Abstract
Animal research suggests that anhedonia is a tobacco withdrawal symptom, but this topic has not been addressed definitively in research with humans. This research sought to determine whether anhedonia is: 1) an element of the tobacco withdrawal syndrome in humans and 2) an impediment to successful tobacco cessation. Data were from 1175 smokers (58.3% women; 85.5% white) participating in a randomized, double blind, placebo-controlled trial of smoking cessation pharmacotherapies. Ecological momentary assessments for 5 days before and 10 days after the target quit day were used to assess anhedonia and other established withdrawal symptoms. Consistent with drug withdrawal, anhedonia showed an inverted-U pattern of change in response to tobacco cessation and was associated with the severity of other withdrawal symptoms and tobacco dependence. Postquit anhedonia was associated with decreased latency to relapse (HR=1.09, 95%CI[1.02,1.17]) and with lower 8-week point prevalence abstinence (OR=.91, 95%CI[.86,.97])—relations that remained significant when other withdrawal symptoms were included as predictors. Finally, nicotine replacement therapy nearly fully suppressed the increase in abstinence-related anhedonia (β = −.66, p<.001), suggesting agonist suppression of withdrawal. Results suggest that anhedonia is a unique and motivationally significant element of the tobacco withdrawal syndrome in humans. These results have implications for defining and assessing tobacco use disorder and for understanding and treating tobacco addiction.
Keywords: anhedonia, tobacco withdrawal, tobacco dependence, smoking cessation
Animal research indicates that both nicotine and nicotine deprivation can affect the capacity to experience pleasure. Research clearly shows that nicotine produces pleasure and effectively rewards instrumental responses (e.g., Corrigall, 1999; Harvey et al., 2004). For instance, conditioned place preference (e.g.,Fudala & Iwamoto, 1985; Le Foll & Goldberg, 2005), human laboratory (Gloria et al., 2009; Perkins, Grobe, & Fonte, 1997; Stein et al., 1998), and brain imaging studies (e.g., Gloria et al., 2009) all demonstrate that nicotine directly produces pleasure. Emerging research suggests that nicotine may also affect the reward and incentive value of non-drug stimuli. Caggiula and colleagues have demonstrated that administration of nicotine to rodents increases their rate of responding for non-drug rewards (e.g., Caggiula et al., 2009; Chaudhri et al., 2006; Donny et al., 2003). Moreover, such apparent reward-enhancing effects of nicotine are nonassociative in both animals (Chaudhri et al., 2006) and humans (Perkins & Karelitz, 2013); thus, smoking could presumably enhance the appetitive effects of virtually any non-drug pleasurable event that a smoker encounters in daily life.
There is also evidence that nicotine deprivation can diminish reward value, leading to anhedonia—the reduced experience of pleasure in response to reward. Animal research provides evidence that nicotine deprivation following prolonged exposure to nicotine results in decreased instrumental responding for rewarding electrical brain stimulation, a putative measure of anhedonia (e.g., Epping-Jordan, Watkins, Koob, & Markou, 1998; Hilario, Turner, & Blendy, 2012; Johnson, Hollander, & Kenny, 2008). Further, animal research suggests that the time course of anhedonia following nicotine deprivation is consistent with nicotine withdrawal (Hilario et al., 2012; Johnson et al., 2008), and it is accompanied by an increase in the reward value of acute nicotine administration (Hilario et al., 2012), consistent with nicotine withdrawal in humans (e.g., Gloria et al., 2009).
Laboratory research with humans also suggests that nicotine deprivation results in anhedonia; e.g., deprived smokers show diminished (1) responding (al-Adawi & Powell, 1997; Dawkins, Powell, West, Powell, & Pickering, 2006; Powell, Dawkins, & Davis, 2002; Powell, Pickering, Dawkins, West, & Powell, 2004); and (2) reports of pleasure (Dawkins, Acaster, & Powell, 2007; Dawkins & Powell, 2011; although cf. Snuggs & Hajek, 2013) to nondrug rewards. Nicotine-deprived smokers also expect to derive less enjoyment from hypothetical pleasurable situations than do satiated smokers (Powell et al., 2002; Powell et al., 2004). Thus, considerable laboratory research is consistent with the hypothesis that anhedonia is an element of nicotine withdrawal in humans (e.g., Dawkins et al., 2007).
Finding that anhedonia is a symptom of the tobacco withdrawal syndrome could have both theoretical and clinical importance. Anhedonia could inform the assessment of tobacco dependence, serve as a target of treatment development, and explain some of the motivational force of dependence. Deprivation-induced anhedonia could affect tobacco motivation via multiple routes. It could blunt pleasurable response to environmental rewards, which could both impact affect and reduce engagement in pleasurable activities. Such a narrowing of environmental rewards could render tobacco deprivation more aversive, and nicotine’s appetitive effects more salient by contrast. In addition, to the extent that tobacco use reverses abstinence-induced anhedonia, dependent tobacco users might resume smoking to restore the rewarding value of nonpharmacologic rewards (Dawkins et al., 2007; Perkins & Karelitz, 2013).
An increase in anhedonia following nicotine deprivation could reflect either: (1) an offset effect whereby appetitive capacity dissipates due to the loss of a direct, agonist effect of nicotine (Hughes, 2007c), or (2) a withdrawal effect whereby relatively tonic neuroadaptations caused by chronic nicotine exposure lead to the expression of appetitive deficits upon nicotine removal. In the case of an offset effect, anhedonia would remain elevated and stable throughout the cessation attempt and thereafter, provided the individual does not return to smoking. In contrast, a withdrawal effect would produce an initial increase in anhedonia following nicotine deprivation, but the anhedonia would diminish over time—its biphasic (inverted-U shaped) nature presumably reflecting homeostatic adjustments (e.g., Koob, Markou, Weiss, & Schulteis, 1993; Siegel, 1983; Solomon & Corbit, 1973).
While prior research has been of great value, additional research is needed to determine whether anhedonia displays the features of a nicotine withdrawal symptom in humans. For instance, almost no research has defined anhedonia based upon diminished response to real-world pleasurable events that occur in smokers’ daily lives (although cf. Snuggs & Hajek, 2013). Moreover, it remains unclear whether anhedonia following tobacco deprivation in humans meets other criteria emblematic of the tobacco withdrawal syndrome such as: (1) exhibiting a curvilinear trajectory consistent with a withdrawal phenomenon (e.g., peaks shortly after drug deprivation followed by gradual decline with continued deprivation; Hughes, 2007c); and (2) reduction by agonist administration (Benowitz, 2010; Siegel, 1983).
The present study sought to determine whether anhedonia is a component of the tobacco withdrawal syndrome in humans. Smokers in a cessation trial (N=1504) reported daily pleasure derived from putative rewards in the natural environment for 5 days before and 10 days after the target quit day. Evaluation of whether anhedonia is a withdrawal symptom was based primarily on whether: (1) anhedonia showed a time-course consistent with withdrawal (i.e., peaking shortly after drug deprivation, followed by return to prequit levels; Hughes, 2007b; Piasecki, Jorenby, Smith, Fiore, & Baker, 2003a, 2003b; Welsch et al., 1999) versus a unidirectional change associated with an offset effect (Hughes, 2007c); and (2) anhedonia was reduced by agonist administration (Benowitz, 2010; Kenny & Markou, 2006; Koob et al., 1993; Malin et al., 1996; Solomon & Corbit, 1973). We also assessed whether anhedonia conformed with several other features of a withdrawal symptom. First, we examined anhedonia’s correlations with other, established withdrawal symptoms given that withdrawal symptoms tend to be meaningfully correlated with one another (Robinson et al., 2011; Zinser, Baker, Sherman, & Cannon, 1992). Second, we examined anhedonia’s relations with tobacco dependence (American Psychiatric Association, 2013; Baker et al., 2012) since withdrawal symptoms tend to be correlated with dependence measures, albeit, often modestly (Baker et al., 2012; Killen, Fortmann, Telch, & Newman, 1988). Finally, we sought to determine if anhedonia, like some other withdrawal symptoms (e.g., negative affect: Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Piasecki et al., 2003b), has motivational properties that could serve as a barrier to tobacco cessation. Specifically, we determined whether anhedonia contributes predictive information regarding cessation outcomes beyond that provided by craving and negative affect, the two withdrawal symptoms most robustly associated with tobacco dependence and abstinence (e.g., Baker et al., 2012; Hendricks, Ditre, Drobes, & Brandon, 2006; Leventhal, Waters, Moolchan, Heishman, & Pickworth, 2010). Therefore, the two primary aims of this research were to determine whether anhedonia meets criteria for a tobacco withdrawal symptom, and to determine whether it provides important information relevant to tobacco motivation (i.e., indexes the likelihood of cessation failure following a quit attempt).
Method
Participants
This study is a secondary analysis of a smoking cessation clinical trial (see Piper et al., 2009). A total of 1504 smokers from South Central Wisconsin participated in the trial. The 1175 participants with complete anhedonia data constituted the final sample used in this analysis. All participants smoked at least 10 cigarettes per day for the past 6 months and were motivated to quit smoking. Exclusion criteria included a contraindication for study medication; a history of psychosis, bipolar disorder, or an eating disorder; or a consumption pattern of six or more alcoholic beverages at least 6 days a week. This study was approved by the University of Wisconsin Health Sciences Institutional Review Board.
Procedure
Participants were recruited through media advertisements and earned media such as TV interviews and press releases. Study candidates who passed an initial phone screen were invited to an Information Session where they provided written informed consent. Participants then attended three baseline assessments during which they underwent multiple screenings, including a medical history screening and a carbon monoxide breath test. Participants also completed demographic, smoking history, and tobacco dependence questionnaires. Participants were then randomized to treatment conditions.
Treatment
Eligible participants were randomized, blocked on gender and ethnicity, to one of six treatment conditions: (1) bupropion SR (9 weeks, starting 1 week prior to the target quit day); (2) nicotine lozenge (12 weeks starting on the target quit day); (3) nicotine patch (8 weeks starting on the quit day); (4) nicotine patch + nicotine lozenge; (5) bupropion SR + nicotine lozenge; or (6) placebo. There were 5 placebo conditions matched to each of the active treatment conditions, such that each constituted one fifth of the placebo control group. All participants received 6 counseling sessions.
Measures
Smoking Status
Daily smoking data were collected with a smoking calendar using the timeline follow-back method (Sobell, Brown, Leo, & Sobell, 1996). Seven-day point-prevalence abstinence was assessed 8-weeks after the target quit day and biochemically confirmed by a carbon monoxide rating of < 10 parts per million.
Cessation Milestone Variables
Three smoking cessation milestone variables were created using the smoking calendar data. The initial abstinence variable indicates whether participants smoked zero cigarettes on at least 1 day in the first 14 days following the target quit day. The lapse variable—computed for those who achieved initial abstinence—indicates the number of days between the first day participants smoked zero cigarettes and the first day they smoked a cigarette. The relapse variable—computed for participants who lapsed—indicates the number of days from the lapse day until the relapse day (the first of 7 consecutive days of smoking). Individuals were censored at the time of their last contact if they did not report an event (i.e, lapse, relapse; Japuntich, Piper, Leventhal, Bolt, & Baker, 2011).
Dependence
Participants completed the 6-item Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), including time-to-first-cigarette, which has been shown to relate strongly to smoking heaviness, withdrawal, and cessation failure (Baker et al., 2007; Bolt et al., 2009; Heatherton, Kozlowski, Frecker, Rickert, & Robinson, 1989). Participants also completed the Wisconsin Inventory of Smoking Dependence Motives (WISDM; Piper et al., 2004), which includes 13 theoretically derived motivational domains thought to reflect elements of tobacco dependence. Variance in overall WISDM score reflects the influence of the measure’s two major factors—the Primary Dependence Motives (PDM) and Secondary Dependence Motives (SDM). The PDM constitutes the core of tobacco dependence (smoking is heavy, automatic, out of control, and related to significant craving) while the SDM assesses instrumental reasons for using tobacco (e.g., smoking to regulate mood or hunger; Piasecki, Piper, Baker, & Hunt-Carter, 2011). We statistically controlled effects of the SDM when examining PDM associations (and vice versa; see: Piasecki, Piper, & Baker, 2010a, 2010b).
Mood Disorder History
Participants were assessed for whether they met diagnostic criteria for a mood disorder in the past 12 months or ever in their life via the Composite International Diagnostic Interview (CIDI; Wittchen, 1994), a structured clinical interview administered by study personnel using Computer Assisted Personal Interviews (CAPI), Version 20.
Ecological Momentary Assessments (EMA)
Personal digital assistants (PDAs) prompted participants to answer questions four times a day (after waking, before bed, and at two other random times during the day) for up to 2 weeks prior to and 2 weeks after their target quit day. This research analyzed data from 5 days before to 10 days after the target quit day.1 As in our previous research (Piper et al., 2011), we included items selected from validated questionnaires such as the Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999) and the Positive Affect Negative Affect Scale (PANAS; Watson, Clark, & Tellegen, 1988). Each EMA prompt asked participants to report on a slider that ranged from “disagree!!” (coded as 0) to “agree!!” (coded as 10) how they felt in the last 15 minutes on the following items: (a) six negative affect items (tense or anxious; impatient; bothered by negative moods such as anger, frustration, or irritability; irritable or easily angered; sad or depressed; and hopeless or discouraged); (b) two craving items—”Bothered by desire to smoke a cigarette” and “Urge to smoke”—the two strongest loading items in a scale derivation factor analysis; (c) two concentration items—”Hard to pay attention” and “Difficult to think clearly”; and (d) two hunger items—”Thinking about food a lot” and “Hungry”. A composite consisting of the mean of the items from each domain (e.g., negative affect, craving) was used in analyses.
During the evening prompt, participants also reported how much pleasure they experienced that day, on a slider that ranged from “no pleasure” (coded as 0) to “extreme pleasure” (coded as 10), from three domains (social, recreation, and performance/accomplishment) that are used in standardized, well validated anhedonia scales (Fawcett, Clark, Scheftner, & Gibbons, 1983; Snaith et al., 1995). For example, for the recreation domain, participants were asked, “Think about the most pleasant thing you did for fun today. How much pleasure did you get from this?” Because scores in the three pleasure domains were highly correlated (prequit r’s= .77−.81; postquit r’s= .75−.77; ps<.01), a mean of the scores from the three domains was used in analyses. Pleasure responses were reverse scored so that higher scores reflected greater anhedonia.
Analytic Plan
The within-subjects, repeated measures withdrawal data were analyzed in growth-curve analyses using a two-level hierarchical linear model with restricted maximum likelihood estimation (HLM 5.04; Raudenbush, Bryk, & Congdon, 2001). A discontinuous piecewise linear model was fit for each withdrawal symptom (anhedonia, craving, negative affect, hunger, and concentration), with the quit day constituting a node of discontinuity and with each variable modeled with regard to time of measurement.
Y = β0 + β1 (pre-post jump) + β2 (prequit day) + β3 (postquit day) + e
The pre–post variable was a dummy variable coded as 0 if the data were collected before the quit day and as 1 if the data were collected on or following the quit day. The days prequit variable indicated the number of days prior to the quit day coded negatively (i.e., –5, −4, etc., to 0 at the quit date and all days post quit), and the days post-quit variable indicated days since the quit day coded positively (i.e., 0 for all days prior to the quit date, and from 0 on the quit date [12:01 AM] up to +10 days post-quit). Therefore, the model represented individual differences in withdrawal change with respect to four parameters: (1) the pre-quit intercept (β0), or the mean level of the symptoms immediately prior to the quit attempt (i.e., prior to Day 1 postquit when all time variables were coded as 0); (2) the jump in symptoms that occurred on the quit day (β1); (3) the pre-quit slope (β2, the expected change in symptoms per day pre-quit); and (4) the post-quit slope (β3, the expected change in symptoms per day postquit). All four of these effects were allowed to vary randomly across persons. In addition, agonist treatment was modeled as a between–subjects dummy factor (1= nicotine replacement therapy [NRT], 0= placebo) and entered as a predictor of all four parameters at level 2 of the model.
Because accounting for postquit smoking is important when examining abstinence effects (Hughes, 2007c), we also examined the anhedonia temporal pattern in a restricted sample of participants who smoked fewer than a mean of 5 cigarettes per day during the first 10 days after the target quit day. The 5 cigarette cut-off represents half of the minimum baseline smoking rate to qualify for study entry (10 cigarettes per day for the last 6 months), suggesting that smokers remaining in the analyses had meaningfully reduced their drug intake and experienced significant withdrawal symptoms even if they were not entirely abstinent (Piasecki et al., 2003a). Only 34% of participants in this study reported no smoking in the first 10 days post-quit, and 80% reported smoking less than one cigarette per day, on average, in the first 10 days post-quit. This suggests that excluding all participants who smoked at all would create an unrepresentative sample. In addition, excluding those who relapsed (smoked daily) would likely exclude smokers who experienced the greatest withdrawal symptoms (Hughes, 2007a; Piasecki et al., 2003a, 2003b).2
Additional analyses were conducted using PASW Statistics 17.0 to relate cessation outcomes and dependence scores with withdrawal symptom and anhedonia scores. To permit comparison with prior findings in this area and because of demonstrated predictive validity (Baker et al., 2012; Piper et al., 2011), symptoms were modeled via mean prequit, mean postquit, and change from pre to postquit (via residualized postquit scores). Relations between anhedonia and lapse and relapse likelihood were modeled with Cox proportional hazard analyses. Individuals were censored at the time of their last contact if they did not report an event (e.g., lapse, relapse). Logistic regression was used to analyze initial abstinence and 8-week point prevalence outcomes. Consistent with the intent-to-treat principle, all randomized participants were retained in the analyzed dataset; participants who did not provide outcome information were assumed to be smoking. Smoking cessation milestones were examined out to 8 weeks postquit because this timeframe is sufficiently proximal to be influenced by early withdrawal symptoms but sufficiently distal to be a good predictor of long-term outcome (e.g., 6 months postquit).
For both point-prevalence and survival models, univariate models were initially run that identified whether anhedonia was significantly associated with smoking outcomes. We then included withdrawal symptom covariates in the models (i.e., negative affect and craving) in order to determine whether anhedonia provided orthogonal predictive validity. Finally, other substantively relevant covariates were added to the models (i.e., type of treatment [0=placebo, 1=monotherapy, 2=combination therapy], tobacco dependence, mood disorder within the past 12 months, and gender).
We examined missing anhedonia EMA data by creating variables for each subject representing the number of completed anhedonia daily EMA assessments during the pre and postquit assessment periods. Of the possible five prequit anhedonia assessments used to derive mean prequit anhedonia, the mean number of completed assessments was 3.61 (SD=1.30). Of the possible ten postquit anhedonia assessments, the mean number of completed assessments was 4.90 (SD = 2.82). Using a missingness moderation method to test the assumption that the missingness was not random (Piper et al., 2011), we controlled for missingness statistically by including the number of completed EMA assessments and the “Anhedonia × Completed Assessments” interaction term in all models examining the relation between anhedonia and smoking cessation outcomes (initial cessation, lapse, relapse, and point-prevalence abstinence). The number of completed EMA assessments was significantly associated with all cessation outcomes (all ps < .01), but the “Anhedonia × Completed Assessments” interaction term was not significantly associated with abstinence. When number of completed EMA assessments was included in each of the cessation models, EMA missingness did not change the patterns of significant findings obtained. In addition, neither prequit anhedonia nor postquit anhedonia was significantly associated with missing abstinence data at 8 weeks postquit (OR= .94, 95%CI[.85,1.04], p=.21 and OR= .99, 95%CI[.86,1.10], p=.82, respectively).
Results
Participant Characteristics
Of the 1175 participants included in this study, 58.3% were women. The majority of participants were white (85.5%); 11.7% were African American; and 2.8% reported another race. Participants’ mean age was 44.80 (SD = 11.05), they smoked 21.46 (SD = 9.05) cigarettes per day at baseline, and they had made 5.69 (SD = 9.21) previous quit attempts. Their mean prequit anhedonia level was 4.37 (SD=1.96) on a 0–10 scale. Men reported higher prequit anhedonia than women (M = 4.70, SD =1.94 vs. M = 4.13, SD=1.75; p < .001). Approximately 17.5% of participants were classified as having a lifetime history of a depressive disorder, 5% in the past year.3 Those with higher levels of prequit anhedonia were more likely than others to report experiencing past-year depression (r= .07, p= .02) but not lifetime depression. In addition, prequit anhedonia was positively associated with cigarettes per day (r= .14, p< .001). Finally, prequit anhedonia was not associated with age or with lifetime or past year substance use disorder.
Anhedonia and Withdrawal Time Course
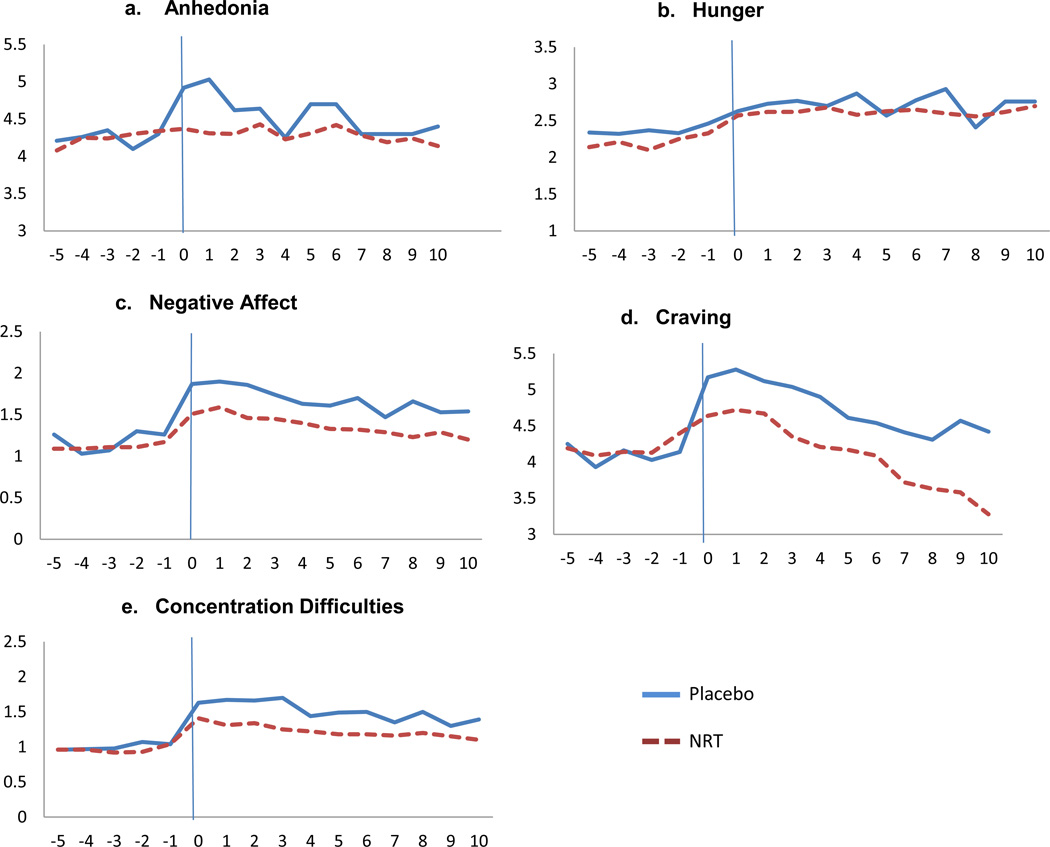
As depicted in Figure 1a, mean anhedonia’s quadratic pattern amongst those receiving placebo resembles a prototypic withdrawal pattern: a rapid increase postquit followed by a return to prequit levels. A similar biphasic pattern was observed for all other withdrawal symptoms except hunger (see Figures 1b – 1e). Moreover, Figures 1a – 1e illustrate that agonist treatment (NRT) essentially eliminated the postquit rise in anhedonia, an effect that was present, but to a lesser degree, for the other withdrawal symptoms.
Figure 1.
Mean waveforms of anhedonia and other withdrawal symptoms from Day -5 day prequit to Day 10 postquit. The quit day occurred on day 0. Anhedonia and all withdrawal symptoms were assessed on 0–10 scales.
Growth curve model results show generally modest prequit slope effects for anhedonia (β=.01, p=.07); hunger (β=.02, p=.002); negative affect (β=−.01, p=.02); craving (β=−.01, p=.15); and concentration (β= .00, p=.62). There was a significant quit day increase (jump) in anhedonia among those who received placebo (β= .69, p= <.001). This effect was nearly fully mitigated among those who received NRT (β = −.66; p<.001). Similar agonist effects were observed for negative affect, concentration, and craving, although to a lesser degree than for anhedonia. For example, there was a significant quit day increase in negative affect (β=.69, p= <.001) that was equal in size to the anhedonia jump. The negative affect jump was partially attenuated amongst those who received NRT (β = −.28; p=.03). Moreover, significant quit day increases in difficulty concentrating (β=.66, p= <.001) and craving (β= 1.23, p < .001) amongst those taking placebo were partially mitigated by NRT (β = −.28; p=.03; β= −.73; p<.001, respectively). The significant jump in hunger (β=.26, p= <.04) was not influenced by NRT (β = .06; p=.69). Finally, linear postquit growth for all symptoms in both treatment conditions was modest (β’s = −.11−.05). When we conducted the growth curve models in a restricted sample of participants who smoked fewer than a mean of 5 cigarettes per day during the first 10 days after the target quit day (n=1457), the same pattern of results emerged.
We also examined pre and postquit means for anhedonia and the other withdrawal symptoms (see Table 1). Consistent with growth curve analysis results, NRT suppresses mean postquit anhedonia and other withdrawal symptom responses. In addition, NRT decreased the prevalence of participants that experienced any increase in anhedonia, craving, negative affect, and concentration (see Table 1).
Table 1.
Pre and Postquit Anhedonia and Withdrawal Symptoms by Treatment Condition
| Placebo | NRT | |||||||
|---|---|---|---|---|---|---|---|---|
| Prequit | Postquit | Prequit | Postquit | |||||
| M (SD) | M (SD) | d | Participants with any increase in postquit symptoms (%) |
M (SD) | M (SD) | d | Participants with any increase in postquit symptoms (%) |
|
| Anhedonia | 4.28 (2.06) | 4.68 (1.95)** | .20 | 62% | 4.31 (2.04) | 4.40 (2.00)* | .04 | 52% |
| Craving | 4.16 (2.42) | 4.82 ( 2.69)** | .25 | 56% | 4.23 (2.32) | 4.30 (2.55) | .03 | 47% |
| Negative Affect | 1.20 (1.32) | 1.81 (1.58)** | .41 | 68% | 1.17 (1.28) | 1.46 (1.45)** | .21 | 60% |
| Concentration | 1.04 (1.32) | 1.59 (1.74)** | .35 | 66% | 1.02 (1.37) | 1.34 (1.63)** | .21 | 56% |
| Hunger | 2.45 (1.94) | 2.70 (1.91)** | .12 | 61% | 2.70 (1.91) | 2.63 (2.08)** | −.03 | 60% |
Note: *p < .05;
p <.01
Relations among Anhedonia, Withdrawal Symptoms, and Dependence
We examined the relations of mean daily postquit anhedonia with established withdrawal symptoms. After controlling for treatment in each univariate model, regression analysis showed that postquit anhedonia was positively associated with postquit craving, negative affect, and concentration difficulties, but was unrelated to hunger (see Table 2). Next, we examined associations between mean postquit anhedonia and withdrawal symptoms and tobacco dependence indices (FTND, time-to-first-cigarette, cigarettes per day, and WISDM PDM and SDM subscales). FTND, time-to-first-cigarette, cigarettes per day, and the WISDM PDM subscale were all positively associated with mean postquit anhedonia (see Table 2). Conversely, anhedonia was negatively associated with the WISDM SDM subscale. With the exception of hunger, which was unrelated to all dependence indices except the WISDM SDM, all other withdrawal symptoms were moderately related to most tobacco dependence measures.
Table 2.
Relations between Postquit Anhedonia, Withdrawal Symptoms, and Dependence Indices, Controlling for Treatment
| Anhedonia | Negative Affect | Craving | Concentration | Hunger | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | β | p | |
| Anhedonia | -- | -- | ||||||||
| Negative Affect | .27 | <.000 | -- | -- | ||||||
| Craving | .19 | <.001 | .42 | <.001 | -- | -- | ||||
| Concentration | .19 | <.01 | .69 | <.001 | .36 | <.001 | -- | -- | ||
| Hunger | .08 | .06 | .35 | <.001 | .19 | <.001 | .32 | <.001 | -- | -- |
| FTND | .12 | <.001 | .14 | <.001 | .28 | <.001 | .15 | <.001 | .06 | .04 |
| TTFC | .12 | <.001 | .10 | <.001 | .23 | <.001 | .09 | .001 | .03 | .26 |
| Cigs/day | .17 | <.001 | .03 | .34 | .14 | <.001 | .03 | .34 | −.01 | .70 |
| PDM | .10 | .004 | .05 | .14 | .27 | <.001 | .007 | .84 | −.01 | .85 |
| SDM | −.09 | .02 | .20 | <.001 | .10 | .003 | .24 | <.001 | .18 | <.001 |
Note: Withdrawal symptom relations were analyzed using mean daily scores during the postquit period. Dependence was assessed during the prequit period. FTND= Fagerström Test for Nicotine Dependence. TTFC=Time to First Cigarette. PDM=Primary Dependence Motives. SDM=Secondary Dependence Motives.
Relations between Anhedonia and Smoking Cessation Outcomes
We tested univariate models examining whether pre and postquit anhedonia and change in anhedonia from pre to postquit predicted smoking cessation “milestones” and 8-week point-prevalence abstinence. Prequit anhedonia was significantly associated with initial abstinence and 8-week point prevalence abstinence, such that smokers with higher prequit anhedonia were more likely than others to be smoking at both time points (see Table 3). There were no significant associations between prequit anhedonia and time to first lapse or relapse. Higher postquit anhedonia was significantly associated with a greater likelihood of smoking at the initial abstinence and 8-week abstinence time points, and it was also significantly associated with earlier lapse and relapse. Finally, greater pre to postquit change in anhedonia was significantly associated with earlier lapse and a greater likelihood of smoking at the 8-week abstinence timepoint.4 Anhedonia change was not associated with initial abstinence or the transition from lapse to relapse (see Table 3).
Table 3.
Individual Models of Anhedonia Predicting Smoking Cessation Milestones and Point-Prevalence Abstinence over the 8 Weeks Following the Target Quit Day
| Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|
| Failure to achieve initial abstinence |
Days from initial abstinence to first lapsea |
Days from first lapse to relapseb |
Point-prevalence abstinence at 8 weeks postquit |
|||||
| Predictor | OR (95% CI)c | p | HR (95% CI)d | p | HR (95% CI)d | p | OR (95% CI)c | p |
| Prequit Anhedonia | 1.14 (1.03, 1.27) | .01 | 1.01 (0.97, 1.06) | .44 | 1.06(0.99, 1.13) | .10 | .92 (0.87, 0.98) | .009 |
| Postquit Anhedonia | 1.16 (1.04, 1.28) | .007 | 1.05 (1.01, 1.10) | .01 | 1.09(1.02, 1.17) | .01 | .91 (0.86, 0.97) | .002 |
| Anhedonia Changee | 0.98 (0.82, 1.18) | .87 | 1.11 (1.03, 1.19) | .004 | 1.05 (0.92, 1.19) | .48 | .88 (0.80, 0.98) | .02 |
Note. For each outcome, separate models were conducted for each predictor. Initial abstinence (0=abstinent, 1=smoking); Lapse and relapse (0=smoking , 1=abstinent); 8-week point prevalence abstinence (0=smoking, 1=abstinent). Significant effects (p < .05) are bolded.
Includes only individuals who achieved initial abstinence (n = 1259).
Includes only individuals who lapsed (n = 930).
Logistic regression model.
Cox proportional hazards regression survival model.
Pre to Postquit anhedonia change.
Next we examined whether anhedonia was significantly associated with smoking cessation outcomes after controlling for variance associated with craving and negative affect. After controlling for prequit negative affect and prequit craving, higher prequit anhedonia significantly increased the likelihood of smoking at the initial abstinence and 8-week abstinence time points. Likewise, postquit anhedonia predicted initial abstinence, days to relapse, and 8-week point-prevalence abstinence, after controlling for postquit craving and postquit negative affect. Finally, greater pre to postquit change in anhedonia predicated earlier lapse, after controlling for change in craving and negative affect.5 When the cessation models including anhedonia, craving and negative affect were adjusted for treatment, FTND, and gender, the same pattern of significant relations between anhedonia and smoking outcomes emerged (see Table 4).6
Table 4.
Multivariate Analyses of Anhedonia Predicting Smoking Cessation Milestones and Point-Prevalence Abstinence over the 8 Weeks Following the Target Quit Day, Controlling for Craving and Negative Affect
| Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Failure to reach initial abstinence |
Days from initial abstinence to first lapsea |
Days from first lapse to relapseb |
Point-prevalence abstinence at 8 weeks postquit |
||||||
| Step | Predictor | OR (95% CI)c | p | HR(95% CI)d | p | HR(95% CI)d | p | OR (95% CI)c | p |
| Prequit | |||||||||
| 1 | Craving | 1.11 (1.01,1.23) | .04 | 1.01 (0.97,1.05) | .59 | 1.05 (.99,1.21) | .13 | 0.97 (.92, 1.03) | .38 |
| Negative Affect | 1.23 (1.06,1.44) | .008 | 1.08 (1.00,1.15) | .04 | 1.07 (0.96,1.19) | .23 | 0.92 (.83, 1.02) | .10 | |
| 2 | Anhedonia | 1.14 (1.02, 1.27) | .02 | 1.02 (0.98,1.07) | .26 | 1.06 (0.99,1.14) | .11 | 0.92 (.87, .98) | .009 |
| Postquit | |||||||||
| 1 | Craving | 1.17 (1.06, 1.30) | .002 | 1.12 (1.08, 1.16) | <.001 | 1.07 (1.00,1.14) | .05 | 0.87 (.82, .92) | <.001 |
| Negative Affect | 1.10 (.94, 1.26) | .21 | 1.03 (.97, 1.09) | .34 | 1.09 (.99,1.19) | .09 | 0.97 (.88, 1.07) | .53 | |
| 2 | Anhedonia | 1.13 (1.01, 1.26) | .04 | 1.04 (1.00, 1.08) | .08 | 1.08 (1.00,1.16) | .04 | 0.92 (.87, .98) | .01 |
| Change | |||||||||
| 1 | Craving | 1.25 (1.10, 1.42) | .001 | 1.17 (1.12, 1.22) | <.001 | 1.04 (0.96,1.12) | .37 | 0.83 (.77, .89) | <.001 |
| Negative Affect | 0.92 (0.70–1.19) | .52 | 1.01 (.92, 1.11) | .88 | 1.10 (0.94,1.28) | .22 | 0.96 (.82, 1.13) | .64 | |
| 2 | Anhedonia | 0.94 (0.78–1.14) | .54 | 1.09 (1.01, 1.16) | .03 | 1.02 (0.90,1.16) | .72 | 0.91 (.82, 1.02) | .10 |
Note. All models are adjusted for treatment, FTND, and gender. Initial abstinence (0=abstinent, 1=smoking); Lapse and relapse (0=smoking , 1=abstinent); 8-week point prevalence abstinence (0=smoking, 1=abstinent). Significant effects (p < .05) are bolded.
Includes only individuals who achieved initial abstinence (n = 1259).
Includes only individuals who lapsed (n = 930).
Logistic regression model.
Cox proportional hazards regression survival model.
Discussion
These results show that anhedonia meets criteria for a clinically significant and independent tobacco withdrawal symptom. First, consistent with the biphasic pattern emblematic of withdrawal symptoms (Hughes, 2007b), anhedonia increased abruptly upon cessation and then returned to prequit levels. Although anhedonia peaked somewhat earlier than has been reported for other withdrawal symptoms (1 day postquit versus 2–3 days postquit), other valid tobacco withdrawal symptoms peak within a 1–7 day postquit period (Hughes 2007b). Second, the increase in anhedonia was greatly attenuated by agonist therapy, conforming to theory and data on the nature of withdrawal symptoms (e.g., Benowitz, 2010; Siegel, 1983). It is possible that cessation-related anhedonia could reflect merely the loss of nicotine’s agonist incentive/reinforcing properties. However, anhedonia’s quadratic pattern suggests that NRT effects are more consistent with alleviating a withdrawal versus an offset effect (Hughes, 2007c). Third, consistent with the nature of a syndrome element (American Psychiatric Association, 2013), anhedonia was correlated with other tobacco withdrawal symptoms. However, associations with other withdrawal symptoms were modest in size, as has been found in other research (Snuggs & Hajek, 2013). Importantly, anhedonia’s modest interrelations with other withdrawal symptoms permit it to convey information about withdrawal that may not be provided by other withdrawal symptoms. Fourth, anhedonia was meaningfully associated with core tobacco dependence measures. This is not only consistent with theory, but also mirrors the pattern of interrelations of other core withdrawal symptoms (craving and negative affect) with dependence (e.g., Baker et al., 2012). Finally, anhedonia predicted several cessation outcomes, suggesting that it possesses motivational significance as do some other core withdrawal symptoms (i.e., craving and negative affect; Baker et al., 2004; Swan, Jack, Javitz, McAfee, & McClure, 2008).
In sum, the findings of this research are consistent with prior human laboratory (e.g., Dawkins et al., 2007; Powell et al., 2002; Powell et al., 2004) and neurobiological research (e.g., D'Souza & Markou, 2010; Epping-Jordan et al., 1998) that showed that suspension of nicotine delivery results in diminished pleasure and instrumental responding for nonpharmacologic rewards (e.g., Epping-Jordan et al., 1998). The present research extends such findings by showing that anhedonia meets the key criteria of a withdrawal symptom and it does so amongst a large group of smokers as they smoke, report affective responses to nonpharmacologic pleasurable events, experience tobacco deprivation, and respond to agonist administration, in a research context with substantial clinical and real-world relevance.
Contrary to our findings, Snuggs and Hajik (2013) found that anhedonia decreased following quitting and was not associated with smoking cessation outcomes. However, that research differed from the present research in the nature of the cessation outcome, power, and the assessment of anhedonia, both in terms of the measure used and time course. For example, anhedonia was not assessed until 1-week postquit, when our study showed that anhedonia had already returned to baseline. Another study yielded inconclusive data, showing some decrease in anhedonia across the first 7 days post quit. However, the decrease in anhedonia did not vary as a function of abstinence versus continued smoking, and baseline values were not reported (Dawkins, Powell, Pickering, Powell, & West, 2009). Thus, such studies may be viewed as inconclusive with regards to the status of anhedonia being a tobacco withdrawal symptom.
The present study is the first, to our knowledge, to demonstrate that loss in post-cessation pleasure in response to daily activities is a significant barrier to quitting smoking. If participants’ reports of anhedonia following quitting are valid (reflect an actual insensitivity to appetitive stimulus effects), then anhedonia may well provide a strong motive for withdrawing smokers to resume smoking. As Perkins and colleagues noted (Perkins et al., 2013), lapsing back to smoking would not only allow the smoker to re-experience the appetitive effects of smoking per se, but the ingested nicotine would restore the pleasure-enhancing effects of nondrug stimuli as well (e.g., al-Adawi & Powell, 1997). Moreover, in the current research, postquit anhedonia predicted risk of initial lapse as well as risk for transition from lapse to full relapse. Thus, postquit anhedonia may have increased the desire to return to smoking to restore pleasurable response to appetitive stimuli, and, once a lapse had occurred, the restoration of pleasure may have reinforced a return to daily smoking.
We also found that prequit anhedonia predicted cessation outcome, consistent with other research (Cook, Spring, McChargue, & Doran, 2010; Leventhal, Ramsey, Brown, LaChance, & Kahler, 2008). The relation between prequit anhedonia and cessation is consistent with the fact that other pre and postquit withdrawal symptoms tend to be highly inter-correlated (e.g., Piper et al., 2011). Even during periods of active drug use, dependent individuals typically display meaningful levels of withdrawal (e.g., Isbell, Fraser, Wikler, Belleville, & Eisenman, 1955; Mello & Mendelson, 1970). Such reports likely reflect the fact that active drug use (e.g., smoking) provides only temporary withdrawal suppression (e.g., Hendricks et al., 2006).
Consistent with longstanding theories of addiction (e.g., Siegel, 1983; Solomon & Corbit, 1973), research has demonstrated that withdrawal symptoms (e.g., craving and negative affect) are more severe amongst highly dependent smokers (Baker et al., 2012). Similarly, we found a modest, positive association between withdrawal-related anhedonia and core dependence features reflecting heavy, compulsive, and automatic smoking patterns. Specifically, anhedonia was positively associated with Primary Dependence Motives (the PDM) and inversely associated with Secondary Dependence Motives (i.e., smoking for instrumental reasons such as regulation of negative moods, the SDM). This finding is notable since one might assume that anhedonia reflects neuroticism or negative affectivity as opposed to nicotine dependence per se. However, substantial psychopathology symptom data show that anhedonia is psychometrically distinct from negative affect forms of distress (Cook, Spring, McChargue, & Hedeker, 2004; Leventhal, Chasson, Tapia, Miller, & Pettit, 2006; Shafer, 2006). Further, research shows that negative affect variables—e.g., the Positive and Negative Affect Scale (Watson et al., 1988) and the MPQ Negative Emotionality Scale (Patrick, Curtin, & Tellegen, 2002)—are more strongly associated with the SDM than with the PDM (Baker et al., 2012). Thus, the current data suggest that anhedonia is related to a dependence dimension (i.e., the PDM) that is especially reflective of heavy, automatic smoking, intolerance to interruptions in smoking, decreased likelihood of cessation, and risk alleles on the chromosome 15 CHRNA5A3B4 nicotinic receptor gene associated with heightened tobacco dependence and inability to quit smoking (e.g., Baker et al., 2012; Baker et al., 2009; Chen et al., 2012; Piasecki et al., 2003a; Piasecki et al., 2011).
Treatment implications
These results certainly suggest that reversal of anhedonia may be an important mechanism of NRT. Other agents might also mitigate withdrawal-related anhedonia (e.g., fluoxetine: Cook, Spring, McChargue, Borrelli, et al., 2004). In addition, behavioral interventions such as Behavioral Activation (BA) might be effective in treating tobacco withdrawal. BA is an efficacious treatment for major depressive disorder, one that increases engagement in reinforcing activities (Dimidjian et al., 2006). By increasing exposure to alternative sources of non-smoking reinforcement, BA may counteract withdrawal-related anhedonia and increase quit rates (MacPherson et al., 2010).
Finally, these results may have implications for smokers with mental health disorders that are characterized by anhedonia (e.g., major depressive disorder, schizophrenia; Kashdan, Barrios, Forsyth, & Steger, 2006; Treadway & Zald, 2013). Because such smokers have reward processing deficits prior to quitting smoking, any postquit rise in anhedonia could be particularly aversive, spurring cessation failure. Therefore, such smokers may especially benefit from smoking cessation treatments that enhance the pleasure derived from daily activities and rewards (Leventhal, Piper, Japuntich, Baker, & Cook, 2013).
Limitations
This research has several limitations that should be considered. First, this sample comprised only individuals motivated to quit and who received intense treatment, which may reduce generalizability. Moreover, the anhedonia assessments relied on self-reports and may reflect error intrinsic to self-report (e.g., incorrect attributions, broad attitudinal factors). For instance, the reports of event-related pleasure may reflect different rates of encounters with pleasurable events and not the capacity to experience pleasure per se. Thus, the results do not permit precise characterization of type of reward (or incentive) related deficit and most likely reflect some combination of processes associated with anhedonia (e.g., reward anticipation, reward value and costs; Der-Avakian & Markou, 2012). Anhedonia may also be related to other important clinical phenomena such as positive affect, which differs from anhedonia conceptually in that it is not contingent on the occurrence of a pleasurable event. In addition, the study did not measure trait anhedonia, which also has been shown to influence smoking outcomes (e.g., Cook et al., 2010). Finally, the effects observed in this study may reflect some admixture of nicotine agonist (i.e, offset) and withdrawal effects that would be difficult to distinguish in the present context.
Conclusion
In summary, our results suggest that anhedonia is a key, independent tobacco withdrawal symptom. This conclusion is based on anhedonia’s dynamic waveform, its attenuation through agonist administration, and its relations with other tobacco withdrawal symptoms, with core tobacco dependence features, and with tobacco cessation. However, further research is needed to establish definitively whether anhedonia is a symptom of the tobacco withdrawal syndrome, including providing a more precise characterization and assessment of withdrawal-related pleasure deficits. If replicated, these results could have implications for the diagnostic criteria for tobacco use disorder. Moreover, anhedonia could become an important target of tobacco dependence treatment and treatment development.
Acknowledgements
This research was supported in part by grant 9P50CA143188 from the National Cancer Institute to the University of Wisconsin-Center for Tobacco Research and Intervention; by grant M01 RR03186 from the General Clinical Research Centers Program of the National Center for Research Resources, NIH; by grants K08DA02131, K08DA025041, and K05CA139871 from NIH; by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine; and by the Wisconsin Partnership Program. Medication was provided to participants at no cost under a research agreement with GlaxoSmithKline; no part of this manuscript was written or edited by anyone employed by GlaxoSmithKline.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
Responses outside this window were eliminated from analyses because not all participants completed the full assessment period based on appointment scheduling, resulting in insufficient data for analysis of treatment subgroups.
As an additional test of the influence of smoking on the anhedonia trajectory, smoking was included as a time varying covariate; the anhedonia growth curve remained unchanged.
Prequit characteristics did not differ as a function of treatment assignment.
Including EMA-assessed positive affect in all cessation outcome models did not change the pattern of significant results obtained.
The pattern of significant relations remained the same when hunger and concentration difficulties were added to the smoking outcome models along with anhedonia, craving, and negative affect.
Depression in the past 12 months was trimmed from the models because it significantly predicted only 8 week point prevalence abstinence. When depression was added to the full 8 week abstinence models, anhedonia still predicted 8 week point-prevalence abstinence outcomes (p’s<.05).
References
- al-Adawi S, Powell J. The influence of smoking on reward responsiveness and cognitive functions: a natural experiment. Addiction. 1997;92(12):1773–1782. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- Baker, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, Toll BA. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9(Suppl 4):S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, Schlam TR, Cook JW, Smith SS, Loh WY, Bolt D. Are tobacco dependence and withdrawal related amongst heavy smokers? Relevance to conceptualizations of dependence. J Abnorm Psychol. 2012;121(4):909–921. doi: 10.1037/a0027889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Weiss RB, Bolt D, von Niederhausern A, Fiore MC, Dunn DM, Cannon DS. Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob Res. 2009;11(7):785–796. doi: 10.1093/ntr/ntp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt DM, Piper ME, McCarthy DE, Japuntich SJ, Fiore MC, Smith SS, Baker TB. The Wisconsin Predicting Patients' Relapse questionnaire. Nicotine Tob Res. 2009;11(5):481–492. doi: 10.1093/ntr/ntp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebraska Symposium on Motivation. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 2006;189(1):27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Chen LS, Baker TB, Grucza R, Wang JC, Johnson EO, Breslau N, Bierut LJ. Dissection of the phenotypic and genotypic associations with nicotinic dependence. Nicotine Tob Res. 2012;14(4):425–433. doi: 10.1093/ntr/ntr231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression: a brief report. Nicotine Tob Res. 2010;12(9):978–982. doi: 10.1093/ntr/ntq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine Tob Res. 2004;6(1):39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue DE, Borrelli B, Hitsman B, Niaura R, Kristeller J. Influence of fluoxetine on positive and negative affect in a clinic-based smoking cessation trial. Psychopharmacology. 2004;173(1–2):153–159. doi: 10.1007/s00213-003-1711-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA. Nicotine self-administration in animals as a dependence model. Nicotine Tob Res. 1999;1(1):11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- D'Souza MS, Markou A. Neural substrates of psychostimulant withdrawal-induced anhedonia. Current topics in behavioral neurosciences. 2010;3:119–178. doi: 10.1007/7854_2009_20. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Acaster S, Powell JH. The effects of smoking and abstinence on experience of happiness and sadness in response to positively valenced, negatively valenced, and neutral film clips. Addict Behav. 2007;32(2):425–431. doi: 10.1016/j.addbeh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell J. Effects of nicotine and alcohol on affective responses to emotionally toned film clips. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2197-4. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell J, Pickering A, Powell J, West R. Patterns of change in withdrawal symptoms, desire to smoke, reward motiation and response inhibition across 3 months of smoking abstinence. Addiction. 2009;104:850–858. doi: 10.1111/j.1360-0443.2009.02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I--Effects on incentive motivation. Psychopharmacology (Berl) 2006;189(3):355–367. doi: 10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends in Neurosciences. 2012;35(1):68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006;74(4):658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169(1):68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393(6680):76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing anhedonia in psychiatric patients. Arch Gen Psychiatry. 1983;40(1):79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET. Nicotine-induced conditioned place preference (CPP): dose-response, temporal requirements and lack of tolerance development. Soc Neuroscience Abstr. 1985;11(12):378–378. [Google Scholar]
- Gloria R, Angelos L, Schaefer HS, Davis JM, Majeskie M, Richmond BS, Baker TB. An fMRI investigation of the impact of withdrawal on regional brain activity during nicotine anticipation. Psychophysiology. 2009;46:681–693. doi: 10.1111/j.1469-8986.2009.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor CM, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1300–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology (Berl) 2004;175(2):134–142. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction. 1989;84(7):791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology (Berl) 2006;187(3):385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hilario MR, Turner JR, Blendy JA. Reward sensitization: effects of repeated nicotine exposure and withdrawal in mice. Neuropsychopharmacology. 2012;37(12):2661–2670. doi: 10.1038/npp.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: etiology, animal models, epidemiology, and significance: a subjective review. Nicotine Tob Res. 2007a;9(3):329–339. doi: 10.1080/14622200701188927. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007b;9(3):315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Measurement of the effects of abstinence from tobacco: a qualitative review. Psychol Addict Behav. 2007c;21(2):127–137. doi: 10.1037/0893-164X.21.2.127. [DOI] [PubMed] [Google Scholar]
- Isbell H, Fraser HF, Wikler A, Belleville RE, Eisenman AJ. An experimental study of the etiology of rum fits and delirium tremens. Quarterly Journal of Studies on Alcohol. 1955;16(1):1–33. [PubMed] [Google Scholar]
- Japuntich SJ, Piper ME, Leventhal AM, Bolt DM, Baker TB. The effect of five smoking cessation pharmacotherapies on smoking cessation milestones. J Consult Clin Psychol. 2011;79(1):34–42. doi: 10.1037/a0022154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Hollander JA, Kenny PJ. Decreased brain reward function during nicotine withdrawal in C57BL6 mice: evidence from intracranial self-stimulation (ICSS) studies. Pharmacology, Biochemistry and Behavior. 2008;90(3):409–415. doi: 10.1016/j.pbb.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan TB, Barrios V, Forsyth JP, Steger MF. Experiential avoidance as a generalized psychological vulnerability: comparisons with coping and emotion regulation strategies. Behaviour Research and Therapy. 2006;44(9):1301–1320. doi: 10.1016/j.brat.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31(6):1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Telch MJ, Newman B. Are heavy smokers different from light smokers? A comparison after 48 hours without cigarettes. JAMA : the journal of the American Medical Association. 1988;260(11):1581–1585. [PubMed] [Google Scholar]
- Koob GF, Markou A, Weiss F, Schulteis G. Opponent process and drug dependence: Neurobiological mechanisms. Semin Neurosci. 1993;5:351–358. [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 2005;178(4):481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: A psychometric analysis of three anhedonia scales. Journal of Clinical Psychology. 2006;62(12):1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine Tob Res. 2008;10(3):507–517. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Piper ME, Japuntich SJ, Baker TB, Cook JW. Anhedonia, depressed mood, and smoking cessation outcome. J Consult Clin Psychol. 2014;82(1):122–129. doi: 10.1037/a0035046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, Pickworth WB. A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addict Behav. 2010;35(12):1120–1130. doi: 10.1016/j.addbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Tull MT, Matusiewicz AK, Rodman S, Strong DR, Kahler CW, Lejuez CW. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. J Consult Clin Psychol. 2010;78(1):55–61. doi: 10.1037/a0017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Payne MC, Short PE, Carter VA, Cunningham JS, Wilson OB. Nicotine alleviation of nicotine abstinence syndrome is naloxone-reversible. Pharmacology, Biochemistry and Behavior. 1996;53(1):81–85. doi: 10.1016/0091-3057(95)00202-2. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Experimentally induced intoxication in alcoholics: a comparison between programed and spontaneous drinking. J Pharmacol Exp Ther. 1970;173(1):101–116. [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychol Assess. 2002;14(2):150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe J, Fonte C. Influence of acute smoking exposure on the subsequent reinforcing value of smoking. Experimental and Clinical Psychopharmacology. 1997;5(3):277–285. doi: 10.1037//1064-1297.5.3.277. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology (Berl) 2013;228(3):479–486. doi: 10.1007/s00213-013-3054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. J Abnorm Psychol. 2003a;112(1):3–13. [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. J Abnorm Psychol. 2003b;112(1):14–27. [PubMed] [Google Scholar]
- Piasecki TM, Piper ME, Baker TB. Refining the tobacco dependence phenotype using the Wisconsin Inventory of Smoking Dependence Motives: II. Evidence from a laboratory self-administration assay. J Abnorm Psychol. 2010a;119(3):513–523. doi: 10.1037/a0020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Piper ME, Baker TB. Tobacco dependence: Insights from investigations of self-reported smoking motives. Curr Dir Psychol Sci. 2010b;19(6):395–401. doi: 10.1177/0963721410389460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Piper ME, Baker TB, Hunt-Carter EE. WISDM primary and secondary dependence motives: Associations with self-monitored motives for smoking in two college samples. Drug Alcohol Depend. 2011;114(2–3):207–216. doi: 10.1016/j.drugalcdep.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) J Consult Clin Psychol. 2004;72(2):139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh WY, Baker TB. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology. 2011;216(4):569–578. doi: 10.1007/s00213-011-2250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, Baker TB. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66(11):1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J, Dawkins L, Davis RE. Smoking, reward responsiveness, and response inhibition: tests of an incentive motivational model. Biol Psychiatry. 2002;51(2):151–163. doi: 10.1016/s0006-3223(01)01208-2. [DOI] [PubMed] [Google Scholar]
- Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addict Behav. 2004;29(7):1407–1426. doi: 10.1016/j.addbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, Congdon N. HLM5: Hierarchical linear modeling. Lincolnwood, IL: Scientific Software International; 2001. [Google Scholar]
- Robinson JD, Lam CY, Carter BL, Minnix JA, Cui Y, Versace F, Cinciripini PM. A multimodal approach to assessing the impact of nicotine dependence, nicotine abstinence, and craving on negative affect in smokers. Exp Clin Psychopharmacol. 2011;19(1):40–52. doi: 10.1037/a0022114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. Journal of Clinical Psychology. 2006;62(1):123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- Siegel S. Classical conditioning, drug tolerance, and drug dependence. In: Smart RG, Glaser FB, Israel Y, Kalant R, Popham E, Schmidt W, editors. Research advances in alcohol and drug problems. 7th ed. New York: Plenum; 1983. [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Snuggs S, Hajek P. Responsiveness to reward following cessation of smoking. Psychopharmacology (Berl) 2013;225(4):869–873. doi: 10.1007/s00213-012-2874-y. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42(1):49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. II. Cigarette addiction. J Abnorm Psychol. 1973;81(2):158–171. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Bloom AS. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155(8):1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Markou A. Withdrawal from chronic cocaine administration induces deficits in brain reward function in C57BL/6J mice. Behavioural Brain Research. 2011;223(1):176–181. doi: 10.1016/j.bbr.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Javitz HS, McAfee T, McClure JB. Predictors of 12-month outcome in smokers who received bupropion sustained-release for smoking cessation. CNS Drugs. 2008;22(3):239–256. doi: 10.2165/00023210-200822030-00004. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Parsing anhedonia: translational models of reward-processing deficits in psychopathology. Curr Dir Psychol Sci. 2013;22(3):244–249. doi: 10.1177/0963721412474460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wittchen HU. Reliability and validity studies of the WHO--Composite International Diagnostic Interview (CIDI): a critical review. Journal of Psychiatric Research. 1994;28(1):57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Zinser MC, Baker TB, Sherman JE, Cannon DS. Relation between self-reported affect and drug urges and cravings in continuing and withdrawing smokers. J Abnorm Psychol. 1992;101(4):617–629. doi: 10.1037//0021-843x.101.4.617. [DOI] [PubMed] [Google Scholar]