Abstract
Falls are a major source of hospitalization, long-term institutionalization, and death in older adults and patients with Parkinson’s disease (PD). Limited attentional resources are a major risk factor for falls. In this review, we specify cognitive–behavioral mechanisms that produce falls and map these mechanisms onto a model of multi-system degeneration. Results from PET studies in PD fallers and findings from a recently developed animal model support the hypothesis that falls result from interactions between loss of basal forebrain cholinergic projections to the cortex and striatal dopamine loss. Striatal dopamine loss produces inefficient, low-vigor gait, posture control, and movement. Cortical cholinergic deafferentation impairs a wide range of attentional processes, including monitoring of gait, posture and complex movements. Cholinergic cell loss reveals the full impact of striatal dopamine loss on motor performance, reflecting loss of compensatory attentional supervision of movement. Dysregulation of dorsomedial striatal circuitry is an essential, albeit not exclusive, mediator of falls in this dual-system model. Because cholinergic neuromodulatory activity influences cortical circuitry primarily via stimulation of α4β2* nicotinic acetylcholine receptors, and because agonists at these receptors are known to benefit attentional processes in animals and humans, treating PD fallers with such agonists, as an adjunct to dopaminergic treatment, is predicted to reduce falls. Falls are an informative behavioral endpoint to study attentional–motor integration by striatal circuitry.
Keywords: Falls, Acetylcholine, Dopamine, Striatum, Basal forebrain
Introduction
Falls are the sixth leading cause of death in the elderly and in patients with PD. Approximately a third of adults aged 65 years or older, and about two thirds of patients with PD, experience at least one fall per year (Balash et al., 2005; Wood, 2002). Survivors often require long-term hospitalization and rehabilitation (Baker and Harvey, 1985; Dellinger and Stevens, 2006; Grimbergen et al., 2004; Tinetti et al., 1988).
The propensity for falls in the elderly and PD patients is associated with cognitive impairments, specifically reduced capacities to sustain and divide attention. Extensive evidence from experiments using dual task paradigms support the hypothesis that limited attentional resources are further taxed by the presence of a secondary cognitive task, instigating impairments in gait, balance, and movement control and precipitating falls (Allcock et al., 2009; Hausdorff et al., 2006; Holtzer et al., 2007; Hsu et al., 2012; Kang et al., 2009; LaPointe et al., 2010; Nagamatsu et al., 2013; Shumway-Cook and Woollacott, 2000; Theill et al., 2011).
The following objectives guide this review. First, we synthesize the clinical evidence indicating correlations between decline in cholinergic systems and increased fall propensity in patients with PD (Bohnen and Albin, 2011; Bohnen et al., 2009b; Muller et al., 2013) and, to a lesser degree, healthy elderly (Bohnen et al., 2009a; Grothe et al., 2012), with research from a new animal model that has begun to reveal the nature of cholinergic–dopaminergic interactions causing falls (Kucinski et al., 2013). This synthesis leads to specific hypotheses about the cognitive–motor interactions and underlying forebrain circuitry dysfunctions mediating falls. Falls in PD patients and possibly in the healthy elderly are essentially, but not exclusively, mediated by dysfunction of a dorsomedial striatal region that is defined by its afferent projections from prefrontal regions. Specifically, we propose that loss of cortical cholinergic inputs impairs the attentional processing of gait, posture, and movement-related cues. The striatal circuitry, which normally would receive information about these cues via cortico-striatal projections, is thus “deprived” of this information, which it would normally use to select and sequence motor actions. In other words, dual cholinergic–dopaminergic loss attenuates the (compensatory) attentional supervision of striatal circuitry and thereby ‘unmasks’ the consequences of striatal dopaminergic denervation on gait, balance and complex movements.1 In the presence of an intact cholinergic system, performance errors activate the neuromodulatory component of cortical cholinergic activity to enhance the detection of cues and errors to stabilize and recover performance (St Peters et al., 2011). Such compensatory attentional control (see also Sarter et al., 2006) is attenuated as a result of loss of basal forebrain cholinergic neurons, further revealing the impact of striatal dopaminergic deafferentation. Falls in dual task conditions might thus be interpreted as reflecting limited attentional resources that are further constrained because of cholinergic system decline. However, we propose a potential alternative framework: Impaired cholinergic modulation of the frontal cortex biases the calculation of the relative utilities of competing tasks so that subjects more likely disengage from the primary motor task. In summary, we describe a cognitive neuroscience-oriented conceptualization of falls that provides a new framework to investigate the nature of cognitive–motor, cortico- and midbrain-striatal integration.
Aging and PD-associated impairments in attention and loss of cholinergic neurons
There is an extensive and complex literature on the attentional impairments associated with normal aging and due to structural or functional decline of the basal forebrain cholinergic system. The complexities of this literature reflect the enormous variability of the effects of age on cognition as well as the traditional focus on post mortem markers of the structural integrity of cholinergic neurons to demonstrate aging effects. Such markers likely represent the end stages of long-term functional declines in neuronal systems and thus likely underestimate decades of prior functional decline.
Impairments in the ability to divide attention, as typically assessed by dual task performance, are generally correlated with chronological age (Crossley and Hiscock, 1992; Salthouse et al., 1995; for review see Sarter and Turchi, 2002). This main effect of age is typically interpreted as reflecting a decrease in the overall capacity for information processing or a decline in the executive management of such resources (Craik et al., 2010; Tombu and Jolicæeur, 2003). In healthy older adults as well as patients with PD, impairments in a range of measures of divided as well as sustained attentional performance are a robust predictor for freezing of gait and falls (Allcock et al., 2009; Lord et al., 2010; Naismith et al., 2010; O’Halloran et al., 2011; Persad et al., 2008; Woollacott and Shumway-Cook, 2002; Yarnall et al., 2011).
In unimpaired middle aged and older adults, cholinergic axons in the cortex begin to exhibit structural abnormalities thought to reflect the particular and early vulnerability of these neurons (e.g., Geula and Mesulam, 1989; Geula et al., 2008; Grothe et al., 2013). Evidence for cholinergic decline in healthy aging is somewhat mixed, but studies correlating imaging-derived measures of basal forebrain cholinergic structural decline with lower performance in older adults (Düzel et al., 2010; Fernández et al., 2011; Hall et al., 2011; Wolf et al., 2013) support the “cholinergic hypothesis” of age-related cognitive decline (Bartus et al., 1982). Likewise, in rodents, aging per se does not consistently correlate with a decline in cholinergic function in laboratory rodents, but when decline occurs, attention and related encoding functions are generally impaired (Parikh et al., 2012; Sarter and Bruno, 1998; Schliebs and Arendt, 2011).
In PD, the loss of basal forebrain cholinergic neurons and associated cortical cholinergic innervation is more extensive than in the brain of older adults and reaches and possibly exceeds basal forebrain cholinergic cell loss in Alzheimer’s disease. The exact time of onset of cholinergic cell loss relative to degeneration of midbrain dopamine cells in PD remains disputed but it may occur as early as the loss of midbrain dopaminergic neurons (Bohnen and Albin, 2009; Bohnen et al., 2003; Candy et al., 1983; Nakano and Hirano, 1984; Shimada et al., 2009). When PD patients also develop dementia, such cholinergic loss progresses most severely (Bohnen and Frey, 2007; Bohnen et al., 2003, 2012; Whitehouse et al., 1983; Ziegler et al., 2013).
Bohnen and colleagues demonstrated that lower levels of cortical and thalamic acetylcholinesterase (AChE), which likely reflect the reduced density of cholinergic synapses, differentiate PD fallers from nonfallers. Nigrostriatal dopaminergic terminal densities did not separate these groups (Bohnen and Albin, 2011; Bohnen et al., 2009a,b). Together, these findings suggest that falls in aged subjects and PD are closely associated with limited attentional resources that result from the loss of basal forebrain cholinergic neurons (see also Yarnall et al., 2011). The cognitive–motor, cholinergic–dopaminergic mechanisms that underlie falls are further detailed below.
Cholinergic–dopaminergic interactions: hypotheses derived from a new animal model for falls in PD
PD patients prone to falls exhibit postural control and balance deficits when confronted with complex surfaces, such as stairs, and they have slower walking speeds, shorter strides, and poor postural sway and frontal plane instability (Bohnen et al., 2013; Cole et al., 2010, 2011; Kurz et al., 2013; Marchese et al., 2003; Paul et al., 2013). In the presence of a limited attentional capacity for monitoring gait, balance, and movement, falls arise from freezing of movement, loss of balance, and poor rebalancing after movement errors. These precursors for falls often occur in response to distractors (e.g., Cowie et al., 2012) and secondary tasks.
We developed a behavioral instrument to test the ability of rats to move across complex surfaces, e.g., rotating squared rods, to determine fall propensity (the Michigan Complex Movement Control Task; MCMCT; Kucinski et al., 2013). The animal is required to traverse a rotating rod (Fig. 1), which is thought to tax the attentional supervision of motor performance, just as an older adult or a PD patient would recruit attentional supervision when traversing unfamiliar surfaces, descending an unfamiliar staircase, or negotiating an obstacle (e.g., Uemura et al., 2011). To directly demonstrate relationships between attentional impairments and falls, attentional performance was also measured using a well-characterized sustained attention task (SAT; e.g., Demeter et al., 2008).
Fig. 1.
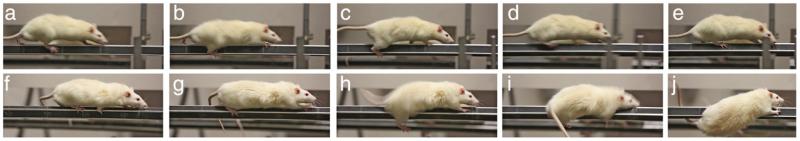
Example of slips and recovery versus non-recovery from a slip in a control and a lesioned rat (still photos taken from video). a–e show a sham-operated control rat and f–j a rat with cholinergic loss as well as striatal dopamine loss traversing a rotating rod (10 rpm) at 0° incline. Both rats exhibited a slip (b, h) but the lesioned rat fails to initiate correcting steps and compensatory postural control and thus falls (into a net).
We assessed animals with bilateral cortical cholinergic and/or partial striatal dopaminergic deafferentation, produced by intracranial infusions of neurotoxins (192-IgG saporin, SAP and 6-hydroxydopamine, 6-OHDA, respectively). The extent of the cholinergic lesions in animals was guided by the results from AChE-PET imaging studies that indicated moderate decreases in cortical and thalamic cholinergic input in PD fallers (Bohnen et al., 2009b). However, we reasoned that PET-AChE as well histochemical measures of AChE likely underestimate loss of cholinergic terminals when compared with post mortem determination of cortical choline acetyltransferase (ChAT) content and basal forebrain cell counts (e.g., Ikonomovic et al., 2005). Post mortem cortical ChAT activity in PD is decreased by 50–60% (Mattila et al., 2001). Such loss of ChAT activity reflects the loss of cholinergic inputs required to significantly impair SAT performance in rats (Burk et al., 2002; McGaughy and Sarter, 1998; McGaughy et al., 1996). We removed, consequently, on average about 50% of the cholinergic inputs to the cortex.
In our experiments, striatal dopamine lesions targeted the prefrontal projection field in the dorsomedial caudate nucleus (Mailly et al., 2013; Reep et al., 2003), reflecting our goal to determine interactions between dopamine loss and the impact of loss of cholinergic deafferentation of primarily prefrontal regions on striatal function. This subdivision of the striatum in rodents is considered homologous to the human caudate nucleus (Deumens et al., 2002; Haber, 2003).
Performances on the SAT and the MCMCT, the latter involving an increasingly demanding series of traversal conditions (e.g., stationary planks and rods; rotating beams placed at an incline) were compared between rats with sham-surgeries (SHAMS), single cholinergic deafferentation (SAP), single dopaminergic deafferentation (6-OHDA), or dual cholinergic and dopaminergic deafferentation (DL). The main results and conclusions were:
SAT performance was similarly impaired in SAP and DL rats, indicating that cholinergic loss alone impaired attentional performance and that striatal dopamine loss did not exacerbate such impairment.
MCMCT performance was unimpaired in 6-OHDA rats. Furthermore, SAT performance in 6-OHDA animals was superior compared to all other groups. As the relationship between levels of cholinergic neuromodulatory activity and SAT performance is fairly well understood (Paolone et al., 2013; St Peters et al., 2011), performance-associated cortical cholinergic activity in 6-OHDA rats likely increases beyond those seen on control rats.
Fall rates were moderately but significantly increased in SAP rats and DL rats exhibited more than twice the SAP group’s number of falls. In DL rats, higher fall rates in the MCMCT were correlated with poorer attentional performance in the SAT. Furthermore, falls in DL rats were associated with slower traversal speed, lower step frequency,“slouched posture”, micropauses while traversing (freezing of forward movement for less than 1 s), and less active rebalancing after slips (Fig. 1). These risk factors for falls may be considered analogous to those observed in PD patients, such as slower and more reluctant gait, freezing of gait, delayed arm movements, and impaired efficacy of arm movements to push the center of gravity away from impending fall direction (for review see Grimbergen et al., 2004).
None of the three lesion conditions (striatal dopaminergic loss, cortical cholinergic deafferentation, or combined dopaminergic-cholinergic loss) resulted in impairments in tests of basic limb coordination, hind limb control, and dexterous forepaw function.
Quantitative histological analyses indicated that in DL rats, but not in rats with dopaminergic deafferentation alone, larger and more precisely placed dorsomedial striatal dopamine loss predicted higher fall rates. Cholinergic cell loss was not correlated with measures of MCMCT performance. This finding suggests that the impact of striatal dopamine loss was ‘unmasked’ by loss of cortical cholinergic inputs.
The demonstration that PD patients exhibit greater activity in cortical, including prefrontal regions while performing automatic movements likewise suggests recruitment of cognitive–attentional networks even for relatively undemanding gait and posture control (Wu and Hallett, 2005). If these cortices were to lack cholinergic input such compensatory recruitment would remain largely ineffective. The recent finding that slow gait speed in PD patients is correlated with comorbid cholinergic cell loss, but is not affected in patients with relatively isolated dopamine loss (Bohnen et al., 2013) is also consistent with the hypothesis that cholinergic cell loss unmasks the low-vigor gait resulting from striatal dopamine loss (Mazzoni et al., 2007; Niv et al., 2007).
Partial striatal dopamine loss alone did not result in impairments in MCMCT performance, suggesting that compensatory attentional mechanisms contributed to the prevention of falls in these animals. However, striatal dopamine loss was previously documented to generally slow responding and decrease response accuracy in tasks involving habitual or automatic responding or tasks requiring shifts between behavioral contingencies (Baunez and Robbins, 1999; Cools et al., 2001; Crofts et al., 2001; Darvas and Palmiter, 2009; Devan et al., 1999; Domenger and Schwarting, 2008; Gauntlett-Gilbert et al., 1999; Hauber and Schmidt, 1994; Lex and Hauber, 2010; Rogers et al., 2001). Furthermore, low striatal dopamine levels in PD patients are associated with movement impairments if the biomechanical costs are high (Gepshtein et al., 2014). The behavioral risk factors for falls seen in DL rats (see point 3 above), particularly the slowing of traversal and the presence of micropauses, may reflect impairments in the planning and sequencing of movements and, more generally, low “motor motivation”. These risk factors may reflect primarily the impact of striatal dopamine loss (see also Mazzoni et al., 2007) that could not be compensated for by heightened cholinergic-attentional mechanisms.
Risk factors for falls resulting from striatal dopamine decline
Impairments in gait and posture control are risk factors for falls in the elderly and in PD patients (Bridenbaugh and Kressig, 2011; Coelho et al., 2012; Granacher et al., 2011; Uemura et al., 2011; for review see Ayers et al., 2013; Gepshtein et al., 2014; Montero-Odasso et al., 2012a,b). Our evidence from the animal model supports the hypothesis that striatal dopaminergic decline is a contributor to the manifestation of these risk factors. Measures of striatal dopamine functions decline with age and, while per se not differentiating fallers from non-fallers, reduced striatal dopamine was shown to be associated with impairments in gait and gait correction. Recurrent fallers had lower levels of striatal dopamine than patients who fell only once (Bohnen et al., 2009a; Cham et al., 2008, 2011; Troiano et al., 2010). Although age-related striatal dopamine decline may not match the loss seen in PD (Darbin, 2012), reduced striatal dopamine mediates relatively low levels of motor motivation in both older adults and PD patients prone to fall (Gepshtein et al., 2014; Hurley et al., 2011; Yue et al., 2012).
Essential for falls: striatal convergence of cortical cholinergic deafferentation and dopamine loss
Loss of cortical cholinergic inputs is hypothesized to interact with striatal dopamine loss to increase falls. This interaction between attenuated attentional compensatory control and impairments in gait, balance and complex movement control may be based in part on functionally complementary decline in largely separate neuronal systems. Because, however, the evidence from the animal model indicated that higher fall rates were predicted by the size and accuracy with which dopamine innervation was removed from the prelimbic projection field in the dorsomedial caudate nucleus, the trans-synaptic striatal consequences of frontal cholinergic deafferentation may interact with dopaminergic deafferentation to disrupt dorsomedial striatal function (Fig. 2). We begin evaluating this hypothesis by detailing our current knowledge of the organization and functions of cholinergic projections to the cortex. We will then extrapolate the consequences of cholinergic cell loss for the corticostriatal function.
Fig. 2.
Schematic illustration of essential neurocircuitry underlying attentional–motor interactions in the intact brain (a), and following the dual loss of basal forebrain cholinergic neurons and striatal dopamine (b) that is hypothesized to be essential for falls. The figure is not designed to provide a comprehensive illustration of known circuitry, including the synaptic organization within individual regions. Rather, it represents the major anatomical–functional interactions deduced from research in PD fallers and, to a lesser degree, older adults prone to fall, and in an animal model of PD falling (Kucinski et al., 2013). In the intact brain (a), cholinergic projections to the cortex arise from the nucleus basalis of Meynert (nbM), the substantia innominate (SI) and the horizontal nucleus of the diagonal band (HDB) of the basal forebrain. The precise origin of cholinergic projections in these regions depends on the cortical target region but all subregions contribute to cortical innervation (e.g., Luiten et al., 1987; Zaborszky et al., 2012, 2013). In prefrontal cortex (PFC), cholinergic neurons contact GABAergic inhibitory interneurons and pyramidal cells and, as illustrated, both innervation patterns may contribute to corticostriatal output. Muscarinic (m)AChRs may primarily mediate the effects of cholinergic activity on cortical output (e.g., Nelson et al., 2005). In the cortex, two types of cholinergic activity likely originate from separate neurons in the basal forebrain. First, as detailed in the main text, for certain cues to be detected, the cues need to evoke a brief cholinergic release event (“transient”; Howe et al., 2013). Furthermore, cue-evoked glutamate release from mediodorsal thalamic (MD) input is necessary but not sufficient to evoke such a cholinergic transient (Parikh et al., 2008, 2010). The exact mechanisms linking this glutamatergic–cholinergic transient interaction are unknown and the figure indicates a parsimonious direct contact at cholinergic terminals. Cholinergic transients are thought to be the primary source for cholinergic stimulation of prefrontal output. The second, neuromodulatory component of cholinergic activity influences glutamatergic–cholinergic transients via stimulation of α4β2* nAChR expressed by glutamatergic terminals (references above; see also Lambe et al., 2003) (Note that other thalamic inputs to cortical neurons are not shown). Cortical projections to medium spiny neurons (MSNs) in the striatum preferentially make contact at the head of spines that are also contacted, as illustrated, by dopaminergic afferents (DA) from the midbrain (CPu, caudate-putamen). In the rat model, converging dopamine loss and the functional impact of cholinergic deafferentation of prefrontal cortex for cortico-striatal function was found to be essential for generating high rates of falls. This finding primarily implicates dopamine D1 receptor-expressing MSNs of the direct projection pathway to the midbrain (SNr, substantia nigra, pars reticulata). As illustrated in (b), falling in older adults and, more severely, PD, is a result of striatal dopamine loss and cortical cholinergic deafferentation, yielding striatal circuitry that lacks information about the efficacy of gait, posture, and movement and that is impaired in selecting and sequencing motor actions, resulting in slow and reluctant movements or fails to initiate movement altogether (see main text).
The basal forebrain contains multiple populations of neurons, including cholinergic, glutamatergic, and GABAergic neurons which project to telencephalic and diencephalic regions (Gritti et al., 2006; Zaborszky, 2002; Zaborszky et al., 2005, 2008, 2012, 2013). In contrast to the evidence concerning functions of cholinergic projections to the cortex, the role of non-cholinergic neurons arising from the basal forebrain is less clear (Lin et al., 2006). Beginning with experiments assessing the impact of lesions of the cholinergic system (reviewed in McGaughy et al., 2000), the necessity of the cortical cholinergic input system for a wide range of attentional functions and capacities has been extensively demonstrated. Cholinergic lesions disrupt sustained, selective and divided attention performance (Bucci et al., 1998; Dalley et al., 2004; McGaughy et al., 1996) and impair performance involving cross-modal feature-binding (Botly and De Rosa, 2009; Newman and McGaughy, 2008; Turchi and Sarter, 1997) and attention-dependent learning and memory (Sarter et al., 2001, 2003; for review see Hasselmo and Sarter, 2011).
Two modes of cortical cholinergic activity contribute to attention performance. First, brief cholinergic release events (“transients”), occurring on the scale of hundreds of milliseconds to seconds, are evoked by cues in specific trials in the sustained attention task, namely trials involving a shift from monitoring for cues to acting on cues. Cholinergic transients are necessary to act on cue in this context (‘detection’ in Posner et al., 1980). The causal role of those transients for cue detection was confirmed by the finding that optogenetically-evoked transients increased the number of false claims that a cue was present during trials without a cue (false alarms; Gritton et al., 2012). Cortical cholinergic deafferentation abolishes such transients, resulting in missed cues in 50–70% of trials, with no recovery over extended periods of practice. Such deterministic contributions of cholinergic transients to other forms of attention remain unknown; however, it is plausible to speculate that, for example, the performance in a cross-modal divided attention task likewise depends on cholinergic transients during trials indicating a modality switch (Sarter et al., in press; Turchi and Sarter, 1997).
Second, a neuromodulatory component of cholinergic activity occurs on the scale of tens of seconds to minutes and influences the cortical target circuitry that contributes to the generation of cholinergic transients (Guillem et al., 2011; Parikh and Sarter, 2008; Parikh et al., 2007). Levels of this slower cholinergic neuromodulation are associated with goal-directed or top-down control of attention. For example, higher levels of cholinergic neuromodulatory activity are seen in response to presenting a distractor (St Peters et al., 2011). Conversely, animals that exhibit dampened neuromodulatory cholinergic activity as a trait show relatively poor and highly fluctuating levels of attentional performance (Paolone et al., 2013). Thus, removal of the cortical cholinergic input system attenuates both cue-driven (or bottom-up) and goal-driven (or top-down) aspects of attentional performance. Partial cholinergic lesions produce poor and fluctuating performance while more complete lesions produce performance that is unresponsive to manipulations that previously elicited increases in attentional effort (Sarter et al., 2006).
The postsynaptic targets of cholinergic projections to the cortex include nicotinic acetylcholine receptors (nAChRs) expressed on the terminals of thalamic glutamatergic projections. Neuromodulatory effects of ACh influence the generation of cholinergic transients via this target (Aracri et al., 2013; Dickinson et al., 2008; Guillem et al., 2011; Howe et al., 2010; Parikh and Sarter, 2008; Parikh et al., 2008, 2010). Muscarinic (m) AChRs mediate a complex and layer-specific array of inhibitory and excitatory effects at cortical interneurons and output neurons (Eggermann and Feldmeyer, 2009; Egorov et al., 2002; Gulledge and Stuart, 2005; Hasselmo and Bower, 1992). The specific role of frontal cortical mAChRs in attention is not well understood (Disney and Aoki, 2008; for evidence on the role of these receptors in temporal and sensory regions during attention and encoding see Disney et al., 2012; Herrero et al., 2008; Newman et al., 2013; Thiele et al., 2012). However, mAChR stimulation is essential for transferring the effects of cortical cholinergic activity to downstream regions (Nelson et al., 2005) including, presumably, to the striatum (Fig. 2). Consistent with this hypothesis, cue detection was found to be associated with high-frequency gamma oscillations in prefrontal cortex, and gamma power was attenuated by blocking muscarinic M1 AChRs in the prelimbic cortex (Howe et al., 2011). M1 stimulation-induced high frequency synchronicity is thought to coordinate larger cortical and subcortical networks in part to organize the recruitment of efferent, or downstream, regions to complete and execute behavioral responses (Deco and Thiele, 2009; Fries, 2005; Ossandón et al., 2011).
These considerations have implications for the consequences of cortical cholinergic input loss on cortico-striatal information transfer. In attentional contexts, the occurrence of a cholinergic transient and thus the decision to report the presence of the cue is forwarded to the striatum, specifically to synapses on dendritic spines of medium spiny neurons (MSNs; Dubé et al., 1988), including direct pathway neurons projecting preferentially to the substantia nigra pars reticulata and the internal globus pallidus (Wall et al., 2013). Following removal of the cholinergic system, cues are detected at a very low rate and the residual hits occur mostly in trials that do not require cholinergic transients (that is, occasional strings of consecutive hits; unpublished observations). Thus, following cholinergic cell loss, the striatum may be largely deprived of information that normally reports the presence of behaviorally relevant cues, including cues normally detected and employed to support complex movement, such as successful limb placement onto a dynamic surface or slips that would normally trigger corrective action.
For the direct pathway projecting neurons that are preferentially contacted by prefrontal efferents (Wall et al., 2013), dopaminergic afferents modulate cortico-striatal signaling via D1 receptors expressed on the neck of the spines that are contacted also by corticostriatal terminals (Pickel et al., 1981). Dopamine is suggested to select certain corticostriatal inputs over others and, therefore, dopaminergic deafferentation may disrupt selection or filtering of cortical inputs (Bamford et al., 2004; Devan et al., 1999; Guthrie et al., 2013; Kim et al., 2013; Matell et al., 2007; Strafella et al., 2005; van Schouwenburg et al., 2012). In the context of ongoing complex movements, such as traversing a rotating rod, impaired cortico-striatal input selection may therefore slow and even stop complex movement sequences (Kim et al., 2013; e.g., Bhutani et al., 2013; Yin, 2014), yielding the sensorimotor risk factors for falls described above.
Dual cortical cholinergic and striatal dopaminergic deafferentation therefore disrupts the monitoring of complex movements and movement errors as well as the selection of residual detection information. The inefficiency with which DL rats corrected slips and fell most closely reflected the consequences of converging striatal dopamine loss and impaired cortico-striatal information transfer (Kucinski et al., 2013). Likewise, falls associated with stepping errors or impaired inspection of obstacles (Uemura et al., 2012; Young and Hollands, 2010) are mediated via dorsomedial striatal circuitry that does not receive adequate information about the presence of instrumental cues. The consequences of striatal dopamine loss are impaired selection and sequencing of actions (Kim et al., 2013), including those needed to stabilize gait and posture and to move around obstacles or recover from a slip (see also Koop et al., 2013), and reduced vigor for initiating movement (Mazzoni et al., 2007).
In addition to the consequences of dual cholinergic–dopaminergic deafferentation for the dorsomedial striatum (Fig. 2b), falls in humans and the animal model may reflect broader impairments in sustaining and dividing attention that result from cholinergic cell loss, and also broader effects of more widespread striatal dopamine loss on gait and balance. However, we hypothesize that the dual dysregulation of dorsomedial caudate function is a major basis for falls. As a result of the functional cholinergic and anatomical dopaminergic, deafferentation of this region, the direct projection pathway is dysfunctional and initiation and execution of motor programs are disrupted (Freeze et al., 2013). The key role of the dorsomedial striatum has been confirmed by the finding that dopaminergic deafferentation of more lateral and ventral regions of the striatum, combined with cortical cholinergic input loss, does not reproduce the high fall rate reported for DL rats with dorsomedial striatal dopamine loss (Kucinski et al., 2013).
Dual task-associated falls in subjects with low cholinergic levels: de-prioritization of gait and movement control
In older adults and PD patients, the precision of gait and postural stability are readily impaired in the presence of a secondary task (above). The consumption of limited attentional resources by the secondary task, and thus withdrawing of such resources from supervising gait, balance and complex movement, is hypothesized to be a major cause of falls in older adults and PD patients (Amboni et al., 2013; Brown et al., 1999; Crossley and Hiscock, 1992; Hausdorff et al., 2006; Plotnik et al., 2011; Springer et al., 2006; Tombu and Jolicæur, 2003; Yogev-Seligmann et al., 2013). In the presence of cholinergic cell loss attentional resources are already markedly reduced (Sarter and Turchi, 2002) and thus additional taxation nearly completely abolishes the attentional monitoring of motor action. As a result, gait freezes, postural imbalance, error-prone movements, and eventually, falls, occur (see above; see also Uemura et al., 2011). Humans prioritize gait and movement control over other tasks (“posture first”; Li et al., 2001; Mersmann et al., 2013), and thus measures of gait, posture, movement speed and errors serve as an indirect measure of attentional resource limitation.
The concept of cognitive resource limitations is unsatisfactory (Navon, 1984). A more useful conceptualization proposes that experiencing attentional fatigue and exhaustion reflects the ongoing computation of the costs and benefits of continuing performing the current task relative to terminating such performance and engaging in alternative action (Kurzban et al., 2013). As these “opportunity costs” increase, staying on task becomes a more aversive choice and this is experienced as a depletion of cognitive resources that then motivates alternative action, such as engaging in a secondary task. As humans prioritize movement control (above), this is therefore the “depleting” task that will be de-prioritized in the presence of low cholinergic neuromodulatory activity. In other words, cholinergic cell loss favors disengagement from gait, balance and movement control and fosters engagement in an alternative action, be it daydreaming or an actual alternative task. This hypothesis allows one to predict the conditions under which falls will occur and minimizes the need to speculate about task difficulty and complexity, cognitive load, or dynamic changes in attentional resource levels. De-prioritization of the motor task would also attenuate the prioritization of the processing of movement errors. Finally, as dorsal striatal dopamine loss reduces the vigor for demanding motor actions in PD patients and renders patients reluctant to move (see also Gepshtein et al., 2014; Niv et al., 2007; Wang et al., 2013), such loss may interact with low levels of cortical cholinergic activity to deprioritize the planning and execution of movements.
Cholinergic–dopaminergic, cognitive–motor interactions: pharmacological opportunities
Antimuscarinic cholinergic agents are used, albeit with decreasing frequency, primarily to treat tremor in PD. Such compounds are expected to adversely affect gait and posture, particularly when attentional resources need to be recruited to support complex movements and to correct movement errors. Indeed, in the elderly, the greater fall risk associated with anticholinergic drug use is well documented (Aizenberg et al., 2002; Berdot et al., 2009; Wilson et al., 2011). This issue has not been systematically investigated in PD patients.
Evidence in support of potential beneficial effects of acetylcholinesterase inhibitors on PD-associated falling is inconclusive (Chung et al., 2010; Possin et al., 2013). The extraordinarily high levels of extracellular acetylcholine generated by these compounds inhibit presynaptic cholinergic activity and tonically stimulate synaptic and extrasynaptic, muscarinic and nicotinic acetylcholine receptors (m, nAChRs). Because of the non-specific effects of cholinesterase inhibitors it is difficult to conceive of robust and reliable therapeutic effects with these compounds (reviewed in Hasselmo and Sarter, 2011).
Falls result from interactions between error-prone gait and imperfect balance, due to impaired striatal dopamine and failure of a damaged cholinergic system to provide attentional compensatory support. Consistent with the clinical evidence, this hypothesis predicts that levodopa treatment benefits aspects of gait and balance but does not prevent falls (McNeely et al., 2013; Sethi, 2008). Our hypothesis further predicts that drugs that benefit attentional performance also reduce fall rates, likely requiring co-administration of levodopa. In our previous studies, one group of compounds that reliably enhanced attentional performance in intact rats were agonists at α4β2* nAChRs. Stimulation of these receptors in the cortex mimics and amplifies the cholinergic neuromodulatory effects on cortical cue detection circuitry (see Fig. 2; Howe et al., 2010; Parikh et al., 2010; Sarter et al., 2009). Stimulation of α4β2* nAChRs enhances the top-down control of attention or, in terms of the theoretical alternative discussed above, decreases opportunity costs and therefore sustains engagement with the primary (motor) task. As predicted by the attentional effects in animals, α4β2* nAChRs agonists benefit the symptoms of adult ADHD (see results from Phase II trials in Apostol et al., 2011; Bain et al., 2013).
Previous studies in monkeys generated support for the hypothesis that stimulation of α4β2* nAChRs, in combination with potentially relatively low doses of levodopa, benefits the non-motor symptoms of PD (Decamp and Schneider, 2009; Schneider et al., 1998, 1999, 2003). In our rat model (Kucinski et al., 2013), co-administration of the α4β2* nAChR agonist ABT-089 (Arneric et al., 1997), levodopa and benserazide, reduced falls across testing conditions on the MCMCT by approximately 50% (Kucinski et al., 2012). Clearly, the efficacy of α4β2* nAChR agonists will need to be further demonstrated using other compounds. We are aware of one clinical trial of one of these compounds in PD, the α4β2* nAChR agonist SIB-1508Y. The clinical efficacy of this compound, assessed in levodopa-naïve patients, was determined using the Unified Parkinson’s Disease Rating Scale (UPDRS) and a cognitive test battery. The drug was ineffective (Parkinson Study Group, 2006). Future studies on the potential of α4β2* nAChR agonists to treat non-motor symptoms in PD should focus on PD fallers or patients with low levels of cholinergic activity in the cortex and thalamus and to test α4β2* nAChR agonists as an adjunctive to pro-dopaminergic therapy. Evidence indicating that α4β2* nAChR agonist treatment also reduce dyskinesias (Huang et al., 2011; Quik et al., 2008) may further motivate such trials.
Conclusions
Falls in older adults and in PD arise from dysregulation and degeneration of multiple neuronal systems including, primarily and perhaps essentially, the basal forebrain cholinergic projection system and the striatal dopamine system. Falls in PD are also associated with loss of cholinergic projections from the pedunculopontine nucleus (PPN) in the brain stem (Bohnen et al., 2009b; Hirsch et al., 1987; Karachi et al., 2010; Muller et al., 2013; Pahapill and Lozano, 2000); however, the cognitive–behavioral contributions of the widespread, ascending and descending PPN cholinergic projections to gait, posture and complex movement control remain unclear and thus beyond the scope of the article.
We propose that in the presence of sensorimotor risk factors for falls, reflecting the impact of loss of striatal dopamine, subjects with impaired basal forebrain cholinergic systems are impaired in the attentional control of gait, posture, movement and the detection of movement errors. Cholinergic cortical deafferentation indirectly disrupts the function of striatal circuitry to select and sequence motor actions; furthermore, such loss limits any potential compensatory recruitment of the attention system to stabilize and recover movement errors. Thus, striatal dopamine loss and cortical cholinergic deafferentation converge to dysregulate dorsomedial striatal output and the initiation of movement (Freeze et al., 2013).
Our hypothesis provides the basis for a rational pharmacological treatment for reducing falls. Because of available tolerability evidence from Phase II studies on the effects of α4β2* nAChR agonists in adult ADHD, some of these compounds are likely to be safe for testing their effects in PD fallers. In older adults prone to falls, a test of these drugs may also be encouraged by the relatively benign side effect spectrum of this group of compounds.
Falls arise from detrimental interactions between multiple cognitive and sensorimotor risk factors. Although disruption of dorsomedial caudate function is hypothesized to be a major basis for falls, we lack information about the role of converging thalamo–striatal interactions (Bradfield et al., 2013), as well as an understanding of the mechanistic impact of loss of cholinergic innervation of the thalamus from both the basal forebrain and PPN. Furthermore, we do not understand, in behavioral and neuronal-mechanistic terms, the contributions of the loss of the PPN projections to the mid- and forebrain, cerebellum and spinal cord (Fasano et al., 2012). There is also limited information about the role of dopamine loss on the direct versus indirect pathway for impairing motor action (e.g., Freeze et al., 2013). As falls represent the results of impaired integration of cognitive and motor functions, they may constitute a fruitful behavioral endpoint to guide research on the role of striatal and other circuitry in integrating information from these two domains.
Finally, we acknowledge potential clinical complications in our understanding of the cognitive–motor conditions that enhance the propensity for falls and also relevant for proposals for treating fall propensity in PD patients. Levodopa treatment may indirectly increase the risk for falls by improving the patient’s overall mobility. Furthermore, higher doses of levodopa treatment may have detrimental effects on patient’s (residual) cognitive abilities. As the clinical usefulness of adjunctive treatments to levodopa is explored, these potential complications will need to be investigated.
Acknowledgments
Aspects of research discussed in this article were supported in part by the Michael J. Fox Foundation for Parkinson’s Research and PHS grants 1R01MH086530 and 1PO1 DA031656.
Footnotes
Complex movements are required to circumvent obstacles or move over unfamiliar or unstable surfaces or, in the animal model, traverse rotating rods. In these cases, the regular frequency-based patterning of limb movements that defines gait (e.g., Bridenbaugh and Kressig, 2011) is challenged and often disrupted, involving changes in the direction of travel, the rapid development of torque and postural muscle activity, including upper limb (or forelimb) movements to correct for imbalance, imperfect limb placements or stepping errors. Complex movements demand attentional monitoring, specifically the detection of limb placement and of gait and posture errors, as demonstrated by the efficacy of distractors or dual-task demands to disrupt complex movement (e.g., Chen et al., 1996). Notably, however, even postural corrections, such as recovering upright stance following a perturbation, are sensitive to attentional distraction (Brown et al., 1999).
References
- Aizenberg D, Sigler M, Weizman A, Barak Y. Anticholinergic burden and the risk of falls among elderly psychiatric inpatients: a 4-year case–control study. Int. Psychogeriatr. 2002;14:307–310. doi: 10.1017/s1041610202008505. [DOI] [PubMed] [Google Scholar]
- Allcock LM, Rowan EN, Steen IN, Wesnes K, Kenny RA, Burn DJ. Impaired attention predicts falling in Parkinson’s disease. Parkinsonism Relat. Disord. 2009;15:110–115. doi: 10.1016/j.parkreldis.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Amboni M, Barone P, Hausdorff JM. Cognitive contributions to gait and falls: evidence and implications. Mov. Disord. 2013;28:1520–1533. doi: 10.1002/mds.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol G, Abi-Saab W, Kratochvil CJ, Adler LA, Robieson WZ, Gault LM, et al. Efficacy and safety of the novel α(4)β(2) neuronal nicotinic receptor partial agonist ABT-089 in adults with attention-deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled crossover study. Psychopharmacology (Berl.) 2011;219:715–725. doi: 10.1007/s00213-011-2393-2. [DOI] [PubMed] [Google Scholar]
- Aracri P, Amadeo A, Pasini ME, Fascio U, Becchetti A. Regulation of glutamate release by heteromeric nicotinic receptors in layer V of the secondary motor region (fr2) in the dorsomedial shoulder of prefrontal cortex in mouse. Synapse. 2013;67:338–357. doi: 10.1002/syn.21655. [DOI] [PubMed] [Google Scholar]
- Arneric SP, Campbell JE, Carroll S, Daanen JF, Holladay MW, Johnson P, et al. ABT-089 [3-(2 (S)-pyrrolidinylmethoxy)-2-methyl-pyridine]: an orally effective cholinergic channel modulator with potential once-a-day dosing and cardiovascular safety. Drug Dev. Res. 1997;41:31–43. [Google Scholar]
- Ayers EI, Tow AC, Holtzer R, Verghese J. Walking while talking and falls in aging. Gerontology. 2013;60:108–113. doi: 10.1159/000355119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain EE, Robieson W, Pritchett Y, Garimella T, Abi-Saab W, Apostol G, et al. A randomized, double-blind, placebo-controlled phase 2 study of α4β2 agonist ABT-894 in adults with ADHD. Neuropsychopharmacology. 2013;38:405–413. doi: 10.1038/npp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SP, Harvey AH. Fall injuries in the elderly. Clin. Geriatr. Med. 1985;1:501–512. [PubMed] [Google Scholar]
- Balash Y, Peretz C, Leibovich G, Herman T, Hausdorff JM, Giladi N. Falls in out-patients with Parkinson’s disease: frequency, impact and identifying factors. J. Neurol. 2005;252:1310–1315. doi: 10.1007/s00415-005-0855-3. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine modulates release from corticostriatal terminals. J. Neurosci. 2004;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Baunez C, Robbins TW. Effects of dopamine depletion of the dorsal striatum and further interaction with subthalamic nucleus lesions in an attentional task in the rat. Neuroscience. 1999;92:1343–1356. doi: 10.1016/s0306-4522(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Berdot S, Bertrand M, Dartigues JF, Fourrier A, Tavernier B, Ritchie K, et al. Inappropriate medication use and risk of falls—a prospective study in a large community-dwelling elderly cohort. BMC Geriatr. 2009;9:30. doi: 10.1186/1471-2318-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutani N, Sureshbabu R, Farooqui A, Behari M, Goyal V, Murthy A. Queuing of concurrent movement plans by basal ganglia. J. Neurosci. 2013;33:9985–9997. doi: 10.1523/JNEUROSCI.4934-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL. Cholinergic denervation occurs early in Parkinson disease. Neurology. 2009;73:256–257. doi: 10.1212/WNL.0b013e3181b0bd3d. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav. Brain Res. 2011;221:564–573. doi: 10.1016/j.bbr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Frey KA. Imaging of cholinergic and monoaminergic neurochemical changes in neurodegenerative disorders. Mol. Imaging Biol. 2007;9:243–257. doi: 10.1007/s11307-007-0083-6. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, et al. Cortical cholinergic function is more severely affected in Parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch. Neurol. 2003;60:1745–1748. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Muller ML, Kuwabara H, Cham R, Constantine GM, Studenski SA. Age-associated striatal dopaminergic denervation and falls in community-dwelling subjects. J. Rehabil. Res. Dev. 2009a;46:1045–1052. doi: 10.1682/jrrd.2009.03.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Müller ML, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009b;73:1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Müller MLTM, Kotagal V, Koeppe RA, Kilbourn MR, Gilman S, et al. Heterogeneity of cholinergic denervation in Parkinson’s disease without dementia. J. Cereb. Blood Flow Metab. 2012;32:1609–1617. doi: 10.1038/jcbfm.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, Scott PJ, et al. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology. 2013;81:1611–1616. doi: 10.1212/WNL.0b013e3182a9f558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botly LC, De Rosa E. Cholinergic deafferentation of the neocortex using 192 IgG-saporin impairs feature binding in rats. J. Neurosci. 2009;29:4120–4130. doi: 10.1523/JNEUROSCI.0654-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield L, Bertran-Gonzalez J, Chieng B, Balleine B. The thalamostriatal pathway and cholinergic control of goal-directed action: interlacing new with existing learning in the striatum. Neuron. 2013;79:153–166. doi: 10.1016/j.neuron.2013.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridenbaugh SA, Kressig RW. Laboratory review: the role of gait analysis in seniors’ mobility and fall prevention. Gerontology. 2011;57:256–264. doi: 10.1159/000322194. [DOI] [PubMed] [Google Scholar]
- Brown LA, Shumway-Cook A, Woollacott MH. Attentional demands and postural recovery: the effects of aging. J. Gerontol. A Biol. Sci. Med. Sci. 1999;54:M165–M171. doi: 10.1093/gerona/54.4.m165. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J. Neurosci. 1998;18:8038–8046. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk JA, Herzog CD, Porter MC, Sarter M. Interactions between aging and cortical cholinergic deafferentation on attention. Neurobiol. Aging. 2002;23:467–477. doi: 10.1016/s0197-4580(01)00315-3. [DOI] [PubMed] [Google Scholar]
- Candy JM, Perry RH, Perry EK, Irving D, Blessed G, Fairbairn AF, et al. Pathological changes in the nucleus of Meynert in Alzheimer’s and Parkinson’s diseases. J. Neurol. Sci. 1983;59:277–289. doi: 10.1016/0022-510x(83)90045-x. [DOI] [PubMed] [Google Scholar]
- Cham R, Studenski SA, Perera S, Bohnen NI. Striatal dopaminergic denervation and gait in healthy adults. Exp. Brain Res. 2008;185:391–398. doi: 10.1007/s00221-007-1161-3. [DOI] [PubMed] [Google Scholar]
- Cham R, Perera S, Studenski SA, Bohnen NI. Age-related striatal dopaminergic denervation and severity of a slip perturbation. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:980–985. doi: 10.1093/gerona/glr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Schultz AB, Ashton-Miller JA, Giordani B, Alexander NB, Guire KE. Stepping over obstacles: dividing attention impairs performance of old more than young adults. J. Gerontol. A Biol. Sci. Med. Sci. 1996;51:M116–M122. doi: 10.1093/gerona/51a.3.m116. [DOI] [PubMed] [Google Scholar]
- Chung KA, Lobb BM, Nutt JG, Horak FB. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology. 2010;75:1263–1269. doi: 10.1212/WNL.0b013e3181f6128c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho FG, Stella F, de Andrade LP, Barbieri FA, Santos-Galduróz RF, Gobbi S, et al. Gait and risk of falls associated with frontal cognitive functions at different stages of Alzheimer’s disease. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2012;19:644–656. doi: 10.1080/13825585.2012.661398. [DOI] [PubMed] [Google Scholar]
- Cole MH, Silburn PA, Wood JM, Worringham CJ, Kerr GK. Falls in Parkinson’s disease: kinematic evidence for impaired head and trunk control. Mov. Disord. 2010;25:2369–2378. doi: 10.1002/mds.23292. [DOI] [PubMed] [Google Scholar]
- Cole MH, Silburn PA, Wood JM, Kerr GK. Falls in Parkinson’s disease: evidence for altered stepping strategies on compliant surfaces. Parkinsonism Relat. Disord. 2011;17:610–616. doi: 10.1016/j.parkreldis.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb. Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cowie D, Limousin P, Peters A, Hariz M, Day BL. Doorway-provoked freezing of gait in Parkinson’s disease. Mov. Disord. 2012;27:492–499. doi: 10.1002/mds.23990. [DOI] [PubMed] [Google Scholar]
- Craik FI, Luo L, Sakuta Y. Effects of aging and divided attention on memory for items and their contexts. Psychol. Aging. 2010;25:968–979. doi: 10.1037/a0020276. [DOI] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, et al. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb. Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Crossley M, Hiscock M. Age-related differences in concurrent-task performance of normal adults: evidence for a decline in processing resources. Psychol. Aging. 1992;7:499–506. doi: 10.1037//0882-7974.7.4.499. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Bouger P, Chudasama Y, Cardinal RN, Robbins TW. Cortical cholinergic function and deficits in visual attentional performance in rats following 192 IgG-saporin-induced lesions of the medial prefrontal cortex. Cereb. Cortex. 2004;14:922–932. doi: 10.1093/cercor/bhh052. [DOI] [PubMed] [Google Scholar]
- Darbin O. The aging striatal dopamine function. Parkinsonism Relat. Disord. 2012;18:426–432. doi: 10.1016/j.parkreldis.2011.11.025. [DOI] [PubMed] [Google Scholar]
- Darvas M, Palmiter RD. Restriction of dopamine signaling to the dorsolateral striatum is sufficient for many cognitive behaviors. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14664–14669. doi: 10.1073/pnas.0907299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decamp E, Schneider JS. Interaction between nicotinic and dopaminergic therapies on cognition in a chronic Parkinson model. Brain Res. 2009;1262:109–114. doi: 10.1016/j.brainres.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Thiele A. Attention: oscillations and neuropharmacology. Eur. J. Neurosci. 2009;30:347–354. doi: 10.1111/j.1460-9568.2009.06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger AM, Stevens JA. The injury problem among older adults: mortality, morbidity and costs. J. Saf. Res. 2006;37:519–522. doi: 10.1016/j.jsr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Demeter E, Sarter M, Lustig C. Rats and humans paying attention: cross-species task development for translational research. Neuropsychology. 2008;22:787–799. doi: 10.1037/a0013712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp. Neurol. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate-putamen lesions on place- and cue-guided behaviors in the water maze: relation to thigmotaxis. Behav. Brain Res. 1999;100:5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- Dickinson JA, Kew JN, Wonnacott S. Presynaptic alpha 7- and beta 2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol. Pharmacol. 2008;74:348–359. doi: 10.1124/mol.108.046623. [DOI] [PubMed] [Google Scholar]
- Disney AA, Aoki C. Muscarinic acetylcholine receptors in macaque V1 are most frequently expressed by parvalbumin-immunoreactive neurons. J. Comp. Neurol. 2008;507:1748–1762. doi: 10.1002/cne.21616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney A, Aoki C, Hawken J. Cholinergic suppression of visual responses in primate V1 is mediated by GABAergic inhibition. J. Neurophysiol. 2012;108:1907–1923. doi: 10.1152/jn.00188.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenger D, Schwarting RK. Effects of neostriatal 6-OHDA lesion on performance in a rat sequential reaction time task. Neurosci. Lett. 2008;444:212–216. doi: 10.1016/j.neulet.2008.08.048. [DOI] [PubMed] [Google Scholar]
- Dubé L, Smith AD, Bolam JP. Identification of synaptic terminals of thalamic or cortical origin in contact with distinct medium-size spiny neurons in the rat neostriatum. J. Comp. Neurol. 1988;267:455–471. doi: 10.1002/cne.902670402. [DOI] [PubMed] [Google Scholar]
- Düzel S, Münte TF, Lindenberger U, Bunzeck N, Schütze H, Heinze HJ, et al. Basal forebrain integrity and cognitive memory profile in healthy aging. Brain Res. 2010;1308:124–136. doi: 10.1016/j.brainres.2009.10.048. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Feldmeyer D. Cholinergic filtering in the recurrent excitatory microcircuit of cortical layer 4. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11753–11758. doi: 10.1073/pnas.0810062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransén E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Fasano A, Plotnik M, Bove F, Berardelli A. The neurobiology of falls. Neurol. Sci. 2012;33:1215–1223. doi: 10.1007/s10072-012-1126-6. [DOI] [PubMed] [Google Scholar]
- Fernández PJ, Campoy G, García Santos JM, Antequera MM, García-Sevilla J, Castillo A, et al. Is there a specific pattern of attention deficit in mild cognitive impairment with subcortical vascular features? Evidence from the attention network test. Dement. Geriatr. Cogn. Disord. 2011;31:268–275. doi: 10.1159/000327165. [DOI] [PubMed] [Google Scholar]
- Freeze S, Kravitz V, Hammack N, Berke D, Kreitzer C. Control of basal ganglia output by direct and indirect pathway projection neurons. J. Neurosci. 2013;33:18531–18539. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Gauntlett-Gilbert J, Roberts RC, Brown VJ. Mechanisms underlying attentional set-shifting in Parkinson’s disease. Neuropsychologia. 1999;37:605–616. doi: 10.1016/s0028-3932(98)00049-9. [DOI] [PubMed] [Google Scholar]
- Gepshtein S, Li X, Snider J, Plank M, Lee D, Poizner H. Dopamine function and the efficiency of human movement. J. Cogn. Neurosci. 2014;26:645–657. doi: 10.1162/jocn_a_00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula C, Mesulam MM. Cortical cholinergic fibers in aging and Alzheimer’s disease: a morphometric study. Neuroscience. 1989;33:469–481. doi: 10.1016/0306-4522(89)90399-0. [DOI] [PubMed] [Google Scholar]
- Geula C, Nagykery N, Nicholas A, Wu CK. Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2008;67:309–318. doi: 10.1097/NEN.0b013e31816a1df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granacher U, Bridenbaugh SA, Muehlbauer T, Wehrle A, Kressig RW. Age-related effects on postural control under multi-task conditions. Gerontology. 2011;57:247–255. doi: 10.1159/000322196. [DOI] [PubMed] [Google Scholar]
- Grimbergen YA, Munneke M, Bloem BR. Falls in Parkinson’s disease. Curr. Opin. Neurol. 2004;17:405–415. doi: 10.1097/01.wco.0000137530.68867.93. [DOI] [PubMed] [Google Scholar]
- Gritti I, Henny P, Galloni F, Mainville L, Mariotti M, Jones BE. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience. 2006;143:1051–1064. doi: 10.1016/j.neuroscience.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritton H, Mallory CS, Hetrick VL, Berke JD, Sarter M. Bidirectional optogenetic control of cortical acetylcholine signaling demonstrates vital contributions to attentional performance. Society for Neuroscience Abstracts. 2012 abstract # 913.16. [Google Scholar]
- Grothe M, Heinsen H, Teipel SJ. Atrophy of the cholinergic basal forebrain over the adult age range and in early stages of Alzheimer’s disease. Biol. Psychiatry. 2012;71:805–813. doi: 10.1016/j.biopsych.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe M, Heinsen H, Teipel S. Longitudinal measures of cholinergic forebrain atrophy in the transition from healthy aging to Alzheimer’s disease. Neurobiol. Aging. 2013;34:1210–1220. doi: 10.1016/j.neurobiolaging.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Bloem B, Poorthuis RB, Loos M, Smit AB, Maskos U, et al. Nicotinic acetylcholine receptor β2 subunits in the medial prefrontal cortex control attention. Science. 2011;333:888–891. doi: 10.1126/science.1207079. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Cholinergic inhibition of neocortical pyramidal neurons. J. Neurosci. 2005;25:10308–10320. doi: 10.1523/JNEUROSCI.2697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie M, Leblois A, Garenne A, Boraud T. Interaction between cognitive and motor cortico-basal ganglia loops during decision making: a computational study. J. Neurophysiol. 2013;109:3025–3040. doi: 10.1152/jn.00026.2013. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hall D, Echt V, Wolf L, Rogers A. Cognitive and motor mechanisms underlying older adults’ ability to divide attention while walking. Phys. Ther. 2011;91:1039–1050. doi: 10.2522/ptj.20100114. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bower JM. Cholinergic suppression specific to intrinsic not afferent fiber synapses in rat piriform (olfactory) cortex. J. Neurophysiol. 1992;67:1222–1229. doi: 10.1152/jn.1992.67.5.1222. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber W, Schmidt WJ. Differential effects of lesions of the dorsomedial and dorsolateral caudate-putamen on reaction time performance in rats. Behav. Brain Res. 1994;60:211–215. doi: 10.1016/0166-4328(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Doniger GM, Springer S, Yogev G, Simon ES, Giladi N. A common cognitive profile in elderly fallers and in patients with Parkinson’s disease: the prominence of impaired executive function and attention. Exp. Aging Res. 2006;32:411–429. doi: 10.1080/03610730600875817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. PNAS U. S. A. 1987;84:5976. doi: 10.1073/pnas.84.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Friedman R, Lipton RB, Katz M, Xue X, Verghese J. The relationship between specific cognitive functions and falls in aging. Neuropsychology. 2007;21:540–548. doi: 10.1037/0894-4105.21.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Ji J, Parikh V, Williams S, Mocaër E, Trocmé-Thibierge C, et al. Enhancement of attentional performance by selective stimulation of alpha4beta2* nAChRs: underlying cholinergic mechanisms. Neuropsychopharmacology. 2010;35:1391–1401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Gritton H, Berke J, Sarter M. Attention-demanding cues evoke prefrontal gamma oscillations and are differentially modulated by prefrontal muscarinic and nicotinic receptors. Society for Neuroscience Abstracts. 2011 abstract # 197.09. [Google Scholar]
- Howe WM, Berry AS, Francois J, Gilmour G, Carp JM, Tricklebank M, et al. Prefrontal cholinergic mechanisms instigating shifts from monitoring for cues to cue-guided performance: converging electrochemical and fMRI evidence from rats and humans. J. Neurosci. 2013;33:8742–8752. doi: 10.1523/JNEUROSCI.5809-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CL, Nagamatsu LS, Davis JC, Liu-Ambrose T. Examining the relationship between specific cognitive processes and falls risk in older adults: a systematic review. Osteoporos. Int. 2012;23:2409–2424. doi: 10.1007/s00198-012-1992-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LZ, Campos C, Ly J, Ivy Carroll F, Quik M. Nicotinic receptor agonists decrease l-dopa-induced dyskinesias most effectively in partially lesioned Parkinsonian rats. Neuropharmacology. 2011;60:861–868. doi: 10.1016/j.neuropharm.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley PJ, Elsworth JD, Whittaker MC, Roth RH, Redmond DE. Aged monkeys as a partial model for Parkinson’s disease. Pharmacol. Biochem. Behav. 2011;99:324–332. doi: 10.1016/j.pbb.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic MD, Mufson EJ, Wuu J, Bennett DA, DeKosky ST. Reduction of choline acetyltransferase activity in primary visual cortex in mild to moderate Alzheimer’s disease. Arch. Neurol. 2005;62:425–430. doi: 10.1001/archneur.62.3.425. [DOI] [PubMed] [Google Scholar]
- Kang HG, Costa MD, Priplata AA, Starobinets OV, Goldberger AL, Peng CK, et al. Frailty and the degradation of complex balance dynamics during a dual-task protocol. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:1304–1311. doi: 10.1093/gerona/glp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachi C, Grabli D, Bernard FA, Tandé D, Wattiez N, Belaid H, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J. Clin. Investig. 2010;120:2745–2754. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee D, Jung W. Signals for previous goal choice persist in the dorsomedial, but not dorsolateral striatum of rats. J. Neurosci. 2013;33:52–63. doi: 10.1523/JNEUROSCI.2422-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop MM, Hill BC, Bronte-Stewart HM. Perceptual errors increase with movement duration and may contribute to hypokinesia in Parkinson’s disease. Neuroscience. 2013;243:1–13. doi: 10.1016/j.neuroscience.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Kucinski A, Paolone G, Petersen CC, Ronan EA, Albin RL, Sarter M. Deficits in the attentional control of posture and complex movements in a rat model of early state, multisystem Parkinson’s disease. Society for Neuroscience Abstracts. 2012 abstract # 5112. [Google Scholar]
- Kucinski A, Paolone G, Bradshaw M, Albin RL, Sarter M. Modeling fall propensity in Parkinson’s disease: deficits in the attentional control of complex movements in rats with cortical-cholinergic and striatal-dopaminergic deafferentation. J. Neurosci. 2013;33:16522–16539. doi: 10.1523/JNEUROSCI.2545-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz I, Berezowski E, Melzer I. Frontal plane instability following rapid voluntary stepping: effects of age and a concurrent cognitive task. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:1402–1408. doi: 10.1093/gerona/glt040. [DOI] [PubMed] [Google Scholar]
- Kurzban R, Duckworth A, Kable JW, Myers J. An opportunity cost model of subjective effort and task performance. Behav. Brain Sci. 2013;36:661–679. doi: 10.1017/S0140525X12003196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- LaPointe LL, Stierwalt JAG, Maitland CG. Talking while walking: cognitive loading and injurious falls in Parkinson’s disease. Int. J. Speech Lang. Pathol. 2010;12:455–459. doi: 10.3109/17549507.2010.486446. [DOI] [PubMed] [Google Scholar]
- Lex B, Hauber W. The role of dopamine in the prelimbic cortex and the dorsomedial striatum in instrumental conditioning. Cereb. Cortex. 2010;20:873–883. doi: 10.1093/cercor/bhp151. [DOI] [PubMed] [Google Scholar]
- Li KZ, Lindenberger U, Freund AM, Baltes PB. Walking while memorizing: age-related differences in compensatory behavior. Psychol. Sci. 2001;12:230–237. doi: 10.1111/1467-9280.00341. [DOI] [PubMed] [Google Scholar]
- Lin SC, Gervasoni D, Nicolelis MA. Fast modulation of prefrontal cortex activity by basal forebrain noncholinergic neuronal ensembles. J. Neurophysiol. 2006;96:3209–3219. doi: 10.1152/jn.00524.2006. [DOI] [PubMed] [Google Scholar]
- Lord S, Rochester L, Hetherington V, Allcock LM, Burn D. Executive dysfunction and attention contribute to gait interference in ‘off’ state Parkinson’s disease. Gait Posture. 2010;31:169–174. doi: 10.1016/j.gaitpost.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Luiten PG, Gaykema RP, Traber J, Spencer DG. Cortical projection patterns of magnocellular basal nucleus subdivisions as revealed by anterogradely transported Phaseolus vulgaris leucoagglutinin. Brain Res. 1987;413:229–250. doi: 10.1016/0006-8993(87)91014-6. [DOI] [PubMed] [Google Scholar]
- Mailly P, Aliane V, Groenewegen HJ, Haber SN, Deniau JM. The rat prefrontostriatal system analyzed in 3D: evidence for multiple interacting functional units. J. Neurosci. 2013;33:5718–5727. doi: 10.1523/JNEUROSCI.5248-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese R, Bove M, Abbruzzese G. Effect of cognitive and motor tasks on postural stability in Parkinson’s disease: a posturographic study. Mov. Disord. 2003;18:652–658. doi: 10.1002/mds.10418. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH, Lustig C. Not “just” a coincidence: frontal-striatal interactions in working memory and interval timing. Memory. 2007;13:441–448. doi: 10.1080/09658210344000404. [DOI] [PubMed] [Google Scholar]
- Mattila PM, Röyttä M, Lönnberg P, Marjamäki P, Helenius H, Rinne JO. Choline acetyltransferase activity and striatal dopamine receptors in Parkinson’s disease in relation to cognitive impairment. Acta Neuropathol. 2001;102:160–166. doi: 10.1007/s004010100372. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Hristova A, Krakauer JW. Why don’t we move faster? Parkinson’s disease, movement vigor, and implicit motivation. J. Neurosci. 2007;27:7105–7116. doi: 10.1523/JNEUROSCI.0264-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Sustained attention performance in rats with intracortical infusions of 192 IgG-saporin-induced cortical cholinergic deafferentation: effects of physostigmine and FG 7142. Behav. Neurosci. 1998;112:1519–1525. doi: 10.1037//0735-7044.112.6.1519. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical ache-positive fiber density. Behav. Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Everitt BJ, Robbins TW, Sarter M. The role of cortical cholinergic afferent projections in cognition: impact of new selective immunotoxins. Behav. Brain Res. 2000;115:251–263. doi: 10.1016/s0166-4328(00)00262-x. [DOI] [PubMed] [Google Scholar]
- McNeely ME, Duncan RP, Earhart GM. Medication improves balance and complex gait performance in Parkinson disease. Gait Posture. 2013;19:86–91. doi: 10.1016/j.gaitpost.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersmann F, Bohm S, Bierbaum S, Dietrich R, Arampatzis A. Young and old adults prioritize dynamic stability control following gait perturbations when performing a concurrent cognitive task. Gait Posture. 2013;37:373–377. doi: 10.1016/j.gaitpost.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Montero-Odasso M, Muir SW, Speechley M. Dual-task complexity affects gait in people with mild cognitive impairment: the interplay between gait variability, dual tasking, and risk of falls. Arch. Phys. Med. Rehabil. 2012a;93:293–299. doi: 10.1016/j.apmr.2011.08.026. [DOI] [PubMed] [Google Scholar]
- Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J. Am. Geriatr. Soc. 2012b;60:2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MLTM, Albin RL, Kotagal V, Koeppe RA, Scott PJH, Frey KA, et al. Thalamic cholinergic innervation and postural sensory integration function in Parkinson’s disease. Brain. 2013;136:3282–3289. doi: 10.1093/brain/awt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu LS, Munkacsy M, Liu-Ambrose T, Handy TC. Altered visual–spatial attention to task-irrelevant information is associated with falls risk in older adults. Neuropsychologia. 2013;51:3025–3032. doi: 10.1016/j.neuropsychologia.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith SL, Shine JM, Lewis SJ. The specific contributions of set-shifting to freezing of gait in Parkinson’s disease. Mov. Disord. 2010;25:1000–1004. doi: 10.1002/mds.23005. [DOI] [PubMed] [Google Scholar]
- Nakano I, Hirano A. Parkinson’s disease: neuron loss in the nucleus basalis without concomitant Alzheimer’s disease. Ann. Neurol. 1984;15:415–418. doi: 10.1002/ana.410150503. [DOI] [PubMed] [Google Scholar]
- Navon D. Resources: a theoretical soup stone? Psychol. Rev. 1984;91:216. [Google Scholar]
- Nelson CL, Sarter M, Bruno JP. Prefrontal cortical modulation of acetylcholine release in posterior parietal cortex. Neuroscience. 2005;132:347–359. doi: 10.1016/j.neuroscience.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Newman LA, McGaughy J. Cholinergic deafferentation of prefrontal cortex increases sensitivity to cross-modal distractors during a sustained attention task. J. Neurosci. 2008;28:2642–2650. doi: 10.1523/JNEUROSCI.5112-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EL, Gillet SN, Climer JR, Hasselmo ME. Cholinergic blockade reduces theta-gamma phase amplitude coupling and speed modulation of theta frequency consistent with behavioral effects on encoding. J. Neurosci. 2013;33:19635–19646. doi: 10.1523/JNEUROSCI.2586-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl.) 2007;191:507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- O’Halloran AM, Pénard N, Galli A, Fan C, Robertson IH, Kenny R. Falls and falls efficacy: the role of sustained attention in older adults. BMC Geriatr. 2011;11:1–10. doi: 10.1186/1471-2318-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossandón T, Jerbi K, Vidal JR, Bayle DJ, Henaff MA, Jung J, et al. Transient suppression of broadband gamma power in the default-mode network is correlated with task complexity and subject performance. J. Neurosci. 2011;31:14521–14530. doi: 10.1523/JNEUROSCI.2483-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson’s disease. Brain. 2000;123:1767–1783. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J. Neurosci. 2013;33:8321–8335. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple time scales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann. N. Y. Acad. Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- Parikh V, Man K, Decker MW, Sarter M. Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. J. Neurosci. 2008;28:3769–3780. doi: 10.1523/JNEUROSCI.5251-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Ji J, Decker MW, Sarter M. Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. J. Neurosci. 2010;30:3518–3530. doi: 10.1523/JNEUROSCI.5712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Howe WM, Welchko RM, Naughton SX, D’Amore DE, Han DH, et al. Diminished trka receptor signaling reveals cholinergic-attentional vulnerability of aging. Eur. J. Neurosci. 2012;37:278–293. doi: 10.1111/ejn.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson Study Group Randomized placebo-controlled study of the nicotinic agonist SIB-1508Y in Parkinson disease. Neurology. 2006;66:408–410. doi: 10.1212/01.wnl.0000196466.99381.5c. [DOI] [PubMed] [Google Scholar]
- Paul SS, Sherrington C, Canning CG, Fung VS, Close JC, Lord SR. The relative contribution of physical and cognitive fall risk factors in people with Parkinson’s disease: a large prospective cohort study. Neurorehabil. Neural Repair. 2013;28:282–290. doi: 10.1177/1545968313508470. [DOI] [PubMed] [Google Scholar]
- Persad CC, Jones JL, Ashton-Miller JA, Alexander NB, Giordani B. Executive function and gait in older adults with cognitive impairment. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:1350–1355. doi: 10.1093/gerona/63.12.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Beckley SC, Joh TH, Reis DJ. Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 1981;225:373–385. doi: 10.1016/0006-8993(81)90843-x. [DOI] [PubMed] [Google Scholar]
- Plotnik M, Giladi N, Dagan Y, Hausdorff JM. Postural instability and fall risk in Parkinson’s disease: impaired dual tasking, pacing, and bilateral coordination of gait during the “ON” medication state. Exp. Brain Res. 2011;210:529–538. doi: 10.1007/s00221-011-2551-0. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J. Exp. Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- Possin KL, Kang GA, Guo C, Fine EM, Trujillo AJ, Racine CA, et al. Rivastigmine is associated with restoration of left frontal brain activity in Parkinson’s disease. Mov. Disord. 2013;28:1384–1390. doi: 10.1002/mds.25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, O’Leary K, Tanner CM. Nicotine and Parkinson’s disease: implications for therapy. Mov. Disord. 2008;23:1641–1652. doi: 10.1002/mds.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep RL, Cheatwood JL, Corwin JV. The associative striatum: organization of cortical projections to the dorsocentral striatum in rats. J. Comp. Neurol. 2003;467:271–292. doi: 10.1002/cne.10868. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Baunez C, Everitt BJ, Robbins TW. Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behav. Neurosci. 2001;115:799–811. doi: 10.1037//0735-7044.115.4.799. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Fristoe NM, Lineweaver TT, Coon VE. Aging of attention: does the ability to divide decline? Mem. Cogn. 1995;23:59–71. doi: 10.3758/bf03210557. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Age-related changes in rodent cortical acetylcholine and cognition: main effects of age versus age as an intervening variable. Brain Res. Rev. 1998;27:143–156. doi: 10.1016/s0165-0173(98)00003-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Turchi J. Age- and dementia-associated impairments in divided attention: psychological constructs, animal models, and underlying neuronal mechanisms. Dement. Geriatr. Cogn. Disord. 2002;13:46–58. doi: 10.1159/000048633. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res. Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol. Learn. Mem. 2003;80:245–256. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res. Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. NAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem. Pharmacol. 2009;78:658–667. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Howe WM, Gritton H, Berry AS. Deterministic functions of cortical acetylcholine. Eur. J. NeuroSci. 2014 doi: 10.1111/ejn.12515. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011;221:555–563. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Van Velson M, Menzaghi F, Lloyd GK. Effects of the nicotinic acetylcholine receptor agonist SIB-1508Y on object retrieval performance in mptp-treated monkeys: comparison with levodopa treatment. Ann. Neurol. 1998;43:311–317. doi: 10.1002/ana.410430308. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Tinker JP, Van Velson M, Menzaghi F, Lloyd GK. Nicotinic acetylcholine receptor agonist SIB-1508Y improves cognitive functioning in chronic low-dose MPTP-treated monkeys. J. Pharmacol. Exp. Ther. 1999;290:731–739. [PubMed] [Google Scholar]
- Schneider JS, Tinker JP, Menzaghi F, Lloyd GK. The subtype-selective nicotinic acetylcholine receptor agonist SIB-1553A improves both attention and memory components of a spatial working memory task in chronic low dose 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated monkeys. J. Pharmacol. Exp. Ther. 2003;306:401–406. doi: 10.1124/jpet.103.051912. [DOI] [PubMed] [Google Scholar]
- Sethi K. Levodopa unresponsive symptoms in Parkinson disease. Mov. Disord. 2008;23:S521–S533. doi: 10.1002/mds.22049. [DOI] [PubMed] [Google Scholar]
- Shimada H, Hirano S, Shinotoh H, Aotsuka A, Sato K, Tanaka N, et al. Mapping of brain acetylcholinesterase alterations in lewy body disease by PET. Neurology. 2009;73:273–278. doi: 10.1212/WNL.0b013e3181ab2b58. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott M. Attentional demands and postural control: the effect of sensory context. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55:M10–M16. doi: 10.1093/gerona/55.1.m10. [DOI] [PubMed] [Google Scholar]
- Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov. Disord. 2006;21:950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- St Peters M, Demeter E, Lustig C, Bruno JP, Sarter M. Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J. Neurosci. 2011;31:9760–9771. doi: 10.1523/JNEUROSCI.1902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Ko JH, Grant J, Fraraccio M, Monchi O. Corticostriatal functional interactions in Parkinson’s disease: A rtms/[11C]raclopride PET study. Eur. J. Neurosci. 2005;22:2946–2952. doi: 10.1111/j.1460-9568.2005.04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theill N, Martin M, Schumacher V, Bridenbaugh SA, Kressig RW. Simultaneously measuring gait and cognitive performance in cognitively healthy and cognitively impaired older adults: the Basel motor-cognition dual-task paradigm. J. Am. Geriatr. Soc. 2011;59:1012–1018. doi: 10.1111/j.1532-5415.2011.03429.x. [DOI] [PubMed] [Google Scholar]
- Thiele A, Herrero JL, Distler C, Hoffmann KP. Contribution of cholinergic and gabaergic mechanisms to direction tuning, discriminability, response reliability, and neuronal rate correlations in macaque middle temporal area. J. Neurosci. 2012;32:16602–16615. doi: 10.1523/JNEUROSCI.0554-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Tombu M, Jolicæur P. A central capacity sharing model of dual-task performance. J. Exp. Psychol. Hum. Percept. Perform. 2003;29:3–18. doi: 10.1037//0096-1523.29.1.3. [DOI] [PubMed] [Google Scholar]
- Troiano AR, Schulzer M, de la Fuente-Fernandez R, Mak E, McKenzie J, Sossi V, et al. Dopamine transporter PET in normal aging: dopamine transporter decline and its possible role in preservation of motor function. Synapse. 2010;64:146–151. doi: 10.1002/syn.20708. [DOI] [PubMed] [Google Scholar]
- Turchi J, Sarter M. Cortical acetylcholine and processing capacity: effects of cortical cholinergic deafferentation on crossmodal divided attention in rats. Cogn. Brain Res. 1997;6:147–158. doi: 10.1016/s0926-6410(97)00027-x. [DOI] [PubMed] [Google Scholar]
- Uemura K, Yamada M, Nagai K, Ichihashi N. Older adults at high risk of falling need more time for anticipatory postural adjustment in the precrossing phase of obstacle negotiation. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:904–909. doi: 10.1093/gerona/glr081. [DOI] [PubMed] [Google Scholar]
- Uemura K, Yamada M, Nagai K, Tanaka B, Mori S. Impaired choice stepping in response to a visual-spatial attention demanding task among older adults at high risk of falling: a pilot study. Aging Clin. Exp. Res. 2012;24:361–364. doi: 10.1007/BF03325266. [DOI] [PubMed] [Google Scholar]
- van Schouwenburg R, O’Shea J, Mars B, Rushworth MFS, Cools R. Controlling human striatal cognitive function via the frontal cortex. J. Neurosci. 2012;32:5631–5637. doi: 10.1523/JNEUROSCI.6428-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall N, De La Parra M, Callaway E, Kreitzer A. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron. 2013;79:347–360. doi: 10.1016/j.neuron.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AY, Miura K, Uchida N. The dorsomedial striatum encodes net expected return, critical for energizing performance vigor. Nat. Neurosci. 2013;16:639–647. doi: 10.1038/nn.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse PJ, Hedreen JC, White CL, Price DL. Basal forebrain neurons in the dementia of Parkinson disease. Ann. Neurol. 1983;13:243–248. doi: 10.1002/ana.410130304. [DOI] [PubMed] [Google Scholar]
- Wilson NM, Hilmer SN, March LM, Cameron ID, Lord SR, Seibel MJ, et al. Associations between drug burden index and falls in older people in residential aged care. J. Am. Geriatr. Soc. 2011;59:875–880. doi: 10.1111/j.1532-5415.2011.03386.x. [DOI] [PubMed] [Google Scholar]
- Wolf D, Grothe M, Fischer FU, Heinsen H, Kilimann I, Teipel S, et al. Association of basal forebrain volumes and cognition in normal aging. Neuropsychologia. 2013;53C:54–63. doi: 10.1016/j.neuropsychologia.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Wood BH. Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. J. Neurol. Neurosurg. Psychiatry. 2002;72:721–725. doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005;128:2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson’s disease. Mov. Disord. 2011;26:2496–2503. doi: 10.1002/mds.23932. [DOI] [PubMed] [Google Scholar]
- Yin HH. Action, time and the basal ganglia. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20120473. doi: 10.1098/rstb.2012.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Giladi N, Gruendlinger L, Hausdorff JM. The contribution of postural control and bilateral coordination to the impact of dual tasking on gait. Exp. Brain Res. 2013;226:81–93. doi: 10.1007/s00221-013-3412-9. [DOI] [PubMed] [Google Scholar]
- Young WR, Hollands MA. Can telling older adults where to look reduce falls? Evidence for a causal link between inappropriate visual sampling and suboptimal stepping performance. Exp. Brain Res. 2010;204:103–113. doi: 10.1007/s00221-010-2300-9. [DOI] [PubMed] [Google Scholar]
- Yue F, Zeng S, Wu D, Yi D, Alex Zhang Y, Chan P. Age-related decline in motor behavior and striatal dopamine transporter in cynomolgus monkeys. J. Neural Transm. 2012;119:943–952. doi: 10.1007/s00702-012-0770-6. [DOI] [PubMed] [Google Scholar]
- Zaborszky L. The modular organization of brain systems. Basal forebrain: the last frontier. Prog. Brain Res. 2002;136:359–372. doi: 10.1016/s0079-6123(02)36030-8. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Buhl DL, Pobalashingham S, Bjaalie JG, Nadasdy Z. Three-dimensional chemoarchitecture of the basal forebrain: spatially specific association of cholinergic and calcium binding protein-containing neurons. Neuroscience. 2005;136:697–713. doi: 10.1016/j.neuroscience.2005.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage. 2008;42:1127–1141. doi: 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, van den Pol A, Gyengesi E. The basal forebrain cholinergic projection system in mice. The mouse nervous system. Elsevier; 2012. pp. 684–718. [Google Scholar]
- Zaborszky L, Csordas A, Mosca K, Kim J, Gielow R, Vadasz C, et al. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DA, Wonderlick JS, Ashourian P, Hansen LA, Young JC, Murphy AJ, et al. Substantia nigra volume loss before basal forebrain degeneration in early Parkinson disease. JAMA Neurol. 2013;70:241–247. doi: 10.1001/jamaneurol.2013.597. [DOI] [PMC free article] [PubMed] [Google Scholar]



