Pavlovcic S, Ferluga D. Histologic and immunohistologic study and clinical presentation of ANCA-associated glomerulonephritis with correlation to ANCA antigen specificity. Am J Kidney Dis. 2003;41:539–49. doi: 10.1053/ajkd.2003.50142. [DOI] [PubMed] [Google Scholar]
- 59.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noel LH, Pusey CD, Waldherr R, Bruijn JA, Bajema IM. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–36. doi: 10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- 60.Kain R, Matsui K, Exner M, Binder S, Schaffner G, Sommer EM, Kerjaschki D. A novel class of autoantigens of anti-neutrophil cytoplasmic antibodies in necrotizing and crescentic glomerulonephritis: the lysosomal membrane glycoprotein h-lamp-2 in neutrophil granulocytes and a related membrane protein in glomerular endothelial cells. J Exp Med. 1995;181:585–97. doi: 10.1084/jem.181.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen M, Yu F, Wang SX, Zou WZ, Zhao MH, Wang HY. Antineutrophil cytoplasmic autoantibody-negative Pauci-immune crescentic glomerulonephritis. J Am Soc Nephrol. 2007;18:599–605. doi: 10.1681/ASN.2006091021. [DOI] [PubMed] [Google Scholar]
- 62.Ronco P, Debiec H. Pathogenesis of membranous nephropathy: recent advances and future challenges. Nat Rev Nephrol. 2012;8:203–13. doi: 10.1038/nrneph.2012.35. [DOI] [PubMed] [Google Scholar]
- 63.Janeway CAJ, Travers P, Walport M, Sclomchik MJ. Immunobiology: The Immune System in Health and Disease. 5th edition Garland Science; New York: 2001. [Google Scholar]
- 64.Mollnes TE, Haga HJ, Brun JG, Nielsen EW, Sjoholm A, Sturfeldt G, Martensson U, Bergh K, Rekvig OP. Complement activation in patients with systemic lupus erythematosus without nephritis. Rheumatology (Oxford) 1999;38:933–40. doi: 10.1093/rheumatology/38.10.933. [DOI] [PubMed] [Google Scholar]
- 65.Savage CO. Pathogenesis of anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Clin Exp Immunol. 2011;164(Suppl 1):23–6. doi: 10.1111/j.1365-2249.2011.04362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alenzi FQ, Salem ML, Alenazi FA, Wyse RK. Cellular and molecular aspects of Goodpasture syndrome. Iran J Kidney Dis. 2012;6:1–8. [PubMed] [Google Scholar]
- 67.Endo M, Ohi H, Ohsawa I, Fujita T, Matsushita M. Complement activation through the lectin pathway in patients with Henoch-Schonlein purpura nephritis. Am J Kidney Dis. 2000;35:401–7. doi: 10.1016/s0272-6386(00)70192-2. [DOI] [PubMed] [Google Scholar]
- 68.Matsuda M, Shikata K, Wada J, Sugimoto H, Shikata Y, Kawasaki T, Makino H. Deposition of mannan binding protein and mannan binding protein-mediated complement activation in the glomeruli of patients with IgA nephropathy. Nephron. 1998;80:408–13. doi: 10.1159/000045212. [DOI] [PubMed] [Google Scholar]
- 69.West CD. The complement profile in clinical medicine. Inherited and acquired conditions lowering the serum concentrations of complement component and control proteins. Complement Inflamm. 1989;6:49–64. [PubMed] [Google Scholar]
- 70.Farber JL, Kyle ME, Coleman JB. Mechanisms of cell injury by activated oxygen species. Lab Invest. 1990;62:670–9. [PubMed] [Google Scholar]
- 71.Sedor JR, Carey SW, Emancipator SN. Immune complexes bind to cultured rat glomerular mesangial cells to stimulate superoxide release. Evidence for an Fc receptor. J Immunol. 1987;138:3751–7. [PubMed] [Google Scholar]
- 72.Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H2023–31. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- 73.Touyz RM. Reactive oxygen species as mediators of calcium signaling by angiotensin II: implications in vascular physiology and pathophysiology. Antioxid Redox Signal. 2005;7:1302–14. doi: 10.1089/ars.2005.7.1302. [DOI] [PubMed] [Google Scholar]
- 74.Nath KA, Fischereder M, Hostetter TH. The role of oxidants in progressive renal injury. Kidney Int Suppl. 1994;45:S111–5. [PubMed] [Google Scholar]
- 75.Boucher A, Droz D, Adafer E, Noel LH. Relationship between the integrity of Bowman's capsule and the composition of cellular crescents in human crescentic glomerulonephritis. Lab Invest. 1987;56:526–33. [PubMed] [Google Scholar]
- 76.Drew AF, Tucker HL, Liu H, Witte DP, Degen JL, Tipping PG. Crescentic glomerulonephritis is diminished in fibrinogen-deficient mice. Am J Physiol Renal Physiol. 2001;281:F1157–63. doi: 10.1152/ajprenal.2001.281.6.F1157. [DOI] [PubMed] [Google Scholar]
- 77.Song D, Wu LH, Wang FM, Yang XW, Zhu D, Chen M, Yu F, Liu G, Zhao MH. The spectrum of renal thrombotic microangiopathy in lupus nephritis. Arthritis Res Ther. 2013;15:R12. doi: 10.1186/ar4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu LH, Yu F, Tan Y, Qu Z, Chen MH, Wang SX, Liu G, Zhao MH. Inclusion of renal vascular lesions in the 2003 ISN/RPS system for classifying lupus nephritis improves renal outcome predictions. Kidney Int. 2013;83:715–23. doi: 10.1038/ki.2012.409. [DOI] [PubMed] [Google Scholar]
- 79.Brennan DC, Yui MA, Wuthrich RP, Kelley VE. Tumor necrosis factor and IL-1 in New Zealand Black/White mice. Enhanced gene expression and acceleration of renal injury. J Immunol. 1989;143:3470–5. [PubMed] [Google Scholar]
- 80.Tomosugi NI, Cashman SJ, Hay H, Pusey CD, Evans DJ, Shaw A, Rees AJ. Modulation of antibody-mediated glomerular injury in vivo by bacterial lipopolysaccharide, tumor necrosis factor, and IL-1. J Immunol. 1989;142:3083–90. [PubMed] [Google Scholar]
- 81.Le Hir M, Haas C, Marino M, Ryffel B. Prevention of crescentic glomerulonephritis induced by anti-glomerular membrane antibody in tumor necrosis factor-deficient mice. Lab Invest. 1998;78:1625–31. [PubMed] [Google Scholar]
- 82.Khan SB, Cook HT, Bhangal G, Smith J, Tam FW, Pusey CD. Antibody blockade of TNF-alpha reduces inflammation and scarring in experimental crescentic glomerulonephritis. Kidney Int. 2005;67:1812–20. doi: 10.1111/j.1523-1755.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 83.Ryffel B, Eugster H, Haas C, Le Hir M. Failure to induce anti-glomerular basement membrane glomerulonephritis in TNF alpha/beta deficient mice. Int J Exp Pathol. 1998;79:453–60. doi: 10.1046/j.1365-2613.1998.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karkar AM, Smith J, Pusey CD. Prevention and treatment of experimental crescentic glomerulonephritis by blocking tumour necrosis factor-alpha. Nephrol Dial Transplant. 2001;16:518–24. doi: 10.1093/ndt/16.3.518. [DOI] [PubMed] [Google Scholar]
- 85.Timoshanko JR, Sedgwick JD, Holdsworth SR, Tipping PG. Intrinsic renal cells are the major source of tumor necrosis factor contributing to renal injury in murine crescentic glomerulonephritis. J Am Soc Nephrol. 2003;14:1785–93. doi: 10.1097/01.asn.0000073902.38428.33. [DOI] [PubMed] [Google Scholar]
- 86.Bethunaickan R, Sahu R, Liu Z, Tang YT, Huang W, Edegbe O, Tao H, Ramanujam M, Madaio MP, Davidson A. Anti-tumor necrosis factor alpha treatment of interferon-alpha-induced murine lupus nephritis reduces the renal macrophage response but does not alter glomerular immune complex formation. Arthritis Rheum. 2012;64:3399–408. doi: 10.1002/art.34553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Exp Cell Res. 2003;283:1–16. doi: 10.1016/s0014-4827(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 88.Lang A, Benke D, Eitner F, Engel D, Ehrlich S, Breloer M, Hamilton-Williams E, Specht S, Hoerauf A, Floege J, von Bonin A, Kurts C. Heat shock protein 60 is released in immune-mediated glomerulonephritis and aggravates disease: in vivo evidence for an immunologic danger signal. J Am Soc Nephrol. 2005;16:383–91. doi: 10.1681/ASN.2004040276. [DOI] [PubMed] [Google Scholar]
- 89.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 90.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 91.Oyama Y, Hashiguchi T, Taniguchi N, Tancharoen S, Uchimura T, Biswas KK, Kawahara K, Nitanda T, Umekita Y, Lotz M, Maruyama I. High-mobility group box-1 protein promotes granulomatous nephritis in adenine-induced nephropathy. Lab Invest. 2010;90:853–66. doi: 10.1038/labinvest.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bruchfeld A, Qureshi AR, Lindholm B, Barany P, Yang L, Stenvinkel P, Tracey KJ. High Mobility Group Box Protein-1 correlates with renal function in chronic kidney disease (CKD). Mol Med. 2008;14:109–15. doi: 10.2119/2007-00107.Bruchfeld. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wibisono D, Csernok E, Lamprecht P, Holle JU, Gross WL, Moosig F. Serum HMGB1 levels are increased in active Wegener's granulomatosis and differentiate between active forms of ANCA-associated vasculitis. Ann Rheum Dis. 2010;69:1888–9. doi: 10.1136/ard.2009.119172. [DOI] [PubMed] [Google Scholar]
- 94.Bruchfeld A, Wendt M, Bratt J, Qureshi AR, Chavan S, Tracey KJ, Palmblad K, Gunnarsson I. High-mobility group box-1 protein (HMGB1) is increased in antineutrophilic cytoplasmatic antibody (ANCA)-associated vasculitis with renal manifestations. Mol Med. 2011;17:29–35. doi: 10.2119/molmed.2010.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sato F, Maruyama S, Hayashi H, Sakamoto I, Yamada S, Uchimura T, Morita Y, Ito Y, Yuzawa Y, Maruyama I, Matsuo S. High mobility group box chromosomal protein 1 in patients with renal diseases. Nephron Clin Pract. 2008;108:c194–201. doi: 10.1159/000118942. [DOI] [PubMed] [Google Scholar]
- 96.Frosch M, Vogl T, Waldherr R, Sorg C, Sunderkotter C, Roth J. Expression of MRP8 and MRP14 by macrophages is a marker for severe forms of glomerulonephritis. J Leukoc Biol. 2004;75:198–206. doi: 10.1189/jlb.0203076. [DOI] [PubMed] [Google Scholar]
- 97.Myllymaki J, Honkanen T, Syrjanen J, Helin H, Rantala I, Pasternack A, Mustonen J. Uric acid correlates with the severity of histopathological parameters in IgA nephropathy. Nephrol Dial Transplant. 2005;20:89–95. doi: 10.1093/ndt/gfh584. [DOI] [PubMed] [Google Scholar]
- 98.Zhou Y, Fang L, Jiang L, Wen P, Cao H, He W, Dai C, Yang J. Uric acid induces renal inflammation via activating tubular NF-kappaB signaling pathway. PLoS One. 2012;7:e39738. doi: 10.1371/journal.pone.0039738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107:9813–8. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–5. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, Shealy D, Denny MF, Plumas J, Chaperot L, Kretzler M, Bruce AT, Kaplan MJ. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–52. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aleyd E, van Hout MW, Ganzevles SH, Hoeben KA, Everts V, Bakema JE, van Egmond M. IgA Enhances NETosis and Release of Neutrophil Extracellular Traps by Polymorphonuclear Cells via Fcalpha Receptor I. J Immunol. 2014;192:2374–83. doi: 10.4049/jimmunol.1300261. [DOI] [PubMed] [Google Scholar]
- 103.Mareninova OA, Sung KF, Hong P, Lugea A, Pandol SJ, Gukovsky I, Gukovskaya AS. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem. 2006;281:3370–81. doi: 10.1074/jbc.M511276200. [DOI] [PubMed] [Google Scholar]
- 104.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–11. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 105.Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, Becker C. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–9. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, Haase I, Pasparakis M. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity. 2011;35:572–82. doi: 10.1016/j.immuni.2011.08.014. [DOI] [PubMed] [Google Scholar]