Abstract
Purpose
A retrospective comparison of refractive outcomes of a new, aspherically optimized profile with an enhanced energy correction feature (Triple-A) and the conventionally used aspherically optimized profile (ASA, or aberration smart ablation) for correction of low-to-high myopia.
Setting
Augen-OP-Centrum, Cologne, Germany
Design
Retrospective nonrandomized comparative study
Methods
A central database at the Augen-OP-Centrum was used to gather retrospective data for low-to-high myopia (up to −10 D). One hundred and seven eyes (56 patients) were treated with the ASA profile, and 79 eyes (46 patients) were treated with the Triple-A profile. Postoperative outcomes were evaluated at 1 month, 3 months, 6 months, and 1 year follow-up time points.
Results
The Triple-A profile showed better predictability indicated by a significantly lower standard deviation of residuals (0.32–0.34 vs 0.36–0.44, Triple-A vs ASA) in the 6-month to 1-year period. The Triple-A group had better stability across all time intervals and achieved better postoperative astigmatism improvements with significantly lower scatter. This group achieved better safety at 1 year, with 100% of eyes showing no change or gain in Snellen lines, compared with 97% in the ASA group. A better safety index was observed for the Triple-A group at later time points. The Triple-A group had a better efficacy index and a higher percentage of eyes with an uncorrected Snellen visual acuity of 20/20 or greater at all investigated follow-up time points.
Conclusion
The new aspherically optimized Triple-A profile can safely and effectively correct low-to-high myopia. It has demonstrated superiority over the ASA profile in most refractive outcomes.
Keywords: Triple-A, wavefront measurements, corneal aberrations, corneal asphericity, ablation profile
Introduction
Laser in situ keratomileusis (LASIK) is one of the most commonly performed ophthalmic surgeries worldwide. Its popularity lies in the safety, efficacy, visual recovery, and patient comfort during and after the procedure.1 A successful surgery depends on many factors, such as the design of the ablation profile, precise delivery of laser energy to the corneal surface, and an understanding of the biomechanical processes involved in the postoperative outcome. The latest advancements in technology have markedly improved LASIK outcomes. The introduction of femtosecond laser flap creation has significantly reduced flap-related complications.2 More recently, “customized” treatments have been developed. Customization of the ablation procedure is possible either using wavefront measurements of the whole eye3 (obtained by Hartmann–Shack wavefront sensors) or using corneal topography-derived wavefront,4 topography-guided,5 wavefront-guided,6 wavefront-optimized,7 and asphericity-preserving analyses as well as Q-factor profiles,8 all of which have been considered as possible solutions for optimized refractive treatment.
Marcos et al9 showed that changes in total spherical aberrations are not fully accounted for by changes in the anterior corneal surface. Total spherical aberration in eyes increased slightly less than corneal aberrations, most likely due to a significant change in the posterior corneal shape, shifting into more negative values of spherical aberration. The increment in total spherical aberration shows a strong correlation to the amount of correction in spherical error and is associated with increased corneal asphericity.
Several reasons have been stated as possible causes for a consistent increase in corneal asphericity (and associated spherical aberration) after refractive surgery:
Assumptions used for the theoretical ablation profile,
Laser efficiency changes associated with the corneal curvature, and
Biomechanical changes in the corneal surface after surgery associated with the process of wound healing.
Mrochen and Seiler10 proposed that the increased asphericity associated with refractive surgery occurs due to changes in ablation efficiency as the laser spot moves from the center of the cornea to the periphery. The increase in the angle of incidence at the periphery increases the energy reflected from the surface of the cornea and, at the same time, also increases the total corneal area illuminated. This results in a decreased ablation depth per laser pulse.
Several studies have estimated the changes in corneal asphericity expected from the theoretical application of the standard ablation pattern, using both the formula reported by Munnerlyn et al11 (which assumes that both pre- and postoperative corneal shapes are spherical) and a parabolic approximation of the Munnerlyn formula. In previous studies, Marcos et al12 and Cano et al13 simulated postoperative corneal surfaces by a subtraction of the standard Munnerlyn ablation pattern and a parabolic approximation of the Munnerlyn pattern from real preoperative corneas. They also compared the estimated postoperative asphericity (Q) and spherical aberration with the real postoperative values.
Most ablation profiles are an intellectual property of the laser companies. Although the profiles are generally based on Munnerlyn’s formula, they are not identical, as shown experimentally with research involving certain laser systems.14 The profiles are optimized to perform as good as Munnerlyn’s basic formula. When simulated, Munnerlyn’s ablation profiles show good results without increasing the incidence of higher-order aberrations. However, the real clinical data demonstrate a higher incidence of spherical aberrations (Z4,0) when the ablation profile is Munnerlyn-based.
Today, different modern excimer lasers have reached an optimal status of high performance. Thus, for further optimization, only a small room is expected to be left. In this study, we compare a new aspherically optimized profile (Triple-A, or Advanced Ablation Algorithm) with the conventional aspherically optimized profile (ASA, or Aberration Smart Ablation) used on the MEL®80 excimer laser platform (Carl Zeiss Meditec AG, Jena, Germany). Triple-A is a new optimized profile that consists of a basic profile in combination with a compensation algorithm for an enhanced energy correction. The enhanced energy correction in the Triple-A profile is stronger than that in ASA and tissue-saving ablation (TSA). The combined profile has the same central depth of ablation, which assists with tissue saving in myopia treatments.
Triple-A, the new profile, can be applied for a complete range of corrections, whereas among the previously known profiles, ASA is recommended for medium and high myopic corrections and TSA is generally used for small myopic corrections up to −3 D. The reason for using TSA instead of the aspherically optimized ASA profile is that at low corrections, ASA leads to a higher ablation depth than does a pure Munnerlyn-based profile. The Triple-A profile overcomes these difficulties as it combines the aspherical optimization and TSA at lower corrections.
Materials and methods
Patient population
In this retrospective, comparative study, data were gathered from patients 19 years and older routinely visiting Augen-OP Centrum in Cologne, Germany, from October 2004 to July 2007 (ASA) and from August 2009 to April 2011 (Triple-A). Patient selection criteria were based on the availability of a full case report and a smaller variation between the two groups (Triple-A and ASA) with respect to the spherical equivalent (SE) distribution and cylinder distribution. Patients are not monitored for aberrations during routine visits, and therefore, the data on aberrations were not collected. LASIK was used for correction of myopia (sphere up to −10.5 D), with and without astigmatism (up to −4.5 D). In all cases, the residual stromal thickness was a minimum of 280 μm. To fulfill this criterion in cases of high myopic patients, the preoperative pachymetry was checked to be sufficiently high, and if justifiable with respect to the pupil diameter, the optical zone diameter was reduced slightly from the standard value of 6.5 mm to a smaller value, which was at minimum 5.75 mm (ASA group) or 6 mm (Triple-A group). Keratoconus formefruste cases were specifically excluded from this study. All surgeries were performed by the same surgeon (Bertram Meyer).
Preoperative examination
The preoperative examinations included uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), autorefractometer measurement with and without cycloplegia, determination of binocular status, corneal topography (including the front side and back side of the cornea), wavefront analysis, pupillometry, endothelial cell measurement, IOP (intraocular pressure) measurement, slit lamp examination of the anterior and posterior segments, and a proof against retinal abnormalities.
Surgical technique
All patients had LASIK surgery. Patients were divided into two groups: Triple-A (79 eyes) and ASA (107 eyes). All surgeries were performed on eyes with a low-to-high degree of myopia and astigmatism. All flaps for the Triple-A group were created using the VisuMax® femtosecond laser (Carl Zeiss Meditec AG). The flap thickness was 110–120 μm, and the flap diameter was 7.9 mm with the treatment pack size S and 8.7 mm with the treatment pack size M. In the ASA group, most flaps were created using the Moria M2 microkeratome (Moria SA, Antony, France) except for eleven eyes, for which the flap was created with the VisuMax® femtosecond laser (Carl Zeiss Meditec AG). The flap thickness parameter for the microkeratome was 90 μm. Excimer laser ablation was performed with the MEL 80 Excimer Laser System (Carl Zeiss Meditec AG) at a pulse repetition rate of 250 Hz. The MEL 80 includes a 1 kHz eye tracker. The system determines the pupil center from an infrared image of the patient’s eye, refreshed and processed at 1 kHz.
The two groups were subjected to different ablation profiles. The ASA group was treated with the conventional aspherically optimized profile that implements the use of a standard energy correction. The optimization is based on the target surface with a constant asphericity. Therefore, the aspherical component of the profile is nearly constant when correction strength is increased.
The Triple-A group was treated with the new Triple-A profile, which is designed as a basic profile combined with an enhanced energy correction function. It is based on the following considerations: To accomplish favorable outcomes, laser ablation profiles must increase the energy targeted at the periphery of the treatment zone to account for losses in radial energy across the dome-shaped surface of the cornea (so-called energy correction). The multiplication of the radially varying energy correction function (which is equal to 1 in the center) with the basic profile is responsible for the maintenance of the same central depths. By further increasing the energy correction at the periphery, the multiplication with the basic profile leads to aspherical components, which increase linearly with the correction strength. The aspherical component of the Triple-A profile is larger than that of the ASA profile, especially at higher corrections.
The mean optical zone sizes for the Triple-A group and the ASA group were 6.46±0.12 and 6.28±0.27 mm, respectively, with a minimum optical zone of 6 mm for the Triple-A group and 5.75 mm for the ASA group. The maximum optical zone was 6.5 mm for both groups. The frequencies of optical zones of 6, 6.25, and 6.5 mm were 3, 8, and 68, respectively, for the Triple-A group. For the ASA group, the frequencies of optical zones of 5.75, 6, and 6.5 mm were 8, 35, and 64, respectively.
In all cases, the postoperative topical medication was dexamethasone eye drops 4 times/day for 2 weeks, ofloxacin eye drops 4 times/day for 3 days, and lubricants as and when required.
Postoperative evaluation
Postoperative examinations included the determination of refraction (sphere, cylinder, and axis) and the uncorrected and corrected visual acuities. The postoperative data analyses were performed on primary cases only.
Postoperative full-case analyses were performed at 1 month, 3 months, 6 months, and 1 year follow-up time points. Postoperative examinations included a standard analysis for predictability of the spherical equivalent (SE), accuracy of the SE, efficacy, safety, and refractive astigmatism and stability as described by Waring et al.15 The vertex distance during the measurement of refraction was 12 mm. Standard optotypes were used, and the reading charts were at a distance of 5 m. For visual acuity, the lines were accepted when read fluently by the patients.
The safety and efficacy indices were also determined for the two groups. These indices are defined in two ways in literature. They can be described either as a ratio of the mean values of visual acuities16 or as the mean value of the ratio of visual acuities (per eye).17 We decided to calculate both values: the “safety/efficacy index” (ratio of mean values) and the “safety/efficacy index per eye” (mean of ratios). For the safety/efficacy index, we averaged the visual acuity data in logMAR units and then converted into decimal units. The safety/efficacy index per eye is better suited for an analysis using statistical tests, and therefore, the statistical analysis was performed using the second method alone (ie, the safety/efficacy index per eye).
The safety index was defined as postoperative CDVA divided by the preoperative CDVA; the efficacy index was defined as postoperative UDVA divided by the preoperative CDVA.
The predictability analysis was performed with respect to attempted SE (attempted spherical equivalent = Preoperative manifest SE [MRSE] - SE target). The accuracy analysis of SE was performed with respect to postoperative SE (residual refractive error) minus the target SE. This also accounts for the deviations from the intended refractive error.
Cylinder distribution was analyzed as follows: The distribution of the absolute values of postoperative cylinder minus the target cylinder was calculated. Using this method, the cases involving an intended partial correction of astigmatism were also taken into account (four cases for the ASA group and one case for the Triple-A group).
For stability analysis, in the standard diagram the postoperative MRSE minus SE target was calculated for the different postoperative follow-up time points.
Parameters used to define different outcome measures were as follows: predictability was defined as standard deviation (SD) of residuals of regression analysis (scatter in predictability diagram), slope, and intercept of predictability diagram. Accuracy was defined as the percentage of eyes within ±0.5 D, percentage of eyes within ±1.0 D, and the mean and SD of postoperative MRSE minus SE target. Safety was defined as the percentage of lines gained, safety index, and the mean and SD of safety index per eye. For efficacy: the percentage of eyes with UDVA ≥20/20, efficacy index, and the mean and SD of efficacy index per eye. For cylinder distribution: the percentage of eyes within ±0.5 D, the percentage of eyes within ±1.0 D, and the mean and SD of the absolute values of postoperative cylinder minus target cylinder. For stability: the percentage of eyes with a change of MRSE by more than 0.5 D in the time interval 1–6 months and 6 months to 1 year, and the mean and SD of the postoperative SE in the time interval 1–6 months, 3–6 months, and 6 months to 1 year.
Statistical evaluation
Statistical analysis was performed using the SAS software (SAS Institute Inc., Cary, NC, USA) and the Microsoft Excel software (Microsoft, Redmond, WA, USA).
The Fisher’s exact test was used to analyze the frequencies (percentages). To analyze the mean and SDs, the following approach was taken: The test of normality of distributions was performed using the Kolmogorov test. Most of the analyzed variables were not normally distributed, and therefore, nonparametric tests were used to compare variables according to data distribution. The two-sided Siegel–Tukey test was used to investigate the significance of the difference of the variances between the ASA and Triple-A groups. If the differences of variances were not significant, the means were analyzed by the two-sided Wilcoxon two-sample test (identical to Mann–Whitney U-test). If the variances were found to be statistically significantly different, the two-sided median two-sample test was used for the analysis of the means.
For a comparison of the results of linear regression analysis (predictability plots), 95% confidence intervals were calculated for the slope and the intercept parameters. Unless otherwise stated, P<0.05 was considered as statistically significant.
Results
Patient data
The patient demographics are summarized in Table 1. Mean patient age was 38±10 years (range, 20–57 years) for the Triple-A group and 34±9 years (range, 19–59 years) for the ASA group.
Table 1.
Summary of patient demographics
| Parameter | ASA | Triple-A | P-value | |
|---|---|---|---|---|
| General | Number of eyes | 107 | 79 | – |
| Right eyes | 48% (51) | 46% (36) | – | |
| Left eyes | 52% (56) | 54% (43) | – | |
| Average age at surgery (years) | ||||
| Mean ± SD | 34±9 | 38±10 | P=0.024a | |
| Range | (19, 59) | (20, 57) | ||
| Male | 40% | 39% | – | |
| Female | 60% | 61% | – | |
| Preoperative | Sphere (D) | |||
| Mean ± SD | −4.86±2.24 | −4.09±2.64 | P=0.055b | |
| Range | (−0.75, −10.5) | (0.5, −9.25) | ||
| Cylinder (D) | ||||
| Mean ± SD | −1.07±1.00 | −1.28±1.02 | P=0.084a | |
| Range | (0, −4.25) | (0, −4.50) | ||
| SE (D) | ||||
| Mean ± SD | −5.39±2.27 | −4.73±2.65 | P=0.159b | |
| Range | (−1.125, −11.75) | (−0.25, −9.75) | ||
| CDVA (logMAR) | ||||
| Mean ± SD | 0.017±0.052 | 0.022±0.042 | P=0.194a | |
| Range | (−0.097, 0.301) | (0.000, 0.176) | ||
Notes:
Two-sided Wilcoxon two-sample test
two-sided median two-sample test.
Abbreviations: ASA, aberration smart ablation; SD, standard deviation; SE, spherical equivalent; CDVA, corrected distance visual acuity.
The ASA group and the Triple-A group were not significantly different with respect to the preoperative values of sphere, cylinder, SE, and CDVA. However, the Triple-A group had a significantly higher mean age (38 years), compared with ASA group (34 years). A higher age may contribute to a lower accommodation capability and might result in slightly lower UDVA values, when overcorrected.
Predictability
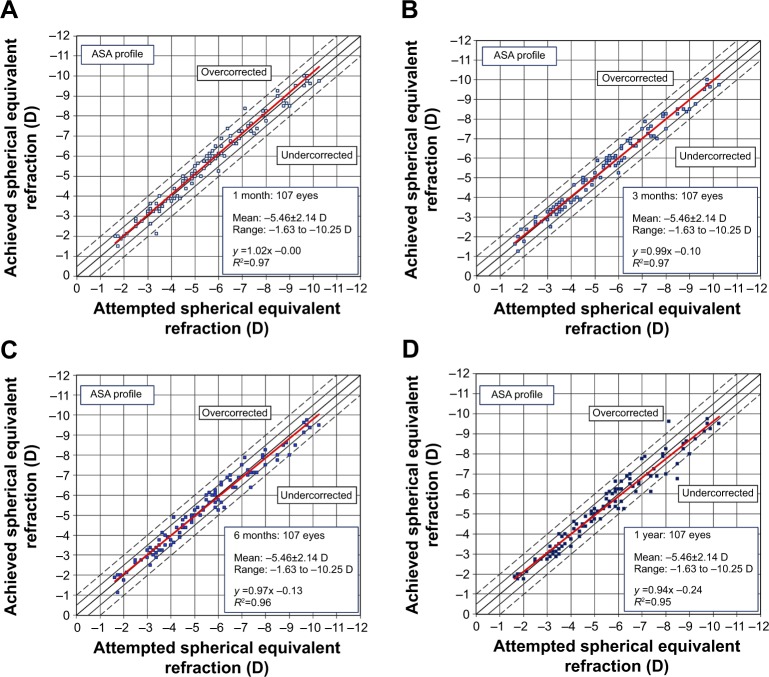
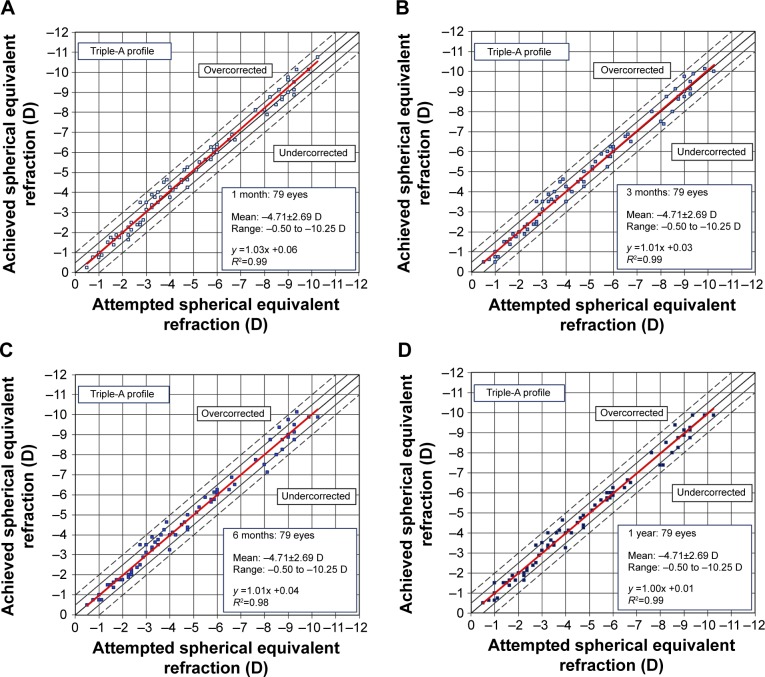
We plotted scattergrams for the achieved SE versus the attempted ones (both at a vertex distance of 12 mm) and analyzed the slope and intercept of the linear regression. The slopes of the fitted curves were comparable for both ASA (Figure 1) and the Triple-A (Figure 2) profiles, with a more stable slope for the Triple-A group, indicating a better predictability pattern. A comparison of the parameters for predictability, as summarized in Table 2, shows that the Triple-A group displays a lower scatter when compared with the ASA profile. The SD of residuals showed no significant difference for the Triple-A and the ASA groups at 1 and 3 months. However, a statistically significant difference in the SD of residuals was observed at 6 months (P=0.0303) and 1 year (P=0.0219), at which time points the Triple-A group had a lower value for SD of residuals. As the SD of residuals is indicative of deviation from the best-fit plot, a lower residual for the Triple-A group indicates a better predictability in the Triple-A group.
Figure 1.
Predictability scattergrams of overall achieved correction versus the attempted correction for the ASA profile at 1 month (A), 3 months (B), 6 months (C), and 1 year (D).
Note: The confidence bands are marked at ±0.5 and ±1 D.
Abbreviation: ASA, aberration smart ablation.
Figure 2.
Predictability scattergrams of overall achieved correction versus the attempted correction for the Triple-A profile at 1 month (A), 3 months (B), 6 months (C), and 1 year (D).
Note: The confidence bands are marked at ±0.5 and ±1 D.
Table 2.
Postoperative analyses of predictability
| Parameter | Time points | ASA (n=107 eyes) | Triple-A (n=79 eyes) | P-value |
|---|---|---|---|---|
| SD of residuals (root MSE) (D) | 1 mo | 0.40 | 0.31 | 0.5462 |
| 3 mo | 0.36 | 0.33 | 0.3404 | |
| 6 mo | 0.40 | 0.34 | 0.0303* | |
| 1 yr | 0.44 | 0.32 | 0.0219* | |
| Slopes (CI) | 1 mo | 1.021 (−0.211; 0.208) | 1.034 (1.009; 1.060) | – |
| 3 mo | 0.988 (0.956; 1.021) | 1.011 (0.983; 1.039) | ||
| 6 mo | 0.966 (0.930; 1.002) | 1.007 (0.978; 1.036) | ||
| 1 yr | 0.939 (0.899; 0.978) | 0.995 (0.968; 1.022) | ||
| Intercept (CI) (D) | 1 mo | 0.00 (−0.21; 0.21) | 0.06 (−0.08; 0.20) | – |
| 3 mo | −0.10 (−0.30; 0.09) | 0.03 (−0.12; 0.18) | ||
| 6 mo | −0.13 (−0.35; 0.08) | 0.04 (−0.11; 0.20) | ||
| 1 yr | −0.24 (−0.47; −0.01) | 0.01 (−0.14; 0.16) |
Notes:
P<0.05 was considered to be significant. For SD of residuals comparison, the Siegel–Tukey test was used.
Abbreviations: ASA, aberration smart ablation; SD, standard deviation; root MSE, root mean square error; CI, confidence interval; Mo, months; yr, year.
The regression slopes (Table 2) were comparable within the Triple-A and ASA groups for 1 and 3 months. At later time points (6 months and 1 year), the slopes were closer to the ideal slope of 1.0 for the Triple-A group (1.007 and 0.995, respectively), when compared with the ASA group (0.966 and 0.939, respectively). As expected, the achieved refractive outcome correlated well with the attempted correction in both the ASA and Triple-A groups.
The 1-year postoperative slope for the Triple-A group (0.995) was better than that of the ASA group (0.939), indicating a higher undercorrection of 6.1% for the ASA group in comparison to an undercorrection of only 0.5% for the Triple-A group.
The scattergrams for the ASA group (Figure 1) indicated a slight decrease in the correlation coefficient R2 with time. The R2 values for both the 1 month and 3 months follow-up time points were 0.97, which decreased to 0.96 and 0.95, respectively, at 6 months and 1 year. On the other hand, the R2 values for the Triple-A group (Figure 2) indicated a higher correlation coefficient between the attempted and the achieved correction levels. The R2 for the Triple-A group was stable at all time points and had a higher value of 0.99 at 1 year when compared to the R2 value of 0.95 for the ASA group at the same time period.
Accuracy
The distribution of postoperative SE refraction is shown in Figure 3. Most eyes (≥90%) treated in the Triple-A group were corrected within the range of ±0.5 D. In concordance with these results, the preoperative mean MRSE changed from −4.73±2.65 to −0.03±0.32 D at 1 year (MRSE – SE target). The MRSE minus SE target values for the Triple-A group remained consistent (≥90% of eyes) within ±0.5 D (Table 3).
Figure 3.
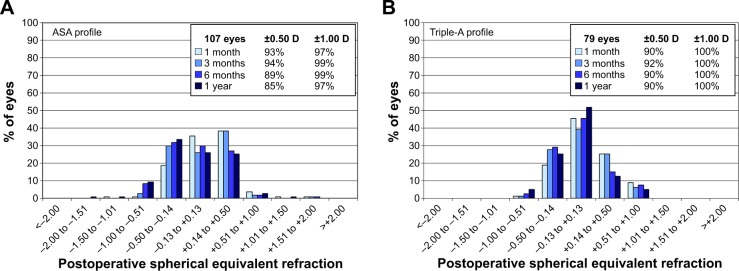
Accuracy plots for the ASA group (A) and the Triple-A group (B).
Note: The insets indicate the percentage of eyes corrected within ±0.5 and ±1.0 D.
Abbreviation: ASA, aberration smart ablation.
Table 3.
Accuracy comparison between ASA and Triple-A profiles
| Parameter | Time points | ASA (n=107 eyes) | Triple-A (n=79 eyes) | P-value | |
|---|---|---|---|---|---|
| % with SE within ±0.5 D (%) | 1 mo | 93 | 90 | 0.4215a | |
| 3 mo | 94 | 92 | 0.7641a | ||
| 6 mo | 89 | 90 | 1.0000a | ||
| 1 yr | 85 | 90 | 0.3820a | ||
| % with SE within ±1.0 D (%) | 1 mo | 97 | 100 | 0.2630a | |
| 3 mo | 99 | 100 | 1.0000a | ||
| 6 mo | 99 | 100 | 1.0000a | ||
| 1 yr | 97 | 100 | 0.2630a | ||
| P (mean) | P (variance) | ||||
| Postoperative SE – target (mean ± SD) | 1 mo | 0.11±0.40 D | 0.10±0.32 D | 0.731b | 0.784c |
| 3 mo | 0.04±0.36 D | 0.03±0.33 D | 0.760b | 0.427c | |
| 6 mo | −0.05±0.41 D | −0.01±0.34 D | 0.591b | 0.062c | |
| 1 yr | −0.09±0.46 D | −0.03±0.32 D | 0.518d | 0.007c | |
Notes:
Statistical analysis between the Triple-A and ASA groups was performed using Fisher’s exact test
two-sided Wilcoxon two-sample test
two-sided Siegel–Tukey test
two-sided median two-sample test.
Abbreviations: ASA, aberration smart ablation; SE, spherical equivalent; SD, standard deviation.
For the ASA group, the accuracy with respect to percentage within ±0.5 D was initially better at 1 and 3 months, as 93% and 94% of respective treated eyes showed a correction in the mean MRSE within ±0.5 D. At 6 months and 1 year, the percentage of eyes corrected within ±0.5 D decreased to 89% and 85%, respectively.
As seen in Figure 3B, in the Triple-A group, all eyes achieved a postoperative SE refraction within ±1 D for all time periods in comparison to 97%, 99%, 99%, and 97% of eyes for ASA (Figure 3A) at 1 month, 3 months, 6 months, and 1 year, respectively. However, this difference was not statistically significant. The same holds true for the percentage of eyes within ±0.5 D after 6 months and 1 year.
The variance of 1-year postoperative MRSE minus SE target (Table 3) for the Triple-A group (−0.03±0.32 D) was significantly lower than for the ASA group (−0.09±0.46 D) (P=0.007), indicating a lower scatter and better accuracy for the Triple-A group.
Stability
As seen in Figure 4, the percentage of eyes showing a change in SE refraction of >0.5 D between 1 and 6 months postoperatively was 7% for the ASA group (Figure 4A) and 5% for the Triple-A group (Figure 4B). However, these changes in SE refraction were not statistically significant. For 6 months to 1 year, the percentage of eyes with a deviation of >0.5 D was 3% for both groups.
Figure 4.
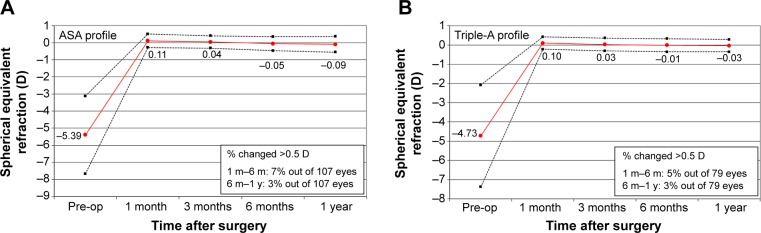
Stability plots for the ASA group (A) and the Triple-A group (B).
Abbreviation: ASA, aberration smart ablation.
Both groups displayed a similar pattern of initial overcorrection at 1 month. This progressively changed to a slight undercorrection for both groups at 1 year, whereas the regression was lower for the Triple-A group.
Table 4 shows differences in MRSE minus SE target per month. The Δ (MRSE – SE target) per month values for the Triple-A group were better than those for the ASA group across all time intervals. Best stability was observed at 6 months and 1 year, at which time points there was a small change in the Δ for both groups.
Table 4.
Comparison of stability parameters of ASA and Triple-A groups
| Parameter | Time interval | ASA (n=107 eyes) | Triple-A (n=79 eyes) |
P-value
|
|
|---|---|---|---|---|---|
| P (mean) | P (SD) | ||||
| Δ (SE – target)/month (mean ± SD) | 1 yr–6 mo | −0.04±0.22 | −0.02±0.16 | 0.3790a | 0.1563b |
| 6–3 mo | −0.09±0.22 | −0.04±0.15 | 0.0885a | 0.0882b | |
| 6–1 mo | −0.16±0.33 | −0.11±0.23 | 0.0917a | 0.1595b | |
| Changed >0.5 D (%) | 1–6 mo | 7 | 5 | 0.762c | |
| 6 mo–1 yr | 3 | 3 | 1.000c | ||
Notes:
P<0.05 was considered significant
two-sided Wilcoxon two-sample test
two-sided Siegel–Tukey test
Fisher’s exact test.
Abbreviations: ASA, aberration smart ablation; SE, spherical equivalent; SD, standard deviation.
Astigmatism
Figure 5 indicates pre- and postoperative refractive astigmatism for the ASA (Figure 5A) and Triple-A (Figure 5B) groups. The ranges for preoperative cylinder, as indicated in Table 1, were [0, −4.50] and [0, −4.25] D for the Triple-A and ASA groups, respectively. Although the preoperative mean cylinder was slightly higher for the Triple-A group (eg, −1.28±1.02 D vs −1.07±1.00 D for ASA), the postoperative results indicate a better outcome in the Triple-A group compared with the postoperative outcomes in the ASA group.
Figure 5.
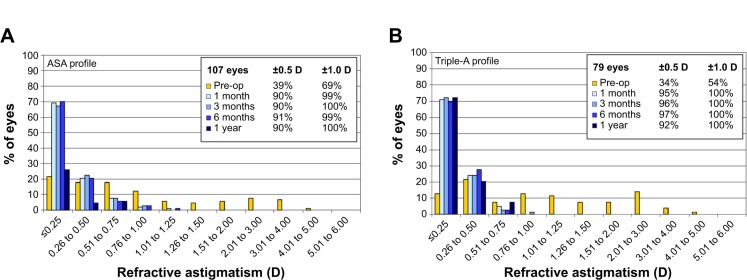
Refractive astigmatism profiles of the ASA group (A) and the Triple-A group (B).
Abbreviation: ASA, aberration smart ablation.
All eyes in the Triple-A group achieved a refractive astigmatism of ≤1.0 D for all postoperative follow-up time points. These results were comparable to the ASA group, in which 99%, 100%, 99%, and 100% of treated eyes reported a refractive astigmatism of ≤1.0 D after 1 month, 3 months, 6 months, and 1 year, respectively. In the time period 1 month to 1 year, the Triple-A group showed a better percentage of eyes with a postoperative cylinder in the range of ±0.5 D.
The Triple-A group showed better astigmatism improvements at all time points. The results of pre- and postoperative cylinder in the Triple-A and the ASA groups are indicated in Table 5.
Table 5.
Comparison of pre- and postoperative cylinder
| Parameter | Time points | ASA (n=107 eyes) | Triple-A (n=79 eyes) | P-value | |
|---|---|---|---|---|---|
| % with cylinder within ±0.5 D | Preoperative | 39 | 34 | – | |
| 1 mo | 90 | 95 | 0.278a | ||
| 3 mo | 90 | 96 | 0.158a | ||
| 6 mo | 91 | 97 | 0.074a | ||
| 1 yr | 90 | 92 | 0.613a | ||
| % with cylinder within ±1.0 D | Preoperative | 69 | 54 | – | |
| 1 mo | 99 | 100 | 1.000a | ||
| 3 mo | 100 | 100 | NA | ||
| 6 mo | 99 | 100 | 1.000a | ||
| 1 yr | 100 | 100 | NA | ||
|
| |||||
| P (mean) | P (SD) | ||||
| Postoperative CYL – target (mean ± SD) | 1 mo | 0.22±0.29 D | 0.23±0.23 D | 0.0602b | 0.0197c,* |
| 3 mo | 0.21±0.29 D | 0.21±0.24 D | 0.1233b | 0.0127c,* | |
| 6 mo | 0.21±0.30 D | 0.22±0.23 D | 0.3622d | 0.0507c | |
| 1 yr | 0.25±0.31 D | 0.23±0.24 D | 0.1243b | 0.0014c,* | |
Notes:
P<0.05 was considered significant.
Fisher’s exact test
two-sided median two-sample test
two-sided Siegel–Tukey test
two-sided Wilcoxon two-sample test.
Abbreviations: ASA, aberration smart ablation; SD, standard deviation; Cyl, cylinder; NA, not applicable.
The mean cylinder in both groups was not significantly different. However, the standard deviations of the means were significantly higher for the ASA group at 1 month (P=0.0197), 3 months (P=0.0127), and 1 year (P=0.0014). A lower standard deviation of the means indicates a lower scatter in the Triple-A group in comparison to the ASA group, implying that the Triple-A group showed better astigmatism improvements over time.
Safety
No eye in either group lost more than one Snellen line of corrected distance visual acuity (Figure 6). In the ASA group, the percentage of eyes losing one Snellen line was 1%, 2%, 0%, and 3% at 1 month, 3 months, 6 months, and 1 year, respectively (Figure 6A). The Triple-A group showed a loss of one Snellen line in 1%, 0%, 1%, and 0% of eyes at 1 month, 3 months, 6 months, and 1 year, respectively (Figure 6B).
Figure 6.
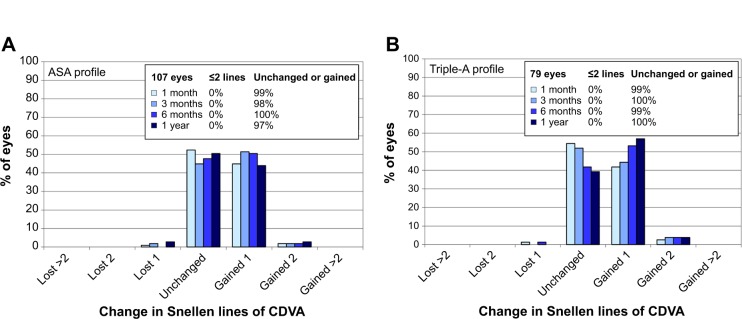
Safety profiles for the ASA group (A) and the Triple-A group (B).
Note: The safety profiles are indicated by the change in Snellen lines of corrected distance visual acuity.
Abbreviations: ASA, aberration smart ablation; CDVA, corrected distance visual acuity.
As shown in Table 6, in the Triple-A group, 43%, 48%, 57%, and 61% of eyes showed a gain of Snellen lines at 1 month, 3 months, 6 months, and 1 year, respectively. The respective percentages were 47%, 53%, 52%, and 47% for the ASA group.
Table 6.
Safety parameters for the ASA and Triple-A groups
| Parameter | Time points | ASA (n=107 eyes) | Triple-A (n=79 eyes) | P-value | |
|---|---|---|---|---|---|
| Lines gained (%) | 1 mo | 47 | 43 | 0.6565a | |
| 3 mo | 53 | 48 | 0.5534a | ||
| 6 mo | 52 | 57 | 0.5545a | ||
| 1 yr | 47 | 61 | 0.0746a | ||
| Safety index | 1 mo | 1.12 | 1.11 | – | |
| 3 mo | 1.13 | 1.13 | – | ||
| 6 mo | 1.13 | 1.15 | – | ||
| 1 yr | 1.11 | 1.16 | – | ||
| P (mean) | P (SD) | ||||
| Safety index per eye | 1 mo | 1.11±0.14 | 1.11±0.14 | 0.7048b | 0.4365c |
| 3 mo | 1.13±0.15 | 1.13±0.14 | 0.6069b | 0.3044c | |
| 6 mo | 1.14±0.14 | 1.15±0.14 | 0.5490b | 0.9950c | |
| 1 yr | 1.12±0.16 | 1.16±0.14 | 0.0498b,* | 0.0869c | |
Notes:
P<0.05 was considered significant
Fisher’s exact test
two-sided Wilcoxon two-sample test
two-sided Siegel–Tukey test.
Abbreviations: ASA, aberration smart ablation; SD, standard deviation.
One hundred and seven eyes in the ASA group and 79 eyes in the Triple-A group were analyzed for safety index calculations (Table 6). The safety index was higher for the Triple-A group at 6 months and 1 year, compared with the ASA group. At the earlier time points of 1 and 3 months, the safety indices of both groups were comparable. The Triple-A profile achieved a significantly better safety index per eye (1.16±0.14) at 1 year, compared with the ASA group (1.12±0.16, P=0.0498).
Efficacy
Figure 7 shows the efficacy expressed as Snellen visual acuity. A preoperative CDVA of 20/20 or better was observed in 85% and 78% eyes in the ASA (Figure 7A) and the Triple-A (Figure 7B) groups, respectively. A postoperative UDVA of 20/20 or better at 1 month, 3 months, 6 months, and 1 year was observed in 76%, 75%, 75%, and 73% of eyes, respectively, in the Triple-A group and 64%, 68%, 66%, and 72% of eyes, respectively, in the ASA group.
Figure 7.
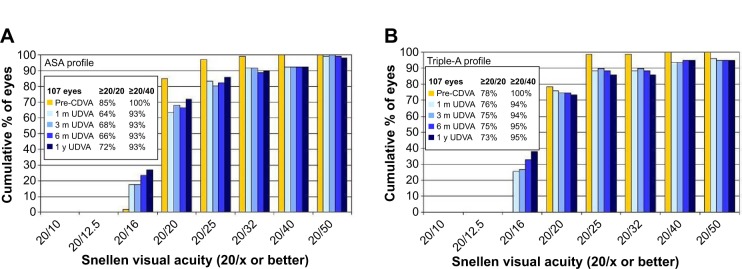
Snellen visual acuity of the ASA group (A) and the Triple-A group (B).
Abbreviation: ASA, aberration smart ablation.
A preoperative CDVA of 20/40 or better was observed in 100% of eyes in both groups. In the ASA group, a postoperative UDVA of 20/40 or better was consistently at 93% across all follow-up time points. In the Triple-A group, a postoperative UDVA of 20/40 or better at 1 and 3 months was observed in 94% of the treated eyes, which showed a slight increase to 95% at both 6 months and 1 year.
The efficacy index of the Triple-A group was better than that of the ASA group across all follow-up time points (Table 7). For the Triple-A group, the efficacy index was 0.96 at 1 and 3 months and 0.97 at 6 months and 1 year. In the ASA group, the efficacy index at 1 month, 3 months, 6 months, and 1 year was 0.92, 0.93, 0.93, and 0.95, respectively.
Table 7.
Efficacy index and efficacy index per eye for ASA and Triple-A groups
| Parameter | Time points | ASA (n=107 Eyes) | Triple-A (n=79 Eyes) | P-value | |
|---|---|---|---|---|---|
| % with UDVA ≥20/20 | Pre-CDVA | 85 | 78 | – | |
| 1 mo | 64 | 76 | 0.0798a | ||
| 3 mo | 68 | 75 | 0.4143a | ||
| 6 mo | 66 | 75 | 0.2590a | ||
| 1 yr | 72 | 73 | 0.8691a | ||
| Efficacy index | 1 mo | 0.92 | 0.96 | – | |
| 3 mo | 0.93 | 0.96 | – | ||
| 6 mo | 0.93 | 0.97 | – | ||
| 1 yr | 0.95 | 0.97 | – | ||
| P (mean) | P (SD) | ||||
| Efficacy index per eye | 1 mo | 0.954±0.239 | 1.018±0.258 | 0.0186b,* | 0.7777c |
| 3 mo | 0.963±0.243 | 1.018±0.272 | 0.0651b | 0.9236c | |
| 6 mo | 0.968±0.252 | 1.032±0.281 | 0.0323b,* | 0.9766c | |
| 1 yr | 0.992±0.250 | 1.041±0.277 | 0.0959b | 0.5694c | |
Notes:
P<0.05 was considered significant
Fisher’s exact test
two-sided Wilcoxon two-sample test
two-sided Siegel–Tukey test.
Abbreviations: ASA, aberration smart ablation; UDVA, uncorrected distance visual acuity; CDVA, corrected distance visual acuity; SD, standard deviation.
Additionally, the mean efficacy index per eye was significantly higher for the Triple-A group at 1 month (1.018±0.258 vs 0.954±0.239, Triple-A vs ASA, P=0.0186) and 6 months (1.032±0.281 vs 0.968±0.252, Triple-A vs ASA, P=0.0323). Overall, the efficacy index as well as the efficacy index per eye showed an increasing trend for both the Triple-A and the ASA group, whereas the Triple-A group had more stability.
Discussion
Aspherical ablation profiles aim to reduce spherical aberrations, which have been associated with corneal refractive surgical procedures. In this retrospective study using the MEL 80 excimer laser (Carl Zeiss Meditec AG), we analyzed the outcomes using two different ablation profiles (ASA and Triple-A). The traditionally used ASA profile is an aspherically optimized profile with an energy correction function generally used for moderate-to-high corrections. The new Triple-A profile combines the properties of the ASA profile with an enhanced energy correction, which increases the aspherical component with the correction strength that can be implemented for corrections in all ranges.
In our retrospective analysis, patient demographics were similar for the ASA and Triple-A profiles, with no significant differences in parameters except age. The mean age for the Triple-A group was higher than that for the ASA group. Some studies report that age influences the outcomes, as younger patients report better outcomes because the accommodation capacity reduces with increased age, resulting in slightly worse UDVA outcomes.18 Other studies report no association between the age and outcome. In our results, we observed a better UDVA outcome with Triple-A than with ASA despite the mean older age.
A comparison of predictability plots indicated a significantly lower scatter for the Triple-A group at 6 months and 1 year. The Triple-A group had excellent refractive predictability demonstrated by a significantly lower residual SD ranging from 0.32 to 0.34 D in this period, which was significantly lower than the SD of residuals for the ASA group (0.40–0.44 D). The Triple-A group also had better accuracy, with more than 90% of eyes corrected within ±0.5 D across the 6-month to 1-year time interval. Also, 100% of eyes in the Triple-A group were corrected within ±1.0 D in comparison to 97%–99% for the ASA group in the 6-month to 1-year time interval. Furthermore, the Triple-A group also displayed a significantly lower variance of the means for postoperative MRSE minus SE target for the 6-month and 1-year postoperative follow-up time points.
A comparison between the stability for the two groups indicated a lower Δ (MRSE – SE target) per month for the Triple-A group across all pairs of time intervals. Also, a significantly lower scatter for the standard deviation was observed for the Triple-A group when compared to the ASA group.
The Triple-A group also displayed a better correction in astigmatism across all follow-up time points, as indicated by the standard deviations of the postoperative cylinder (Cyl-target), which were statistically significant at 1 month, 3 months, and 1 year.
The Triple-A group had a significantly better safety index 1 year postoperatively and a comparable safety index at all other time points with the ASA group.
A better efficacy index was also observed for the Triple-A group at all follow-up time points, and statistical significance was achieved at 1 and 6 months. The UDVA comparison indicated a better profile for the Triple-A group, in which 76% of eyes achieved a 1-month postoperative visual acuity of 20/20 or better in comparison to 64% for the ASA group. This trend, however, showed improvement over time for the ASA profile, while the Triple-A profile indicated a slight decrease with time. At 1 year, an uncorrected visual acuity of 20/20 or better was achieved in 72% and 73% eyes, respectively, for the ASA and Triple-A groups. At 1 year, a UDVA of 20/40 or better was achieved in 95% and 97% eyes in the ASA and Triple-A groups, respectively.
Taken together, our data indicate a significant improvement in outcomes with the Triple-A profile when compared to the conventional aspherically optimized ASA profile.
In the past, comparative studies have been reported between the Technolas® 217z (Bausch + Lomb Technolas, Munich, Germany) and Wavelight® Allegretto Wave® Eye-Q 400 Hz excimer lasers (WaveLight GmbH, Erlangen, Germany), in which comparable outcomes were achieved in terms of uncorrected visual acuity, MRSE, and the safety and efficacy indices. In addition, the Wavelight® Allegretto produced slightly lower astigmatism and greater refractive predictability than did the Technolas® excimer laser. In our study, we achieved at 3 months an efficacy index per eye of 1.02±0.27 with the new Triple-A profile, which is better than the efficacy indices achieved in a comparison of Allegretto (0.97±0.13) and Technolas® (0.97±0.17) excimer laser platforms.17 In general, modern excimer lasers show very good results, with differences only in specific details.
The postoperative percentage of eyes with UDVA >20/20 ranged from 73% to 76% with the Triple-A profile in our study. This value is lower than those reported by several studies.17,19–21 The UDVA results from different studies can be compared in terms of the efficacy index instead of a direct comparison of UDVA, as UDVA results are subject to individual differences introduced by physicians. Physicians determine the point at which the patient cannot read properly, introducing a subjective element in the results, which is largely responsible for huge disparities in reported values.
The efficacy index per eye with the Triple-A profile is better than that reported in a comparison of the Wavelight® Allegretto Wave® and Technolas® by Han et al17 and with the Schwind Amaris® platform (SCHWIND eye-tech-solutions GmbH, Kleinostheim, Germany) used by Alio et al.19 The efficacy index per eye with the Triple-A profile (1.02–1.04) in our study is also slightly better than the efficacy index per eye calculated with data from the US Food and Drug Administration (FDA) clinical trial on the Carl Zeiss MEL 80 platform (0.99).21
The postoperative mean residual astigmatism reported in a comparative study of Wavelight® Allegretto and Technolas® was 0.33±0.3 and 0.44±0.52 D, respectively, with Wavelight® Allegretto performing significantly better than the Technolas® platform. At 3 months in our study, the Triple-A profile had a mean residual astigmatism of 0.21±0.24 D, which was much lower than the residual astigmatism reported for the Wavelight® Allegretto. However, Arbelaez et al20 reported an astigmatism of 0.17±0.21 D at 6 months on a Schwind Amaris® 500 platform, which is lower than the values for the Triple-A group. It is worthwhile to mention that the mean preoperative cylinder for the Arbelaez study was 0.69 D, which is much lower than the mean preoperative cylinder for the Triple-A group (1.3 D). Another study by Alio et al19 conducted on the Schwind Amaris® 500 platform reported a 3- to 6-month postoperative mean astigmatism of −0.36±0.47 D, which is worse than the outcomes achieved with the Triple-A profile. Additionally, the mean residual astigmatism is also better in comparison to the results of the FDA trial of MEL 80, in which a postoperative cylinder of 0.36±0.35 was reported.21
The Triple-A profile has excellent cylinder outcomes also in terms of percentages within ±0.5 and ±1.0 D, which are better than some of the more advanced devices with the latest profile designs. The cylinder data for the Triple-A profile are better than the outcomes reported by Schumacher et al21 using a Wavelight® Concerto (WaveLight GmbH) with Allegretto ray tracing for the treatment of astigmatism. The outcomes of the cylinder data are also better than the outcomes of a comparative study reported by Han et al17 comparing the Wavelight® Allegretto wavefront-guided platform with the Technolas® platform. With respect to astigmatism correction, the Triple-A profile showed 100% of eyes with a postoperative cylinder within ≤1.0 D and 92%–97% of eyes within ≤0.5 D. A previous study by Schallhorn et al22 reported 90% of treated eyes with a postoperative cylinder of ≤0.5 D at 1 month. The Triple-A profile in our study has a slightly better percentage of 95% at 1 month. However, in the study by Schallhorn et al22 on a wavefront-guided profile with a new aberrometer, a better postoperative mean astigmatism (0.16±0.25 D) was observed than with the Triple-A profile in our study (0.23±0.23 D) at 1 month, whereas the Triple-A has a slightly lower scatter.
The Triple-A profile used in our study shows better accuracy than the ASA profile, with 90%–92% of eyes within ±0.5 D and 100% of eyes within ±1.0 D. These results are indicative of better accuracy for the Triple-A profile in comparison to that with the latest ray-tracing profile reported by Schumacher et al21 (87.2%–87.4% within ±0.5 D and 96.7%–97.7% within ±1.0 D). Published data from the Wavelight® Allegretto clinical trial by the FDA23 showed 84.8%–85.6% and 96.7%–97.7% of analyzed eyes within ±0.5 and ±1.0 D, respectively. With the new Triple-A profile, we also achieved a better accuracy than those in the following published reports: The MEL 80 trial from the FDA24 (at 6 months, 76.8% and 95.5% within ±0.5 D and within ±1.0 D, respectively), the VISX Star trial from the FDA18 (76.4%–79.1% and 95.5%–97.4% within ±0.5 D and within ±1.0 D, respectively), and the study published by Alio et al19 (69%–84.3% and 89%–90.2% within ±0.5 and ±1.0 D, respectively) on the Schwind Amaris® platform. In another study on the Schwind Amaris® 500 platform, Arbelaez et al20 reported better accuracy, with 96% of treated eyes within ±0.5 D, in comparison to 90% for the Triple-A group at 6 months; however, in the same study, only myopic cases up to −7.50 D were treated, whereas in our study using Triple-A, myopic cases up to −9.25 D were treated.
Also, in our study, the 3- to 6-month postoperative stability of the Triple-A profile (−0.04±0.15 D) was slightly better than the stability reported in the FDA trial with Allegretto (−0.05±0.3 D)23 and in the FDA trial with MEL 80 (−0.06±0.38 D).24 However, Arbelaez et al20 using the same platform, showed a better stability (0.03 D) for the 3- to 6-month time interval postoperation.
Taken together, the new aspherically optimized Triple-A profile, using an enhanced energy correction feature, produces better refractive outcomes in comparison to the conventionally used ASA profile. However, the retrospective, nonrandomized nature of this study is a limitation. Another notable difference between the two groups is the method of flap creation: The flap was created using the VisuMax® femtosecond laser for the Triple-A group and with a microkeratome for the ASA group for all but eleven eyes. We did not evaluate the contribution of the method of flap creation on the refractive outcomes. The microkeratome is not expected to have a large impact on the refractive outcomes.25 Future studies would overcome limitations of data analyses due to differences in the surgical protocol.
Conclusion
In conclusion, the new aspherically optimized Triple-A profile showed better outcomes compared to the conventional aspherically optimized ASA profile based on better predictability as a result of a significantly lower scatter, better accuracy (with 100% of eyes corrected within ±0.5 D at 1 year), excellent cylinder corrections, better safety index per eye at later time points, and better efficacy index across all time points. In addition, the new aspherically optimized Triple-A profile also demonstrated outcomes comparable with or better than other platforms reported in literature.
Our study was based on myopic treatments with an excimer laser platform running on a repetition rate of 250 Hz. An interesting subject of future studies will be to investigate the outcomes of the Triple-A profile on a platform with a higher repetition rate of 500 Hz (MEL®90) and to investigate hyperopic and mixed astigmatism corrections, or to conduct a detailed analysis of induced spherical aberrations for Triple-A.
Footnotes
Disclosure
Matthias Wottke and Dr Georg Sluyterman van Langeweyde are employees of Carl Zeiss Meditec AG (Jena, Germany), and Dr Bertram Meyer is a consultant and clinical investigator for Carl Zeiss Meditec AG (Jena, Germany). The authors report no other conflicts of interest in this work.
References
- 1.Schallhorn SC, Amesbury EC, Tanzer DJ. Avoidance, recognition, and management of LASIK complications. Am J Ophthalmol. 2006;141(4):733–739. doi: 10.1016/j.ajo.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 2.Moreno-Barriuso E, Lloves JM, Marcos S, Navarro R, Llorente L, Barbero S. Ocular aberrations before and after myopic corneal refractive surgery: LASIK-induced changes measured with laser ray tracing. Invest Ophthalmol Vis Sci. 2001;42(6):1396–1403. [PubMed] [Google Scholar]
- 3.Thibos LN, Hong X, Bradley A, Applegate RA. Accuracy and precision of objective refraction from wavefront aberrations. J Vis. 2004;23:4. doi: 10.1167/4.4.9. (4):329–351. [DOI] [PubMed] [Google Scholar]
- 4.Mrochen M, Jankov M, Bueeler M, Seiler T. Correlation between corneal and total wavefront aberrations in myopic eyes. J Refract Surg. 2003;19:104–112. doi: 10.3928/1081-597X-20030301-04. [DOI] [PubMed] [Google Scholar]
- 5.Alió JL, Belda JI, Osman AA, Shalaby AM. Topography-guided laser in situ keratomileusis (TOPOLINK) to correct irregular astigmatism after previous refractive surgery. J Refract Surg. 2003;19:516–527. doi: 10.3928/1081-597X-20030901-06. [DOI] [PubMed] [Google Scholar]
- 6.Mrochen M, Kaemmerer M, Seiler T. Clinical results of wavefront-guided laser in situ keratomileusis 3 months after surgery. J Cataract Refract Surg. 2001;27:201–207. doi: 10.1016/s0886-3350(00)00827-0. [DOI] [PubMed] [Google Scholar]
- 7.Mrochen M, Donitzky C, Wüllner C, Löffler J. Wavefront-optimized ablation profiles: theoretical background. J Cataract Refract Surg. 2004;30:775–785. doi: 10.1016/j.jcrs.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Koller T, Iseli HP, Hafezi F, Mrochen M, Seiler T. Q-factor customized ablation profile for the correction of myopic astigmatism. J Cataract Refract Surg. 2006;32:584–589. doi: 10.1016/j.jcrs.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 9.Marcos S, Barbero S, Llorente L, Merayo-Lloves J. Optical response to LASIK surgery for myopia from total and corneal aberration measurements. Invest Ophthalmol Vis Sci. 2001;42:3349–3356. [PubMed] [Google Scholar]
- 10.Mrochen M, Seiler T. Influence of corneal curvature on calculation of ablation patterns used in photorefractive laser surgery. J Refract Surg. 2001;17:S584–S587. doi: 10.3928/1081-597X-20010901-15. [DOI] [PubMed] [Google Scholar]
- 11.Munnerlyn CR, Koons SJ, Marshall J. Photorefractive keratectomy: a technique for laser refractive surgery. J Cataract Refract Surg. 1988;14:46–52. doi: 10.1016/s0886-3350(88)80063-4. [DOI] [PubMed] [Google Scholar]
- 12.Marcos S, Cano D, Barbero S. Increase in corneal asphericity after standard laser in situ keratomileusis for myopia is not inherent to the Munnerlyn algorithm. J Refract Surg. 2003;19:S592–S596. doi: 10.3928/1081-597X-20030901-17. [DOI] [PubMed] [Google Scholar]
- 13.Cano D, Barbero S, Marcos S. Comparison of real and computer-simulated outcomes of LASIK refractive surgery. J Opt Soc Am A Opt Image Sci Vis. 2004;21:926–936. doi: 10.1364/josaa.21.000926. [DOI] [PubMed] [Google Scholar]
- 14.Canals M, Elies D, Costa-Vila J, Coret A. Comparative study of ablation profiles of six different excimer lasers. J Refract Surg. 2004;20:106–109. doi: 10.3928/1081-597X-20040301-01. [DOI] [PubMed] [Google Scholar]
- 15.Waring GO, 3rd, Reinstein DZ, Dupps WJ, Jr, et al. Standardized graphs and terms for refractive surgery results. J Refract Surg. 2011;27:7–9. doi: 10.3928/1081597X-20101116-01. [DOI] [PubMed] [Google Scholar]
- 16.Taneri S, Feit R, Azar DT. Safety, efficacy, and stability indices of LASEK correction in moderate myopia and astigmatism. J Cataract Refract Surg. 2004;30:2130–2137. doi: 10.1016/j.jcrs.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 17.Han DC, Chen J, Htoon HM, Tan DT, Mehta JS. Comparison of outcomes of conventional WaveLight(®) Allegretto Wave(®) and Technolas(®) excimer lasers in myopic laser in situ keratomileusis. Clin Ophthalmol. 2012;6:1159–1168. doi: 10.2147/OPTH.S29660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.FDA Trial VISX Star Excimer Laser System Models S2 and S3, PMA P930016/S14, Summary of Safety and Effectiveness. Data for a Supplemental Premarket Approval Application. [Accessed March 26, 2014]. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf/P930016S014b.pdf.
- 19.Alio JL, Vega-Estrada A, Piñero DP. Laser-assisted in situ keratomileusis in high levels of myopia with the amaris excimer laser using optimized aspherical profiles. Am J Ophthalmol. 2011;52:954–963. doi: 10.1016/j.ajo.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Arbelaez MC, Aslanides IM, Barraquer C, et al. LASIK for myopia and astigmatism using the SCHWIND AMARIS excimer laser: an international multicenter trial. J Refract Surg. 2010;26(2):88–98. doi: 10.3928/1081597X-20100121-04. [DOI] [PubMed] [Google Scholar]
- 21.Schumacher S, Seiler T, Cummings A, Maus M, Mrochen M. Optical ray tracing-guided laser in situ keratomileusis for moderate to high myopic astigmatism. J Cataract Refract Surg. 2012;38:28–34. doi: 10.1016/j.jcrs.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Schallhorn SC, Venter JA. One-month outcomes of wavefront-guided LASIK for low to moderate myopia with the VISX STAR S4 laser in 32,569 eyes. J Refract Surg. 2009;25:S634–S641. doi: 10.3928/1081597X-20090611-02. [DOI] [PubMed] [Google Scholar]
- 23.FDA MEL 80 PMA P060004. [Accessed March 26, 2014]. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf2/P020050S004b.pdf.
- 24.FDA MEL 80 PMA P060004. [Accessed March 26, 2014]. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf6/P060004b.pdf.
- 25.Cosar CB, Gonen T, Moray M, Sener AB. Comparison of visual acuity, refractive results and complications of femtosecond laser with mechanical microkeratome in LASIK. Int J Ophthalmol. 2013;6(3):350–355. doi: 10.3980/j.issn.2222-3959.2013.03.18. [DOI] [PMC free article] [PubMed] [Google Scholar]