Abstract
Background
The Academic Model Providing Access To Healthcare (AMPATH) program provides comprehensive HIV care and treatment services. Approximately 30% of patients have become lost to follow-up (LTFU). We sought to actively trace and identify outcomes for a sample of these patients.
Methods
LTFU was defined as missing a scheduled visit by ≥ 3 months. A randomly selected sample of 17% of patients identified as LTFU between January 2009 and June 2011 was generated, with sample stratification on age, antiretroviral therapy (ART) status at last visit, and facility. Chart reviews were conducted followed by active tracing. Tracing was completed by trained HIV-positive outreach workers July 2011 to February 2012. Outcomes were compared between adults and children and by ART status.
Results
Of 14,811 LTFU patients, 2,540 were randomly selected for tracing (2,179 adults, 1,071 on ART). The chart reviews indicated that 326 (12.8%) patients were not actually LTFU. Outcomes for 71% of sampled patients were determined including 85% of those physically traced. Of those with known outcomes, 21% had died while 29% had disengaged from care for various reasons. The remaining patients had moved away (n=458, 25%) or were still receiving HIV care (n=443 total, 25%).
Conclusions
Our findings demonstrate the feasibility of a large scale sampling-based approach. A significant proportion of patients were found not to be LTFU and further, high numbers of patients who were LTFU could not be located. Over a quarter of patients disengaged from care for various reasons including access challenges and familial influences.
Keywords: Lost to Follow-Up, Sampling, Outreach, Tracing, HIV/AIDS
Introduction
Improved access to HIV care and especially antiretroviral therapy (ART) globally has resulted in decreases in HIV-related morbidity and mortality (1-5). Among people living with HIV/AIDS (PLWHA), retention in HIV care programs is critical for achieving timely treatment initiation and viral suppression. Continuous engagement in care is also programmatically critical for positively impacting HIV incidence (1, 5). Disruption in HIV care through missed visits/appointments can undermine clinical outcomes (6); retention in HIV care programs remains a major challenge across settings (7-10). A 2010 review of 39 sub-Saharan ART cohorts reported that approximately 25% (11-32%) of patients were no longer in care after 2 years of treatment with ART. After adjusting for variable follow-up among the various cohorts in sensitivity analysis, median attrition at 2 years was 30% (27-33%). Attrition was mostly due to losses to follow-up (LTFU) followed by death (9).
The dynamic complexities individuals face during the course of their HIV care (e.g., logistical challenges) (11-25) can impact upon their ability to return to the clinic for scheduled follow-up visits. This, in turn, places individuals at high-risk for disease progression, drug resistance and death (11, 26-30). At the same time, program planners remain uncertain about how and where to direct outreach and return-to-care efforts (6, 11, 31, 32). Large numbers of losses to follow-up can indicate poorly designed programs that do not meet patient needs as well as ineffective or inefficient use of program resources. Patient tracing through outreach activities is commonly used to track individuals who miss scheduled visits, in order to determine their status and encourage their return to care (32, 33). This occurs through direct contact with the patient but can also include discussions with neighbors, family members and friends when the patient can't be found or is known to have died. True outcomes of adults and children LTFU are difficult to assess and HIV care clinics continue to face operational challenges when it comes to finding patients who miss visits. Studies that identify outcomes of patients who are traced are an important way of improving quality of care (34). In spite of increasing numbers of individuals in HIV care and on ART (35), health worker shortages, organizational challenges and high costs continue to limit the ability of HIV programs to trace all patients who are missing or LTFU. Attempting to trace all patients can result in biased estimates particularly if a large proportion of those lost could not actually be located. However, tracing only a sample of patients may be considered as a ‘scalable alternative’ (36), as analyses of data obtained on patients who are actually located can allow for the adjustment of mortality and LTFU estimates. We have previously demonstrated the effectiveness of sampling-based approaches for improving estimates of patient retention and survival (36-39).
The Academic Model Providing Access to Healthcare (AMPATH) program was initiated in 2001 in response to the HIV epidemic in western Kenya. It has enrolled over 130,000 HIV-infected patients and 21,000 HIV-exposed infants in > 65 Ministry of Health facilities throughout western Kenya (Figure 1). Approximately 30% of these patients have become LTFU since 2001; however tracing all patients known to be LTFU in our setting is difficult for the reasons highlighted above (36). Therefore, the objective of the present study was to trace only a sample of those LTFU in order to document their reasons for LTFU and inform program improvement and patient monitoring.
Figure 1. Study Sites in western Kenya.
Methods
Academic Model Providing Access to Healthcare (AMPATH)
As of 2012, an estimated 5.6% of Kenyans aged 15-64 were estimated to be HIV positive (40) although prevalence varies geographically, with the highest prevalence (15%) demonstrated in the western region of Nyanza (41). Antiretroviral therapy has been provided through the public sector since 2006 and as of 2012, it is estimated that 61% of treatment-eligible adults were receiving ART (42, 43).
The AMPATH Consortium, based in Eldoret, Kenya (about 350km north-west of Nairobi) was initiated in 2001 as a joint partnership between Moi University School of Medicine, Moi Teaching and Referral Hospital (MTRH) (44, 45), and a consortium of North American universities led by Indiana University (IU) School of Medicine. With financial support from United States Agency for International Development (USAID), the USAID-AMPATH Partnership was established in 2004. The AMPATH Consortium provides technical support, mentorship and training to Kenyan medical faculty and staff with the aim of developing healthcare services in Kenya. AMPATH has enrolled over 130,000 HIV-infected adults and children plus 21,000 HIV-exposed infants in >65 Ministry of Health facilities around western Kenya. Currently, nearly 80,000 patients are actively followed, 83% of whom are on combination ART (cART); 21% are aged ≤14 years. All HIV and tuberculosis (TB)-related care and treatment are free at the point of service for patients. Patients are managed according to National Kenyan protocols, which are consistent with World Health Organization (WHO) guidelines. Clinic visits occur monthly for all patients on ART unless alternative arrangements have been made with their healthcare provider. Patients who are not yet eligible for treatment are seen monthly or bi-monthly depending on their immunologic status and other factors in their health profile. Standard paper data collection forms are used at enrolment to the program and at each subsequent visit. Data from these forms are entered into the AMPATH electronic Medical Record System (AMRS) (46, 47) by data entry technicians.
Outreach Program
AMPATH has a robust mechanism for following-up patients who miss clinic visits. Trained and remunerated HIV-positive peers with records of perfect clinic and/or treatment adherence contact patients by phone or through home visits if they miss a scheduled clinic visit (48). Active outreach of patients who miss scheduled visits started in January 2005 at two of the AMPATH Clinics: Moi Teaching and Referral Hospital (MTRH), an urban referral hospital located in Eldoret and Mosoriot, a rural health center which serves a catchment area of approximately 6,000 located about 30 km from Eldoret. The program now covers all AMPATH clinics. AMRS produces a daily list of patients scheduled for visits and patients that miss theirs are listed for outreach. Adult patients on ART for less than three months are given priority with outreach efforts beginning within 24 hours of a missed visit. Ideally, patients will be found within seven days. Outreach for patients who have been on ART for more than 3 months begins within seven days after a missed visit. Tracing for pre-ART individuals is not initiated until 28 days after a missed visit. At the time of this study, the outreach program maintained a standalone MS Access database that contained data pertaining to every outreach encounter including vital status of located patients and date of death for patients found to be deceased. This database has since become part of the AMRS. Mortality ascertainment is determined through a program wide Standard Operating Procedure and Form for Death Reporting in which all deaths are recorded and reported to the central data system for documentation in the AMRS, including deaths identified in the course of patient-tracing. Note that all patients are asked to provide telephone and locator information at every visit for the purposes of tracing. This includes home-visits in the event a patient misses a scheduled visit. They provide verbal consent in the context of the care program.
Definition of LTFU
LTFU was defined as absence from clinic, without known death or transfer to another facility, for at least 3 months since last scheduled visit.
Sample
All patients (including adults and children as well as those on and off ART) enrolled in AMPATH who had made a visit between January 2009 and July 2011, were identified as the population of interest. From this cohort, we identified patients who were LTFU. We stratified the sample on clinic site, ART status at last clinic visit and age (categorized as adults and children) to ensure adequate representation within each of these strata. We selected 17% of LTFU patients within each of these categories based on an assessment of practical, theoretical and statistical considerations. Previous work suggests that a 10-20% sample provides optimal precision gained per patient sampled (36). Within this range, we took the largest sample we felt was feasible to trace with the given resources.
Measures
First, chart reviews were undertaken, while the second step involved active tracing of patients who after chart review, were still deemed LTFU. Standardized data extraction forms were used for data collection. Outreach workers fill out a locator card for all patients who enroll into the clinical care program which includes the patients contact information as well as a map to get to their residence including landmarks. This information is used to find the patient in the event of a missed appointment. If sampled patients were untraceable, information was obtained from an informant familiar with the patient (e.g., family member, neighbour). Outreach workers will attempt to locate the patient at least 3 times. Reasons why patients stopped going to any clinic for HIV care are captured and divided into 5 main categories: Access to Care (6 items), Clinic Quality (5 items), Work and Family (3 items) Medical (6 items) and Alternative Treatment and Advice (4 items). Importantly, patients could provide more than one reason for disengaging. Tracing of patients was initiated in July 2011 and was completed in February 2012. This study was reviewed and approved by institutional review bodies of all participating sites and universities including Moi University College of Health Sciences, Moi Teaching and Referral Hospital, Indiana University and the University of California, San Francisco.
Analysis
Data analysis was performed using SAS version 9.3. Categorical variables were summarized as frequencies and the corresponding percentages. Association between categorical variables was assessed using Pearson's Chi Square test and the associated p-values were reported. Fisher's exact test was used when the expected cell frequencies in the constructed Chi-Square tables were less than 5. Results were considered statistically significant when p < 0.05. The data for a total of 2,540 participants, adults and children, were included for analysis. Comparisons were explored for adults versus children as well as between individuals on ART and individuals not on ART.
Results
Summary of findings
Of the 14,811 patients identified as LTFU during the study period, 2,540 were randomly selected for tracing including 2,179 (85.8%) adults and 361 (14.2%) children. A total of n=1,071 (42%) were on ART. The median time on ART was 432 days (IQR: 124-827). Figure 2 demonstrates the flow of patients and their outcomes through this study. Over 70% of all patients in this study (n=1,800) were successfully traced and outcomes could be determined for 85% of those who were physically traced. Table 1 presents the summary of patient tracing outcomes. Of those successfully traced, 881/1,800 (49%) had their whereabouts obtained via an informant whereas 919/1,800 (51%) patients were communicated with directly. Significantly, more children (190/361, 52.6%) were communicated to directly (via a guardian and/or parent) compared with adults (729/2,179, 33.5%, p<0.001).
Figure 2. Flow of Patient Outcomes through the Study.
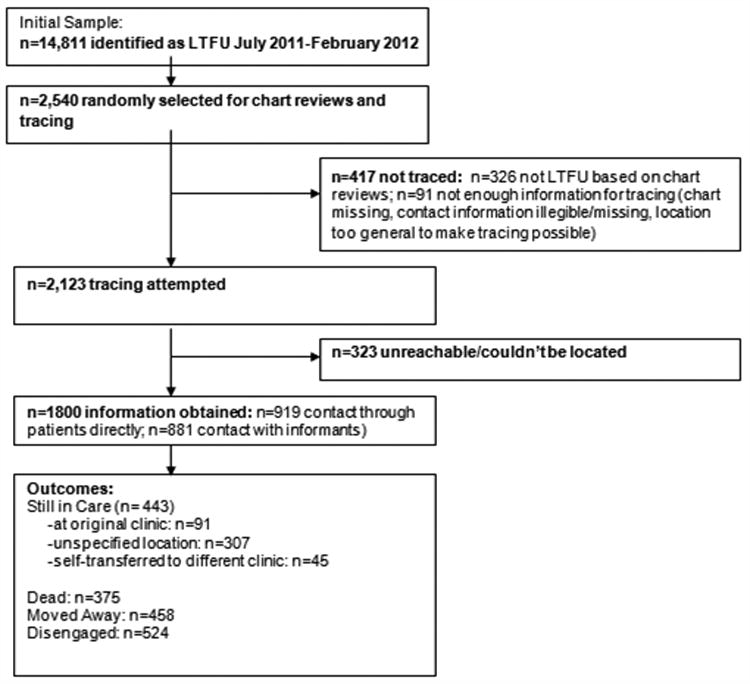
Table 1. Summary of Tracing Results (n=2,540).
| Patient type n (%) | ART status n (%) | ||||||
|---|---|---|---|---|---|---|---|
| All | Adults | Children | p-value | Off | On | p-value | |
| Successfully traced | 1,800 (70.9) | <0.0001 | <0.0001 | ||||
| Communicated with patient | 919 (51.1) | 729 (33.5) | 190 (52.6) | 563 (38) | 356 (33) | ||
| Communicated with informant | 881 (48.9) | 811 (37.2) | 70 (19.4) | 496 (34) | 385 (36) | ||
| Unreachable | 740 (29.1) | ||||||
| Attempted but couldn't trace | 323 (43.6) | 287 (13.2) | 36 (10) | 213 (15) | 110 (10) | ||
| Not attempted (see Table 2) | 417 (56.4) | 352 (16.2) | 65 (18) | 195 (13) | 220 (21) | ||
| 2,540 (100) | 2,179 (100) | 361 (100) | 1,467 (100) | 1,071 (100) | |||
Outcomes of Chart Review
The chart reviews demonstrated that 326/2,540 (12.8%) patients were not actually LTFU: n=50/326 (15.3%), had died n=16/326 (4.9%) were HIV negative, n=47/326 (14.4%) had a recent visit at their original AMPATH clinic, n=45/326 (14.0%) were in care at another AMPATH clinic and n=168/326 (51.5%) had transferred out of AMPATH. Among the n=326 patients found not to be LTFU, significantly more children (28.1% of all children not LTFU) were found to be HIV-negative compared to adults (0% of all adults not LTFU). A higher proportion of adults had a recent visit at their original AMPATH clinic (15.6% of all adults not LTFU versus (vs.) 8.8% of all children not LTFU) or had transferred out (52.3% vs. 38.6%). Significantly more patients on ART had died compared to pre-ART patients (19% of all ART patients not LTFU vs. 10% of all non-ART patients not LTFU).
Tracing Process Outcomes
A total of 1,800/2,540 (70.8%) patients were successfully traced: 881/1,800 (49%) had their whereabouts obtained via an informant whereas 919/1,800 (51.0%) patients were communicated with directly. Of all patients, a total of 740/2,540 (29.1%) patients were not traced. Tracing was attempted but not successful for n=323/740 (43.6%) patients. Tracing was not attempted for 417/740 (56.4%) patients: the chart was missing (n=2, 0.5%), contact information was illegible (n=4, 1.0%), missing (n=30, 7.2%), or too general (n=36, 8.6%) to make tracing possible (Table 2). There was a significant association between ART status and tracing results (p<0.001) with tracing not attempted in a higher proportion of ART patients compared to pre-ART patients (21% vs. 13%). A higher proportion of pre-ART patients had missing contact information needed to make tracing possible (13% vs. 2%) while a higher proportion of patients on ART (190/220, 86%) were found not actually to have been LTFU compared to pre-ART patients (135/195, 69%).
Table 2. Reasons for not tracing (n=417).
| Patient type n (%) | ART Status n (%) | ||||||
|---|---|---|---|---|---|---|---|
| All | Adults | Children | p-value | Off | On | p-value | |
| Chart missing | 2 (<1) | 2 (0.6) | 0 | 0.397 | 1 (0.5) | 1 (0.5) | 0.0001 |
| Contact information illegible | 4 (<1) | 3 (0.9) | 1 (1.5) | 3 (1.5) | 1 (0.5) | ||
| Contact information Missing | 30 (7.2) | 27 (7.7) | 3 (4.6) | 25 (13) | 5 (2) | ||
| Location listed to general to make tracing possible | 36 (8.6) | 33 (9.4) | 3 (4.6) | 20 (10) | 16 (7) | ||
| Other | 19 (4.6) | 18 (5.1) | 1 (1.5) | 11 (6)* | 7 (3) | ||
| Not lost to follow up | 326 (78.2) | 269 (76.4) | 57 (87.7) | 135 (69.0) | 190 (86.0) | ||
| Dead | 50 (15.3) | 42 (15.6) | 8 (14.0) | 0.0001 | 14 (10) | 36 (19) | <0.001 |
| HIV negative | 16 (4.9) | 0 | 16 (28.1) | 16 (12) | 0 (0) | ||
| Has a recent visit at original AMPATH clinic | 47 (14.4) | 42 (15.6) | 5 (8.8) | 18 (13) | 28 (15) | ||
| Transferred to another AMPATH clinic | 45 (14) | 39 (14.5) | 6 (10.5) | 19 (14) | 26 (14) | ||
| Transferred out of AMPATH | 168 (51.5) | 146 (52.3) | 22 (38.6) | 68 (50) | 100 (53) | ||
| Total | 417 (100) | 352 (100) | 65 (100) | 195 (100) | 220 (100) | ||
n=1 missing
Outcomes of Tracing
Outcomes of patients whose status was successfully determined through contact with patients themselves or through informants are presented in Table 3 (n=1,800). A total of 375/1,800 (20.8%) patients had died. A higher proportion of adults (23.6% vs. 4.6%) and patients on ART (26.0% vs. 17.0%) had died compared to children and pre-ART patients respectively (p<0.001). Among those found alive, 458/1,425 (32.1%) had moved away, while 443/1,425 (31.1%) reported that they had received care in the last 3 months. Of these, n=91/443 (20.5%) were in care at their original clinic, n=45/443 (10.2%) had self-transferred to another clinic and n=307/443 (69.3%) were in care at an unspecified location. The remaining patients had disengaged from care (n=524/1,800, 29.1% of those successfully traced). A higher proportion of children (119/260, 45.8%) and ART patients (347/1,059, 33%) had disengaged from care compared to adults (405/1,540, 26.3%) and pre-ART patients (177/741, 24%) (p<0.001).
Table 3. Outcomes of patients who were successfully traced (n=1,800).
| Patient type n (%) | ART Status n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| All | Adults | Children | p-value | No | Yes | p-value | ||
|
Still in Care n=443
(24.6%) Other n=1357 (75.4%) |
Getting care in the original clinic | 91 (20.5.) | 79 (5.1) | 12 (4.6) | <0.001 | 46 (4.0) | 45 (6.0) | <0.001 |
| Other/not specified | 307 (69.3) | 250 (16.2) | 57 (21.9) | 174 (16.0) | 133 (18.0) | |||
| Self transferred (still in care) | 45 (10.2) | 40 (2.6) | 5 (1.9) | 24 (2) | 21 (3) | |||
| Moved away | 458 (25.4) | 403 (26.2) | 55 (21.2) | 286 (27) | 172 (23) | |||
| Dead | 375 (20.8) | 363 (23.6) | 12 (4.6) | 182 (17) | 193 (26) | |||
| Disengaged (See Table 4) | 524 (29.1) | 405 (26.3) | 119 (45.8) | 347 (33) | 177 (24) | |||
| Total | 1800 (100) | 1540 (100) | 260 (100) | 1059 (100) | 741 (100) | |||
Reasons for Disengaging from Care
Table 4 outlines the reasons for disengaging from care. Access to Care (reported by n=219 patients); Clinic Factors (n=132 patients); Work and Family (n=247 patients); Medical (n=209 patients) and those related to Alternative Treatment and Advice (n=37 patients). The most commonly reported reasons why patients disengaged from care were: felt well so didn't need care (n=140 patients), transport was too difficult or expensive (n=84 patients), work or need for money interfered with picking up medicine (n=64 patients).
Table 4. Reasons for disengaging from care (n=524 patients provided reasons)*.
| Patient Type n (%) | ART n (%) | ||||
|---|---|---|---|---|---|
| Category | Total | Adult | Children | Off | On |
| Access To Care | n=219 | n=179 | n=40 | n=150 | n=69 |
| Transport too difficult or expensive | 84 (38.4) | 61 (34.1) | 23 (57.5) | 53 (35.3) | 31 (44.9) |
| Transport too difficult or expensive or no money to access care | 54 (24.7) | 46 (25.7) | 8 (20.0) | 42 (28.0) | 12 (17.4) |
| Spent too much time in clinic | 27 (12.3) | 26 (14.5) | 1 (2.5) | 19 (12.7) | 8 (11.6) |
| Clinic Factor | n=132 | n=117 | n=15 | n=86 | n=46 |
| Afraid of scolding | 43 (32.6) | 32 (27.4) | 11 (73.3) | 23 (26.7) | 20 (43.4) |
| Attending clinic risked disclosure to community | 37 (28.0) | 35 (29.9) | 2 (13.3) | 28 (32.6) | 9 (19.6) |
| Staff was not nice | 12 (9.1) | 12 (10.3) | 0 (0) | 7 (8.1) | 5 (10.9) |
| Work & Family | n=247 | n=206 | n=41 | n=161 | n=86 |
| Work or need for money interfered with picking up medicine | 64 (25.9) | 56 (27.2) | 8 (19.5) | 41 (25.5) | 23 (26.7) |
| Care for family | 42 (17.0) | 31 (15.1) | 11 (26.8) | 26 (16.2) | 16 (18.6) |
| Family conflict | 31 (12.6) | 21 (10.2) | 10 (24.4) | 24 (14.9) | 7 (8.1) |
| Attending clinic risked disclosure to family that I had HIV | 31 (12.6) | 27 (13.1) | 4 (9.8) | 19 (11.8) | 12 (14.0) |
| Work or need for money interfered with picking up medicine or Care for family | 18 (7.3) | 15 (7.3) | 3 (7.3) | 9 (5.6) | 9 (10.5) |
| Care for family and family conflict | 14 (5.7) | 13 (6.3) | 1 (2.4) | 9 (5.6) | 5 (5.8) |
| Medical | n=206 | n=159 | n=47 | n=133 | n=73 |
| Felt well so did not need care | 140 (68.0) | 103 (64.8) | 37 (78.7) | 114 (85.7) | 26 (35.6) |
| Alternative Treatment and Advice | n=37 | n=31 | n=6 | n=20 | n=17 |
| Went to someone who tried/is trying to cure me by prayer/religious rituals | 15 (40.5) | 15 (48.4) | 0 (0) | 6 (30.0) | 9 (52.9) |
| Family person or important person asked me to stop from going to clinic | 611 (29.7) | 8 (25.8) | 3 (50.0) | 6 (30.0) | 5 (29.4) |
percentages calculated separately for each category. Only most common reasons for each category are presented.
Differences between adults and children and between ART and pre-ART patients are presented in Table 4. Among those who reported Access to Care challenges, a higher proportion of children (57.5%) and individuals on ART (44.9%) reported that transport was too difficult or expensive compared to adults (34.1%) and individuals not on ART (35.3%), respectively. For those reporting Clinic Factors, a higher proportion of children and individuals on ART reported that they were afraid of scolding compared to adults (73.3% vs. 27.4%) and individuals not on ART (43.4% vs. 26.7%). A higher proportion of adults reported staff not being nice as a reason for disengaging from care compared to children (10.3% vs. 0%). A higher proportion of individuals not on ART reported that they felt so well they did not need care compared to ART patients (85.7% vs. 35.6%). Of those citing wanting to access Alternative Treatment and Advice, a higher proportion of adults (48.4%) and ART patients (52.9%) reported that they went to someone trying to cure HIV by prayer/religious rituals compared to children (0%) and individuals not on ART (30%), respectively.
Discussion
In the present study, we successfully identified outcomes for 71% of sampled patients initially identified as being LTFU and 85% of those physically traced. These findings suggest that a large scale sampling-based outreach program can be both feasible and effective in locating patients suspected of being LTFU and determining their status. Of those with known outcomes, 21% had died while another 25% of patients were not actually LTFU and still were receiving care within AMPATH or elsewhere. Related to this is the high proportion of individuals who could not be traced suggesting accurate and up-to-date information on patient status is needed at each follow-up. Finally, over a quarter of patients who were found had chosen to disengage from care for various reasons. Compared to adults, a higher proportion of children were found not to be LTFU, and of those successfully traced, a higher proportion were ascertained as being still in care at an AMPATH clinic.
Our findings reinforce the challenges associated with obtaining and maintaining up-to-date information on the locations and status of patients who miss their scheduled visits. A recent Malawian study noted that poor documentation was amongst the top reasons for explaining why patients may become LTFU (49). The quality of collected data varies widely across large longitudinal cohorts (50, 51) with data management being particularly challenging in many resource-poor settings (50, 52-55). This can be due to poor infrastructure, a shortage of trained personnel, an unbalanced provider-patient ratio (54, 56) and a lack of investment by implementers and funders in electronic health records systems. It is worth noting that HIV programs with electronic monitoring capabilities (50, 51), such as AMPATH's AMRS, have demonstrated better-quality data. Regardless, the findings of this study indicate that 25% of sampled patients were not even LTFU (i.e., were still in care). While there were patients who truly did miss their visits, poor documentation led some patients to be incorrectly labeled as LTFU. A significant proportion of patients in this study originally considered LTFU were later confirmed to have transferred out, either to another AMPATH clinic or out of the program entirely (Table 3). Similar findings have been found elsewhere (56). Therefore, missing patients may have transferred to another clinic and thus are only LTFU from the perspective of their original clinic (50). Similarly, patients may be misclassified as LTFU (56) when patient files were lost or the visit itself was not recorded. Findings from the chart review suggest that 47 patients had actually had a recent visit (Table 2) and another 25% of patients found through tracking reported that were still in care (Table 3). A higher proportion of pre-ART patients had missing contact information making tracing difficult compared to ART patients. This may be partially explained by the frequency of visits with the latter group being expected to come to the clinic more frequently (and thus have more opportunities to collect information) than those not yet on ART. Identifying where (e.g., which clinics) and when (e.g., data entry, filing) errors occur is needed. An accurate record of the date of the next expected visit is needed in order to calculate the discrepancy between the expected return date and an actual return date. This can help program planners and clinicians to identify situations where patients may require additional support and management.
Patients who miss scheduled visits need to be followed-up in order to ascertain their status (e.g., alive, in care, died etc) and to adjust program LTFU and mortality estimates (57, 58). In the present study, death was a primary outcome of patients LTFU suggesting that death reporting clearly needs improvement. In Kenya, while mortality is reported using routine health facility reports, these do not include deaths that occur at home and as a result, mortality estimates are likely underestimated in this context (59). A 2013 review of ART programs in low- and middle-income settings reported that programs that incorporated physical tracing had lower estimates of LTFU and higher estimates of mortality (33). Since risk factors for losses to follow-up and mortality can be similar (e.g., lower CD4 count at enrollment), estimates that do not account for patients considered lost but who have actually died can severely underestimate the true extent of mortality and conversely overestimate the positive impact of a care and treatment program (36). As successful outreach can lead to increased “re-engagement with care” (33), accurate information on patient locations is needed to ensure patients can actually be found. In the present study, 85% of the individuals confirmed to be LTFU (e.g., based on chart reviews) could be physically traced. Importantly, one of the strengths of the AMPATH outreach program is the level of detail captured on patient physical locations at the time of enrollment. Phone numbers are also verified at each visit. Importantly a working phone number is one of the strongest predictors of successfully finding patients (34, 60). Since 2009, AMPATH has captured geographic coordinates using GPS during home-based testing and counseling (HBCT) (61). This can further assist outreach workers in locating patients in the future. Home visits, however, can lead to involuntary disclosure of one's HIV status (62) emphasizing the need to consider not only who physically traces patients but also how outreach efforts are implemented in general.
Over a quarter of sampled patients disengaged from care on their own. Challenges accessing care were amongst the most frequently reported reasons why patients missed their visits. This may be particularly challenging for parents/guardians who need to travel with their child/children, adding to transport costs. Financial constraints (12-14) and transport-related costs (11, 13, 15-18) have been shown to be important for losses to follow-up particularly when individuals have to choose between using their limited income on transport, or food to feed themselves and their families (19). Negotiating transport continues to present challenges and while arranging for transport (on behalf of the patient) and/or reimbursing patients for travel costs (20, 54) may not be a feasible long-term solution, other strategies need to be investigated. This can include less frequent visits for patients deemed stable (20) or arranging for one individual to pick-up medication for a larger group. Regardless, ethical considerations are needed with respect to the provision of incentives in settings with widespread poverty. Stigma and fear of disclosure (11, 20-22) was reported as a reason for disengagement with fear of scolding and mistreatment by healthcare staff and family influences being particularly important. Poor patient-provider relationships (11, 14, 49) are an important reason why individuals choose to disengage from care. Frustrations with the healthcare they receive and the use of exposing language (20, 49) that essentially ‘outs’ their positive HIV status to others in the clinic have been previously reasons for become LTFU. Family influences can also discourage patients from staying in care (12-14, 16, 21, 22, 49); here, almost 30% of interviewed patients reporting issues related to Alternative Treatment and Advice specifically indicated that ‘family or close friends asked them to stop going to the clinic’. Importantly, healthcare delivery models that acknowledge patient fears are critical as these can undermine relationships which are essential for survival (23, 24).
The most commonly reported reason why patients in this study disengaged from care were that they felt well. Not surprisingly, a higher proportion of pre-ART patients reported that they felt well enough to not require care. Previous studies have demonstrated that ART patients may become lost for similar reasons, particularly if they have been on ART for longer periods of time. ART can not only provide individuals with a renewed sense of life (12, 25), feeling better and experiencing an improvement in health can lead to an increased risk of stopping ART and disengaging from care (11, 20, 49). This can lead to a resurgence in viral load, and increase the risk of opportunistic infections and/or death (11, 26-30). Indeed, the health-seeking behaviors of many African populations, including Kenyans, suggest that individuals may only seek care when symptomatic and/or when health becomes a top priority over other life challenges (63-65). In addition to continued patient education on treatment literacy (e.g., understanding the need to take medication as prescribed) (66, 67), creative strategies involving community-based approaches can also work to engage asymptomatic HIV-positive individuals with care.
This study has numerous strengths including the large study sample and the high proportion of patients who could be physically traced pointing to the feasibility of large-scale outreach programs. Furthermore, by including both adults and children as well individuals on and off ART, we were able to generate a snapshot of outcomes in our setting. The broad inclusion criteria also increase the generalizability of our findings to other settings and programs. However, there were several limitations in the present study. The reasons why patients disengaged may be subject to social desirability responding particularly if patients fear disclosing their frustrations around the care they receive. Patients who are LTFU are, by definition, a hard group to follow-up. Patients successfully found in this study may be different from those who were not traceable and therefore the reasons why patients disengage from care may not be generalizable to the latter group. However, by including both children and adults on and off treatment in the present study, we were able to generate a population-wide snapshot identifying outcomes of patients LTFU in our setting. Individuals not successfully found through outreach efforts may have died, therefore, in order to correct for mortality and LTFU estimates, there is a clear need to distinguish patients who have died from individuals that have become LTFU for other reasons. Other reasons for why patients may disengage from care on their own accord may not be captured in this study. For example, reasons specific to ART such as experiencing side effects are not captured nor explored in the present study.
In the present study, we were able to successfully identify outcomes for a high proportion of individuals reported to be LTFU. Importantly, we found that a proportion of patients initially identified as LTFU were not actually LTFU indicating the importance of maintaining up-to-date information on patient status as well as the need for accurate details on visit history and patient locations, in order to assist with timely tracing. Future research should work to identify where and when errors occur and how to improve coordination between clinics when there are transfers involved. Related to this is the need to capture details on deaths as they occur, at the national level, in order to correct mortality and LTFU estimates. The findings of the present study have implications for the development and implementation of healthcare delivery and outreach program models that acknowledge patient realities and needs.
Acknowledgments
This study was made possible through joint support of the United States Agency for International Development (USAID).The contents are the sole responsibility of AMPATH and do not necessarily reflect the views of USAID or the United States Government.
Sources of funding: Funded in part through a supplement to the National Institutes of Allergy and Infectious Diseases (NIAID) award 2U01AI069911-06 and NIH award P30 AI027763 and a supplement to the East Africa International epidemiologic Databases to Evaluate AIDS (IeDEA) Consortium (NIH Award U01 AI069911). Funded in part by the Office of the Global AIDS Coordinator through the NIAID award 2U01AI069911-06.
Footnotes
Meetings at which part of data were presented: The XIX International AIDS Conference, Washington DC, USA July 2012
Conflicts of Interests: None
References
- 1.Tanser F, Barnighausen T, Grapsa E, et al. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–71. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mamlin J, Kimaiyo S, Lewis S, et al. Integrating nutrition support for food-insecure patients and their dependents into an HIV care and treatment program in Western Kenya. Am J Public Health. 2009;99:215–21. doi: 10.2105/AJPH.2008.137174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkatesh KK, Flanigan TP, Mayer KH. Is expanded HIV treatment preventing new infections? Impact of antiretroviral therapy on sexual risk behaviors in the developing world. AIDS. 2011;25:1939–49. doi: 10.1097/QAD.0b013e32834b4ced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams B, Wood R, Dukay V, et al. Treatment as prevention: preparing the way. J Int AIDS Soc. 2011;14(Suppl 1):S6. doi: 10.1186/1758-2652-14-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen M. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. New Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geng EH, Nash D, Kambugu A, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/ADS Rep. 2010;7:234–244. doi: 10.1007/s11904-010-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eholie SP, Eba Aussi F, Songda Ouattara I, et al. HIV treatment and care in resource-constrained environments: challenges for the next decade. J Int AIDS Soc. 2012;15:17334. doi: 10.7448/IAS.15.2.17334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15:1–16. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: a systematic review and meta-analysis. PLoS One. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGuire M, Munyenembe T, Szumilin E, et al. Vital status of pre-ART and ART patients defaulting from care in rural Malawi. Trop Med Int Health. 2010;15(Suppl 1):55–62. doi: 10.1111/j.1365-3156.2010.02504.x. [DOI] [PubMed] [Google Scholar]
- 12.Dalal RP, MacPhail C, Mghayi M, et al. Characteristics and outcomes of adult patients lost to follow-up at antiretroviral treatment clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;47:101–7. doi: 10.1097/QAI.0b013e31815b833a. [DOI] [PubMed] [Google Scholar]
- 13.Deribe K, Hailekiros F, Biadgilgn S, et al. Defaulters from antiretroviral treatment in Jimma University Specialized Hospital, southwest Ethiopia. Trop Med Int Health. 2008;13:328–33. doi: 10.1111/j.1365-3156.2008.02006.x. [DOI] [PubMed] [Google Scholar]
- 14.Dahab M, Charalambous S, Hamilton R, et al. That is why I stopped the ART': patients' and providers' perspectives on barriers to and enablers of HIV treatment adherence in a South African workplace program. BMC Public Health. 2008;8:63. doi: 10.1186/1471-2458-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palombi L, Marazzi MC, Guidotti G, et al. Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral-treated patients in sub-Saharan African sites with comprehensive monitoring availability. Clin Infect Dis. 2009;48:115–22. doi: 10.1086/593312. 2009. [DOI] [PubMed] [Google Scholar]
- 16.Brinkhof MW, Dabis F, Myer I, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Org. 2008;86:559–67. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng EH, Glidden DV, Emenyonu N, et al. Tracking a sample of patients lost to follow-up has a major impact on understanding determinants of survival in HIV-infected patients on antiretroviral therapy in Africa. Trop Med Int Health. 2010;15(Suppl 1):63–9. doi: 10.1111/j.1365-3156.2010.02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu JKL, Chen SCC, Wang KY, et al. True outcomes for patients on antiretroviral therapy are ‘lost to follow up’ in Malawi. Bull World Health Org. 2007;85:550–54. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuller DM, Bangsberg DR, Senkungu J, et al. Transportation costs impede sustained adherence and access to HAART in a clinic population in Southwestern Uganda: a qualitative study. AIDS Behav. 2010;14:778–784. doi: 10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller CM, Ketlhapile M, Rybasack-Smith, et al. Why are antiretroviral treatment patients lost to follow-up? A qualitative study from South Africa. Trop Med Int Health. 2010;15:48–54. doi: 10.1111/j.1365-3156.2010.02514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell C, Skovdal M, Madanhire C, et al. “We, the AIDS people…”: How antiretroviral therapy enables Zimbabweans living with AIDS to cope with stigma. Am J Public Health. 2011;101:1004–1010. doi: 10.2105/AJPH.2010.202838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray LK, Semaru K, McCurley E, et al. Barriers to acceptance and adherence of antiretroviral therapy in urban Zambian women: a qualitative study. AIDS Care. 2009;21:78–86. doi: 10.1080/09540120802032643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ncama BP, McInerney PA, Bhengu BR, et al. Social support and medication adherence in HIV disease in KwaZulu-Natal, South Africa. Int J Nurs Studies. 2008;45:1757–1763. doi: 10.1016/j.ijnurstu.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Ware NC, Idoko J, Kaaya S, et al. Explaining adherence successes in sub-Saharan Africa: an ethnographic study. PLoS Med. 2009;6:e1000011. doi: 10.1371/journal.pmed.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alamo ST, Colebunders R, Ouma J, et al. Return to normal life after AIDS as a reason for lost to follow-up in a community-based antiretroviral treatment program. J Acquir Immune Defic Syndr. 2012;60:e36–345. doi: 10.1097/FTD.0b013e3182526e6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bisson GP, Stringer JSA. Lost but not forgotten-the economics of improving patient retention treatment programs. PLoS Med. 2009;6:e1000174. doi: 10.1371/journal.pmed.1000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skiest DJ, Su Z, Havlir D, et al. Interruption of antiretroviral treatment in HIV infected patients with preserved immune function is associated with a low rate of clinical progression: a prospective study by AIDS Clinical Trials Group 5170. J Infect Dis. 2007;195:1426–36. doi: 10.1086/512681. [DOI] [PubMed] [Google Scholar]
- 28.Chalker J, Andualem T, Minzi A, et al. Monitoring adherence and defaulting for antiretroviral therapy in 5 east African countries: an urgent need for standards. J Int Ass Physicians AIDS Care. 2008;7:193–99. doi: 10.1177/1545109708320687. [DOI] [PubMed] [Google Scholar]
- 29.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. El-Sadr WM, Lundgren JD, et al. CD4+ count-guided interruptions of antiretroviral treatment. New Engl J Med. 2006;355:397–405. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 30.Bangsberg DR, Charlebois ED, Grant RM, et al. High levels of adherence do not prevent accumulation of HIV drug resistance mutations. AIDS. 2003;17:1925–32. doi: 10.1097/00002030-200309050-00011. [DOI] [PubMed] [Google Scholar]
- 31.Harries AD, Zachariah R, Lawn SD, et al. Strategies to improve retention on antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health. 2010;15:70–75. doi: 10.1111/j.1365-3156.2010.02506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tweya H, Gareta D, Chagwera F, et al. Early active follow-up of patients on antiretroviral therapy (ART) who are lost to follow-up: the ‘Back-to-Care’ project in Lilongwe Malawi. Trop Med Int Health. 2010;15(Suppl 1):82–9. doi: 10.1111/j.1365-3156.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 33.McMahon JH, Elliot JH, Hong SY, et al. Effects of physical tracing on estimates of loss to follow-up, mortality and retention in low and middle income country antiretroviral therapy programs: a systematic review. PLoS One. 2013;8:e56047. doi: 10.1371/journal.pone.0056047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weigel R, Hochgesang M, Brinkhof MW, et al. Outcomes and associated risk factors of patients traced after being lost to follow-up from antiretroviral treatment in Lilongwe, Malawi. BMC Infect Dis. 2011;11:31. doi: 10.1186/1471-2334-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global Report: UNAIDS report on the global AIDS epidemic 2012. Geneva, Switzerland: UNAIDS; 2012. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_with_annexes_en.pdf. [Google Scholar]
- 36.Yiannoustos CT, An MW, Frangakis CE, et al. Sampling-based approaches to improve estimation of mortality among patient drop-outs: experience from a large PEPFAR-funded program in Western Kenya. PLoS One. 2008;3:e38–43. doi: 10.1371/journal.pone.0003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geng EH, Emenyonu N, Bwana MB, et al. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA. 2008;300:506–7. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braitstein P, Songok J, Vreeman RC, et al. “Wamepotea” (they have become lost): outcomes of HIV-positive and HIV-exposed children lost to follow-up from a large HIV treatment program in western Kenya. J Acquir Immune Defic Syndr. 2011;57:e40–6. doi: 10.1097/QAI.0b013e3182167f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yiannoutsos CT, Ming-Wen A, Frangakis CE, et al. Patient outreach and statistical modeling in improving patient care, monitoring and evaluation in HIV treatment programs: Experience of a large PEPFAR-funded program in western Kenya. PLoS Med. 2008;3:e.3843. doi: 10.1371/journal.pone.0003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimanga DO, Ogola S, Umuro M, et al. Prevalence and incidence of HIV infection, trends, and risk factors among persons aged 15-64 year in Kenya: results from a nationally representative study. J Acquir Immune Defic Syndr. 2014;66(Suppl 1):S13–26. doi: 10.1097/QAI.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National AIDS and STI Control Programme. Ministry of Health. Kenya AIDS Indicator Survey, 2012. Preliminary Report. 2013 Sep; Available at: http://nascop.or.ke/library/3d/Preliminary%20Report%20for%20Kenya%20AIDS%20indicator%20survey%202012.pdf.
- 42.Odhiambo J, Kellogg TA, Kim AA. Antiretroviral treatment scale-up among persons living with HIV in Kenya: results from a nationally representative survey. J Acquir Immune Defic Syndr. 2014;66(Suppl 1):S116–122. doi: 10.1097/QAI.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wafula R, Masyuko S, Ng'ang'a L, Kim AA, Gichangi A, Mukui I, Batuka J, Ngugi E, Maina WK, Schwarcz S for the KAIS Study Group. Engagement in HIV care among Kenyan adults and adolescents: results from a national population-based survey. J Acquir Immune Defic Syndr. 2014;66(Suppl 1):S98–105. doi: 10.1097/QAI.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Einterz RM, Kimaiyo S, Mengech HN, et al. Responding to the HIV pandemic: the power of an academic medical partnership. Acad Med. 2007;82:812–8. doi: 10.1097/ACM.0b013e3180cc29f1. [DOI] [PubMed] [Google Scholar]
- 45.Mamlin JJ, Kimaiyo S, Nyandiko W, et al. W H Organization, Editor. World Health Organization; Geneva: 2004. Academic institutions linking access to treatment and prevention, in Perspectives and Practice in Antiretroviral Treatment. [Google Scholar]
- 46.Tierney WM, Rotich JK, Hannan TJ, et al. The AMPATH medical record system: creating, implementing, and sustaining an electronic medical record system to support HIV/AIDS care in western Kenya. Medinfo. 2007;12(Pt 1):372–6. [PubMed] [Google Scholar]
- 47.Mamlin BW, Biondich PG. AMPATH Medical Record System (AMRS): collaborating toward an EMR for developing countries. AMIA Annu Symp Proc. 2005:490–4. [PMC free article] [PubMed] [Google Scholar]
- 48.Ochieng-Ooko V, Ochieng D, Sidle JE, et al. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bull World Health Org. 2010;88:681–8. doi: 10.2471/BLT.09.064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rachlis B, Ahmad F, van Lettow M, et al. Using concept mapping to explore why patients become lost to follow-up from an antiretrovral therapy program in the Zomba Distrct of Malawi. BMC Health Serv Res. 2013;13:210. doi: 10.1186/1472-6963-13-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mate KS, Bennett B, Mphatswe W, et al. Challenges for Routine Health System Data Management in a Large Public Programme to Prevent Mother-to-Child HIV Transmission in South Africa. PLoS One. 2009;4:e583. doi: 10.1371/journal.pone.0005483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw V. Health information system reform in South Africa: developing an essential dataset. Bull World Health Org. 2005;83:632–36. [PMC free article] [PubMed] [Google Scholar]
- 52.Egger M, Ekouevi DK, Williams C, et al. Cohort profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int Epidemiology. 2011;40:1–9. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garrib A, Sttops N, McKenzie A, et al. Evaluation of the district health information system in rural South Africa. South Afr Med. 2008;98:549–552. [PubMed] [Google Scholar]
- 54.Makombe SD, Hochgesang M, Jahn A, et al. Assessing the quality of data aggregated by antiretroviral treatment clinics in Malawi. Bull World Health Org. 2008;86:310–314. doi: 10.2471/BLT.07.044685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forster M, Bailey C, Brinkhof MW. Electronic medical record systems, data quality, and lost to follow-up: survey of antiretroviral therapy programmes in resource-limited settings. Bull World Health Org. 2008;86:939–47. doi: 10.2471/BLT.07.049908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tweya H, Feldacker C, Estill J, et al. Are they really lost? ‘True” status and reasons for treatment discontinuation among HIV infected patients on antiretroviral therapy considered lost to follow up in urban Malawi. PLoS One. 2013;8:e75761. doi: 10.1371/journal.pone.0075761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Cutsem G, Ford N, Hildebrand K, et al. Correcting for mortality among patients lost to follow up on antiretroviral therapy in South Africa: a cohort analysis. PLoS ONE. 2011;6:e14684. doi: 10.1371/journal.pone.0014684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Egger M, Spycher BD, Sidle J, et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med. 2011;8:e1000390. doi: 10.1371/journal.pmed.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Republic of Kenya Health Sector. Accessing health information system performance: never mind the quality, feel the width. HIS of Kenya. 2008 Jun; Available at: http://www.who.int/healthmetrics/library/countries/HMN_KEN_Assess_Final_2008_06_en.pdf.
- 60.Maskew M, Macphail C, Menezes C, et al. Lost to follow-up: contributing factors and challenges in South African patients on antiretroviral therapy. South Afr J. 2007;97:853–857. [PubMed] [Google Scholar]
- 61.Kimayo S, Were MC, Shen C, et al. Home-based HIV counseling and testing in western Kenya. E African Med J. 2010;87:100–108. doi: 10.4314/eamj.v87i3.62195. [DOI] [PubMed] [Google Scholar]
- 62.Merten S, Kenter E, McKenzie O, et al. Patient-reported barriers and drivers of adherence to antiretrovirals in sub-Saharan Africa: a meta- ethnography. Trop Med Int Health. 2010;15(Suppl 1):16–33. doi: 10.1111/j.1365-3156.2010.02510.x. [DOI] [PubMed] [Google Scholar]
- 63.Schilling K, Person B, Faith SH, et al. The challenge of promoting interventions to prevent disease in impoverished populations in rural western Kenya. Am J Public Health. 2013;103:2131–5. doi: 10.2105/AJPH.2013.301459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Medley A, Ackers M, Amolloh M, et al. Early uptake of HIV clinical care after testing HIV-positive during home-based testing and counseling in western Kenya. AIDS Behav. 2013;17:224–34. doi: 10.1007/s10461-012-0344-5. [DOI] [PubMed] [Google Scholar]
- 65.Negussie T, Chepng'eno D. Determinants of health care seeking for childhood iillnesses in Nairobi slums. Trop Med Int Health. 2005;10:240–245. doi: 10.1111/j.1365-3156.2004.01381.x. [DOI] [PubMed] [Google Scholar]
- 66.Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3:e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rachlis BS, Mills EJ, Cole DCC. Livelihood security and adherence to antiretroviral therapy in low and middle income settings: a systematic review. PLoS One. 2011;6:e18948. doi: 10.1371/journal.pone.0018948. [DOI] [PMC free article] [PubMed] [Google Scholar]


