Abstract
In addition to providing complete postnatal nutrition, breast milk is a complex biofluid that delivers bioactive components for the growth and development of the intestinal and immune systems. Lactation is a unique opportunity to understand the role of diet in shaping the intestinal environment including the infant microbiome. Of considerable interest is the diversity and abundance of milk glycans that are energetically costly for the mammary gland to produce yet indigestible by infants. Milk glycans comprise free oligosaccharides, glycoproteins, glycopeptides, and glycolipids. Emerging technological advances are enabling more comprehensive, sensitive, and rapid analyses of these different classes of milk glycans. Understanding the impact of inter- and intraindividual glycan diversity on function is an important step toward interventions aimed at improving health and preventing disease. This review discusses the state of technology for glycan analysis and how specific structure-function knowledge is enhancing our understanding of early nutrition in the neonate.
Keywords: bifidobacteria, breast milk glycans, glycomics, immunity, intestinal barrier function
INTRODUCTION
The first several months of life are a unique window in time to understand how diet affects the growth, development, and protection of the neonate. The gastrointestinal tract is a focal point for the remarkable transitions that occur from birth through weaning. As a result of 120 million years of evolution, mammals have acquired a means of providing complete postnatal nutrition and delivery of bioactive components for growth and maturation—lactation (13). Notably, lactation is providing all these components precisely during the period in which infants develop their innate immunity and gut microbiota.
Breastfeeding is associated with overt benefits to the neonate. In a thorough review of over 400 individual studies, breastfeeding was associated with a reduction in the risk of acute ear infections, asthma (in young children), atopic dermatitis, gastrointestinal infections, respiratory tract diseases, obesity, type 1 and 2 diabetes, childhood leukemia, sudden infant death syndrome in term infants, and necrotizing enterocolitis (NEC) in preterm infants (66). However, one of the long-standing research challenges has been to understand how specific structures delivered within breast milk can explain its diverse functions in vivo. The era of integrating multi-omic technologies and data sets is enabling more comprehensive analyses. Understanding the triad of diet, immunity, and the intestinal microbial ecosystems represents a new frontier for early infant nutrition.
One of the most striking features of breast milk is the diversity and abundance of complex glycans that include free human milk oligosaccharides (HMOs): glycoproteins, glycopeptides, and glycolipids. More striking is that these diverse glycan structures are indigestible to the infant and can reach the large intestine; they can often be found in the stool (32). These diverse milk glycans serve many functions, including protection and development ranging from selectively enriching gut bifidobacteria; prophylactically binding bacteria, viruses, and toxins; promoting the immune system; and enhancing intestinal epithelial barrier function. Taken together, milk glycan functions shape the intestinal microbiome, from the sterile uterine environment through the chaotic introduction of environmental bacteria at birth, through a stable milk-oriented microbiome (MOM) prior to the transition to a more adult-like phenotype after weaning (152). This presents an opportunity to study the structure-function relationships between human milk glycans and their impact on specific aspects of intestinal development. However, elucidating these relationships cannot be achieved without the comprehensive and accurate measurement of the structures and compositions of milk, examination of how these elements are degraded in the gastrointestinal tract, and determination of how they interact in the intestinal environment.
Advances in analytical chemistry—which have made it possible to accurately and comprehensively measure the free oligosaccharides and those bound to proteins, peptides, and lipids in milk—coupled to the powerful and enabling tool sets of microbial meta-genomic, metatranscriptomic, and metabolomics in vitro and in vivo provide insight into how the mother-infant dyad functions to protect the vulnerable neonate. Milk glycans are variable across lactation and among women. This milk glycan diversity has been found to influence immunity and microbiota in the neonate (44, 78, 90, 91, 113, 123, 128, 135).
This review highlights the milestones in research on glycan structures in breast milk, the functions of glycans in the infant, and the impact of maternal phenotype on the structure-function relationships; these findings are instructive for nutrition professionals as they begin to unravel the effect of diet on the intestinal microbiome in health and disease.
MILK GLYCOMICS: MEASURING HUMAN MILK GLYCANS
Glycans have long been recognized as an important determinant of health or disease states. However, the hallmark of glycans is their complexity compared with other polymeric biomolecules such as DNA and proteins, whose primary structures are linear with predictable linkages. The complexity of a glycan refers to several different factors including its nonlinear nature, the number of different sugars, the molecular structure of each sugar residue, the linkages between those sugars leading to multiple isomers for a single mass, and whether they are free or bound to proteins, peptides, or lipids in a heterogeneous manner. This complexity contributes to the ongoing research in glycomic technologies and methodologies to routinely and comprehensively measure the glycans in biological and clinical samples. Although breastfeeding has been linked to a variety of functional benefits associated with glycan structures, initial studies provided limited or no structural information on milk-borne oligosaccharides and/or glycoconjugates. Early studies on the glycobiology of milk focused on measuring individual sugars, and the technology platforms provided a piece of the story but were not comprehensive or rapid enough for routine application. Thus, glycomics is defined as the systematic study of the total complement of sugars present in an organism in their free or bound state (6). Glycomics represents a critical gap in knowledge in nutrition and clinical research, especially in an era of applied omic technologies. Elucidating the glycan structures that are covalently bound to peptides, proteins, and lipids requires merging the fields of glycomics with proteomics and lipidomics to truly elucidate molecular structure (108). Documenting structural diversity through comprehensive glycan analysis provides an important opportunity for understanding diet and health in the neonate.
State of Technology: Human Milk Oligosaccharides
HMOs are a particularly interesting class of molecules that have gained considerable attention because they are an abundant (1–2% w/v) and structurally diverse component of breast milk, yet they are indigestible by the neonate (30, 31). The widespread application of comprehensive milk glycan analysis is dependent on sensitivity, reproducibility, high-throughput and speed of the technologies used to identify specific structural isomers in complex biological samples. Monitoring HMO abundances have traditionally been performed with several separation methods including various types of high-performance liquid chromatography (HPLC) and capillary electrophoresis using standard compounds (31, 121). Liquid chromatographic (LC) methods such as hydrophilic interaction LC, reverse phase (C18), and porous graphitized carbon have been used to separate oligosaccharide isomers (147, 148). Porous graphitized carbon on native compounds has emerged as the best method for separating isomers (147, 148). However, LC methods are severely limited by the small number of HMO standards that are commercially available. The lack of standards is complicated by the difficulty in elucidating HMO structures. Nuclear magnetic resonance has previously been the major method for structural elucidation (122). However, it requires relatively large amounts of pure compounds making it impractical for all except the most abundant species.
Mass spectrometry (MS) is both a sensitive detector for LC methods and a tool for structural elucidation (58). Structural elucidation provides information on the linkages between sugar residues within each HMO molecule to determine the structural isomers present, whereas HMO composition provides information on the different sugar residues present but lacks linkage information. Tandem MS methods provide structural information and have been used for the structural elucidation of many types of oligosaccharides. MS has been used to obtain complete structures (7, 132); however, this method also relies on known standard structural features. Linkages can be obtained with tandem MS, but distinguishing stereoisomers, such as glucose (Glc) versus galactose (Gal) versus mannose, is not possible. In general, de novo structural elucidation with tandem MS is not feasible with current methods. An effective method for the structural elucidation of HMOs employs the combination of MS, tandem MS, and exoglycosidase digestion. In a systematic study of neutral milk oligosaccharides (147) and anionic oligosaccharides (148), 75 HMO structural isomers were determined and annotated. This library has been extended to include more than 200 complete structures, including milks from other mammals. It also has been shown that only 50 structures represent 99% of the abundances in human milks (4, 97, 147, 148). This approach leverages on oligosaccharide analysis by using accurate mass, reproducible retention times through effective isomer separation (HPLC) and tandem MS (for less than 5% of the structures).
The quantitation of HMO structures in a complex mixture, i.e., biofluids, is necessary for phenotyping health and disease in the field of nutrition. Relative abundances of structures (or compositions) provide quantitative information that can be used to obtain fold changes between groups of samples because of the high precision of the method. Absolute quantitation can be obtained by comparing peak areas to known standards. LC/MS quantitation is performed using a number of methods. The most direct is to use ion count, which can be calibrated to some known amount of compound. This approach assumes that all ionization efficiencies are equal, which is not strictly true for oligosaccharides. Nonetheless, the method is sufficient for determining specific changes in abundances. HMOs are often labeled with chromophoric tags such as anthranilic acid or 2-aminobenzamide for quantitation. Deuterium labeling has also been used for HMOs. Nano-LC/MS is useful for performing quantitation using both deuterium labeling and total ion counts methods. Whereas deuterium labeling provides very precise and accurate values (99), total ion count by chromatography produces a suitable level of quantitation, as validated by deuterium labeling (124). More recently, multiple reaction monitoring using triple quadrupole MS has been used for bovine milk oligosaccharides (43). The method was used to monitor a small number of sialylated structures, which make up the major portion of bovine milk oligosaccharides. Multiple reaction monitoring of oligosaccharides is still relatively new but will be used for many more applications in the future.
Human Milk Oligosaccharide Structures and Compositions
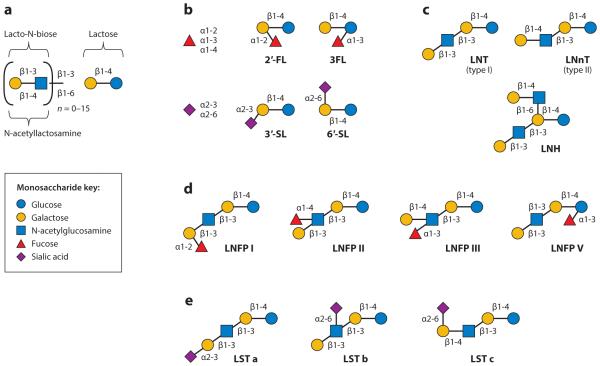
Human milk contains higher amounts and more complex structures of soluble oligosaccharides than any other mammalian milk (127); average amounts range from 7 g/L in mature breast milk to 23 g/L in colostrum (31, 46). The functional implications of the structural diversity are an important area of research. HMOs are soluble complex and diverse sugars derived of Glc, Gal, N-acetylglucosamine (GlcNAc), fucose (Fuc), or sialic acid (Neu5Ac) monosaccharides. The biosynthesis of HMOs in the mammary gland begins with the formation of a lactose core from Gal and Glc catalyzed by β-galactotransferase in the presence of α-lactalbumin. With few exceptions, all HMO structures consist of a lactose core (74). Lactose can be elongated enzymatically by β1-3 linkage to lacto-N-biose or by β1-6 linkage to N-acetyllactosamine. The core HMO structure can be further elongated by the addition of lacto-N-biose and N-acetyllactosamine units by β1-3 and β1-6 linkages; Fuc connected with α1-2, α1-3, or α1-4 linkages; and/or sialic acid residues attached by α2-3 or α2-6 linkages at the terminal positions (Figure 1). The proportion of fucosylated, sialylated, and nonfucosylated neutral HMOs in term breast milk was recently reported as 35–50%, 12–14%, and 42–55%, respectively (131).
Figure 1.
An example of the structural diversity of human milk oligosaccharides (HMOs). (a) With few exceptions, all HMO structures consist of a lactose core linked to lacto-N-biose or to N-acetyllactosamine with n = 0–15 units. (b) Lactose can be fucosylated or sialylated by different linkages. (c) Lactose can be elongated enzymatically in repeats of lacto-N-biose (type I) or N-acetyllactosamine (type II). (d) Elongated type I or II chains can be fucosylated in different linkages to form structures that phenotypically describe secretor status and the Lewis blood group. (e) The elongated core HMO structures can be sialylated by α2-3 or α2-6 linkages at the terminal positions forming structural isomers. Abbreviations: 2′-FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3′-SL, 3′ sialyllactose; 6′-SL, 6′-sialyllactose; LNFP I, II, III, V, lacto-N-fucopentaose I, II, III, V; LNH, lacto-N-hexaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; LST a, b, c, sialyl-lacto-N-tetraoses a–c. Adapted from Reference 15 with permission.
State of Technology: Milk Glycoproteins and Glycopeptides
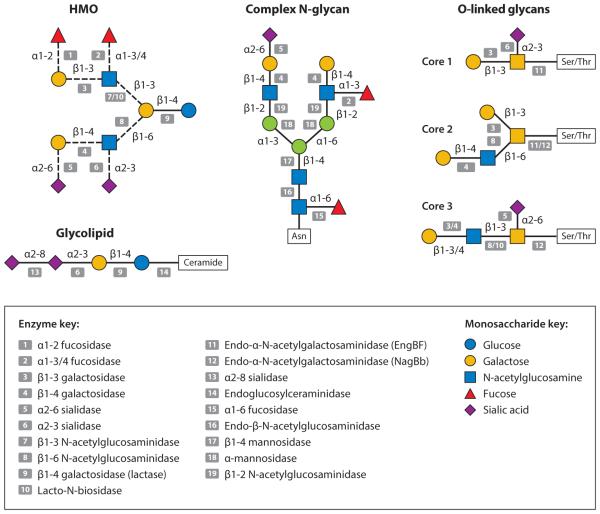
Protein glycosylation is a common and complicated type of posttranslational modification that directly affects glycoprotein structure and protective functions through cell signaling and cell-to-cell recognition events, enrichment of protective gut microbiota, modulation of pathogen adhesion and invasion of the infant intestinal mucosa, and neutralization of antigens (1). The protein portion is cleaved by digestive proteases in the neonatal intestine, yet its glycosylation restricts digestion such that the digestive products and their absorption rates are dependent on the diversity, abundance, and location of oligosaccharide structures present on the protein. The level of proteolytic cleavage therefore has both nutritive and biological consequences. The release of bioactive peptides from proteins may be affected by glycosylation in at least two ways. Glycosylation limits access to protease and peptidase cleavage sites and determines the ability of the remaining glycopeptide to persist and transit to lower parts of the intestine. The glycosylation of immune protective proteins in milk renders them partially resistant to digestion and supports their biological functions in the distal gut. In addition, these milk glycoproteins (72) and glycopeptides (79, 103) possess glycans, which structurally resemble HMOs and can serve as growth substrates for infant-borne bifidobacteria. Indeed, some bifidobacteria possess specific endoglycosidases that enable these strains to cleave the glycan portion away from the cognate glycoproteins, thus freeing the glycan substrate for consumption (Figure 2) (50, 72).
Figure 2.
Infant gut-associated bifidobacteria cleave a diverse range of specific linkages within human milk glycans using a variety of glycosyl hydrolases. Legend at bottom indicates the monosaccharide composition and corresponding potential glycolytic enzymes in bifidobacteria that cleave the specific linkages. The figure depicts the structure of HMO, a complex N-glycan, three different cores found in human O-linked glycans, and the glycolipid structure of ganglioside GD3. Adapted from Reference 51 with permission.
Unlike free milk oligosaccharides, glycoconjugates are complicated due to the respective protein or lipid moieties. Two types of protein glycosylation exist: N-linked and O-linked. N-glycans are found on proteins with a consensus sequence NXT/S where N is asparagine, X is any amino acid except proline, and the third amino acid can be either threonine (T) or serine (S) and, in rare cases, cysteine. O-glycosylation may occur at any serine or threonine residue with no single common core structure or consensus protein sequence. Because glycosylation is dictated by a set of competing glycosyltransferases, the glycosylation patterns of glycoproteins are very complicated. The population of glycans occurring at a given glycosylation site is often heterogeneous such that a specific N-glycosylation and O-glycosylation site may be occupied by a number of structurally distinct glycans and described as microheterogeneous (4). For example, a protein containing three glycosylation sites with 10 different glycans in each site can result in approximately 1,000 different glycoforms of the protein.
Quantitative methods that monitor both protein abundances and site-specific glycosylation remain a significant analytical challenge; however, solving this problem will provide greater understanding of protein functions in vivo. Several approaches are used to study the complexities involved in protein glycosylation. The first is to enzymatically release glycan moieties from the protein molecule to observe the total glycan structures present. This method provides the glycan heterogeneity. This method can also yield site occupancy when the peptide is captured before glycan release. However, relating glycan structure to specific sites is not possible. Another approach is to determine site-specific glycosylation where the intact glycopeptide is measured and is used to retain information on location and microheterogeneity. Although this glycoproteomic method is complicated considerably by the analysis of glycopeptides, it may ultimately provide the level of detail required to measure biological differences in milk across lactation and among women and to determine glycan metabolism within the infant (34).
The large number of glycoforms and the presence of multiple glycan-protein linkage sites have made comprehensive glycoproteomics—the simultaneous determination of glycosites and site-specific microheterogeneity—currently impossible. For this reason, the focus has been on either determining the site of glycosylation on many proteins or determining the site-specific heterogeneity of a few proteins. It is important to determine the glycan microheterogeneity of the protein to understand the role of glycosylation in protein function. Common glycoproteomic methods used for determining site occupancy rely on methods that enrich tryptic glycopeptides (2). To identify the peptide, the glycans are released by the enzyme peptide-N-glycosidase F, thereby losing all information regarding glycan structures. Peptide-N-glycosidase F is an amidase that cleaves between the innermost GlcNAc and asparagine residues of high mannose, hybrid, and complex oligosaccharides from N-linked glycoproteins. The alternative is to determine site-specific glycosylation with microheterogeneity of a targeted group of proteins. Site-specific mapping can be performed using a procedure with nonspecific proteases to digest the proteins into short glycopeptide chains (101). Structural elucidation is possible by coupling accurate mass, tandem MS with bioinformatic tools (125). This highly targeted analysis can be performed on a small number of proteins in a complex sample.
Several proteomic studies have attempted to examine the breadth of proteins in human milk. However, there are very few glycoproteomic analyses of milk. Mechref et al. (89) examined the N-glycans on bile salt–stimulated lipase (BSSL). The glycosylation varied both in absolute quantity of monosaccharide residues and their composition between the first and the sixth month of lactation. BSSL was found to eventually lose all glycosylation during late lactation. Charlwood et al. (23) reported the N-glycosylation of four abundant proteins in milk fat globule membrane. Froehlich et al. (44) have shown that several human milk glycoproteins including lactoferrin, BSSL, tenascin, immunoglobulin A (IgA), and xanthine dehydrogenase exhibit dynamic glycosylation behavior during the lactation period.
State of Technology: Glycolipids
In milk, glycolipids are found almost exclusively in the outer part of the milk fat globule membrane. These glycolipids occur mainly in the form of glycosphingolipids with a dominance of Neu5Ac-containing gangliosides. The combination of the polar head (the oligosaccharide) and the nonpolar lipid tail (ceramide) provides technical challenges from either the free oligosaccharides or the glycoproteins. The catalog of glycolipid structures has been achieved through the release and separation of the polar head group from the nonpolar tail using multiple chromatographic methods. The lipid moieties are best separated by reverse-phase LC, whereas the glycan head group is best separated by hydrophilic interaction LC. The different methods separate oligosaccharides or the lipid moiety, but not both simultaneously. Numerous reviews have published information on human milk glycolipids (see, e.g., 94, 105, 140). These structures have been elucidated through complex and laborious analytical workflows that have been the status quo in the field. However, to achieve high-throughput, routine analysis in determining structural diversity in complex biological fluids including milk, new methods must take fundamentally different approaches to separate, detect, and identify the intact glycolipids. There is a risk of underestimating sialic acid residues because they can be labile and lost as a result of sample preparation. Furthermore, measurement of low-abundant gangliosides that might be functionally active could be missed owing to the presence of more abundant lipids that are more easily ionized. Rapid profiling of breast milk glycolipids is possible using matrix-assisted desorption/ionization Fourier transform ion cyclotron resonance tandem MS (76). Comprehensive methods that overcome the inherent challenges of glycolipid structure will improve our understanding of biological variation and increase the ability to monitor changes in these specific molecules as a function of time or health status or postconsumption.
Human Milk Glycolipids Structures and Compositions
The heterogeneity of the oligosaccharide structures conjugated to milk lipids is lower compared to free oligosaccharides and glycoproteins and is limited to a few known structures. The variations in glycolipids are primarily due to the heterogeneity of the lipid tail, which varies in carbon length and in the number and location of double bonds (16). The concentration of gangliosides (sialylated glycolipids) in human colostrum and mature human breast milk is roughly the same ~9 mg/L (104), with GD3 [Neu5Ac(α2-8)Neu5Ac(α2-3)Gal(β1-4)Glc(β1-1) ceramide] and GM3 [Neu5Ac(α2-3)Gal(β1-4)Glc(β1-1) ceramide] as the most abundant structures (76). Yet, the composition of sialylated gangliosides varies across lactation. The ganglioside GD3 is highest in human colostrum, whereas GM3 is highest in mature breast milk (105). The gangliosides GM2 [GalNAc(β1-4)Neu5Ac(α2-3)Gal(β1-4)Glc(β1-1) ceramide] and GM1 [Gal(β1-3)GalNAc(β1-4)Neu5Ac(α2-3)Gal(β1-4)Glc(β1-1) ceramide] are found in human milk in microgram concentrations (94).
ESTABLISHING STRUCTURE-FUNCTION RELATIONSHIPS OF HUMAN MILK GLYCANS
As discussed previously, HMOs are abundant in human milk and are indigestible by humans, yet evidence suggests at least three major functions for these sugars. First, HMOs promote growth of a milk-oriented microbiota (152), often dominated by bifidobacteria (149). Second, HMOs (especially fucosylated structures) resemble the host epithelial cell surface glycans and thus function as soluble receptor analogs that compete for bacterial binding against the intestinal mucosa, preventing intestinal pathogen adhesion to epithelial surfaces and translocation (95). Third, milk glycans improve host defense by modulating immunity and promoting intestinal barrier function (27). Enrichment of intestinal bifidobacteria, pathogen deflection, and direct cell-surface mediated regulatory events involving the growth and maintenance of epithelia all contribute to host protection and are mediated by milk glycans.
Glycosylation and glycan diversity are directly related to the modulation of microbial adhesion and invasion during infection (87). Indeed, the first step in bacterial infection is the recognition of host glycans by bacterial lectins or vice versa. Thus, glycans of human milk proteins can block or modulate pathogen association to epithelial surfaces, which, given the large amount of glycans delivered to the infant, partially explains the protection of breastfed infants against gastrointestinal tract infections. Human milk lactoferrin, the major milk glycoprotein, binds to pathogenic gram-positive (107) and gram-negative (102) bacteria, exerting antimicrobial activity via iron-depletion and/or bacterial membrane disruption. Human lactoferrin contains three N-linked glycosylation sites at asparagine 138, 479, and 624 (138), and its glycosylation influences the glycoprotein’s susceptibility to proteolysis (137), thus affecting the production of potent active peptides (e.g., lactoferricin) and glycopeptides involved in its biological activities (9, 77). Gangliosides are widely distributed in cellular membranes including the milk fat globule membrane. With their oligosaccharide head group facing the external environment, gangliosides function in host-pathogen interactions, cell-cell recognition, and modulation of membrane protein function. Additionally, milk gangliosides are proposed to modulate immunity and to prevent infection by acting as decoys that interfere with pathogenic binding to host cell receptors of the intestinal mucosa (110).
Prebiotics for Infant Gut Bifidobacteria
Breastfed infants are typically colonized by protective strains of bacteria that are thought to protect, feed, and communicate with the developing intestine (38). More than 100 years ago, Henry Tissier first demonstrated that the feces of breastfed infants contained a bacterial isolate he termed Bacillus bifidus communis (130). Since that time, numerous culture-based studies, and more recently, DNA-based culture-independent methods (61, 109, 114, 149), clearly demonstrated a predominance of bifidobacterial species within the first months of breastfeeding prior to weaning and a transition to a more adult-like microbiota profile (62). Of the bifidobacterial species common to the breastfed intestinal tract, Bifidobacterium longum and B. breve are most frequently observed; B. bifidum, B. pseudocatenulatum, and B. catenulatum are found less often (134).
György and colleagues (57) first showed that B. bifidum (then termed Lactobacillus bifidus) was uniquely able to grow on human milk glycan fractions. Humans lack the various glycolytic enzymes that break down HMOs, and various researchers have shown that these glycans reach the colon intact (25, 30, 39). Thus enrichment of bifidobacteria is believed to be driven in part through the prebiotic effect of free and bound glycans present in human milk (53, 75). Ward et al. (142, 143) first demonstrated the selective growth of bifidobacterial species on intact HMO in vitro. Subsequent studies have confirmed that only certain bifidobacterial species vigorously consume HMOs (53, 83, 115, 123, 133). B. longum subsp. infantis (B. infantis) (54, 77, 114, 137, 139) and select B. breve (111, 149) preferentially consume smaller fucosylated and sialylated HMOs. It is clear the bifidobacterial strains that grow well on HMOs have acquired these specific genetic adaptations for select growth on human milk glycans (111, 117). Supporting a prebiotic concept for HMOs, Yu et al. (150) recently showed that certain HMO species promote bifidobacterial growth within in vitro fecal enrichment assays.
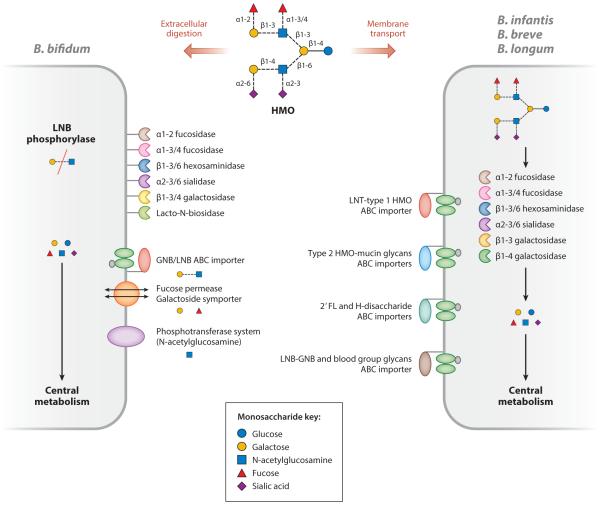
A number of studies have characterized the bifidobacterial moieties that specifically bind and catabolize HMOs (51, 82, 86, 111). Different bifidobacterial species grow on HMO by different catalytic mechanisms. For example, B. infantis, a predominant bifidobacterial species in the breastfed infant colon, possesses a 43-kb gene cluster that encodes transport systems (49) and intracellular glycosyl hydrolases (49, 117–119), which suggests that HMOs are internalized and degraded by this subspecies (51, 52). However, B. bifidum employs a different mode of catalytic activity toward HMO consumption by exporting sialidases, fucosidases, and a lacto-N-biosidase to liberate lacto-N-biose from HMO structures; lacto-N-biose is then transported and metabolized (71) (Figure 3).
Figure 3.
Possible strategies for human milk oligosaccharide (HMO) consumption in Bifidobacterium bifidum, B. infantis, B. breve, and B. longum. Dashed lines in the HMO panel represent potential linkages. Abbreviations: GNB, galacto-N-biose; LNB, lacto-N-biose. Adapted from Reference 48 with permission.
Milk-derived secretory IgA (sIgA), lactoferrin, and haptocorrin are generally believed to be partially resistant to proteolysis and remain partially intact through the gastrointestinal tract (84). Numerous researchers have shown that milk glycoproteins provide some enrichment for bifidobacteria in vitro (8, 63, 70, 106) and in vivo (26, 141). However, it is not always clear if the protein or glycan components (or both) are responsible for the enrichment. Studies have identified milk peptides with bifidobacterial growth-enhancing capacities (79, 103); however, enrichment via these peptides remains to be determined in vivo. The contribution of the glycan portion of these glycoconjugates to the ascribed activities has not been extensively studied because of methodological limitations for the comprehensive structural elucidation and quantitation. Degradation of the glycan portion of complex human milk glycoproteins requires a repertoire of endo- and exoglycosidases and cognate transport systems to make milk glycans available to their central metabolic pathways. Recently, specific cell wall–associated endoglycosidases that are employed by different bifidobacteria initially to degrade O- and N-linked glycoproteins have been identified. Garrido et al. (50) showed that select infant-borne bifidobacteria possess an endo-β-N-acetylglucosaminidase that releases glycans from N-linked glycoproteins at the chitobiose core. An endo-β-acetylgalactosaminidase that cleaves O-linked glycans has also been identified in B. bifidum and select other bifidobacterial strains (72). This latter enzyme is likely involved in both milk and mucin degradation. These endoglycosidases release the free glycans, which are then catabolized via the endogenous HMO consumption pathways for a particular bifidobacterial strain.
Growth on milk glycans confers a specific HMO phenotype to B. infantis that is mechanistically linked to its success in establishing itself and persisting in the infant intestine. Chichlowski et al. (27) showed that growth of B. infantis ATCC15697 on HMOs increases binding to intestinal epithelial cells in vitro, decreases release of inflammatory cytokines, and increases release of anti-inflammatory cytokines in response to an inflammatory stimulus. A similar increase in binding was determined using sialyllactose (69). These studies suggest that the specific growth phenotype of milk glycan–enriched bifidobacterial populations promotes persistence in situ and positively modulates the host epithelium. The promotion of the milk glycan–enriched bifidobacterial population is also supported by in vivo administration of B. infantis to premature infants fed either formula or breast milk (136). Importantly, when delivered in combination with breastfeeding, B. infantis was shown to dominate the premature infant gastrointestinal tract, whereas B. lactis, a strain that does not grow on HMO, did not persist at all. This is the first evidence of the importance of HMO catabolism in bifidobacterial persistence in vivo. Moreover, the combination of B. infantis supplementation and breastfeeding led to decreases in Gammaproteobacteria compared with a matched formula-fed group. Follow-up clinical trials are warranted in premature infants who are at risk for developing NEC, an inflammatory bowel disease, to determine if proliferation of milk glycan–enriched bifidobacterial populations leads to the anti-inflammatory and intestinal barrier functions established in vitro.
Pathogen Deflection
In addition to their prebiotic functions, HMOs also compete for specific pathogen binding with sites in the infant gut (93). Many viral, bacterial, or protozoan pathogens need to adhere to intestinal epithelial surfaces to colonize or invade the host and cause disease. Human milk glycans and intestinal epithelial glycans are synthesized by similar glycosyltransferases and thus have common epitopes. Ingested milk glycans act as pathogen decoys by binding to pathogens and their toxins, thereby limiting their binding to intestinal epithelial mucosal surfaces (95). Milk glycans bind viruses such as HIV (64) and rotavirus (65), pathogens such as Vibrio cholerae, Salmonella fyris, and enteropathogenic Escherichia coli (33), and enterotoxigenic Escherichia coli and caliciviruses (93) and Streptococcus pneumoniae (5). The large diversity of HMO structures suggests a large diversity of decoy functions (Figure 1) (17). Separated HMO fractions have been shown to have differing activities. For instance, fucosylated HMOs inhibit the binding of Campylobacter jejuni to intestinal cells (93), whereas sialylated HMOs block the adhesion of E. coli to human erythrocytes (88).
Milk glycoproteins are well recognized for their protective functions in the neonate (84). Recently, human milk lactoferrin was found to significantly inhibit pathogen adhesion to colonic epithelial cells, and purified human milk lactoferrin glycans significantly reduced Salmonella invasion of colonic epithelial cells to levels associated with noninvasive deletion mutants (9). These data suggest that glycan variation of human milk lactoferrin is involved in modulating pathogen association. sIgA, the predominant immunoglobulin in human milk (28), provides protection to neonates by coating microorganisms and inhibiting colonization and neutralizing viral and bacterial endotoxins (19). sIgA is heavily glycosylated with N- and O-linked oligosaccharides (151), which vary according to the isotype and allotype of the immunoglobulin. Its oligosaccharide structural complexity includes its role in immune protection in cell signaling, cell-cell recognition, and microbial adhesion and invasion (1). sIgA is essential in providing passive immunity to infants against infections (60). For example, specific binding between sialylated glycans of sIgA and pathogens protects newborns from sepsis and meningitis caused by infection by S-fimbriated E. coli (115). In particular, the large diversity of N-glycan structures at seven potential sites on the secretory component of the immunoglobulin creates many glycan epitopes that are potent decoys for lectins on bacterial surfaces, thereby inhibiting infection through attachment with epithelial surfaces (18, 41, 146).
The human milk fat globule membrane contains many membrane-associated glycoconjugates proposed to protect the nursing infant by offering an alternate pathogen binding site (96). Ganglioside composition of milk is implicated in binding and neutralizing various pathogens and their toxins such as enteropathogenic E. coli, C. jejuni, L. monocytogenes, Salmonella enterica (Typhi), S. sonnei, Helicobacter pylori vacuolating toxin, and cholera toxin (36, 105). The interaction between the membrane-associated ganglioside GM1 and cholera toxin protein subunit B is a well-studied system and is frequently employed for assessing the capabilities of any new analytical technique (20, 36). For example, gangliosides from pooled human milk exhibited the ability to reduce the binding of cholera toxin to GM1 by 93%, and structures from goat milk completely inhibited the binding of botulinum type A neurotoxin to trisialoganglioside-GT1b (67). Gangliosides also exert immune-modulating effects on immune cells such as dendritic cells. Treatment of bone marrow–derived dendritic cells with GD3 before lipopolysaccharide-induced maturation significantly reduced the production of various proinflammatory cytokines and reduced the activation of CD4+ cell proliferation compared with treatment with GM3 (21). These data suggest that GD3, which is more abundant in colostrum than GM3, may function to reduce the excessive immune responses against the large amounts of foreign antigens, albeit harmless, the infant encounters during the first weeks of life. Human studies designed to confirm the effects of glycan structures for their antiadhesive and anti-inflammatory and immunomodulatory functions are warranted.
Intestinal Barrier Function and Immune Modulation
The gastrointestinal tract is a complex organ system with local and regional morphological and physiological differences that lead to digestion and absorption of nutrients while providing a barrier against foreign agents. However, at birth the neonate’s gastrointestinal tract is functionally immature and immune incompetent (112), although it is confronted with an increasing antigenic load in the form of dietary proteins, commensal organisms, and pathogens. The single layer of enterocytes that line the intestinal epithelium forms a functional barrier between the luminal contents of the gut and the infant’s circulatory system. Permeability across the intestinal epithelium is determined in part by the rate-limiting barrier of the paracellular pathway involving tight junctions (100). This critical barrier is responsible for allowing nutrients and beneficial macromolecules to cross through the intestinal epithelium while preventing paracellular translocation of bacteria and bacterial products.
Human milk glycans support intestinal barrier function and modulate immunity through a variety of mechanisms. First, the abundance of infant-dominated gut bifidobacteria that results from the prebiotic function of HMOs and glycans leads to lower gastrointestinal pH through the production of short-chain fatty acids (in particular, acetate), which enhances gut barrier function (45). Second, binding of B. infantis grown on HMOs to intestinal cells in vitro enhanced tight junction protein expression and immunomodulatory interleukin (IL)-10 (27). Third, B. infantis– conditioned media prevented tumor necrosis factor alpha and interferon gamma reduction in transepithelial resistance (a measurement of intestinal barrier function) and rearrangement of tight junction proteins in intestinal epithelial cells and attenuated inflammation, normalized colonic permeability, and decreased colonic and splenic interferon gamma secretion in a colitis mouse model (40). Ganguli et al. (47) demonstrated that B. infantis and Lactobacillus acidophilus conditioned media-attenuated lipopolysaccharide- and IL-1β-induced IL-8 and IL-6 expression; decreased toll-like receptor 2 and 4 mRNA; and increased specific negative regulators of inflammation of immature human enterocytes, immature human intestinal xenografts, and primary enterocyte cultures of NEC tissue, with superior effects by B. infantis. These data suggest strain specificity in reducing intestinal inflammation and could explain one mechanism for the reduced rates of NEC in low-birth-weight infants fed breast milk and supplemented with probiotics containing B. infantis (81). Fourth, one of the major milk-fat globule membrane-associated proteins, milk-fat globule EGF factor 8 (MFG-E8) protein, a 66kDa glycoprotein, plays a central role in cell-surface-mediated regulatory events in the growth and maintenance of epithelia, and binding apoptotic cells and cellular debris (59). For instance, depleting MFG-E8 in mice by administration of anti-MFG-E8 antibody or targeted deletion of the MFG-E8 gene resulted in a slower rate of enterocyte migration along the crypt-villus axis during mucosal injury. Treatment with recombinant MFG-E8 restored enterocyte migration, whereas deletion of MFG-E8 impeded mucosal healing in mice with sepsis (22). The improved barrier function exerted by human milk glycans is likely a result of their complex structures and multifarious targets.
UNDERSTANDING THE DIVERSITY OF MILK GLYCANS
The Value of Glycomics for Nutrition Research
The glycans of breast milk are recognized as the vital structures that deliver protection to and promote development of the neonate. Major advances in the field of glycomics in milk research, including high mass accuracy and identification and quantitation of the diverse glycan structures (10, 85, 126, 127, 147), and novel methods for elucidating site-specific glycosylation of glycopeptides (4, 37, 116), including nano-chip technology (11, 98), have enabled our understanding of the functional relationships of breast milk glycans on gut microbiota, pathogen binding, and intestinal barrier function in vitro. These analytical advances offer nutrition research powerful tools to elucidate the in vivo functional outcomes of breast milk glycan structure heterogeneity and variation due to lactation stage, maternal diet, genetics, and phenotype on infant health outcomes. Maternal phenotype largely influences milk composition and structure. Yet functional in vivo out-come studies in infants are scarce and are required to fully understand the mechanistic link between glycan structural variation and infant intestinal health. Such health outcomes in the neonate should encompass gut microbiota composition and function, protection against pathogens, and support of barrier and immune functions. Characterizing, annotating, and evaluating the structure-function of the mother-infant dyad is dependent on documenting and comparing the variable structures within breast milk prospectively for nutritional and health outcomes. Cataloguing the structural and compositional diversity of breast milk as a function of maternal markers, gestational age, and time of lactation provides the experimental controls and depth of information to contribute to robust legacy multi-omic data sets that can truly begin to unravel the impact of structure on function.
Human Milk Oligosaccharides Genetically Determined By Secretor Status and Lewis Blood Group
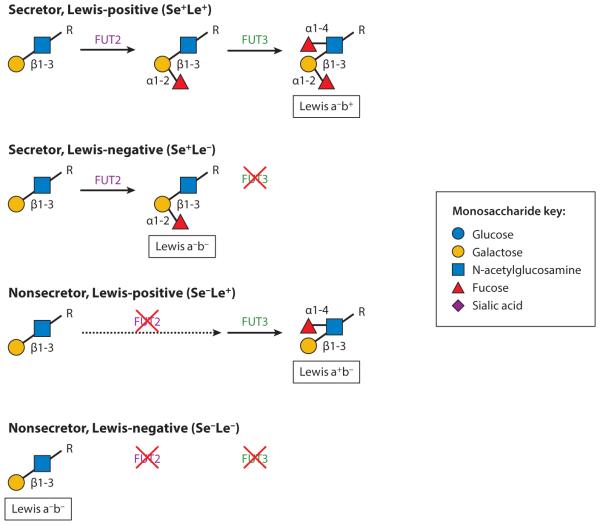
Sensitive and targeted methods are used to capture biological variation and differences in milk glycans concentrations among individuals. For example, the biological variation calculated as the coefficient of variation (% CV) for total HMOs in mature term breast milk is as high as 28% (31, 131). Yet the biological variation is greater for specific structures than for total HMOs. For instance, the % CV ranges from 42% to 84.3% for the specific HMO structures 2′-fucosyllactose (2′-FL), 3-fucosyllactose (3FL), lacto-N-fucopentaose I and II (LNFP I and LNFP II), and lactodifucotetraose (LDFT) in mature term breast milk (122, 131). The variation of HMO-borne Fuc composition is dependent on maternal genetics that encode the activities of three or more distinct fucosyltransferases phenotypically described as secretor (Se) status and Lewis (Le) blood group (74). Fuc residues may be attached by an α1-2 linkage through the action of the secretor gene for α-1,2-fucosyltransferase (FUT2) to a terminal Gal of the type 1 chain of HMOs. Fuc may also be attached by an α1-4 linkage through the action of the Lewis gene for α-1,3/4-fucosyltransferase (FUT3) to a subterminal GlcNAc of the type I chain of HMOs. HMO variation is largely explained by maternal Se and Le blood type. The attachment of Fuc to a subterminal GlcNAc of the type I chain by an α1-4 linkage through FUT3 results in the presence of the Le b sugars in Se milk (Le a−b+) and the Le a sugars in nonsecretor milk (Le a+b−) or an absence of these sugars in Le-negative women (Le a−b−), who can be either secretors or nonsecretors, thus resulting in four groups: Se+Le+, Se−Le+, Se+Le−, and Se−Le− (17) (Figure 4).
Figure 4.
The variation of human milk oligosaccharide (HMO) fucose composition is dependent on maternal genetics that dictate the activities of distinct fucosyltransferases phenotypically described as secretor status and Lewis blood group. Abbreviations: FL, fucosyllactose; LNH, lacto-N-hexaose; LNnT, lacto-N-neotetraose; LNFP I, II, III, V, lacto-N-fucopentaose I, II, III, V; LNT, lacto-N-tetraose; LST a–c, sialyl-lacto-N-tetraoses a–c. Adapted from Reference 17 with permission.
Women who express α-1,2-fucosyltransferase in body fluids such as tears, milk, and saliva and on mucosal surfaces, i.e., people who secrete soluble blood group substances [A, B, and O(H)] that match their specific blood group type, are phenotypically described as secretors, whereas those with undetectable levels are described as nonsecretors (55). Many allelic variants of the Se phenotype have been found across the FUT2 gene worldwide. In 732 individuals from 39 human populations, 55 different single-nucleotide polymorphisms were identified for the FUT2 gene. The percentage of nonsecretors in Europe was 43%; in individuals from the Middle East and North Africa, 54%; in Asians, 42% to 45%; and in sub-Saharan Africans, 26% (42). The allelic variation in the FUT2 gene results in low or undetectable levels of α1,2-linked fucosylated HMOs such as 2′-FL, LDFT, LNFP I, or lacto-N-difucohexaose I (24, 34, 55, 120, 122, 129, 131). Infants who were breastfed by Se women were protected against diarrhea caused by Campylobacter, caliciviruses, and stable toxin of enterotoxigenic E. coli as well as moderate-to-severe diarrhea of all causes. The α1,2-linked fucosylated glycans in human milk act as pathogen antiadhesion agents competing with epithelial cell–receptor binding (93). Milk from non-Se women contains lower amounts of total oligosaccharides than does milk from Se women (131); LNFP III (131), 3FL, and nonfucosylated HMOs such as lacto-N-tetraose (LNT) (122, 129, 131) are higher in milk from non-Se compared with Se women (122, 129, 131). Although breast milk from non-Se women contains less HMO diversity and no or negligible concentrations of α1,2-linked fucosylated HMOs compared to milk from Se women, the increased concentrations of LNFP III, 3FL, and LNT offer protection to infants as prebiotics for specific gut bifidobacterial strains (111) and prevent binding to the host intestinal cell and cytotoxicity by protozoan parasites (68).
Women who are classified as Se+Le+ have higher concentrations of HMOs and more complex profiles than do women who are Se−Le− (46). Additionally, milk from Le− women have undetectable levels of α1,4-linked fucosylated HMOs such as LNFP II and lacto-N-difucohexaose I and II (LNDFH I and II). Yet, Le blood group epitope based on serological tests may not be a reliable marker of HMO composition (14). For example, in 60 Gambian women, the Le sugars Leb, LNDFH I, and LNDFH II were present in 70% of Le-negative Se women and equaled abundances similar to women identified as Le a+b− and Le a−b+. Additionally, LNFP II, which contains an Lea epitope, was found in substantial amounts in nearly 90% of Le-negative women (131). These data suggest that HMO variation is more reliable on maternal Se status than on Le blood type. The growing recognition of the abundant functions and health consequences of oligosaccharides has resulted in studies that are beginning to address the diversity and function of HMOs.
Lactation Stage
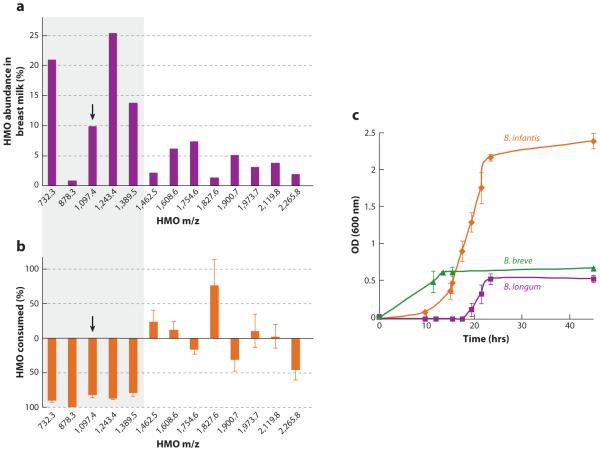
HMO structures and composition vary over the course of lactation (24, 129). HPLC was used in 46 mothers who delivered term infants, and mean total concentration of HMOs was approximately 20 g/L on days 4 and 10 postpartum decreasing to approximately 12 g/L by day 120 postpartum; the concentration of lactose increased from 56 g/L to 69 g/L over the same period (31). The higher concentration of total HMOs in breast milk in the early postpartum period is consistent with the protective functions of colostrum and early milk at a time when the neonate is immunologically immature and the gut microbiota is not yet fully established. Although the total HMO concentration is reduced across lactation, the direction of the change varies among the different HMO compositions. High-performance anion-exchange chromatography showed that concentrations of α1,2-linked fucosyloligosaccharides 2′-FL and LNFP I were highest during early lactation (days 3–4) and were reduced by 37% and 53%, respectively, by day 90. In contrast, 3FL—the structural isomer of 2′-FL—increased across lactation (days 3–4 to day 90 postpartum) by 1.8-fold in Se+Le+ women (129). These data suggest reduced activity of the Se enzyme (FUT2) and increased activity of Se- and Le-independent fucosyltransferases (FUT3, 4, 5, 6, 7, or 9) across lactation; however, human trials involving large numbers of women are required to test this hypothesis. Using MS, investigators determined that the major small neutral HMOs and their isomers (m/z 709.3, 855.3, 1074.4, 1220.4, and 1366.5) were the least varied across lactation (days 3 to 72) (98). These major HMO structures are likely selective growth substrates for specific bifidobacteria in the establishment of an infant’s healthy intestinal microbiota. Glycomic profiling of HMO consumption by bifidobacteria using Fourier transform ion cyclotron resonance MS revealed that B. infantis (58) preferentially consumes small mass oligosaccharides with a degree of polymerization ≤7 (m/z 1389 and below), representing 63.9% of the total HMOs, which are mostly fucosylated (82), whereas other bifidobacterial strains tested—B. longum subsp. longum, B. adolescentis, B. breve, and B. bifidum—showed only low or moderate growth ability (83) (Figure 5). These data suggest the ability of glycans to drive enrichment of a glycan-consuming bifidobacterial population in the infant gut. The development of glycomics analytics has enabled breakthroughs in this research.
Figure 1.
(a) Individual human milk oligosaccharide (HMO) abundance as the normalized percent contribution of each isomeric oligosaccharide species in breast milk. The shaded box indicates the five most abundant oligosaccharide species in the total pool of HMOs analyzed, representing 70% of the overall detectable HMO pool. The arrow shows a particular abundance completely consumed by Bifidobacterium longum subsp. infantis (B. infantis). (b) Nano electrospray ionization Fourier transform ion cyclotron resonance (ESI-FT-ICR) (+) mass spectrometric analysis of B. infantis grown on media initially supplemented with 2.0% (w/v) HMO. Data represent the percent difference of HMO species abundance in the media before and at the end of fermentation, corresponding to 0 h and 94 h. Measurements were conducted in triplicates of individual biological and technical replicates. (c) Growth curves of B. infantis, B. breve, and B. longum on a semisynthetic de Man, Rogosa, and Sharpe (MRS) medium supplemented with 2% (w/v) HMO. Growth was measured as optical density (OD) of the media at 600 nm. Error bars are standard deviations of the mean for each available time point. Abbreviation: m/z, ratio of mass to charge. Adapted from Reference 82 with permission.
Protein glycosylation is also dynamic and varies during lactation. Many breast milk proteins and the extent of their glycosylation as well as the specific glycans vary across lactation (9, 44). The glycosylation composition of lactoferrin influences the function of the glycoprotein in preventing various pathogens from binding to and infecting intestinal epithelial cells (9).
Gestational Age of the Infant
Premature infants are at increased risk for infections compared with infants born at term owing to the immaturity of the gastrointestinal tract and innate and adaptive immune responses. Breast milk consumption by very-low-birth-weight premature infants is protective against NEC and late-onset sepsis but does not meet the metabolic demands of intrauterine growth rates. Nutrients in the breast milk of mothers of premature infants vary widely between and within mothers (56, 144). Although the milk of women who deliver preterm versus term contains higher total HMOs (46), a detailed MS analysis of the fucosylation and sialylation of HMOs using nano-HPLC chip/time-of-flight MS discovered that although LNT is more abundant, it is highly more variable in the milk of women who deliver preterm. Furthermore, fucosylation was not as well regulated, resulting in higher within- and between-mother variations in milk from women delivering preterm versus term. Of particular clinical interest, the concentration of 2′-FL was not consistent across lactation of several mothers who delivered preterm (35). Thus, Se status of preterm milk changed in a given mother. Fluctuations in fucosylated HMOs in mothers’ milk are important because of the role that fucosylated HMOs play in pathogen binding. A high degree of variability suggests immaturity in the regulation of HMO fucosylation in the “premature breast.” Yet, milk from Se women in comparison with non-Se women imparts protection against NEC and gram-negative sepsis (92), which may in part be explained by potential enrichment of gut bifidobacteria. Indeed, milk from Se mothers has specific linkages (α1,2-fucosylated HMOs) and higher amounts of total fucosylated HMOs (131), which are likely to enrich only those bifidobacterial species/strains that can catabolize these glycans (118). Premature infants generally have very low numbers of fecal bifidobacteria (145), and probiotic supplementation with bifidobacteria is protective against NEC (1, 80, 81). Fucosylation of HMOs of preterm milk is unpredictable and raises the interesting question of whether fortification with preterm milk HMOs would further reduce the risk of NEC and late-onset sepsis.
Maternal Health and Phenotype
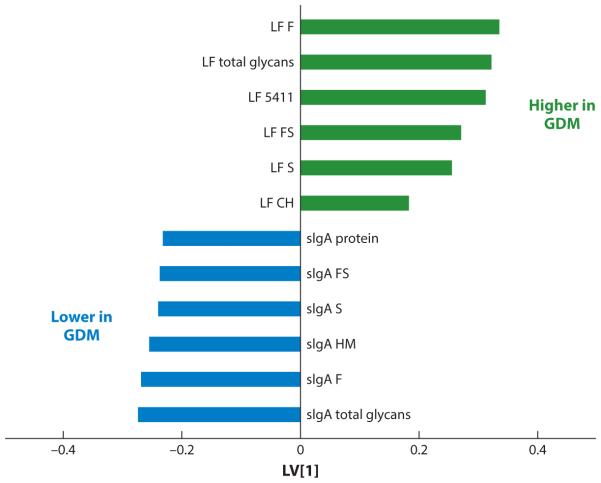
Maternal health ranging from malnutrition to overnutrition and metabolic syndrome leads to long-term health consequences in the neonate. For example, HMO total concentrations were significantly lower in women with a body mass index between 14 and 18 than in women with a body mass index between 24 and 28 (17). In the United States, gestational diabetes mellitus (GDM), a complex disease characterized by elevated blood Glc, affects on average 7% and up to 14% of pregnancies (3). GDM has immediate and lasting consequences in women and to infants exposed to maternal diabetes in utero (12, 29, 73). Hyperglycemia results in aberrant carbohydrate metabolism that contributes to the pathogenesis of the disease in part through increased flux through the hexosamine biosynthetic pathway (38, 62) and altered activities of cellular glycosyltransferases and glycosidases (53, 75). Very little is understood regarding the role of GDM in lactation and breast milk components, and much of what is known about the effects of glycemic dysregulation on milk components comes from research on lactating women diagnosed with insulin-dependent diabetes mellitus and from animal models. Recently, glycomic profiling using nano-HPLC chip/time-of-flight MS and multivariate modeling was used to investigate the effects of GDM on the glycosylation of HMOs, sIgA, and lactoferrin of breast milk (123). Although total HMOs and their composition were not different in milk from women with and without GDM, the N-linked glycosylation of specific milk proteins differed between the two groups. The total N-linked glycosylation, mannose, Fuc, and sialylated residues of sIgA in transitional milk were up to 43% lower in milk from women with GDM in comparison with women without GDM. Reduced sialylation of immunoglobulins secreted by mammary epithelial cells has implications on the nonspecific innate immune defense against pathogen adhesion and infection (115). The total N-linked glycosylation, Fuc, and sialylated residues of lactoferrin were significantly higher, by up to 72% (Figure 6). The higher N-linked glycosylation of lactoferrin observed in milk from women with GDM could result from altered glycan-metabolizing enzymes linked to the pathophysiology of diabetes mellitus during pregnancy (53). The glycosylation level of a glycoprotein influences its susceptibility to proteolysis (137, 139), thus affecting the production of potent active peptides and glycopeptides involved in its biological activities (77). These data suggest that diabetes mellitus during pregnancy alters the glycosylation of protective proteins in milk; future research is needed to discover the effects of these alterations on neonatal outcomes.
Figure 6.
N-Glycosylation of milk proteins is altered in gestational diabetes mellitus (GDM). Milk proteins from mothers with and without GDM were compared using orthogonal signal corrected partial least-squares discriminant analysis. The maximum difference between milk glycans from women with and without GDM was captured in the first dimension or latent variable (LV[1]) of the model. Positive values indicate higher glycosylation in GDM compared to controls; conversely, negative values indicate lower glycosylation in GDM compared to controls. LF 5411 is an N-glycan that contains 5 hexoses, 4 N-acetyl-hexosamines, 1 fucose, and 1 sialic acid. Abbreviations: CH, complex hybrid; F, fucose; FS, fucose and sialic acid; HM, high mannose; LF, lactoferrin; S, sialic acid; sIgA, secretory immunoglobulin A. Adapted from Reference 122 with permission.
CONCLUSIONS AND FUTURE DIRECTIONS
The first proof of principle from integrating the science of glycomics into microbiota research is that stereospecific glycans are key modulators of the intestinal environment in the narrowly defined successful feeding of breastfed infants. The accuracy and specificity of analytical glycomics now enable researchers to determine interindividual and intraindividual milk variations; the ability to track and monitor the fate of glycomic structures assists investigators in understanding how they shape the intestine. The tool sets of systems biology, including genomics, metabolomics, proteomics, and glycomics, which are being used to investigate the complexity of milk and are revealing how colonization and development of the infant microbiota occurs, can now be applied to the more complex challenges of the adult intestinal microbiota. Determining how to standardize, integrate, and use glycomic information in large multi-omic, annotated data sets will be the next milestone in elucidating structure-function relationships in breast milk oligosaccharides.
SUMMARY POINTS.
Milk glycan research is providing a wealth of information regarding the impact of diet on host and intestinal microbiota.
Major advances in the field of glycomics in milk research, including high mass accuracy for the identification and quantitation of diverse glycan structures, contribute to the understanding of the functional relationships between breast milk glycans and gut microbiota, pathogen binding, and intestinal barrier function.
Glycan heterogeneity on milk proteins influences their structures, functions, and digestibility.
Effective tools for the structural elucidation of human milk oligosaccharides, glycolipids, and glycoproteins combine exoglycosidase digestion, separation technologies, MS, structural libraries, and bioinformatics.
Analysis of breast milk glycolipids is now achievable using matrix-assisted desorption/ionization Fourier transform ion cyclotron resonance tandem MS.
The glycans of breast milk are substrates for the selective enrichment of bifidobacterial species in the gastrointestinal tract of breastfed infants.
Growth of B. infantis on human milk glycans promotes intestinal barrier and immunological development and function.
ACKNOWLEDGMENTS
We acknowledge all of the researchers in the UC Davis Foods for Health Institute and the Milk Bioactives Program for their enthusiasm, imagination, and collective contribution to this subject matter. Work by the Milk Bioactives Program has been supported by the UC Davis Research Investments in the Sciences and Engineering Program; the UC Discovery Grant Program; the California Dairy Research Foundation; the Dairy Research Institute; the Bill & Melinda Gates Foundation; and the National Institutes of Health awards R01HD059127, R01HD065122, R01HD061923, R21AT006180, and R01AT007079. D.A.M. acknowledges support as the Peter J. Shields Endowed Chair in Dairy Food Science.
Footnotes
DISCLOSURE STATEMENT
J.B.G. is a past or present consultant for Nestlé, Abbott, Dairy Research Inc., Arla Foods, and FrieslandCampina. The other authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2011;3:CD005496. doi: 10.1002/14651858.CD005496.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Manilla G, Atwood J, III, Guo Y, Warren NL, Orlando R, Pierce M. Tools for glycoproteomic analysis: size exclusion chromatography facilitates identification of tryptic glycopeptides with N-linked glycosylation sites. J. Proteome Res. 2006;5:701–8. doi: 10.1021/pr050275j. [DOI] [PubMed] [Google Scholar]
- 3.Am. Diabetes Assoc. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62–69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An HJ, Froehlich JW, Lebrilla CB. Determination of glycosylation sites and site-specific heterogeneity in glycoproteins. Curr. Opin. Chem. Biol. 2009;13:421–26. doi: 10.1016/j.cbpa.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson B, Porras O, Hanson LÅ, Lagergård T, Svanborg-Edén C. Inhibition of attachment of Streptococcus pneumoniae and Haemophilus influenzae by human milk and receptor oligosaccharides. J. Infect. Dis. 1986;153:232–37. doi: 10.1093/infdis/153.2.232. [DOI] [PubMed] [Google Scholar]
- 6.Aoki-Kinoshita KF. An introduction to bioinformatics for glycomics research. PLoS Comput. Biol. 2008;4:e1000075. doi: 10.1371/journal.pcbi.1000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashline D, Singh S, Hanneman A, Reinhold V. Congruent strategies for carbohydrate sequencing. 1. Mining structural details by MSn. Anal. Chem. 2005;77:6250–62. doi: 10.1021/ac050724z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azuma N, Yamauchi K, Mitsuoka T. Bifidus growth-promoting activity of a glycomacropeptide derived from human K-casein. Agric. Biol. Chem. 1984;48:2159–62. [Google Scholar]
- 9.Barboza M, Pinzon J, Wickramasinghe S, Froehlich JW, Moeller I, et al. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria-host interactions. Mol. Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.015248. M111.015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barboza M, Sela DA, Pirim C, Locascio RG, Freeman SL, et al. Glycoprofiling bifidobacterial consumption of galacto-oligosaccharides by mass spectrometry reveals strain-specific, preferential consumption of glycans. Appl. Environ. Microbiol. 2009;75:7319–25. doi: 10.1128/AEM.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barile D, Marotta M, Chu C, Mehra R, Grimm R, et al. Neutral and acidic oligosaccharides in Holstein-Friesian colostrum during the first 3 days of lactation measured by high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry. J. Dairy Sci. 2010;93:3940–49. doi: 10.3168/jds.2010-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–79. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 13.Blackburn D. Lactation: historical patterns and potential for manipulation. J. Dairy Sci. 1993;76:3195–212. doi: 10.3168/jds.S0022-0302(93)77658-4. [DOI] [PubMed] [Google Scholar]
- 14.Blank D, Dotz V, Geyer R, Kunz C. Human milk oligosaccharides and Lewis blood group: individual high-throughput sample profiling to enhance conclusions from functional studies. Adv. Nutr. 2012;3:440–49S. doi: 10.3945/an.111.001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bode L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology. 2012;22:1147–62. doi: 10.1093/glycob/cws074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bode L, Beermann C, Mank M, Kohn G, Boehm G. Human and bovine milk gangliosides differ in their fatty acid composition. J. Nutr. 2004;134:3016–20. doi: 10.1093/jn/134.11.3016. [DOI] [PubMed] [Google Scholar]
- 17.Bode L, Jantscher-Krenn E. Structure-function relationships of human milk oligosaccharides. Adv. Nutr. 2012;3:383–91S. doi: 10.3945/an.111.001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borén T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–95. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 19.Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J. Pediatr. 2010;156:S8–15. doi: 10.1016/j.jpeds.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Bricarello DA, Smilowitz JT, Zivkovic AM, German JB, Parikh AN. Reconstituted lipoprotein: a versatile class of biologically-inspired nanostructures. ACS Nano. 2011;5:42–57. doi: 10.1021/nn103098m. [DOI] [PubMed] [Google Scholar]
- 21.Brønnum H, Seested T, Hellgren L, Brix S, Frøkier H. Milk-derived GM3 and GD3 differentially inhibit dendritic cell maturation and effector functionalities. Scand. J. Immunol. 2005;61:551–57. doi: 10.1111/j.1365-3083.2005.01566.x. [DOI] [PubMed] [Google Scholar]
- 22.Bu H, Zuo X, Wang X, Ensslin MA, Koti V, et al. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J. Clin. Invest. 2007;117:3673–83. doi: 10.1172/JCI31841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlwood J, Hanrahan S, Tyldesley R, Langridge J, Dwek M, Camilleri P. Use of proteomic methodology for the characterization of human milk fat globular membrane proteins. Anal. Biochem. 2002;301:314–24. doi: 10.1006/abio.2001.5498. [DOI] [PubMed] [Google Scholar]
- 24.Chaturvedi P, Warren CD, Altaye M, Morrow AL, Ruiz-Palacios G, et al. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology. 2001;11:365–72. doi: 10.1093/glycob/11.5.365. [DOI] [PubMed] [Google Scholar]
- 25.Chaturvedi P, Warren CD, Buescher CR, Pickering LK, Newburg DS. Survival of human milk oligosaccharides in the intestine of infants. In: Newburg DS, editor. Bioactive Components of Human Milk. Springer; New York: 2001. pp. 315–24. [DOI] [PubMed] [Google Scholar]
- 26.Chen Q, Cao J, Jia Y, Liu X, Yan Y, Pang G. Modulation of mice fecal microbiota by administration of casein glycomacropeptide. Microbiol. Res. 2012;3:e3. [Google Scholar]
- 27.Chichlowski M, De Lartigue G, German JB, Raybould HE, Mills DA. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J. Pediatr. Gastroenterol. Nutr. 2012;55:321–27. doi: 10.1097/MPG.0b013e31824fb899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chirico G, Marzollo R, Cortinovis S, Fonte C, Gasparoni A. Antiinfective properties of human milk. J. Nutr. 2008;138:1801–6S. doi: 10.1093/jn/138.9.1801S. [DOI] [PubMed] [Google Scholar]
- 29.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes. Diabetes Care. 2008;31:340–46. doi: 10.2337/dc07-1596. [DOI] [PubMed] [Google Scholar]
- 30.Coppa G, Pierani P, Zampini L, Bruni S, Carloni I, Gabrielli O. Characterization of oligosaccharides in milk and feces of breast-fed infants by high-performance anion-exchange chromatography. In: Newburg DS, editor. Bioactive Components of Human Milk. Springer; New York: 2001. pp. 307–14. [DOI] [PubMed] [Google Scholar]
- 31.Coppa GV, Gabrielli O, Pierani P, Catassi C, Carlucci A, Giorgi PL. Changes in carbohydrate composition in human milk over 4 months of lactation. Pediatrics. 1993;91:637–41. [PubMed] [Google Scholar]
- 32.Coppa GV, Pierani P, Zampini L, Bruni S, Carloni I, Gabrielli O. Characterization of oligosaccharides in milk and feces of breast-fed infants by high-performance anion-exchange chromatography. Adv. Exp. Med. Biol. 2001;501:307–14. doi: 10.1007/978-1-4615-1371-1_38. [DOI] [PubMed] [Google Scholar]
- 33.Coppa GV, Zampini L, Galeazzi T, Facinelli B, Ferrante L, et al. Human milk oligosaccharides inhibit the adhesion to Caco-2 cells of diarrheal pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatr. Res. 2006;59:377–82. doi: 10.1203/01.pdr.0000200805.45593.17. [DOI] [PubMed] [Google Scholar]
- 34.De Leoz ML, Wu S, Strum JS, Ninonuevo MR, Gaerlan SC, et al. A quantitative and comprehensive method to analyze human milk oligosaccharide structures in the urine and feces of infants. Anal. Bioanal. Chem. 2013;405:4089–105. doi: 10.1007/s00216-013-6817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Leoz MLA, Gaerlan SC, Strum JS, Dimapasoc LM, Mirmiran M, et al. Lacto-N-tetraose, fucosylation, and secretor status are highly variable in human milk oligosaccharides from women delivering preterm. J. Proteome Res. 2012;11:4662–72. doi: 10.1021/pr3004979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desai PT, Walsh MK, Weimer BC. Solid phase capture of pathogenic bacteria using gangliosides and detection with real time PCR. Appl. Environ. Microbiol. 2008;74:2254–58. doi: 10.1128/AEM.02601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dodds ED, Seipert RR, Clowers BH, German JB, Lebrilla CB. Analytical performance of immobilized pronase for glycopeptide footprinting and implications for surpassing reductionist glycoproteomics. J. Proteome Res. 2009;8:502–12. doi: 10.1021/pr800708h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du X-L, Edelstein D, Rossetti L, Fantus IG, Goldberg H, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. 2000;97:12222–26. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engfer MB, Stahl B, Finke B, Sawatzki G, Daniel H. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am. J. Clin. Nutr. 2000;71:1589–96. doi: 10.1093/ajcn/71.6.1589. [DOI] [PubMed] [Google Scholar]
- 40.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G1025–34. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 41.Falk P, Roth KA, Boren T, Westblom TU, Gordon JI, Normark S. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage specific tropism in the human gastric epithelium. Proc. Natl. Acad. Sci. USA. 1993;90:2035–39. doi: 10.1073/pnas.90.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrer-Admetlla A, Sikora M, Laayouni H, Esteve A, Roubinet F, et al. A natural history of FUT2 polymorphism in humans. Mol. Biol. Evol. 2009;26:1993–2003. doi: 10.1093/molbev/msp108. [DOI] [PubMed] [Google Scholar]
- 43.Fong B, Ma K, McJarrow P. Quantification of bovine milk oligosaccharides using liquid chromatography-selected reaction monitoring-mass spectrometry. J. Agric. Food Chem. 2011;59:9788–95. doi: 10.1021/jf202035m. [DOI] [PubMed] [Google Scholar]
- 44.Froehlich J, Dodds E, Barboza M, McJimpsey E, Seipert R, et al. Glycoprotein expression in human milk during lactation. J. Agric. Food Chem. 2010;58:6440–48. doi: 10.1021/jf100112x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–47. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 46.Gabrielli O, Zampini L, Galeazzi T, Padella L, Santoro L, et al. Preterm milk oligosaccharides during the first month of lactation. Pediatrics. 2011;128:e1520–31. doi: 10.1542/peds.2011-1206. [DOI] [PubMed] [Google Scholar]
- 47.Ganguli K, Meng D, Rautava S, Lu L, Walker WA, Nanthakumar N. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304:G132–41. doi: 10.1152/ajpgi.00142.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garrido D, Barile D, Mills DA. A molecular basis for bifidobacterial enrichment in the infant gastrointestinal tract. Adv. Nutr. 2012;3:415–21S. doi: 10.3945/an.111.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garrido D, Kim JH, German JB, Raybould HE, Mills DA. Oligosaccharide binding proteins from Bifidobacterium longum subsp. infantis reveal a preference for host glycans. PLoS ONE. 2011;6:e17315. doi: 10.1371/journal.pone.0017315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garrido D, Nwosu C, Ruiz-Moyano S, Aldredge D, German JB, et al. Endo-β-N-acetylglucosaminidases from infant gut-associated bifidobacteria release complex N-glycans from human milk glycoproteins. Mol. Cell Proteomics. 2012;11:775–85. doi: 10.1074/mcp.M112.018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garrido D, Ruiz-Moyano S, Jimenez-Espinoza R, Eom HJ, Block DE, Mills DA. Utilization of galactooligosaccharides by Bifidobacterium longum subsp. infantis isolates. Food Microbiol. 2013;33:262–70. doi: 10.1016/j.fm.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garrido D, Ruiz-Moyano S, Mills DA. Release and utilization of N-acetyl-D-glucosamine from human milk oligosaccharides by Bifidobacterium longum subsp. infantis. Anaerobe. 2012;18:430–35. doi: 10.1016/j.anaerobe.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Georgieff M, Petry C, Mills M, McKay H, Wobken J. Increased N-glycosylation and reduced transferrin-binding capacity of transferrin receptor isolated from placentae of diabetic women. Placenta. 1997;18:563–68. doi: 10.1016/0143-4004(77)90011-x. [DOI] [PubMed] [Google Scholar]
- 54.Gordon JI, Dewey KG, Mills DA, Medzhitov RM. The human gut microbiota and undernutrition. Sci. Transl. Med. 2012;4:137ps12. doi: 10.1126/scitranslmed.3004347. [DOI] [PubMed] [Google Scholar]
- 55.Grollman EF, Ginsburg V. Correlation between secretor status and the occurrence of 21-fucosyllactose in human milk. Biochem. Biophys. Res. Commun. 1967;28:50–53. doi: 10.1016/0006-291x(67)90404-4. [DOI] [PubMed] [Google Scholar]
- 56.Gross SJ, Geller J, Tomarelli R. Composition of breast milk from mothers of preterm infants. Pediatrics. 1981;68:490–93. [PubMed] [Google Scholar]
- 57.György P, Norris RF, Rose CS. Bifidus factor. I. A variant of Lactobacillus bifidus requiring a special growth factor. Arch. Biochem. Biophys. 1954;48:193–201. doi: 10.1016/0003-9861(54)90323-9. [DOI] [PubMed] [Google Scholar]
- 58.Haarman M, Knol J. Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 2005;71:2318–24. doi: 10.1128/AEM.71.5.2318-2324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–87. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 60.Hanson LA. Breastfeeding provides passive and likely long-lasting active immunity. Ann. Allergy Asthma Immunol. 1998;81:523–37. doi: 10.1016/S1081-1206(10)62704-4. [DOI] [PubMed] [Google Scholar]
- 61.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 2000;30:61–67. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 62.Hebert LF, Jr, Daniels MC, Zhou J, Crook ED, Turner RL, et al. Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J. Clin. Invest. 1996;98:930–36. doi: 10.1172/JCI118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hernandez-Hernandez O, Sanz ML, Kolida S, Rastall RA, Moreno FJ. In vitro fermentation by human gut bacteria of proteolytically digested caseinomacropeptide nonenzymatically glycosylated with prebiotic carbohydrates. J. Agric. Food Chem. 2011;59:11949–55. doi: 10.1021/jf203576g. [DOI] [PubMed] [Google Scholar]
- 64.Hong P, Ninonuevo MR, Lee B, Lebrilla C, Bode L. Human milk oligosaccharides reduce HIV-1-gp120 binding to dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN) Br. J. Nutr. 2009;101:482–86. doi: 10.1017/s0007114508025804. [DOI] [PubMed] [Google Scholar]
- 65.Huang P, Xia M, Tan M, Zhong W, Wei C, et al. Spike protein VP8* of human rotavirus recognizes histo-blood group antigens in a type-specific manner. J. Virol. 2012;86:4833–43. doi: 10.1128/JVI.05507-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ip S, Chung M, Raman G, Chew P, Magula N, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid. Rep. Technol. Assess. 2007;153:1–186. [PMC free article] [PubMed] [Google Scholar]
- 67.Iwamori M, Takamizawa K, Momoeda M, Iwamori Y, Taketani Y. Gangliosides in human, cow and goat milk, and their abilities as to neutralization of cholera toxin and botulinum type A neurotoxin. Glycoconj. J. 2008;25:675–83. doi: 10.1007/s10719-008-9128-6. [DOI] [PubMed] [Google Scholar]
- 68.Jantscher-Krenn E, Lauwaet T, Bliss LA, Reed SL, Gillin FD, Bode L. Human milk oligosaccharides reduce Entamoeba histolytica attachment and cytotoxicity in vitro. Br. J. Nutr. 2012;108:1839–46. doi: 10.1017/S0007114511007392. [DOI] [PubMed] [Google Scholar]
- 69.Kavanaugh DW, O’Callaghan J, Buttó LF, Slattery H, Lane J, et al. Exposure of Bifidobacterium longum subsp. infantis to milk oligosaccharides increases adhesion to epithelial cells and induces a substantial transcriptional response. PLoS ONE. 2013;8:e67224. doi: 10.1371/journal.pone.0067224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim W-S, Ohashi M, Tanaka T, Kumura H, Kim G-Y, et al. Growth-promoting effects of lactoferrin on L. acidophilus and Bifidobacterium spp. Biometals. 2004;17:279–83. doi: 10.1023/b:biom.0000027705.57430.f1. [DOI] [PubMed] [Google Scholar]
- 71.Kitaoka M. Bifidobacterial enzymes involved in the metabolism of human milk oligosaccharides. Adv. Nutr. 2012;3:422–29S. doi: 10.3945/an.111.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiyohara M, Nakatomi T, Kurihara S, Fushinobu S, Suzuki H, et al. α-N-acetylgalactosaminidase from infant-associated bifidobacteria belonging to novel glycoside hydrolase family 129 is implicated in alternative mucin degradation pathway. J. Biol. Chem. 2012;287:693–700. doi: 10.1074/jbc.M111.277384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar R, Ouyang F, Story RE, Pongracic JA, Hong X, et al. Gestational diabetes, atopic dermatitis, and allergen sensitization in early childhood. J. Allergy Clin. Immunol. 2009;124:1031–38. doi: 10.1016/j.jaci.2009.06.052. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu. Rev. Nutr. 2000;20:699–722. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- 75.Lee CL, Chiu PCN, Pang PC, Chu IK, Lee KF, et al. Glycosylation failure extends to glycoproteins in gestational diabetes mellitus. Diabetes. 2011;60:909–17. doi: 10.2337/db10-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee H, An HJ, Lerno LA, German JB, Lebrilla CB. Rapid profiling of bovine and human milk gangliosides by matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry. Int. J. Mass Spectrom. 2011;305:138–50. doi: 10.1016/j.ijms.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Legrand D, Pierce A, Elass E, Carpentier M, Mariller C, Mazurier J. Lactoferrin structure and functions. Adv. Exp. Med. Biol. 2008;606:163–94. doi: 10.1007/978-0-387-74087-4_6. [DOI] [PubMed] [Google Scholar]
- 78.Liao Y, Alvarado R, Phinney B, Lönnerdal B. Proteomic characterization of human milk whey proteins during a twelve-month lactation period. J. Proteome Res. 2011;10:1746–54. doi: 10.1021/pr101028k. [DOI] [PubMed] [Google Scholar]
- 79.Liepke C, Adermann K, Raida M, Mägert HJ, Forssmann WG, Zucht HD. Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur. J. Biochem. 2002;269:712–18. doi: 10.1046/j.0014-2956.2001.02712.x. [DOI] [PubMed] [Google Scholar]
- 80.Lin H-C, Hsu C-H, Chen H-L, Chung M-Y, Hsu J-F, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. 2008;122:693–700. doi: 10.1542/peds.2007-3007. [DOI] [PubMed] [Google Scholar]
- 81.Lin H-C, Su B-H, Chen A-C, Lin T-W, Tsai C-H, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005;115:1–4. doi: 10.1542/peds.2004-1463. [DOI] [PubMed] [Google Scholar]
- 82.LoCascio R, Ninonuevo M, Freeman S, Sela D, Grimm R, et al. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J. Agric. Food Chem. 2007;55:8914–19. doi: 10.1021/jf0710480. [DOI] [PubMed] [Google Scholar]
- 83.Locascio RG, Ninonuevo MR, Kronewitter SR, Freeman SL, German JB, et al. A versatile and scalable strategy for glycoprofiling bifidobacterial consumption of human milk oligosaccharides. Microb. Biotechnol. 2009;2:333–42. doi: 10.1111/j.1751-7915.2008.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lönnerdal B. Bioactive proteins in human milk: mechanisms of action. J. Pediatr. 2010;156:S26–30. doi: 10.1016/j.jpeds.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 85.Marcobal A, Barboza M, Froehlich J, Block D, German JB, et al. Consumption of human milk oligosaccharides by gut-related microbes. J. Agric. Food Chem. 2010;58:5334–40. doi: 10.1021/jf9044205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, et al. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe. 2011;10:507–14. doi: 10.1016/j.chom.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat. Rev. Immunol. 2008;8:874–87. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin-Sosa S, Martin MJ, Hueso P. The sialylated fraction of milk oligosaccharides is partially responsible for binding to enterotoxigenic and uropathogenic Escherichia coli human strains. J. Nutr. 2002;132:3067–72. doi: 10.1093/jn/131.10.3067. [DOI] [PubMed] [Google Scholar]
- 89.Mechref Y, Chen P, Novotny MV. Structural characterization of the N-linked oligosaccharides in bile salt-stimulated lipase originated from human breast milk. Glycobiology. 1999;9:227–34. doi: 10.1093/glycob/9.3.227. [DOI] [PubMed] [Google Scholar]
- 90.Molinari CE, Casadio YS, Hartmann BT, Arthur PG, Hartmann PE. Longitudinal analysis of protein glycosylation and β-casein phosphorylation in term and preterm human milk during the first 2 months of lactation. Br. J. Nutr. 2013;110:105–15. doi: 10.1017/S0007114512004588. [DOI] [PubMed] [Google Scholar]
- 91.Molinari CE, Casadio YS, Hartmann BT, Livk A, Bringans S, et al. Proteome mapping of human skim milk proteins in term and preterm milk. J. Proteome Res. 2012;11:1696–714. doi: 10.1021/pr2008797. [DOI] [PubMed] [Google Scholar]
- 92.Morrow AL, Meinzen-Derr J, Huang P, Schibler KR, Cahill T, et al. Fucosyltransferase 2 non-secretor and low secretor status predicts severe outcomes in premature infants. J. Pediatr. 2011;158:745–51. doi: 10.1016/j.jpeds.2010.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morrow AL, Ruiz-Palacios GM, Jiang X, Newburg DS. Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J. Nutr. 2005;135:1304–7. doi: 10.1093/jn/135.5.1304. [DOI] [PubMed] [Google Scholar]
- 94.Newburg DS. Glycobiology of human milk. Biochemistry (Moscow) 2013;78:771–85. doi: 10.1134/S0006297913070092. [DOI] [PubMed] [Google Scholar]
- 95.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu. Rev. Nutr. 2005;25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553. [DOI] [PubMed] [Google Scholar]
- 96.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr. Res. 2007;61:2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 97.Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, et al. A strategy for annotating the human milk glycome. J. Agric. Food Chem. 2006;54:7471–80. doi: 10.1021/jf0615810. [DOI] [PubMed] [Google Scholar]
- 98.Ninonuevo MR, Perkins PD, Francis J, Lamotte LM, LoCascio RG, et al. Daily variations in oligosaccharides of human milk determined by microfluidic chips and mass spectrometry. J. Agric. Food Chem. 2008;56:618–26. doi: 10.1021/jf071972u. [DOI] [PubMed] [Google Scholar]
- 99.Ninonuevo MR, Ward RE, LoCascio RG, German JB, Freeman SL, et al. Methods for the quantitation of human milk oligosaccharides in bacterial fermentation by mass spectrometry. Anal. Biochem. 2007;361:15–23. doi: 10.1016/j.ab.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 100.Nusrat A, Turner J, Madara J. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G851–57. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 101.Nwosu CC, Huang J, Aldredge DL, Strum JS, Hua S, et al. In-gel nonspecific proteolysis for elucidating glycoproteins: a method for targeted protein-specific glycosylation analysis in complex protein mixtures. Anal. Chem. 2012;85:956–63. doi: 10.1021/ac302574f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ochoa TJ, Noguera-Obenza M, Ebel F, Guzman CA, Gomez HF, Cleary TG. Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli. Infect. Immun. 2003;71:5149–55. doi: 10.1128/IAI.71.9.5149-5155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oda H, Wakabayashi H, Yamauchi K, Sato T, Xiao J-Z, et al. Isolation of a bifidogenic peptide from the pepsin hydrolysate of bovine lactoferrin. Appl. Environ. Microbiol. 2013;79:1843–49. doi: 10.1128/AEM.03343-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pan XL, Izumi T. Variation of the ganglioside compositions of human milk, cow’s milk and infant formulas. Early Hum. Dev. 2000;57:25–31. doi: 10.1016/s0378-3782(99)00051-1. [DOI] [PubMed] [Google Scholar]
- 105.Peterson R, Cheah WY, Grinyer J, Packer N. Glycoconjugates in human milk: protecting infants from disease. Glycobiology. 2013;23:1425–38. doi: 10.1093/glycob/cwt072. [DOI] [PubMed] [Google Scholar]
- 106.Petshow BW, Talbottt RD, Batema RP. Ability of lactoferrin to promote the growth of Bifidobacterium spp. in vitro is independent of receptor binding capacity and iron saturation level. J. Med. Microbiol. 1999;48:541–49. doi: 10.1099/00222615-48-6-541. [DOI] [PubMed] [Google Scholar]
- 107.Qiu J, Hendrixson DR, Baker EN, Murphy TF, Geme JWS, Plaut AG. Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae. Proc. Natl. Acad. Sci. 1998;95:12641–46. doi: 10.1073/pnas.95.21.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reinhold V, Zhang H, Hanneman A, Ashline D. Toward a platform for comprehensive glycan sequencing. Mol. Cell. Proteomics. 2013;12:866–73. doi: 10.1074/mcp.R112.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roger LC, McCartney AL. Longitudinal investigation of the faecal microbiota of healthy full-term infants using fluorescence in situ hybridization and denaturing gradient gel electrophoresis. Microbiology. 2010;156:3317–28. doi: 10.1099/mic.0.041913-0. [DOI] [PubMed] [Google Scholar]
- 110.Rueda R. The role of dietary gangliosides on immunity and the prevention of infection. Br. J. Nutr. 2007;98:S68–73. doi: 10.1017/S0007114507832946. [DOI] [PubMed] [Google Scholar]
- 111.Ruiz-Moyano S, Totten SM, Garrido DA, Smilowitz JT, German JB, et al. Variation in consumption of human milk oligosaccharides by infant gut-associated strains of Bifidobacterium breve. Appl. Environ. Microbiol. 2013;79:6040–49. doi: 10.1128/AEM.01843-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rumbo M, Schiffrin EJ. Ontogeny of intestinal epithelium immune functions: developmental and environmental regulation. Cell Mol Life Sci. 2005;62:1288–96. doi: 10.1007/s00018-005-5033-3. [DOI] [PubMed] [Google Scholar]
- 113.Ruvoën-Clouet N, Le Pendu J, Mas E, Marionneau S, Guillon P, Lombardo D. Bile-salt-stimulated lipase and mucins from milk of “secretor” mothers inhibit the binding of Norwalk virus capsids to their carbohydrate ligands. Biochem. J. 2006;393:627–34. doi: 10.1042/BJ20050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sakata S, Tonooka T, Ishizeki S, Takada M, Sakamoto M, et al. Culture-independent analysis of fecal microbiota in infants, with special reference to Bifidobacterium species. FEMS Microbiol. Lett. 2005;243:417–23. doi: 10.1016/j.femsle.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 115.Schroten H, Stapper C, Plogmann R, Köhler H, Hacker J, Hanisch FG. Fab-independent anti-adhesion effects of secretory immunoglobulin A on S-fimbriated Escherichia coli are mediated by sialyloligosaccharides. Infect. Immun. 1998;66:3971–73. doi: 10.1128/iai.66.8.3971-3973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seipert RR, Dodds ED, Clowers BH, Beecroft SM, German JB, Lebrilla CB. Factors that influence fragmentation behavior of N-linked glycopeptide ions. Anal. Chem. 2008;80:3684–92. doi: 10.1021/ac800067y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sela DA, Chapman J, Adeuya A, Kim J, Chen F, et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. USA. 2008;105:18964–69. doi: 10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sela DA, Garrido D, Lerno L, Wu S, Tan K, et al. Bifidobacterium longum subsp. infantis ATCC 15697 α-fucosidases are active on fucosylated human milk oligosaccharides. Appl. Environ. Microbiol. 2012;78:795–803. doi: 10.1128/AEM.06762-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sela DA, Li Y, Lerno L, Wu S, Marcobal AM, et al. An infant-associated bacterial commensal utilizes breast milk sialyloligosaccharides. J. Biol. Chem. 2011;286:11909–18. doi: 10.1074/jbc.M110.193359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shen L, Grollman EF, Ginsburg V. An enzymatic basis for secretor status and blood group substance specificity in humans. Proc. Natl. Acad. Sci. USA. 1968;59:224–30. doi: 10.1073/pnas.59.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shen Z, Warren CD, Newburg DS. High-performance capillary electrophoresis of sialylated oligosaccharides of human milk. Anal. Biochem. 2000;279:37–45. doi: 10.1006/abio.1999.4448. [DOI] [PubMed] [Google Scholar]
- 122.Smilowitz JT, O’Sullivan A, Barile D, German JB, Lönnerdal B, Slupsky CM. The human milk metabolome reveals diverse oligosaccharide profiles. J. Nutr. 2013;143:1709–18. doi: 10.3945/jn.113.178772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Smilowitz JT, Totten SM, Huang J, Grapov D, Durham HA, et al. Human milk secretory immunoglobulin A and lactoferrin N-glycans are altered in women with gestational diabetes mellitus. J. Nutr. 2013;143:1906–12. doi: 10.3945/jn.113.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Strum JS, Kim J, Wu S, De Leoz ML, Peacock K, et al. Identification and accurate quantitation of biological oligosaccharide mixtures. Anal. Chem. 2012;84:7793–801. doi: 10.1021/ac301128s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Strum JS, Nwosu CC, Hua S, Kronewitter SR, Seipert RR, et al. Automated assignments of N- and O-site specific glycosylation with extensive glycan heterogeneity of glycoprotein mixtures. Anal. Chem. 2013;85:5666–75. doi: 10.1021/ac4006556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tao N, Ochonicky KL, German JB, Donovan SM, Lebrilla CB. Structural determination and daily variations of porcine milk oligosaccharides. J. Agric. Food Chem. 2010;58:4653–59. doi: 10.1021/jf100398u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tao N, Wu S, Kim J, An HJ, Hinde K, et al. Evolutionary glycomics: characterization of milk oligosaccharides in primates. J. Proteome Res. 2011;10:1548–57. doi: 10.1021/pr1009367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thakkar SK, Giuffrida F, Cristina CH, Castro CA, Mukherjee R, et al. Dynamics of human milk nutrient composition of women from Singapore with a special focus on lipids. Am. J. Hum. Biol. 2013;25:770–79. doi: 10.1002/ajhb.22446. [DOI] [PubMed] [Google Scholar]
- 129.Thurl S, Munzert M, Henker J, Boehm G, Muller-Werner B, et al. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010;104:1261–71. doi: 10.1017/S0007114510002072. [DOI] [PubMed] [Google Scholar]
- 130.Tissier H. Recherches sur la flore intestinale des nourrissons: état normal et pathologique. G. Carré et C. Naud; Paris: 1900. [Google Scholar]
- 131.Totten SM, Zivkovic AM, Wu S, Ngyuen U, Freeman SL, et al. Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers. J. Proteome Res. 2012;11:6124–33. doi: 10.1021/pr300769g. [DOI] [PubMed] [Google Scholar]
- 132.Tseng K, Hedrick JL, Lebrilla CB. Catalog-library approach for the rapid and sensitive structural elucidation of oligosaccharides. Anal. Chem. 1999;71:3747–54. doi: 10.1021/ac990095r. [DOI] [PubMed] [Google Scholar]
- 133.Turroni F, Bottacini F, Foroni E, Mulder I, Kim JH, et al. Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc. Natl. Acad. Sci. USA. 2010;107:19514–19. doi: 10.1073/pnas.1011100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Turroni F, Van Sinderen D, Ventura M. Genomics and ecological overview of the genus Bifidobacterium. Int. J. Food Microbiol. 2011;149:37–44. doi: 10.1016/j.ijfoodmicro.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 135.Uchiyama S-i, Sekiguchi K, Akaishi M, Anan A, Maeda T, Izumi T. Characterization and chronological changes of preterm human milk gangliosides. Nutrition. 2011;27:998–1001. doi: 10.1016/j.nut.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 136.Underwood MA, Kalanetra KM, Bokulich NA, Lewis ZT, Mirmiran M, et al. A comparison of two probiotic strains of bifidobacteria in premature infants. J. Pediatr. 2013;163:1585–91. doi: 10.1016/j.jpeds.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Van Berkel P, Geerts M, Van Veen H, Kooiman P, Pieper F, et al. Glycosylated and unglycosylated human lactoferrins both bind iron and show identical affinities towards human lysozyme and bacterial lipopolysaccharide, but differ in their susceptibilities towards tryptic proteolysis. Biochem. J. 1995;312:107–14. doi: 10.1042/bj3120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Van Berkel P, Van Veen H, Geerts M, De Boer H, Nuijens J. Heterogeneity in utilization of N-glycosylation sites Asn624 and Asn138 in human lactoferrin: a study with glycosylation-site mutants. Biochem. J. 1996;319:117–22. doi: 10.1042/bj3190117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van Veen H, Geerts M, van Berkel P, Nuijens J. The role of N-linked glycosylation in the protection of human and bovine lactoferrin against tryptic proteolysis. Eur. J. Biochem. 2004;271:678–84. doi: 10.1111/j.1432-1033.2003.03965.x. [DOI] [PubMed] [Google Scholar]
- 140.Varki A. Evolutionary forces shaping the Golgi glycosylation machinery: why cell surface glycans are universal to living cells. Cold Spring Harb. Perspect. Biol. 2011;3:6. doi: 10.1101/cshperspect.a005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Y-Z, Shan T-Z, Xu Z-R, Feng J, Wang Z-Q. Effects of the lactoferrin (LF) on the growth performance, intestinal microflora and morphology of weanling pigs. Anim. Feed Sci. Technol. 2007;135:263–72. [Google Scholar]
- 142.Ward RE, Ninonuevo M, Mills DA, Lebrilla CB, German JB. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Appl. Environ. Microbiol. 2006;72:4497–99. doi: 10.1128/AEM.02515-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ward RE, Ninonuevo M, Mills DA, Lebrilla CB, German JB. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol. Nutr. Food Res. 2007;51:1398–405. doi: 10.1002/mnfr.200700150. [DOI] [PubMed] [Google Scholar]
- 144.Weber A, Loui A, Jochum F, Bührer C, Obladen M. Breast milk from mothers of very low birthweight infants: variability in fat and protein content. Acta Paediatr. 2001;90:772–75. [PubMed] [Google Scholar]
- 145.Westerbeek EA, van den Berg A, Lafeber HN, Knol J, Fetter WP, van Elburg RM. The intestinal bacterial colonisation in preterm infants: a review of the literature. Clin. Nutr. 2006;25:361–68. doi: 10.1016/j.clnu.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 146.Wold AE, Mestecky J, Tomana M, Kobata A, Ohbayashi H. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect. Immun. 1990;58:3073–77. doi: 10.1128/iai.58.9.3073-3077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu S, Tao N, German JB, Grimm R, Lebrilla CB. Development of an annotated library of neutral human milk oligosaccharides. J. Proteome Res. 2010;9:4138–51. doi: 10.1021/pr100362f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu SA, Grimm R, German JB, Lebrilla CB. Annotation and structural analysis of sialylated human milk oligosaccharides. J. Proteome Res. 2011;10:856–68. doi: 10.1021/pr101006u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–27. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yu Z-T, Chen C, Kling DE, Liu B, McCoy JM, et al. The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology. 2013;23:169–77. doi: 10.1093/glycob/cws138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zauner G, Selman MHJ, Bondt A, Rombouts Y, Blank D, et al. Glycoproteomic analysis of antibodies. Mol. Cell. Proteomics. 2013;12:856–65. doi: 10.1074/mcp.R112.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zivkovic AM, Lewis ZT, German JB, Mills DA. Establishment of a milk-oriented microbiota (MOM) in early life: how babies meet their MOMs. Funct. Food Rev. 2013;5:3–12. [Google Scholar]