Abstract
Background
Non-invasive techniques to assess subclinical spread of non-melanoma skin cancer (NMSC) may improve surgical precision. High frequency ultrasound (HIFU) has shown promise to evaluate the extent of NMSC.
Objective
To determine the accuracy of HIFU to assess the margins of basal cell (BCC) and squamous cell carcinomas (SCC) prior to Mohs micrographic surgery (MMS).
Methods
We enrolled 100 patients with invasive SCC or BCC. Prior to the first stage of MMS, a Mohs surgeon delineated the intended surgical margin. Subsequently, a trained ultrasound technologist independently evaluated disease extent using the EPISCAN I-200 to evaluate tumor extent beyond this margin. The accuracy of HIFU was subsequently tested by comparison to pathology from frozen sections.
Results
The test characteristics of the ultrasound were sensitivity= 32%, specificity= 88%, positive predictive value= 47%, and negative predictive value=79%. Subgroup analyses demonstrated improved test characteristics for tumors larger than the median (area >1.74 cm2). Qualitative analyses showed that HIFU was less likely to identify extension from tumors with subtle areas of extension, such as small foci of dermal invasion from infiltrative SCC and micronodular BCC.
Conclusions
HIFU requires additional refinements to improve the preoperative determination of tumor extent prior to surgical treatment of NMSC.
Introduction
Collectively referred to as non-melanoma skin cancer (NMSC), basal cell cancer (BCC) and squamous cell cancer (SCC) affect over one million individuals in the United States every year.[1, 2] NMSC exhausts substantial health care expenditures,[3] impairs health-related quality of life,[4] and may lead to significant cosmetic and functional impairment.
With proper tumor selection and physician experience, both non-excisional and excisional treatment modalities can achieve high-cure rates for NMSC.[5, 6] Either standard excision or Mohs micrographic surgery is indicated when the risk of recurrence or metastases is high, due to large tumor size; location on the head and neck, genitalia, or hands and feet; history of prior treatment; or aggressive histology.[7] For any method of excision, the initial determination of an appropriate surgical margin starts with an accurate assessment of the clinical extent of the tumor. High rates of subclinical spread associated with BCC and SCC frequently make visualization and palpation unreliable to determine of tumor extent.[8, 9] Mohs micrographic surgery addresses this dilemma with complete histologic examination of the surgical cut edge, which allows detection of areas of subclinical tumor extension; and with a meticulous method of tumor mapping, which allows selective excision of subclinical tumor remnants. Despite its high cure rates and optimal tissue sparing, economic and practical matters preclude treatment of all high-risk NMSC with Mohs surgery.
More accurate preoperative assessment of tumor boundaries has important implications to improve care of patients with NMSC. Improved preoperative assessment may facilitate triaging of patients with high-risk NMSC to either standard excision or Mohs surgery. Health care costs may decline, as one might expect a decrease in the incidence of positive margins after standard excision and a decrease in the number of stages with Mohs surgery. Patients may benefit from improved cosmetic and functional outcomes by preventing excision of clinically equivocal areas that are shown preoperatively not to involve tumor.
High resolution ultrasound shows promise as a diagnostic test to improve preoperative delineation of the clinical margins of NMSC. In recent studies, high resolution ultrasound has been shown to determine reliably the depth of BCC.[10, 11] The clinical usefulness of ultrasound in predicting margins of NMSC has not been examined. The purpose of this study was to examine the accuracy of high resolution ultrasound to assess margins preoperatively in patients with NMSC undergoing Mohs surgery.
Methods
Study Design
The study was approved by the University of Pennsylvania Institutional Review Board. A cross sectional study was performed in 100 consecutive patients who met the inclusion/exclusion criteria and provided consent to study participation.
Study population and inclusion/exclusion criteria
Patients ages 18-95 with a biopsy proven invasive squamous cell or basal cell carcinoma were enrolled in this study. Patients were excluded if the tumor was confined to the epidermis (e.g. squamous cell carcinoma in situ and superficial basal cell carcinoma); located in an anatomic area that was difficult to image with the ultrasound probe (e.g. ear, nasal ala, or inner canthus); the surgeon was unable to visualize tumor on clinical examination; or had undergone surgery (e.g. electrodessication and curettage, surgical excision) with the intent to remove the tumor within the past six months.
Study procedures
A fellowship-trained Mohs surgeon (CDS or CJM) preoperatively used a gentian violet pen to delineate the clinical margin of the tumor. The surgeon then drew the proposed surgical margin for the first stage of Mohs surgery, which averaged 1-2 mm margin of clinically normal skin normal skin around the clinical margin of the tumor. The maximum length and width of the area contained within the surgical margin was recorded.
A recent medical school graduate (AJP), was trained over a period of 6 weeks (approximately 10 hours of training) in the application and interpretation of high-frequency ultrasound by staff from Longport, Inc. The ultrasound technologist used a high-frequency ultrasound machine (EPISCAN I-200, 20 MHz-50 MHz; Longport, Inc., Glenn Mills, PA) to examine the extent of the tumor. The ultrasound was set at a frequency of 40 mHz. In particular, the ultrasound was used to detect any areas of tumor extending peripherally beyond the proposed surgical margin for the first stage of Mohs surgery. The ultrasound procedure took approximately five minutes per patient. Uniform hypoechoic areas represented dermal tumor, whereas hyperechoic areas indicated normal dermal tissue. Any areas of tumor extending outside the proposed surgical margin were documented on a tumor map by the ultrasound technologist. The tumor map from the ultrasound examination was precisely oriented to match the surgeon's tumor map from the first stage of Mohs surgery.
The Mohs surgeon was blinded to the ultrasound results when he or she excised the first Mohs stage and interpreted the frozen-section hematoxylin and eosin slides. The Mohs surgeon independently indicated any areas of histologically residual tumor from the margin of the first stage on the Mohs tumor. The first stage Mohs map was then compared to the map from ultrasonographic evaluation. For any discrepancies between the two maps, histopathology from the Mohs margin served as the gold standard.
Data Analysis
The sample size of 100 patients was determined based on predicted correct classification of 80%. This study was powered to estimate a correct classification with a precision of ± 8 based on the 95% confidence interval. Power and sample size was determined using STATA v9.0 (College Station, TX).
If tumor extension was seen on both ultrasound and histology, the Mohs surgeon compared the Mohs and ultrasound diagrams to determine if the location of tumor extension correlated precisely. A case was considered to be a true positive if both ultrasound and histology were positive in the same location. A case was considered to be a false positive if tumor was detected on ultrasound but not on histopathologic evaluation of the Mohs margins. A case was considered to be a false negative if it was negative on ultrasound but positive histologically or if it was positive on ultrasound but not in the same location as the histologically positive area. A case was counted as a true negative when both the ultrasound and histology were negative. Test characteristics were calculated using the following definitions and formulas:
| Term | Definition | Mathematical Equation |
|---|---|---|
| Sensitivity | Probability of margins being positive on ultrasound if margins are truly positive histologically | True Positives/(True Positives+ False Negatives) |
| Specificity | Probability of margins being negative on ultrasound if margins are truly negative histologically | True Negatives/(True Negatives + False Positives) |
| Positive Predictive Value | Probability of having positive margins on histology given that the ultrasound was positive | True Positives/(True Positives+ False Positives) |
| Negative Predictive Value | Probability of having negative margins on histology given that the ultrasound was negative | True Negatives/(True Negatives + False Negatives) |
| Correct Classification | Probability of having positive margins on ultrasound and histology or negative margins on ultrasound and histology | (True Positives + True Negatives)/Total Number Pts |
Results
Of the 112 patients that were eligible to participate in the study, 100 patients consented to study participation, resulting in an 89% participation rate. If a patient presented with more than one tumor, the Mohs surgeon selected the largest tumor as the index case, which was imaged in this study. Thus, only one tumor per patient underwent ultrasonographic margin evaluation. Figure 1 illustrates an example of a tumor photograph taken by ultrasound. In two cases, the Mohs surgeon elected to take a second layer due to the presence of histological tumor remnants located outside the dyed edges of the Mohs tissue section. Because these histologic tumor remnants were suspected to represent a false positive margin due to artifact from tissue processing (i.e. “floaters”), these cases were eliminated from the study. Consequently, 98 cases were left for analysis.
Figure 1.
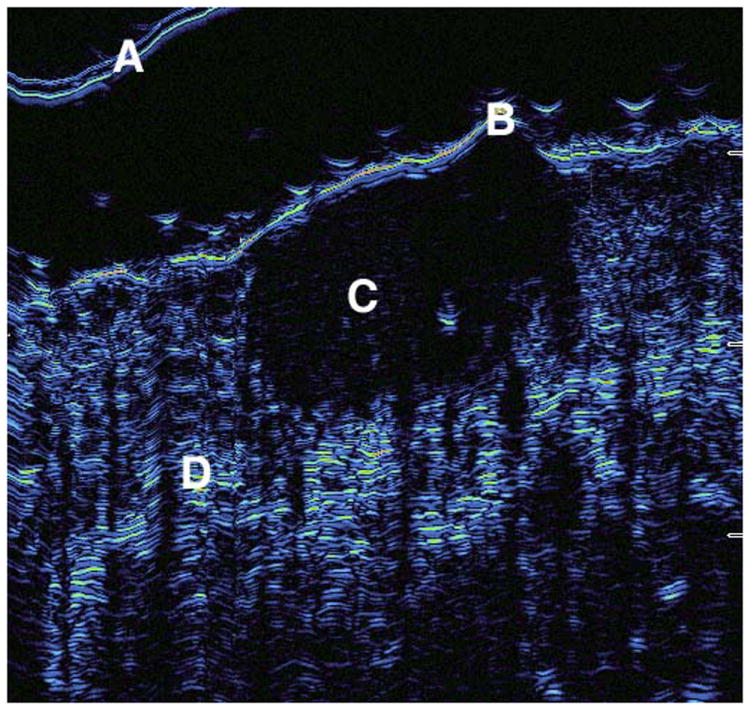
Seen here is a sample of a two-dimensional scan taken by the ultrasound demonstrating tumor findings. A=probe cover, B= epidermis, C= dermal tumor, D= normal dermis.
Table 1 summarizes patient and tumor characteristics for the included cases. Seventy-nine percent (n=77) of tumors were basal cell carcinomas, and 21% (n=21) were squamous cell carcinomas. We are unable to report the histologic subtype of the BCC or SCC, as this is not routinely reported on pathology reports. The majority were comprised of primary tumors (91%) located on the head and neck (82%). The mean and median surface areas of the tumors calculated from length and width of the surface area inside the Mohs surgical margin were 2.6 cm2 and 1.74 cm2 respectively, with an interquartile range of 1.17 cm2 – 3.45 cm2. Twenty-six percent (n=25) of cases were positive histologically. Five of these cases were positive in the subcutaneous fat and nine were positive in the epidermis only. Ten were positive in the dermis, and one was positive in both the dermis and subcutaneous fat.
Table 1.
Description of Patients and Tumor Characteristics
| Number | Mean, Median (25th and 75th percentile) | ||
|---|---|---|---|
| Study Patients | 98 | ||
| Age | 63.27, 64 (54, 73) | ||
| Gender | Male | 72 (73%) | |
| Female | 26 (27%) | ||
| Location | Scalp, Forehead, Temple, Eyebrow | 30 (30.6%) | |
| Nose | 15 (15.3%) | ||
| Ear | 3 (3.1%) | ||
| Lip | 6 (6.1%) | ||
| Cheek | 19 (19.4%) | ||
| Chin/Neck | 7 (7.1%) | ||
| Trunk, Upper Ext, Lower Ext | 18 (18.4%) | ||
| Histology | BCC | 77 (79%) | |
| SCC | 21 (21%) | ||
| Primary vs. Recurrent | Primary | 89 (91%) | |
| Recurrent | 9 (9%) | ||
| Clinical Pre-op Measurements | length (cm) | 1.6, 1.5 (1.1, 2) | |
| width (cm) | 1.4, 1.2 (1, 1.7) | ||
| area (cm2) | 2.6, 1.74 (1.17, 3.45) | ||
| Stages Required to Clear | 1 | 73 (74.5%) | |
| 2 | 23 (23.5%) | ||
| 3 | 2 (2%) |
Patients were classified based on interpretation of ultrasound and histology (Table 2). The overall test characteristics comparing the ultrasonographic margin evaluation compared to histopathology are as follows: sensitivity=32% (95% CI 15-54), specificity = 88% (95% CI 78-94), positive predictive value (PPV) = 47, negative predictive value (NPV) = 79, correct classification (CC) = 0.73 (Table 3). In this study, the prevalence of positive histologic margins was 0.26.
Table 2. How cases were classified.
| Positive Margins on US | Negative Margins on US | |
|---|---|---|
| Positive Margins on frozen sections | 8 (True Positives) | 17 (False Negatives) |
| Negative Margins on frozen sections | 9 (False Positives) | 64 (True Negatives) |
Table 3. Test Characteristics.
| Number | Sensitivity (95% Confidence Interval) | Specificity (95% Confidence Interval) | Positive Predictive Value | Negative Predictive Value | Likelihood ratio | Correct Classification | |||
|---|---|---|---|---|---|---|---|---|---|
| OVERALL | N=98 | 32 (15-54) | 88 (78-94) | 47 | 79 | .73 | |||
| EXCLUDING CASES POSITIVE IN EPIDERMIS OR DEEP MARGIN ONLY | N=85 | 55 (23-83) | 88 (78-94) | 40 | 75 | 4.42 | 0.83 | ||
| Area | Area ≤ 1.74 cm2 | N=41 | 33 (4-78) | 81 (77-98) | 40 | 89 | 3.89 | 0.83 | |
| Area >1.74 cm2 | N=43 | 80 (28-99) | 84 (69-94) | 40 | 97 | 5.07 | 0.84 | ||
In a subgroup analyses, there were no significant differences in the performance of the ultrasound by tumor histology, patient age, gender, or location. Additionally, there was no improvement in sensitivity over the course of the study (data not shown). The ultrasound was more sensitive for larger (area above the median of 1.74 cm2) versus smaller tumors (sensitivity = 55%).
As a qualitative analysis, Mohs slides of the true positive and false negative cases were reviewed to determine if differences in tumor characteristics existed between cases where ultrasound correctly identified tumor (true positives) versus cases where ultrasound failed to identify tumor (false negatives). In cases where the ultrasound correctly identified histologic tumor extension (true positives), the areas of extension were usually in the superficial papillary dermis and were fairly pronounced. Figure 2 represents a Mohs slide and correlated ultrasound image of a true positive case. Of the 17 cases where ultrasound failed to identify histologic tumor extension (false negatives), 11 cases had tumor extension in the epidermis or fat, 5 cases had infiltrative or morpheaform BCC histology (Figure 3), and 1 case was a nodular BCC with extension in the dermis.
Figure 2.
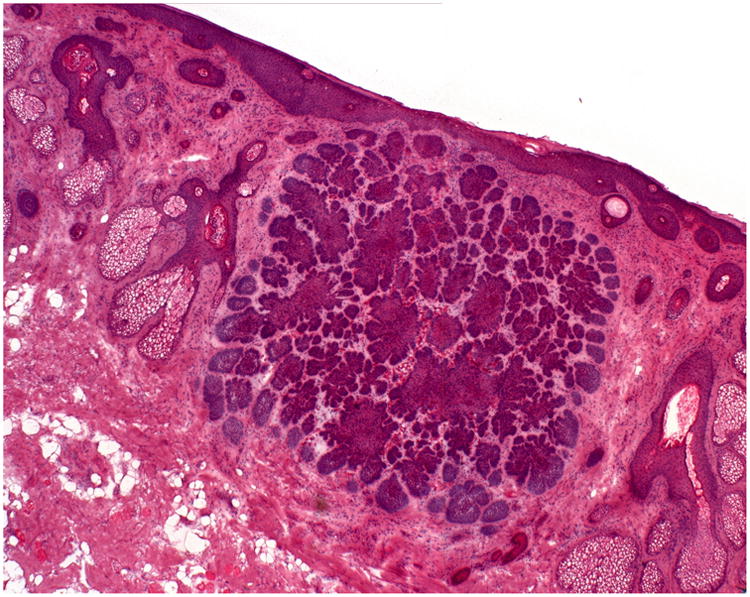
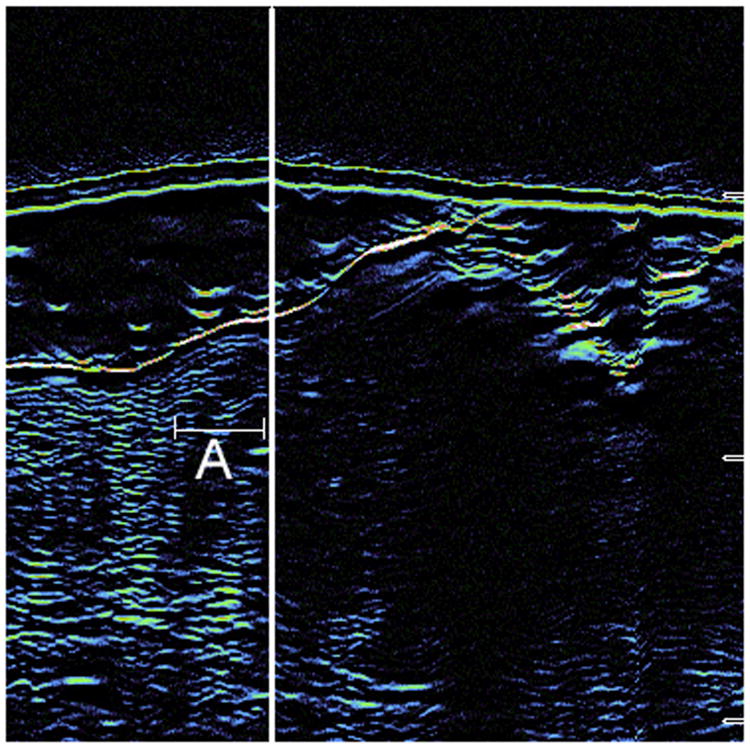
A: Hematoxylin & Eosin staining from Stage I Mohs surgery slides demonstrate a large nodular tumor infiltrating the dermis. The ultrasound was able to detect this area of extension (true positive).
B: Seen here is the correlated ultrasound image from the photographed 2A case. The thick white line represents the Mohs surgeon's delineated surgical margin. The area to the left of the demarcated line (around the letter A) is the area of true positivity.
Figure 3.
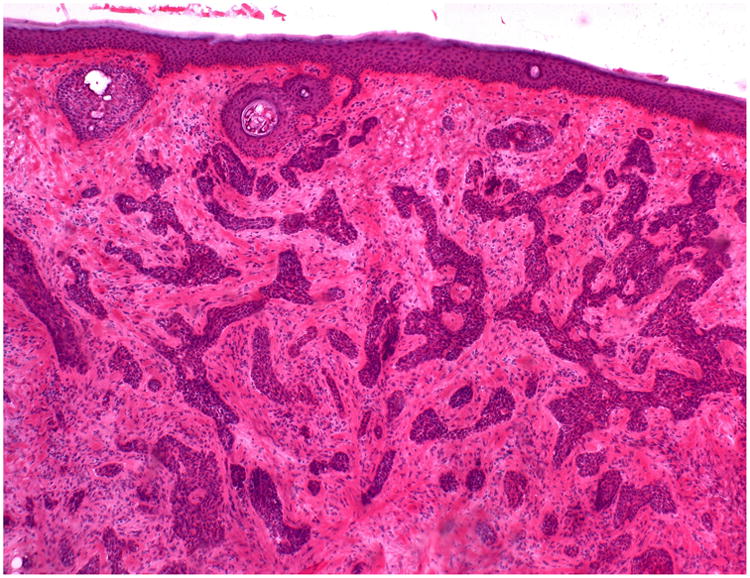
Hematoxylin & Eosin staining from Stage I Mohs surgery slides demonstrate dense strands of infiltrative basal cell carcinoma.
Discussion
The test characteristics of the high resolution ultrasound determined in this study were not found to be clinically useful. The low overall sensitivity (32%) resulted in part from the optical limitations of the device used in this study and limitations of the study design. The ultrasound is optimized to image the dermis with greater resolution compared to the epidermis and thus was expected to provide less accurate imaging of the epidermis. Although the ultrasound has potential to visualize tumor in the subcutaneous tissue (deep margin), this study was designed to only compare peripheral margins of the ultrasound with histopathology. We chose to not assess the deep margins of the tumor with ultrasound since the ultrasound technician would not be able to know how deep the surgeon would excise the tumor, and thus would not be able to determine whether or not there was tumor outside the surgeon's first stage surgical margin. In the qualitative analysis, 65% (11/17) of false negative cases were positive in had histological tumor remnants in either the epidermis or fat. Excluding these tumors with subclinical extension in the epidermis or subcutaneous fat, sensitivity improved from 32 to 56%, a number still too low for clinical application.
When analysis was limited to larger tumors (those with clinical areas greater than the median of 1.74 cm2) with histologically positive margins only in the dermis (n=44), sensitivity of ultrasound was 80% (95% CI 28-99) and specificity 84% (95% CI 69-94) (Table 3). The wide confidence interval around the sensitivity analysis limits its interpretation. However, it appears that ultrasound has the potential to predict margins of larger tumors with subclinical dermal involvement with reasonable accuracy. This finding suggests that ultrasound may be successful in predicting margins of all NMSC as the technology improves.
The characteristics of our study population should be generalizable to populations seen in most Mohs surgery clinics in the United States. The three to one ratio of BCC to SCC in our study is similar to other reports on the epidemiology of non-melanoma skin cancer.[1] Comparable to previous reports, 26% of cases in our study required two or more stages for tumor clearance.[12] Spectrum bias is unlikely, as we enrolled 100 consecutive patients who met our inclusion criteria without regard to disease severity. Verification bias is also unlikely, as we compared ultrasound directly to the gold standard (histopathology) in 98% of cases.
Failure of high resolution ultrasound to detect subclinical tumor margins may have resulted for several potential reasons. First, operator error and incorrect ultrasound interpretation may have prevented accurate assessment of tumor extension. This scenario is unlikely, as test characteristics did not improve during the later stages of the study, when increased experience of the ultrasound technologist could arguably have led to improved ultrasound skills. Second, scar tissue, severe solar elastosis, and inflammation, features very common around tumor sites, produce ultrasound images that are difficult to distinguish from tumor (Figure 4). Additionally, the machine as utilized in this study lacked the resolution necessary to accurately visualize tumor in the epidermis and the study did not assess ultrasound performance in the subcutaneous tissue. In our study, 15 of the 98 included cases (15.3%) had extension in either the epidermis or subcutaneous fat, which the ultrasound was ill-suited to detect. Finally, the ultrasound device failed to detect subclinical tumor in the dermis for six cases. Among these six cases, five tumors exhibited basal cell cancer with infiltrative or morpheaform histology in the dermis. As these represent histological subtypes for which ultrasound would have great potential utility to examine subclinical extent, it was disappointing that the device did not image small tumor extensions with reliability.
Figure 4.
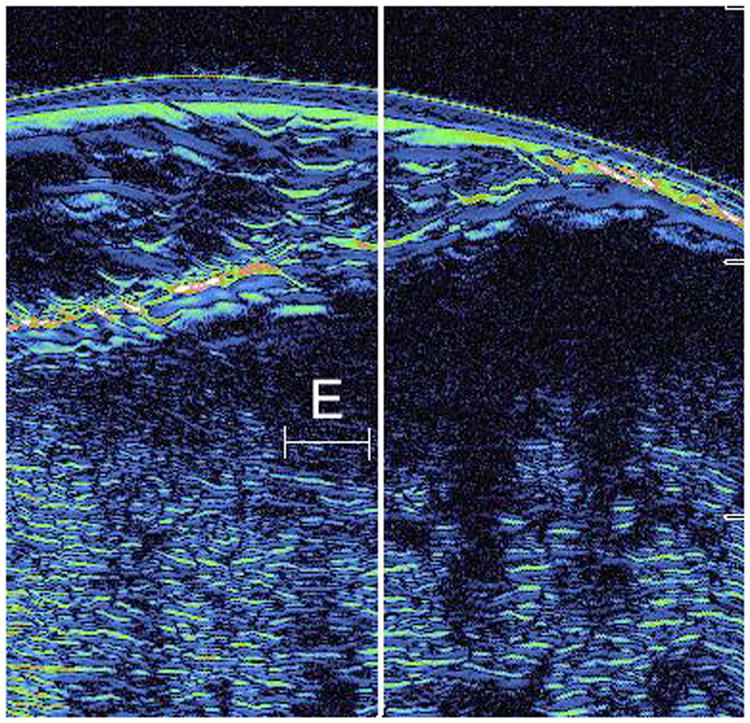
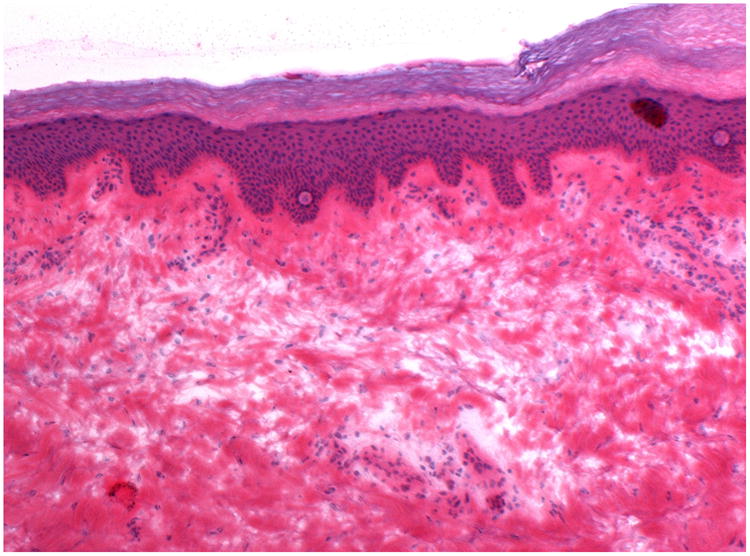
A: This figure is an example of the ultrasound image of a false positive case. The area surrounding letter E represents the area judged to be positive by the ultrasound for subclinical extension, however, no evidence of tumor in this area was observed on histology (false positive). This case was cleared in 1 stage of Mohs surgery.
B: This is the correlated histopathology of the area photographed in Figure 4A. There is significant solar elastosis in the dermis, which may have confounded the ultrasound image.
Conclusions
Non-invasive techniques to improve preoperative assessment of tumor extent have potential to increase the efficiency of surgical therapy and decrease costs associated with NMSC. In our study population, high-frequency ultrasound failed to detect subclinical tumor extensions with accuracy. The utility of high frequency ultrasound will depend on technological improvements that allow high-resolution images of the epidermis, subcutaneous fat, and small tumor strands in the dermis. Higher resolution images must also allow one to distinguish dermal tumor from scar, severe solar elastosis, and inflammation, which frequently accompany NMSC.
Acknowledgments
Funding Support: Dr. Schmults, Dr. Gelfand, and Jennifer Williams received partial salary support from Longport, Inc for a clinical trial conducted using their ultrasound machine.
All Other Relationships: None
Footnotes
Financial Disclosures: This financial relationship is not related to this manuscript. All of the other authors have no other financial disclosures.
References
- 1.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30(5 Pt 1):774–8. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 2.Detailed Guide: Skin Cancer - Basal and Squamous Cell What Are The Key Statistics About Squamous and Basal Cell Skin Cancer? [cited 2007 August 6];2007 http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_statistics_for_skin_cancer_51.asp?sitearea=
- 3.John Chen G, et al. Treatment patterns and cost of nonmelanoma skin cancer management. Dermatol Surg. 2006;32(10):1266–71. doi: 10.1111/j.1524-4725.2006.32288.x. [DOI] [PubMed] [Google Scholar]
- 4.Chren MM, et al. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351–7. doi: 10.1038/sj.jid.5700740. [DOI] [PubMed] [Google Scholar]
- 5.Rowe DE, Carroll RJ, Day CL., Jr Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976–90. doi: 10.1016/0190-9622(92)70144-5. [DOI] [PubMed] [Google Scholar]
- 6.Kopf AW, et al. Curettage-electrodesiccation treatment of basal cell carcinomas. Arch Dermatol. 1977;113(4):439–43. [PubMed] [Google Scholar]
- 7.Miller S. [cited 2007 August 6];NCCN Clinical Practice Guidelines in Oncology: Basal Cell and Squamous Cell Carcinoma. 2007 www.nccn.org.
- 8.Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123(3):340–4. [PubMed] [Google Scholar]
- 9.Leibovitch I, et al. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I. Experience over 10 years. J Am Acad Dermatol. 2005;53(2):253–60. doi: 10.1016/j.jaad.2005.02.059. [DOI] [PubMed] [Google Scholar]
- 10.Moore JV, Allan E. Pulsed ultrasound measurements of depth and regression of basal cell carcinomas after photodynamic therapy: relationship to probability of 1-year local control. Br J Dermatol. 2003;149(5):1035–40. doi: 10.1111/j.1365-2133.2003.05558.x. [DOI] [PubMed] [Google Scholar]
- 11.Gupta AK, et al. High frequency 40-MHz ultrasound. A possible noninvasive method for the assessment of the boundary of basal cell carcinomas. Dermatol Surg. 1996;22(2):131–6. doi: 10.1111/j.1524-4725.1996.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 12.Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5 Pt 1):698–703. doi: 10.1016/s0190-9622(98)70041-6. [DOI] [PubMed] [Google Scholar]